Abstract
Sepsis—leading to septic shock—is the leading cause of death in intensive care units. The systemic inflammatory response to infection, which is initiated by activated myeloid cells, plays a key role in the lethal outcome. Macrophage migration inhibitory factor (MIF) is an upstream immunoregulatory mediator, released by myeloid cells, that underlies a common genetic susceptibility to different infections and septic shock. Accordingly, strategies that are aimed at inhibiting the action of MIF have therapeutic potential. Here, we report the isolation and characterization of tailorable, small, affinity-matured nanobodies (Nbs; single-domain antigen-binding fragments derived from camelid heavy-chain Abs) directed against MIF. Of importance, these bioengineered Nbs bind both human and mouse MIFs with nanomolar affinity. NbE5 and NbE10 inhibit key MIF functions that can exacerbate septic shock, such as the tautomerase activity of MIF (by blocking catalytic pocket residues that are critical for MIF’s conformation and receptor binding), the TNF-inducing potential, and the ability of MIF to antagonize glucocorticoid action. A lead NbE10, tailored to be a multivalent, half-life extended construct (NbE10-NbAlb8-NbE10), attenuated lethality in murine endotoxemia when administered via single injection, either prophylactically or therapeutically. Hence, Nbs, with their structural and pharmacologic advantages over currently available inhibitors, may be an effective, novel approach to interfere with the action of MIF in septic shock and other conditions of inflammatory end-organ damage.—Sparkes, A., De Baetselier, P., Brys, L., Cabrito, I., Sterckx, Y. G.-J., Schoonooghe, S., Muyldermans, S., Raes, G., Bucala, R., Vanlandschoot, P., Van Ginderachter, J. A., Stijlemans, B. Novel half-life extended anti-MIF nanobodies protect against endotoxic shock.
Keywords: Nbs, MIF antagonist, LPS
Cytokines play a critical role in the host response to infection as they both initiate and promote the inflammatory response and coordinate the ensuing cellular and humoral response against the invasive organism; however, excessive cytokine production can culminate in major pathologies for the host and lead to a variety of acute and chronic diseases. Consequently, the identification and neutralization of these regulators of inflammation is considered essential to alleviate end-organ damage (1–3). Multiple clinical studies have supported the role of macrophage migration inhibitory factor (MIF) as a ubiquitously expressed, pleiotropic, immunoregulatory, proinflammatory cytokine that controls numerous processes that are essential for innate and adaptive immunity (4). MIF exists structurally as a homotrimer, and most of its biologic activities require a receptor-based mechanism of action. CD74—the primary transmembrane surface receptor for MIF—is the cell surface–expressed form of the class II invariant chain, but is present on numerous cell types independently of major histocompatibility complex class II expression (5). MIF also exhibits chemokine-like functions and acts as a major regulator of inflammatory cell recruitment via its interaction with CXCR2, CXCR4, and CXCR7 (6–8) In addition, MIF exhibits tautomerase activity, whereby structural features that are imparted by the catalytic proline 1 residue are essential for receptor binding and subsequent signal transduction (9, 10).
MIF is also a biomarker for several diseases with immune involvement, including, but not limited to, arthritis, atherosclerosis, cancer, and endotoxic/septic shock (4, 11–15). With regard to the latter, severe sepsis and septic shock occur with high incidence in emergency and intensive care units (16, 17). Despite the evolution of surgical techniques, extensive methods of supportive care, and a vast array of antimicrobials that are the cornerstones of sepsis therapy, mortality in patients with severe sepsis remains high (18–20). In addition, to date, treatments that are aimed at reducing inflammatory sequelae have not shown benefit despite promising results in preclinical studies. Hence, there is an urgent need for more efficient intervention strategies to prevent septic shock–mediated lethality. Sepsis and septic shock typically result in a widespread activation of the innate immune system and a dysfunction of subsequent immune responses. MIF is believed to play a key regulatory role in this process as a result of the fact that 1) it has a widespread distribution in tissues and is stored preformed in immune cells, barrier epithelium, and stromal cells (21); 2) in response to LPS and stress, MIF is released and promotes macrophage survival, increases TLR4 expression (22, 23), and enhances the secretion of downstream cytokines, such as TNF (i.e., MIF-deficient mice produce lower amounts of TNF in response to LPS stimulation) (4, 24, 25); 3) studies in animal models indicate that the administration of MIF after LPS stimulation enhances mortality, which may be a result, in part, of propagating organ dysfunction (15, 26); 4) serum MIF concentrations are elevated in animals and patients with severe sepsis/endotoxic shock and correlate with disease severity and outcome (27, 28) and play an important role in the development of glucocorticoid resistance (29, 30); and 5) high expression MIF alleles, which occur commonly in the population, have been linked to lethality from bacterial meningitis (31, 32).
Given its involvement in mechanisms that underlie sepsis and septic shock, MIF represents an attractive therapeutic target for the treatment of these pathologies (9, 29, 33). Small-molecule inhibitors, such as ISO-1 [(S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester], and mAbs against MIF or DNA-based MIF vaccination strategies have been shown to attenuate inflammation in animal models of sepsis, which suggests that this approach may also be useful for the treatment of sepsis in humans (34–37). Yet these approaches require either repetitive dosing because of the rapid clearance of the molecules or large amounts of humanized Abs, which could generate undesirable local and systemic side reactions (38); therefore, we investigated the use of nanobodies (Nbs), which are single-domain antigen-binding fragments (∼15 kDa) derived from camelid heavy-chain Abs, as a new approach to block MIF-mediated biologic effects (39). Nbs have several advantageous biologic and physiochemical properties, including low immunogenicity, high solubility, stability across temperature and pH ranges, enhanced tissue penetration because of their small size, the ability to recognize cryptic antigenic sites, and easy tailorability, that make them candidates for the development of biologics with improved potencies and/or multiple valencies and/or specificities (40–42). Moreover, Nbs have already shown potential in the treatment of several types of pathologies, including envenoming, amyloidosis, chronic colitis, viral infections (respiratory syncytial virus), and tumor development (43–45).
In this study, we describe the generation and characterization of anti-MIF Nbs that exhibit cross-reactivity toward mouse and human MIFs, and that are capable of inhibiting MIF-elicited TNF production by monocytes and macrophages after LPS stimulation and of blocking the tautomerase activity of MIF, which is mediated by a region of the protein that is essential for receptor activation. We further designed half-life extended, multivalent constructs of the lead inhibitory candidate (NbE10) that, in an in vivo murine model, mitigated lethal endotoxemia. Collectively, our results demonstrate that Nbs that inhibit the activities of MIF represent a novel approach to reduce endotoxic shock–mediated lethality.
MATERIALS AND METHODS
Immunization, library construction, and screening
Immunization, library construction, and screening were performed by Ablynx (Ghent, Zwijnaarde, Belgium) and carried out as previously described (46). In brief, Nb libraries were generated by using peripheral blood lymphocytes isolated from 3 individual llamas that were immunized 6 consecutive times with recombinant human MIF2–115 (rhMIF; R&D Systems, Minneapolis, MN, USA). Three individual Nb phage libraries were constructed after total RNA extraction and cDNA subcloning into the phagemid vector pAX50. The libraries, which consisted of 3.3–9.5 × 108 transformants (96–100% insert), were subjected to 2 rounds—R1 and R2—of phage display panning using rhMIF. The crude periplasmic extract of individual colonies from R2 was subsequently screened on rhMIF (0.05, 0.5, or 5 μg/ml) and detected by using anti–c-Myc IgG and anti-mouse horseradish peroxidase (Sigma-Aldrich, St. Louis, MO, USA). Selected anti-MIF Nbs were recloned in the pAX100 expression vector and transformed in Escherichia coli WK6 for additional purification or stored at −80°C in Luria-Bertani medium that was supplemented with 50% glycerol.
Expression and purification of anti-MIF Nbs
Expression and purification of Nbs was performed as previously described (47), with the exception that kanamycin (Duchefa Biochemie, Haarlem, The Netherlands) was used instead of ampicillin. In brief, 1 ml of the overnight culture was inoculated in 330 ml Terrific Broth medium that was supplemented with 70 μg/ml kanamycin and 0.1% glucose and subsequently grown at 37°C in shaking flasks (200 rpm). Cultures were grown until OD600nm reached 0.6–0.8, induced by adding 1 mM isopropyl-β-d-thiogalactopyranoside, and additionally incubated at 28°C overnight (200 rpm). Periplasmic extract was obtained by harvesting cells via centrifugation (11,325 g, 8 min, 14°C), resuspension in 4 ml of TES (0.5 mM EDTA, 0.2 M Tris-HCL, 0.5 M sucrose), and incubation on ice for 6 h while shaking at 200 rpm. Eight milliliters of TES/4 per pellet were then added, and after incubation at 4°C overnight (200 rpm), the suspension was centrifuged (30 min, 11,325 g, 4°C) to collect periplasmic extract. Nbs were purified from these extracts by using immobilized metal affinity chromatography on a His-Trap column (GE Healthcare, Waukesha, WI, USA) and elution with 0.5 M imidazole (Sigma-Aldrich) in PBS (pH 7.5). Eluate was additionally purified on a Superdex 75 (10/300) or (16/600) gel filtration column (GE Healthcare) that was equilibrated with PBS by using an Akta Explorer 10S or AktaXpress (GE Healthcare). The purity of all anti-MIF Nbs was evaluated via 12% Bis/Tris gel analysis, and the protein concentration was determined by using Nanodrop (Isogen, Hackensack, NJ, USA). All Nbs were treated with ProSep-Remtox (Immunosource, Schilde, Belgium) and confirmed to be LPS free by using the Limulus Amebocyte Lysate Kinetic-QCL Kit (Cambrex, East Rutherford, NJ, USA) in accordance with the manufacturer’s instructions. Nbs were either used immediately or stored at −20°C.
Generation of multivalent anti-MIF Nb constructs
Multivalent constructs of NbE10 were generated and cloned in the pAX100 vector by Ablynx. The different constructs consisted of bivalent (E10–E10) or serum half-life extended format Nbs by the addition of a crossreactive mouse/human anti-serum albumin Nb (E10-Alb8, E10-E10-Alb8, E10-Alb8-E10). Expression and purification of Nbs was performed as previously described.
ELISA assays
Cytokine ELISA for human and mouse TNF (R&D Systems) on culture supernatants and serum was performed as described by the supplier.
Surface plasmon resonance
Surface plasmon resonance (SPR) experiments were performed on a BIAcore T200 system (GE Healthcare) with rhMIF or recombinant murine MIF (rmMIF; R&D systems) immobilized on a CM5 chip (GE Healthcare). In brief, the carboxylated dextran matrix was activated by an injection of 0.2 M N-ethyl-N′-(3-diethylaminopropyl) carbodiimide and 0.05 M N-hydroxysuccinimide at a flow rate of 5 μl/min for 7 min. An rmMIF solution of 5 μg/ml that was prepared in coupling buffer (50 mM sodium acetate, pH 4.0) was subsequently injected until 200 RU was immobilized. Next, surface immobilization was blocked by an injection of 1 M ethanolamine hydrochloride for 7 min. As a reference, the surface in another flow cell was used and treated only with N-ethyl-N′-(3-diethylaminopropyl) carbodiimide, N-hydroxysuccinimide, and ethanolamine. Data for the rmMIF–Nb interaction were collected in the format of a kinetic titration (48). Sensorgrams for the anti-mMIF Nb constructs were collected at 5 different concentrations (1:2 serial dilution starting from 100, 150, 200, or 750 nM) plus a 0 concentration [injection of running buffer; i.e., HBS (10 mM HEPES, pH 7.5, 150 mM NaCl, 3.5 mM EDTA, 0.005% v/v Tween-20)] at a flow rate of 30 μl/min and a data collection rate of 10 Hz. Analyte injections were performed with association phases of 180 s and a dissociation phase of 600 s. This was followed by 5 pulses of 15 μl regeneration buffer (HBS + 100 mM NaOH). Before data analysis, reference and 0 concentration data were subtracted from the sensorgrams. Data were analyzed with a 1:1 Langmuir binding model (48). Data for the rhMIF–Nb interaction were collected in a multicycle experiment as described in Caljon et al. (49) and analyzed by using a 1:1 Langmuir binding model.
Differential scanning fluorimetry (ThermoFluor)
Differential scanning fluorimetry experiments were carried out on a real-time PCR machine (Bio-Rad, Hercules, CA, USA) in a 96-well plate format (final volume in each well, 25 μl). For the buffer blank, 7.5 μl Sypro Orange Dye (Thermo Fisher Scientific, Waltham, MA, USA) was mixed with 17.5 μl PBS. For Nb samples, 7.5 μl Sypro Orange Dye was mixed with 5 μl of the Nb of interest at a stock concentration 2.5 mg/ml (final concentration, 0.5 mg/ml) and 12.5 μl PBS. Data for all samples were collected in triplicate. To obtain buffer-corrected fluorescence signals for each Nb, the averaged buffer data set was subtracted from the averaged protein data set. The buffer-corrected fluorescence signal (F) was plotted as a function of temperature (T) and fitted with the Boltzmann sigmoidal function to obtain the melting temperature (Tm) using the equation: F = F0 + (Fmax − F0)/{1 + e[(Tm−T)/a)]}. F0 and Fmax are the lowest (pretransitional) and the highest (post-transitional) buffer-corrected fluorescence signals (expressed in arbitrary units) and a is the change in T corresponding to the most significant change in F (also called the slope of the transition region, expressed in arbitrary units/°C).
Epitope mapping
To determine the region within the MIF protein where the anti-MIF Nbs bind, we used an approach similar to that previously described (35). In brief, on the basis of the amino acid sequence of human MIF taken from Swiss-Prot (P14174), synthetic overlapping peptides were designed whereby the N-terminal amino acid (Met) was omitted, as it is cleaved during the processing of the MIF molecule. Peptide sequences were as described previously (35); however, we included a biotin at the N-terminal region of each peptide. Peptides were ordered from Peptide 2.0 (http://peptide2.com) and the BLItz system (Pall Life Sciences, Port Washington, NY, USA) was used to determine the binding potential of the different anti-MIF Nbs. Each peptide (2.5 µM) was coupled to a streptavidin sensor. A single concentration of Nb (0.67 µM) was subsequently added, and binding was observed for 120 s. Washing and regeneration was performed as recommended by the suppliers. For each peptide, a blank run that consisted of PBS only was used to obtain a baseline. The binding signal obtained after 120 s is expressed in nanometers and was calculated as follows: signalNb − signalPBS.
Tautomerase assay
MIF tautomerase assay was performed as previously described (50). In brief, the enzymatic reaction was initiated at 25°C by adding 20 μl of dopachrome methyl ester substrate (2 mM L-3,4-dihydroxyphenylalanine methyl ester and 4 mM sodium periodate) in a 96-well plate that contained 200 μl of either rmMIF (83.3 nM) or rmMIF preincubated for 1 h at 37°C with Nb (0.67 µM) in tautomerase assay buffer (50 mM potassium phosphate, 1 mM EDTA, pH 6.0) or with 10 µM ISO-1 (Merck, Darmstadt, Germany). Activity was determined by the semicontinuous reduction in signal that was measured at OD475nm for 5 min. ELISA plates were read by using an ELX808 Absorbance Microplate Reader (BioTek Instruments, Winooski, VT, USA) and Gen5 1.08 software (BioTek Instruments).
In vitro inhibition assay
Experiments were performed by using either the human monocytic cell line, THP-1 [American Type Culture Collection (ATCC), Manassas, VA, USA], the murine macrophage cell line, RAW264.7 (ATCC), human peripheral blood monocytic cells (PBMCs), or ex vivo peritoneal exudates cells (PECs). Human PBMCs were isolated from buffy coats obtained from health donors (provided by the Belgian Red Cross, Flanders, Mechelen, Belgium) by using Lymphoprep (Axis Shield PoC As, Oslo, Norway) gradient centrifugation. Erythrocyte lysis was performed by using ACK-lysis buffer (Thermo Fisher Scientific) and blocked with 1× HBSS/5% fetal calf serum (FCS). The remaining PBMCs were washed 3 times with HBSS (Thermo Fisher Scientific Life Sciences), resuspended in ME medium (RPMI 1640, 10% FCS, 300 µg/ml l-glutamine, 100 µg/ml penicillin, 100 µg/ml streptomycin, 0.02 mM 2-ME, 1 mM nonessential amino acids, 1 mM sodium pyruvate), counted, and brought at a concentration of 2 × 106 cells/ml (stock solution), followed by 3-h adherence in a Petri dish. Nonadherent cells were then removed and adherent cells were washed with RPMI before adding 3 ml of TrypLE Express Enzyme (1×) and phenol red (Thermo Fischer Scientific) to detach cells. RPMI/5%FCS was subsequently added to neutralize the buffer and cells were pelleted by using an Eppendorf Centrifuge 5810R (Eppendorf, Belgium) at 394 g for 7 min at 4°C. Finally, cells were resuspended in ME medium, counted, and brought at a concentration of 5 × 105/ml (stock solution). PECs were obtained from CO2-euthanized 7- to 8-wk-old female C57BL/6 mice (Janvier, Le Genest-Saint-Isle, France) or MIF-deficient (Mif−/−) C57Bl/6 mice [generated as previously described (51) and bred in our animal facility] in accordance to ethical commission regulations. Cells were harvested by using 10 ml ice-cold PBS and subsequently centrifuged (Eppendorf Centrifuge 5810R) at 394 g for 7 min at 4°C. Cells were then resuspended in ME medium and brought to a final concentration of 5 × 105 cells/ml (stock solution). PBMCs or PECs (1 × 105) were incubated in a Nunc Maxisorp 96-well flat-bottom tissue culture plate either alone or in combination with 10 ng LPS (E. coli 011:B4 or E. coli 011:B5), with or without 1.67 µM of each anti-MIF Nb construct. In addition, 5 × 105 THP-1 or RAW264.7 cells were incubated in a Nunc Maxisorp 96-well flat-bottom tissue culture plate either alone or in combination with 10 ng LPS, with or without 500 nM of anti-MIF Nb and/or 10 nM dexamethasone (Sigma-Aldrich). Cells were incubated for 18 h at 37°C, after which the supernatant was collected and tested in a human or mouse TNF ELISA (R&D Systems).
Animal experiments
All experiments complied with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ECPVA) guidelines [Council of Europe Convention on the Prevention of Terrorism (CETS) No. 123] and were approved by the Vrije Universiteit Brussels Ethical Committee (15-220-11). To induce endotoxic shock, 7- to 8-wk-old C57BL/6 female mice were injected intraperitoneally with 12.5 mg/kg E. coli 011:B4 LPS (Invivogen, San Diego, CA, USA) and monitored for survival over a 72-h period. Treatment experiments consisted of coinjection of LPS with anti-MIF NbE10 constructs at concentrations of 25, 12.5, 6.25, or 2.5 mg/kg. Alternatively, mice received anti-MIF NbE10 constructs (25 mg/kg) 6 h post-LPS injection—that is, the moment mice start showing clinical signs or symptoms as a result of the LPS injection.
Statistics
Statistical analysis (unpaired Student’s t test or 1-way ANOVA) was performed by using Prism 6 (GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Generation and characterization of MIF-binding Nbs
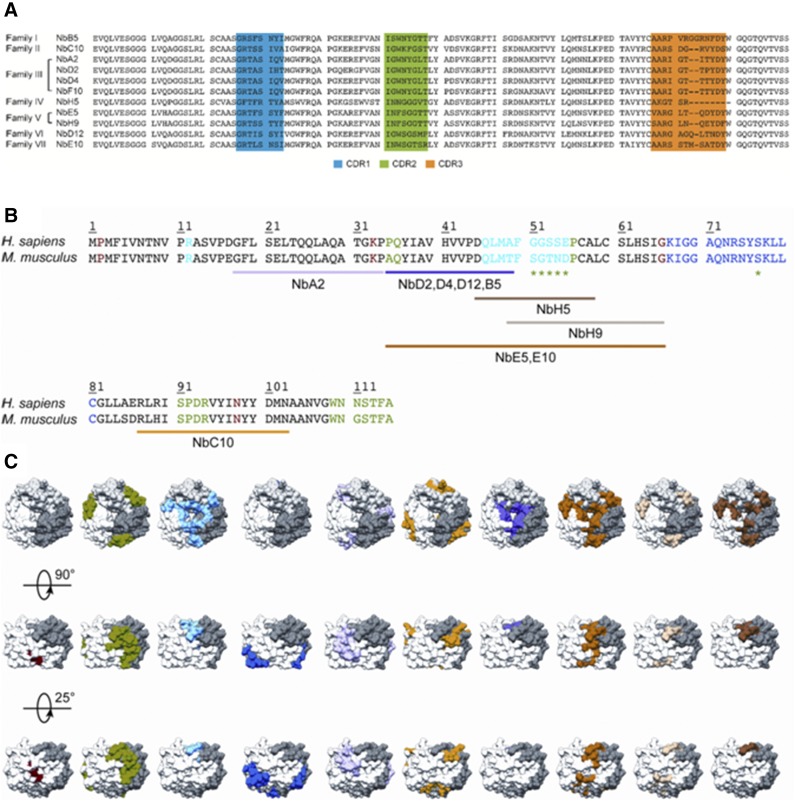
To obtain Nbs against MIF, llamas were immunized with rhMIF, and phage display libraries were generated from their peripheral blood lymphocytes. After 2 rounds of panning and selection on rhMIF, 11 anti-MIF binders were identified that could be separated into 7 different families on the basis of differences in amino acid sequences of the complementarity determinant regions (Fig. 1A). Anti-MIF Nbs were subsequently purified and assessed for binding to rhMIF and rmMIF. SPR analysis revealed that all anti-MIF Nbs, with the exception of NbF10, displayed affinities in the <100-nM range for both rhMIF and rmMIF (Supplemental Figs. 1 and 2, and Tables 1 and 2, respectively). Furthermore, all anti-MIF Nbs, with the exception of NbF10 and NbH5, exhibited good thermal stability, with Tm values ranging between 61 and 72°C (Table 3). Finally, to determine the binding region on MIF, an epitope-mapping study was performed by using overlapping human MIF peptides, as previously described (35). This study demonstrated that the distinct anti-MIF Nbs bind to different MIF peptides (Fig. 1B and Supplemental Table 1). Upon mapping these epitopes on the MIF crystal structure, it is evident that anti-MIF Nbs interact with distinct regions on the MIF protein (Fig. 1C). NbA2 seems to bind most closely to the N-terminal region of MIF (Gly18-Pro34), whereas NbC10 recognizes an epitope close to the C-terminal end of the protein (Arg87-Asn103). A broad region between Pro35-Gly66 contributes to the binding of Nbs E5 and E10, whereas Nbs B5, D2, D4, and D12 bind to a shorter region between Pro35-Ala49. The epitope of NbH5 is confined to a region between Asp45-Ala58. Compared with NbH5, the binding site for NbH9 is slightly shifted and extended toward the C terminus (Ala49-Gly66). Only for NbF10 could no clear binding region be identified, which may be a result of its low affinity for the intact protein (Table 1), or because of the fact that it recognizes a conformational epitope that is not represented in the surface-immobilized peptides.
Figure 1.
Sequences of anti-MIF Nbs and their predicted binding regions on the MIF protein. A) Sequence alignment of the 11 different anti-MIF Nbs, which are organized into different families according to Kabat classification (74). Complementarity determinant region 1 (CDR1), CDR2, and CDR3 are indicated in blue, green, and orange, respectively. B) Sequence alignment of rhMIF (Homo sapiens) and rmMIF (Mus musculus). Residues involved in MIF’s tautomerase activity, CD74 binding, and the interaction with CXCR2 and CXCR4 are colored in red, green (including those indicated by the asterisk), and light and dark blue, respectively. Results of the performed epitope-mapping studies are indicated below the sequence alignment and are color coded (NbA2, pink; Nbs D2, D4, D12 and B5, purple; NbH9, light brown; Nbs E5 and E10, orange; NbC10, yellow; NbH5, dark brown; for NbF10, no definite region could be appointed). C) Results of the epitope-mapping experiment displayed on the structure of human MIF (62). MIF trimer is shown in surface representation, and each constituting monomer is shown in white, gray, and dark gray.
TABLE 1.
Kinetic parameters for Nb–rhMIF interactions as determined by SPR
| Family | Nb clone | Kon (105 M−1 s−1) | Koff (103 s−1) | KD (nM) |
|---|---|---|---|---|
| I | NbB5 | 0.41 | 1.54 | 37.75 |
| II | NbC10 | 0.38 | 1.61 | 41.19 |
| III | NbA2 | 3.11 | 0.92 | 2.960 |
| NbD2 | 0.75 | 1.24 | 16.50 | |
| NbD4 | 0.44 | 1.53 | 35.00 | |
| NbF10 | ND | ND | ND | |
| IV | NbH5 | 0.17 | 0.48 | 27.50 |
| V | NbE5 | 0.78 | 0.74 | 9.410 |
| NbH9 | 0.32 | 2.57 | 80.30 | |
| VI | NbD12 | 0.18 | 2.98 | 166.0 |
| VII | NbE10 | 0.98 | 6.32 | 64.50 |
Data were measured in the format of a multicycle experiment, with rhMIF as the ligand (i.e., coated onto the sensor surface) and anti-MIF Nbs as the analyte. Experimental data were analyzed with a 1:1 Langmuir binding model to obtain the association rates (Kon), dissociation rates (Koff) and affinity constants (KD).
TABLE 2.
Kinetic parameters for Nb–rmMIF interactions as determined by SPR
| Family | Nb clone | Kon (105 M-1 s−1) | Koff (103 s−1) | KD (nM) |
|---|---|---|---|---|
| I | NbB5 | 1.42 | 5.42 | 38.17 |
| II | NbC10 | 1.63 | 2.53 | 15.52 |
| III | NbA2 | 1.43 | 2.00 | 13.98 |
| NbD2 | 1.51 | 1.89 | 12.52 | |
| NbD4 | 0.99 | 2.74 | 27.73 | |
| NbF10 | 0.19 | 4.34 | 228.4 | |
| IV | NbH5 | 1.16 | 2.97 | 25.60 |
| V | NbE5 | 1.63 | 1.91 | 11.72 |
| NbH9 | 0.70 | 2.31 | 33.00 | |
| VI | NbD12 | 0.59 | 5.11 | 85.88 |
| VII | NbE10 | 0.86 | 4.57 | 53.13 |
| Multivalent NbE10 constructs | ||||
| NbE10-NbAlb8 | 0.64 | 5.65 | 88.28 | |
| NbE10-NbE10 | 3.51 | 1.12 | 3.190 | |
| NbE10-NbE10-NbAlb8 | 3.22 | 3.24 | 10.06 | |
| NbE10-NbAlb8-NbE10 | 4.17 | 2.51 | 6.02 | |
Data were measured in the format of a kinetic titration (48), with rmMIF as the ligand (i.e., coated onto the sensor surface) and anti-MIF Nbs as the analyte. Experimental data (Supplemental Fig. 1) were analyzed with a 1:1 Langmuir binding model to obtain the association rates (Kon), dissociation rates (Koff) and affinity constants (KD). For NbE10-NbE10, NbE10-NbAlb8-NbE10, and NbE10-NbE10-NbAlb8, the reported affinity constants should be seen as apparent KD values as a result of avidity effects.
TABLE 3.
Thermostability of anti-MIF Nbs determined by using differential scanning fluorimetry
| Family | Nb clone | Tm (°C) |
|---|---|---|
| I | NbB5 | 68.51 ± 0.18 |
| II | NbC10 | 61.42 ± 0.12 |
| III | NbA2 | 72.16 ± 0.04 |
| NbD2 | 60.81 ± 0.16 | |
| NbD4 | 69.19 ± 0.03 | |
| NbF10 | 47.46 ± 0.04 | |
| IV | NbH5 | 49.34 ± 0.04 |
| V | NbE5 | 66.74 ± 0.41 |
| NbH9 | 61.32 ± 0.19 | |
| VI | NbD12 | 72.82 ± 0.18 |
| VII | NbE10 | 65.81 ± 0.13 |
Data are representative of 2 independent experiments (n = 3) and expressed as means ± sd.
NbE5 and NbE10 inhibit LPS-mediated TNF induction, the tautomerase activity of MIF, and can override the inhibitory effects of MIF on glucocorticoids in vitro
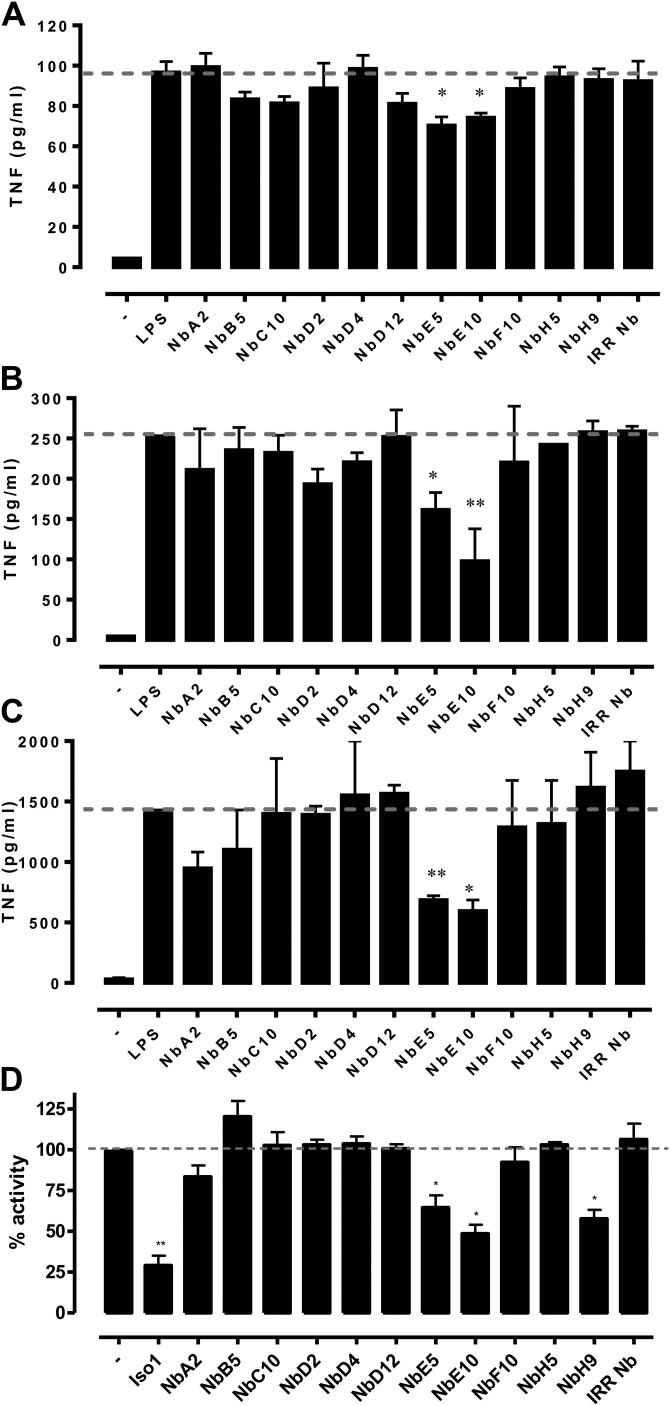
To assess the inhibitory capacity of Nbs on the biologic activity of MIF, we performed different in vitro assays. Stimulation of human THP-1 monocytes with LPS results in MIF secretion, which, in turn, binds to the MIF receptor, CD74, to augment the secretion of TNF (4, 5, 50, 51). Accordingly, we determined the ability of Nbs to block MIF-mediated TNF secretion. As shown in Fig. 2A, only NbE5 and NbE10 were capable of significantly decreasing TNF production by LPS-stimulated THP-1 monocytes. To additionally assess the relevance of these findings, we assessed TNF production by LPS-treated human PBMCs in the presence of anti-MIF Nbs. Again, NbE5 and NbE10 were the only Nbs to potently attenuate TNF production by LPS-stimulated PBMCs (Fig. 2B). Because Nbs are cross-reactive to rmMIF, we also assessed their potential to inhibit MIF-mediated TNF induction by murine RAW264.7 macrophages, the results of which corroborated that only NbE5 and NbE10, and, to a lesser extent, NbA2, were capable of blocking this MIF effect (Fig. 2C). To determine the potency of NbE5 and NbE10 for blocking the proinflammatory activity of MIF, we used TNF production by LPS-stimulated mouse peritoneal macrophages (PEMs) from MIF-deficient (Mif−/−) mice as a control condition in which MIF activity is absent. Of note, as shown in Supplemental Fig. 3, both NbE5 and NbE10 reduced TNF secretion by LPS-stimulated PEMs from wild-type mice to levels observed with Mif−/− PEMs, which illustrated a nearly complete block of MIF activity by these Nbs. Finally, we assessed whether anti-MIF Nbs could interfere with the tautomerase activity of MIF. Small-molecule inhibitors of the tautomerase activity of MIF may block inflammatory activity by interfering with the physical interaction of the tautomerase site with the MIF cognate receptor, CD74 (8, 9). NbE5 and NbE10, as well as NbH9, significantly reduced the tautomerase activity of MIF, although not as strongly as the small-molecule tautomerase inhibitor, ISO-1 (Fig. 2D) (9).
Figure 2.
Inhibition of the biologic activities of MIF by anti-MIF Nbs. A–C) Anti-MIF Nbs were found to reduce TNF secretion from LPS (10 ng/well)-stimulated THP-1 monocytic cells (A), human PBMCs (B), and RAW2647.7 macrophages (C). In brief, cells were treated with 500 nM of each anti-MIF Nb, and an irrelevant Nb was used as negative control. After 18 h of incubation, cell culture supernatants were collected for the determination of TNF concentration. Data are shown as means ± sem. Statistical analysis was performed by comparing TNF levels of Nb-treated LPS-stimulated cells with levels of untreated LPS-stimulated cells. The dashed line represents TNF levels (cutoff level) of mice that were stimulated with LPS alone. D) Inhibition of the d-dopachrome tautomerase activity of MIF by anti-MIF Nbs. ISO-1 was used as positive control. Activity was determined by semicontinuous reduction in signal (tautomerization) measured at OD450nm in the presence and absence of anti-MIF Nbs. Percentage activity was expressed in relationship to the tautomerase activity of rmMIF alone. The dashed line represents 100% activity (i.e., when only MIF was used). Data are shown as means ± sem and representative of 3 independent experiments (n = 2). *P < 0.05, **P < 0.01.
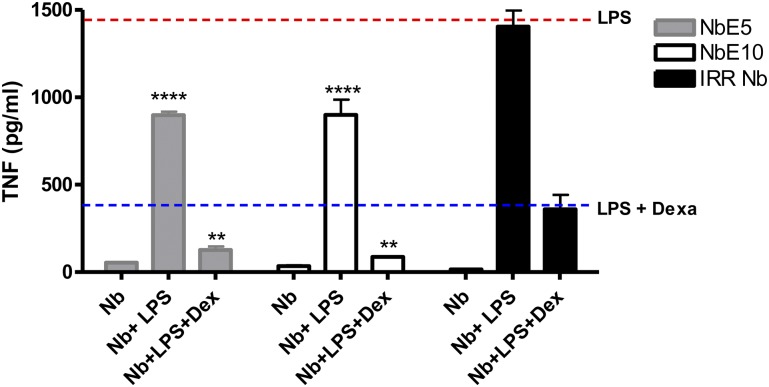
A clinically important aspect of the biologic activity of MIF is its ability to sustain inflammation in the presence of glucocorticoids (52, 53). Of importance, NbE5 and NbE10 could additionally decrease LPS-induced TNF production in the presence of dexamethasone compared with the control condition (blue dotted line) or an irrelevant Nb (Fig. 3), which suggests that these Nbs can override the MIF-mediated resistance to glucocorticoids.
Figure 3.
Anti-MIF Nbs can override the anti-immunosuppressive effects of MIF of glucocorticoids. RAW264.7 cells were treated with 10 ng LPS, with or without 500 nM anti-MIF Nb and/or 10 nM dexamethasone. After 18 h of incubation, cell culture supernatants were collected for the determination of TNF concentration. Data are shown as means ± sem. Statistical analysis was performed by comparing TNF levels of anti-MIF Nb-treated cells [NbE5 (gray bars) or NbE10 (white bars)] with irrelevant Nb-treated cells (black bars) when LPS was added alone or with dexamethasone. The red line represents TNF levels (cutoff level) of mice that were stimulated with LPS in the absence of Nb. The blue line represents TNF levels (cutoff level) of cells that were stimulated with LPS in the presence of dexamethasone. Data are representative of 3 independent experiments ± sem (n = 2). *P < 0.05, **P < 0.01, ****P < 0.0001.
Collectively, the results indicate that NbE5 and NbE10 are promising lead molecules for blocking the proinflammatory activity of MIF.
Multivalent half-life extended NbE10 is protective in a mouse model of endotoxemia
As Nbs exhibit a short serum half-life (54) that might affect their neutralizing potential in in vivo settings, multivalent and/or half-life extended Nbs have to be generated for therapeutic use. Hereby, bi- or multivalent Nb constructs are expected to result in a significant increase in avidity and might allow for blocking multiple sites on the MIF trimer, whereas engineering a bispecific anti-MIF/anti-serum albumin Nb (NbAlb8) should increase circulation half-life in vivo (55–57). As multivalency or half-life extension may affect the functionality of an Nb, we generated different formats of multivalent and serum half-life extended constructs of NbE5 and NbE10 by using a previously reported anti-serum albumin Nb (NbAlb8) (58). Given that NbE10 constructs had superior yield and solubility compared with NbE5 constructs, NbE10 constructs were chosen for in vitro and in vivo studies. These constructs, including NbE10-NbAlb8, NbE10-NbE10, NbE10-NbAlb8-NbE10, and NbE10-NbE10-NbAlb8, were produced, purified, and assessed for their binding capacity to rmMIF via SPR (Supplemental Fig. 4 and Table 2). A linkage to NbAlb8 via a flexible 9GS linker did not significantly affect NbE10 affinity for MIF, whereas the multivalent constructs, NbE10-NbE10, NbE10-NbAlb8-NbE10, and NbE10-NbE10-NbAlb8, exhibited an increased apparent affinity for MIF, likely because of avidity. We next assessed the ability of different NbE10 constructs to reduce MIF-induced TNF production after stimulation of primary murine PEMs with LPS. As shown in Fig. 4, all NbE10 constructs were capable of significantly decreasing TNF production in this setting, which indicated that the linkage to NbAlb8 has no effect on the MIF inhibitory capacity of NbE10. Moreover, NbE10-NbAlb8-NbE10 was found to be the most potent MIF-blocking construct.
Figure 4.
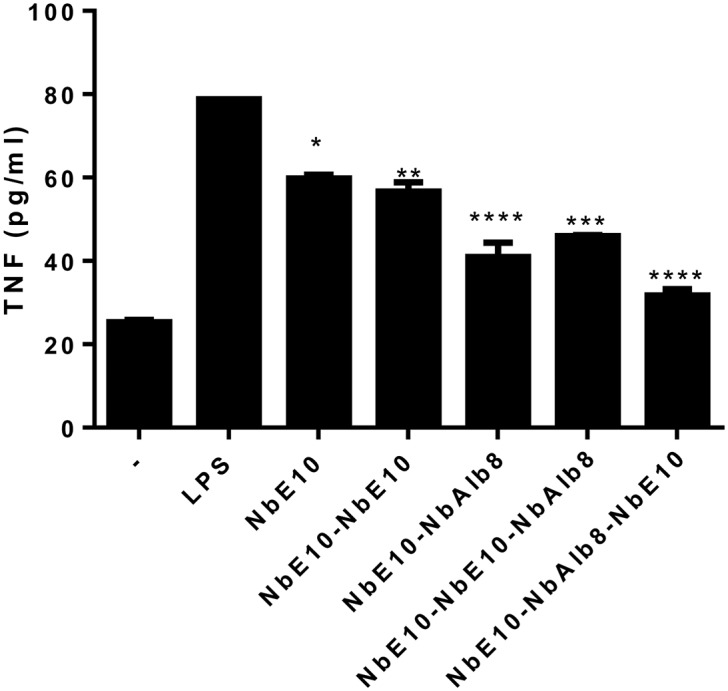
Anti-MIF NbE10 constructs can inhibit TNF secretion from LPS-stimulated macrophages. PEMs from naive mice were treated with 1.67 µM of all anti-MIF NbE10 constructs. After 18 h of incubation, cell culture supernatants were collected for the determination of the TNF concentration. Data are representative of 2 independent experiments (n = 2) and are shown as means ± sem. Statistical analysis was performed by comparing the induction of TNF of LPS-stimulated cells in the presence of anti-MIF Nb constructs with that of LPS-stimulated cells alone. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
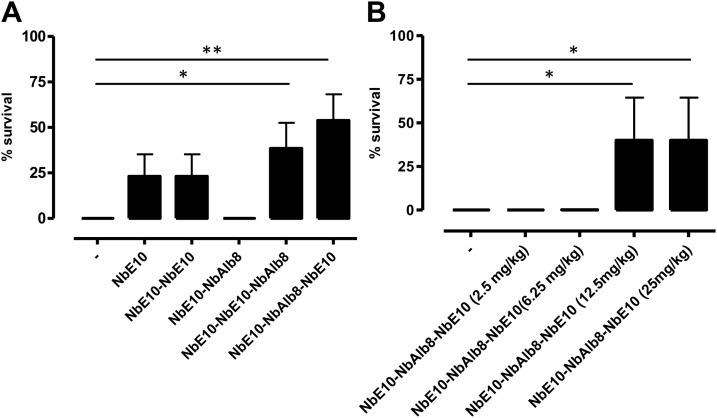
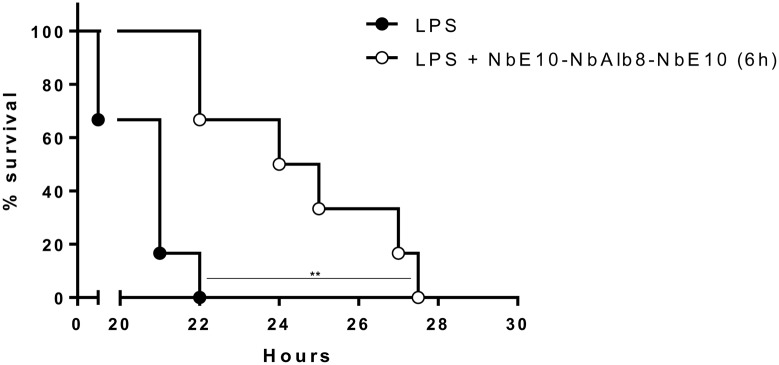
To assess the in vivo MIF-neutralizing efficacy of NbE10 constructs, we subjected mice to endotoxic shock as an experimental model of sepsis (59). To this end, the different anti-MIF NbE10 constructs (25 mg/kg) were coinjected i.p. with a lethal dose of LPS (12.5 mg/kg) and survival was monitored. As shown in Fig. 5A, only the NbE10-NbAlb8-NbE10 and NbE10-NbE10-NbAlb8 constructs significantly enhanced the survival of LPS-treated mice, from which the NbE10-NbAlb8-NbE10 construct was found to be the most efficient. On the basis of this finding, we assessed the minimal NbE10-NbAlb8-NbE10 concentration necessary to rescue mice from endotoxic shock. A serial dilution of NbE10-NbAlb8-NbE10 (25, 12.5, 6.25, and 2.5 mg/kg), coinjected with a lethal LPS dose (12.5 mg/kg), demonstrated that the protective capacity of this construct is lost if <12.5 mg/kg is administered (Fig. 5B). Of interest, NbE10-NbAlb8-NbE10 also reduced peak serum TNF levels (Fig. 6), which is in line with the TNF-reducing capacity of this construct in vitro (Fig. 4). Finally, we assessed whether NbE10-NbAlb8-NbE10 could be used as an acute treatment after the initiation of the systemic inflammatory cascade and when already strong symptoms of morbidity are observed (∼6 h post-LPS injection). As indicated in Fig. 7, NbE10-NbAlb8-NbE10 was indeed able to significantly prolong the survival of LPS-treated and morbid mice in this acute treatment setting.
Figure 5.
A bivalent half-life extended anti-MIF NbE10 construct is protective in a mouse model of endotoxemia. A) C57BL/6 mice were administered a single injection of 12.5 mg/kg LPS alone or in combination with 25 mg/kg of all available NbE10 constructs and monitored for survival. Data are representative of 3 independent experiments (n = 5–13). B) Dose kinetics of NbE10-NbAlb8-NbE10, whereby C57BL/6 mice were administered a single coinjection of 12.5 mg/kg LPS and 25, 12.5, 6.25, or 2.5 mg/kg of the NbE10-NbAlb8-NbE10 construct and monitored for survival. Percentage survival was expressed as the percentage of mice that survived 60 h post-LPS injection. Of note, mice that survived after this time period recovered completely. Data are representative of 2 independent experiments (n = 5) and are shown as means ± sem. *P < 0.05, **P < 0.01.
Figure 6.
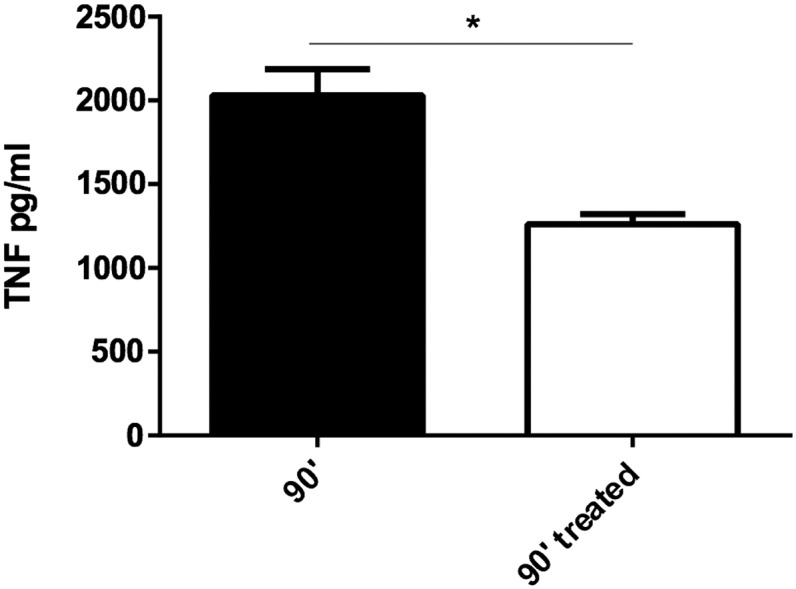
NbE10-NbAlb8-NbE10 reduced peak serum TNF levels in endotoxin-treated mice. C57BL/6 mice were administered a single i.p. injection of 12.5 mg/kg LPS alone or in combination with 25 mg/kg NbE10-NbAlb8-NbE10, and at 90 min, mice were sacrificed and serum tested in a TNF ELISA. Data are representative of 2 independent experiments and expressed as means ± sem (n = 3). *P < 0.05.
Figure 7.
A bivalent half-life extended anti-MIF NbE10 construct administered therapeutically attenuates endotoxemia. C57BL/6 mice were administered a single lethal intraperitoneal injection of 12.5 mg/kg LPS alone (block box) or followed by 25 mg/kg NbE10-NbAlb8-NbE10 administered 6 h post-LPS injection (gray box). Data are representative of 2 independent experiments (n = 5) and are shown as means ± sem. **P < 0.01.
In summary, NbE10-NbAlb8-NbE10 treatment of mice that were injected with a lethal dose of endotoxin rescued ∼40–50% of mice from lethality when administered prophylactically and significantly delayed lethality when administered nonprophylactically during systemic inflammation.
DISCUSSION
MIF has been identified as an upstream regulator of innate immunity (4) and is considered an attractive target to alleviate autoimmune and inflammation-associated disorders (9, 29, 33). Consequently, strategies to inhibit the deleterious effects of MIF might have therapeutic potential and could be administered via a precision medicine approach in patients who are genotypic high MIF expressers (60).
We exploited single-domain antigen-binding fragments—that is, Nbs—as a novel tool to antagonize the inflammatory effects of MIF. Eleven Nbs were identified and found to bind to both rmMIF and rhMIF with nanomolar affinities. This crossreactivity is likely a result of the high sequence and structural similarity between rmMIF and rhMIF (61, 62). The nanomolar apparent affinities of the half-life extended anti-MIF Nbs are within the range of the interaction KD reported for MIF and CD74 (1.5–9 nM) (5). All anti-MIF Nbs, with the exception of NbF10 and NbH5, exhibited good thermal stability, which is a prerequisite for in vivo activity of biologics and an important functional property for the clinical utility of Abs and Ab fragments (63, 64).
Of importance, some Nbs have the ability to potently inhibit endotoxin (LPS)-induced TNF production by human monocytes and murine macrophages, and to inhibit the tautomerase activity of MIF. To additionally rationalize our results, we displayed the outcome of the epitope-mapping experiments on the crystal structure of the MIF trimer (Fig. 1B). NbH9, NbE5, and NbE10 block the tautomerase activity of MIF, which indicates that they bind within or close to the site of the interaction of MIF with CD74 (9). Indeed, as indicated in Fig. 1B, it can be observed that the epitopes of these Nbs on the MIF surface overlap with the tautomerase site substrate-binding pocket. Of note, NbE5 and NbE10 inhibited the tautomerase activity of MIF at a lower concentration than ISO-1 (0.67 µm vs. 10 µM, respectively)—that is, the best-studied small-molecule MIF antagonist (65). Tarasuk et al. (66) have documented that a fully human single-chain Fv directed against MIF also blocks its tautomerase activity, but these authors did not assess the potential for this single-chain Fv construct to inhibit the biologic effects of MIF. Compared with single-chain Fv, Nbs have the additional advantage of being more soluble and stable (67).
Binding of MIF to CD74 leads to the activation of the ETS/AP1 family of transcription factors, which are important for the expression of TLR4, an integral component of the LPS receptor complex (4), and the expression of cell adhesion molecules and inflammatory cytokines, such as TNF (4, 14, 33). In addition, MIF was demonstrated to counteract the immunosuppressive action of glucocorticoids, thereby prolonging inflammatory response (53). By using a murine macrophage cell line (RAW264.7), we demonstrated that NbE5 and NbE10 significantly reduced TNF production after LPS stimulation in vitro in the presence and absence of the glucocorticoid, dexamethasone. Of note, NbE5 and NbE10 seem to be more efficient at inhibiting TNF induction—50% TNF reduction at a concentration of 0.5–1.67 µM—than ISO-1 [50% TNF reduction at 10 µM (9)]. In the same experimental setup, a concentration of 10 µg/ml anti-MIF IgG (∼0.067 µM) reduced TNF output by 50%, which indicated that higher concentrations of Nb are required to reach the same effect. The higher inhibiting potential of conventional anti-MIF IgG might rely on avidity effects that are absent in small-molecule inhibitors, such as Nbs or ISO-1; however, Nbs can easily be tailored into bivalent constructs to increase their avidity and, as a result of the absence of an Fc part, avoid bystander effects that might occur with conventional IgG (41). Thus, NbE5 and NbE10 can efficiently reduce the tautomerase function of MIF, counter its role in LPS-induced TNF production, and inhibit its glucocorticoid overriding effects. Remarkably, although NbH9 also inhibited the tautomerase activity of MIF (Fig. 2B), it had no anti-inflammatory activity in vitro (Fig. 2A). The reason for this is unclear. It is possible that the binding of NbH9 to MIF, which has a comparatively low KD (80.3 nM), may not be sufficient to displace CD74 and interfere with receptor activation. Alternatively, as both MIF and CD74 are trimeric, optimal receptor inhibition may require interference with more than one MIF:CD74 interaction site, which may not be easily predicted from the modeling analysis (59).
A comparison of the regions that are recognized by NbE5 and NbE10 (Pro35-Gly66) with the putative CD74 binding site (68) reveals a strong overlap between both regions, which explains the inhibitory property of these Nbs (Fig. 1B). Of interest, Kerschbaumer and colleagues (35) demonstrated that Abs that bind a similar or adjacent MIF epitope (Phe50-Ile68, BaxB01; Glu86-Met102, BaxD08, respectively) exerted protective effects in models of sepsis. To assess the protective effects of our lead compound (NbE10) in an in vivo model of endotoxic shock, we engineered bivalent half-life extended constructs by using an anti-albumin Nb (40–42), thereby increasing avidity effects and serum half-life for maximal in vivo potential. All NbE10 constructs were demonstrated to efficiently reduce TNF production by LPS-stimulated macrophages compared with the monovalent NbE10, whereby the NbE10-NbAlb8-NbE10 construct was observed to be the most potent. When administered prophylactically in vivo, NbE10-NbAlb8-NbE10 also seemed to be more efficacious than the NbE10-NbE10-NbAlb8 construct, rescuing ∼60% vs. ∼40% of mice that received a lethal LPS dose, where mice recovered completely from the insult 72 h postinjection. The potency difference between both constructs in vitro and in vivo indicates that the position of anti-albumin NbAlb8 in the construct is of relevance. A possible explanation for this may be that the NbE10-NbAlb8-NbE10 construct can bind 2 regions within an MIF trimer or bind 2 trimers simultaneously, whereas for the NbE10-NbE10-NbAlb8 construct, this ability could be impaired because of the close proximity of the 2 NbE10 molecules. In addition, or in concert with the previous hypothesis, the binding of NbAlb8 to albumin might be more of a hindrance in the NbE10-NbE10-NbAlb8 construct compared with the NbE10-NbAlb8-NbE10 construct. Additional experiments using Nb constructs with a longer linker—35GS instead of 9GS—or crystallographic studies will be required to help elucidate this issue, but this is beyond the scope of the current work. Our results also show that, in addition to half-life extension via NbAlb8, bivalent MIF binding has better in vivo blocking potential. Indeed, tailoring the monovalent NbE10 into an NbE10-NbAlb8-NbE10 construct allows more potent blocking of LPS-mediated endotoxemia than ISO-1. Whereas NbE10-NbAlb8-NbE10 can be administered as a single injection of 12.5–25 mg/kg, ISO-1 must be administered in multiple consecutive injections and at high doses of 35 mg/kg to prevent LPS-mediated lethality (9, 33). Moreover, as the affinity of NbAlb8 for mouse serum albumin is suboptimal compared with its human serum albumin binding properties (46), the current construct may underestimate the potential of this approach.
Of importance, NbE10-NbAlb8-NbE10 was found to delay LPS-mediated lethality even when administered 6 h after LPS injection. Eventually, mice still succumbed, which suggests either that a higher dose or multiple and/or continuous injections of the construct might be required or that only the blocking of MIF in the early stages of the insult is crucial in this setting. With regard to the latter possibility, Chagnon et al. (26) demonstrated that inhibition of MIF early in a rat endotoxic shock model partially reverses the imbalance of proapoptotic to prosurvival pathways, thereby reducing acute inflammation and myocardial dysfunction induced by endotoxin. This had been previously observed within 6 h post-LPS/anti-MIF administration and hence indicates that the early blockade of MIF is important.
Whereas several small-molecule inhibitors of the biologic activity of MIF have been documented (65, 69, 70), to our knowledge, this is the first report to establish that an MIF antagonist, when appropriately engineered, can prevent lethal endotoxemia when administered as a single injection. Although monoclonal/polyclonal anti-MIF Abs were also found to exert protective effects (34, 35, 71), Nbs have an advantage in that they can be tailored into multifunctional formats as demonstrated herein. This may be particularly relevant given the recent discovery of the second CD74 ligand, MIF-2, which has a spectrum of action that is similar to that of MIF (72). In contrast to conventional Abs, Nbs also lack an Fc portion, which avoids additional inflammatory reactions, and humanization of the Nb sequence is feasible for clinical translation (73). Thus, anti-MIF Nbs are an as-yet-unexplored avenue for the development of anti-inflammatory agents in MIF-mediated diseases.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Yvon Elkrim, Ella Omasta, Marie-Thérèse Detobel, Maria Slazak, Victor Orimoloye, Natalia Śmiejkowska, Shahid Hussain, and Nadia Abou for technical and administrative assistance, as well as Dr. Alain Beschin for constructive discussions [all from the Vrije Universiteit Brussel (VUB) and the Flanders Institute for Biotechnology (VIB) Center for Inflammation Research]. This work, performed in the frame of an Interuniversity Attraction Pole Program (PAI-IAP N. P7/41; http://www.belspo.be/belspo/iap/index_en.stm), was supported by a grant from the Research Foundation–Flanders (FWO; KaN 1515813N), and a starting budget from Ablynx. B.S. was supported by the Strategic Research Program (SRP3; Vrije Universiteit Brussels). R.B. was supported by the U.S. National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Glossary
- FCS
fetal calf serum
- ISO-1
(S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester
- MIF
macrophage migration inhibitory factor
- Nb
nanobody
- PBMC
peripheral blood monocytic cell
- PEM
peritoneal macrophage
- PEC
peritoneal exudates cell
- rhMIF
recombinant human macrophage migration inhibitory factor
- rmMIF
recombinant murine macrophage migration inhibitory factor
- SPR
surface plasmon resonance
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
I. Cabrito generated the Nbs; P. Vanlandschoot and B. Stijlemans conceived and designed the experiments; A. Sparkes, L. Brys, I. Cabrito, Y. G.-J. Sterckx, S. Schoonooghe, and B. Stijlemans performed the experiments; A. Sparkes, P. De Baetselier, L. Brys, Y. G.-J. Sterckx, S. Schoonooghe, J. A. Van Ginderachter, and B. Stijlemans analyzed the data; P. De Baetselier, G. Raes, R. Bucala, and J. A. Van Ginderachter contributed reagents, materials, analytic tools, and animals; and A. Sparkes, P. De Baetselier, S. Muyldermans, G. Raes, R. Bucala, J. A. Van Ginderachter, and B. Stijlemans wrote the manuscript.
REFERENCES
- 1.Abraham E. (1999) Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 25, 556–566 10.1007/s001340050903 [DOI] [PubMed] [Google Scholar]
- 2.Stijlemans B., Leng L., Brys L., Sparkes A., Vansintjan L., Caljon G., Raes G., Van Den Abbeele J., Van Ginderachter J. A., Beschin A., Bucala R., De Baetselier P. (2014) MIF contributes to trypanosoma brucei associated immunopathogenicity development. PLoS Pathog. 10, e1004414 10.1371/journal.ppat.1004414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stijlemans B., Brys L., Korf H., Bieniasz-Krzywiec P., Sparkes A., Vansintjan L., Leng L., Vanbekbergen N., Mazzone M., Caljon G., Van Den Abbeele J., Odongo S., De Trez C., Magez S., Van Ginderachter J. A., Beschin A., Bucala R., De Baetselier P. (2016) MIF-mediated hemodilution promotes pathogenic anemia in experimental African trypanosomosis. PLoS Pathog. 12, e1005862 10.1371/journal.ppat.1005862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calandra T., Roger T. (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3, 791–800 10.1038/nri1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leng L., Metz C. N., Fang Y., Xu J., Donnelly S., Baugh J., Delohery T., Chen Y., Mitchell R. A., Bucala R. (2003) MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197, 1467–1476 10.1084/jem.20030286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernhagen J., Krohn R., Lue H., Gregory J. L., Zernecke A., Koenen R. R., Dewor M., Georgiev I., Schober A., Leng L., Kooistra T., Fingerle-Rowson G., Ghezzi P., Kleemann R., McColl S. R., Bucala R., Hickey M. J., Weber C. (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587–596 10.1038/nm1567 [DOI] [PubMed] [Google Scholar]
- 7.Alampour-Rajabi S., El Bounkari O., Rot A., Müller-Newen G., Bachelerie F., Gawaz M., Weber C., Schober A., Bernhagen J. (2015) MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J. 29, 4497–4511 10.1096/fj.15-273904 [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran D., Gröning S., Schmitz C., Zierow S., Drucker N., Bakou M., Kohl K., Mertens A., Lue H., Weber C., Xiao A., Luker G., Kapurniotu A., Lolis E., Bernhagen J. (2016) Macrophage migration inhibitory factor-CXCR4 receptor interactions: evidence for partial allosteric agonism in comparison with CXCL12 chemokine. J. Biol. Chem. 291, 15881–15895 10.1074/jbc.M116.717751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Abed Y., Dabideen D., Aljabari B., Valster A., Messmer D., Ochani M., Tanovic M., Ochani K., Bacher M., Nicoletti F., Metz C., Pavlov V. A., Miller E. J., Tracey K. J. (2005) ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J. Biol. Chem. 280, 36541–36544 10.1074/jbc.C500243200 [DOI] [PubMed] [Google Scholar]
- 10.Fingerle-Rowson G., Kaleswarapu D. R., Schlander C., Kabgani N., Brocks T., Reinart N., Busch R., Schütz A., Lue H., Du X., Liu A., Xiong H., Chen Y., Nemajerova A., Hallek M., Bernhagen J., Leng L., Bucala R. (2009) A tautomerase-null macrophage migration-inhibitory factor (MIF) gene knock-in mouse model reveals that protein interactions and not enzymatic activity mediate MIF-dependent growth regulation. Mol. Cell. Biol. 29, 1922–1932 10.1128/MCB.01907-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rex S., Kraemer S., Grieb G., Emontzpohl C., Soppert J., Goetzenich A., Rossaint R., Bernhagen J., Stoppe C. (2014) The role of macrophage migration inhibitory factor in critical illness. Mini Rev. Med. Chem. 14, 1116–1124 10.2174/1389557515666150203143736 [DOI] [PubMed] [Google Scholar]
- 12.Grieb G., Merk M., Bernhagen J., Bucala R. (2010) Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 23, 257–264 10.1358/dnp.2010.23.4.1453629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozza M., Satoskar A. R., Lin G., Lu B., Humbles A. A., Gerard C., David J. R. (1999) Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189, 341–346 10.1084/jem.189.2.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozza M. T., Martins Y. C., Carneiro L. A., Paiva C. N. (2012) Macrophage migration inhibitory factor in protozoan infections. J. Parasitol. Res. 2012, 413052 10.1155/2012/413052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernhagen J., Calandra T., Mitchell R. A., Martin S. B., Tracey K. J., Voelter W., Manogue K. R., Cerami A., Bucala R. (1993) MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365, 756–759 10.1038/365756a0 [DOI] [PubMed] [Google Scholar]
- 16.Nee P. A. (2006) Critical care in the emergency department: severe sepsis and septic shock. Emerg. Med. J. 23, 713–717 10.1136/emj.2005.029934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Qian C., Zhao M., Yu X., Kang Y., Ma X., Ai Y., Xu Y., Liu D., An Y., Wu D., Sun R., Li S., Hu Z., Cao X., Zhou F., Jiang L., Lin J., Mao E., Qin T., He Z., Zhou L., Du B.; China Critical Care Clinical Trials Group . (2014) Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 9, e107181 10.1371/journal.pone.0107181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luan Y.-Y., Dong N., Xie M., Xiao X.-Z., Yao Y.-M. (2014) The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J. Interferon Cytokine Res. 34, 2–15 10.1089/jir.2013.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emonts M., Sweep F. C. G. J., Grebenchtchikov N., Geurts-Moespot A., Knaup M., Chanson A. L., Erard V., Renner P., Hermans P. W. M., Hazelzet J. A., Calandra T. (2007) Association between high levels of blood macrophage migration inhibitory factor, inappropriate adrenal response, and early death in patients with severe sepsis. Clin. Infect. Dis. 44, 1321–1328 10.1086/514344 [DOI] [PubMed] [Google Scholar]
- 20.Esposito S., De Simone G., Boccia G., De Caro F., Pagliano P. (2017) Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J. Glob. Antimicrob. Resist. 10, 204–212 10.1016/j.jgar.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 21.Lai K. N., Leung J. C. K., Metz C. N., Lai F. M., Bucala R., Lan H. Y. (2003) Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J. Pathol. 199, 496–508 10.1002/path.1291 [DOI] [PubMed] [Google Scholar]
- 22.Roger T., David J., Glauser M. P., Calandra T. (2001) MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414, 920–924 10.1038/414920a [DOI] [PubMed] [Google Scholar]
- 23.Roger T., Froidevaux C., Martin C., Calandra T. (2003) Macrophage migration inhibitory factor (MIF) regulates host responses to endotoxin through modulation of Toll-like receptor 4 (TLR4). J. Endotoxin Res. 9, 119–123 10.1177/09680519030090020801 [DOI] [PubMed] [Google Scholar]
- 24.Gao X. M., Liu Y., White D., Su Y., Drew B. G., Bruce C. R., Kiriazis H., Xu Q., Jennings N., Bobik A., Febbraio M. A., Kingwell B. A., Bucala R., Fingerle-Rowson G., Dart A. M., Morand E. F., Du X. J. (2011) Deletion of macrophage migration inhibitory factor protects the heart from severe ischemia-reperfusion injury: a predominant role of anti-inflammation. J. Mol. Cell. Cardiol. 50, 991–999 10.1016/j.yjmcc.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 25.Merk M., Baugh J., Zierow S., Leng L., Pal U., Lee S. J., Ebert A. D., Mizue Y., Trent J. O., Mitchell R., Nickel W., Kavathas P. B., Bernhagen J., Bucala R. (2009) The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor. J. Immunol. 182, 6896–6906 10.4049/jimmunol.0803710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chagnon F., Metz C. N., Bucala R., Lesur O. (2005) Endotoxin-induced myocardial dysfunction: effects of macrophage migration inhibitory factor neutralization. Circ. Res. 96, 1095–1102 10.1161/01.RES.0000168327.22888.4d [DOI] [PubMed] [Google Scholar]
- 27.Beishuizen A., Thijs L. G., Haanen C., Vermes I. (2009) Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J. Clin. Endocrinol. Metab. 86, 2811–2816 [DOI] [PubMed] [Google Scholar]
- 28.Bozza F. A., Gomes R. N., Japiassú A. M., Soares M., Castro-Faria-Neto H. C., Bozza P. T., Bozza M. T. (2004) Macrophage migration inhibitory factor levels correlate with fatal outcome in sepsis. Shock 22, 309–313 10.1097/01.shk.0000140305.01641.c8 [DOI] [PubMed] [Google Scholar]
- 29.Flaster H., Bernhagen J., Calandra T., Bucala R. (2007) The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol. Endocrinol. 21, 1267–1280 10.1210/me.2007-0065 [DOI] [PubMed] [Google Scholar]
- 30.Jing L., Bu M. (2011) Role of macrophage migration inhibitory factor in glucocorticoid release and glucocorticoid receptor function in rats. Ann. Clin. Lab. Sci. 41, 14–19 [PubMed] [Google Scholar]
- 31.Renner P., Roger T., Bochud P. Y., Sprong T., Sweep F. C., Bochud M., Faust S. N., Haralambous E., Betts H., Chanson A. L., Reymond M. K., Mermel E., Erard V., van Deuren M., Read R. C., Levin M., Calandra T. (2012) A functional microsatellite of the macrophage migration inhibitory factor gene associated with meningococcal disease. FASEB J. 26, 907–916 10.1096/fj.11-195065 [DOI] [PubMed] [Google Scholar]
- 32.Savva A., Brouwer M. C., Roger T., Valls Serón M., Le Roy D., Ferwerda B., van der Ende A., Bochud P.-Y., van de Beek D., Calandra T. (2016) Functional polymorphisms of macrophage migration inhibitory factor as predictors of morbidity and mortality of pneumococcal meningitis. Proc. Natl. Acad. Sci. USA 113, 3597–3602 10.1073/pnas.1520727113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morand E. F., Leech M., Bernhagen J. (2006) MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat. Rev. Drug Discov. 5, 399–410 10.1038/nrd2029 [DOI] [PubMed] [Google Scholar]
- 34.Calandra T., Echtenacher B., Roy D. L., Pugin J., Metz C. N., Hültner L., Heumann D., Männel D., Bucala R., Glauser M. P. (2000) Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6, 164–170 10.1038/72262 [DOI] [PubMed] [Google Scholar]
- 35.Kerschbaumer R. J., Rieger M., Völkel D., Le Roy D., Roger T., Garbaraviciene J., Boehncke W.-H., Müllberg J., Hoet R. M., Wood C. R., Antoine G., Thiele M., Savidis-Dacho H., Dockal M., Ehrlich H., Calandra T., Scheiflinger F. (2012) Neutralization of macrophage migration inhibitory factor (MIF) by fully human antibodies correlates with their specificity for the β-sheet structure of MIF. J. Biol. Chem. 287, 7446–7455 10.1074/jbc.M111.329664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Abed Y., VanPatten S. (2011) MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med. Chem. 3, 45–63 10.4155/fmc.10.281 [DOI] [PubMed] [Google Scholar]
- 37.Tohyama S., Onodera S., Tohyama H., Yasuda K., Nishihira J., Mizue Y., Hamasaka A., Abe R., Koyama Y. (2008) A novel DNA vaccine-targeting macrophage migration inhibitory factor improves the survival of mice with sepsis. Gene Ther. 15, 1513–1522 10.1038/gt.2008.112 [DOI] [PubMed] [Google Scholar]
- 38.Martin P., Furman R. R., Rutherford S., Ruan J., Ely S., Greenberg J., Coleman M., Goldsmith S. J., Leonard J. P. (2015) Phase I study of the anti-CD74 monoclonal antibody milatuzumab (hLL1) in patients with previously treated B-cell lymphomas. Leuk. Lymphoma 56, 3065–3070 10.3109/10428194.2015.1028052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muyldermans S., Baral T. N., Retamozzo V. C., De Baetselier P., De Genst E., Kinne J., Leonhardt H., Magez S., Nguyen V. K., Revets H., Rothbauer U., Stijlemans B., Tillib S., Wernery U., Wyns L., Hassanzadeh-Ghassabeh G., Saerens D. (2009) Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 128, 178–183 10.1016/j.vetimm.2008.10.299 [DOI] [PubMed] [Google Scholar]
- 40.Harmsen M. M., De Haard H. J. (2007) Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 77, 13–22 10.1007/s00253-007-1142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Bockstaele F., Holz J.-B., Revets H. (2009) The development of nanobodies for therapeutic applications. Curr. Opin. Investig. Drugs 10, 1212–1224 [PubMed] [Google Scholar]
- 42.Hoefman S., Ottevaere I., Baumeister J., Sargentini-Maier M. L. (2015) Pre-clinical intravenous serum pharmacokinetics of albumin binding and non-half-life extended nanobodies. Antibodies 4, 141–156 10.3390/antib4030141 [DOI] [Google Scholar]
- 43.Hassanzadeh-Ghassabeh G., Devoogdt N., De Pauw P., Vincke C., Muyldermans S. (2013) Nanobodies and their potential applications. Nanomedicine (Lond.) 8, 1013–1026 10.2217/nnm.13.86 [DOI] [PubMed] [Google Scholar]
- 44.Vandenbroucke K., de Haard H., Beirnaert E., Dreier T., Lauwereys M., Huyck L., Van Huysse J., Demetter P., Steidler L., Remaut E., Cuvelier C., Rottiers P. (2010) Orally administered L. lactis secreting an anti-TNF nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 3, 49–56 10.1038/mi.2009.116 [DOI] [PubMed] [Google Scholar]
- 45.Steeland S., Puimège L., Vandenbroucke R. E., Van Hauwermeiren F., Haustraete J., Devoogdt N., Hulpiau P., Leroux-Roels G., Laukens D., Meuleman P., De Vos M., Libert C. (2015) Generation and characterization of small single domain antibodies inhibiting human tumor necrosis factor receptor 1. J. Biol. Chem. 290, 4022–4037 10.1074/jbc.M114.617787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saerens D., Kinne J., Bosmans E., Wernery U., Muyldermans S., Conrath K. (2004) Single domain antibodies derived from dromedary lymph node and peripheral blood lymphocytes sensing conformational variants of prostate-specific antigen. J. Biol. Chem. 279, 51965–51972 10.1074/jbc.M409292200 [DOI] [PubMed] [Google Scholar]
- 47.Conrath K. E., Lauwereys M., Galleni M., Matagne A., Frère J. M., Kinne J., Wyns L., Muyldermans S. (2001) β-lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrob. Agents Chemother. 45, 2807–2812 10.1128/AAC.45.10.2807-2812.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlsson R., Katsamba P. S., Nordin H., Pol E., Myszka D. G. (2006) Analyzing a kinetic titration series using affinity biosensors. Anal. Biochem. 349, 136–147 10.1016/j.ab.2005.09.034 [DOI] [PubMed] [Google Scholar]
- 49.Caljon G., Stijlemans B., Saerens D., Van Den Abbeele J., Muyldermans S., Magez S., De Baetselier P. (2012) Affinity is an important determinant of the anti-trypanosome activity of nanobodies. PLoS Negl. Trop. Dis. 6, e1902 10.1371/journal.pntd.0001902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dios A., Mitchell R. A., Aljabari B., Lubetsky J., O’Connor K., Liao H., Senter P. D., Manogue K. R., Lolis E., Metz C., Bucala R., Callaway D. J., Al-Abed Y. (2002) Inhibition of MIF bioactivity by rational design of pharmacological inhibitors of MIF tautomerase activity. J. Med. Chem. 45, 2410–2416 10.1021/jm010534q [DOI] [PubMed] [Google Scholar]
- 51.Fingerle-Rowson G., Petrenko O., Metz C. N., Forsthuber T. G., Mitchell R., Huss R., Moll U., Müller W., Bucala R. (2003) The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc. Natl. Acad. Sci. USA 100, 9354–9359 10.1073/pnas.1533295100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kudrin A., Scott M., Martin S., Chung C.-W., Donn R., McMaster A., Ellison S., Ray D., Ray K., Binks M. (2006) Human macrophage migration inhibitory factor: a proven immunomodulatory cytokine? J. Biol. Chem. 281, 29641–29651 10.1074/jbc.M601103200 [DOI] [PubMed] [Google Scholar]
- 53.Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., Bucala R. (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377, 68–71 10.1038/377068a0 [DOI] [PubMed] [Google Scholar]
- 54.De Groeve K., Deschacht N., De Koninck C., Caveliers V., Lahoutte T., Devoogdt N., Muyldermans S., De Baetselier P., Raes G. (2010) Nanobodies as tools for in vivo imaging of specific immune cell types. J. Nucl. Med. 51, 782–789 10.2967/jnumed.109.070078 [DOI] [PubMed] [Google Scholar]
- 55.Zhang J., Tanha J., Hirama T., Khieu N. H., To R., Tong-Sevinc H., Stone E., Brisson J. R., MacKenzie C. R. (2004) Pentamerization of single-domain antibodies from phage libraries: a novel strategy for the rapid generation of high-avidity antibody reagents. J. Mol. Biol. 335, 49–56 10.1016/j.jmb.2003.09.034 [DOI] [PubMed] [Google Scholar]
- 56.Coppieters K., Dreier T., Silence K., de Haard H., Lauwereys M., Casteels P., Beirnaert E., Jonckheere H., Van de Wiele C., Staelens L., Hostens J., Revets H., Remaut E., Elewaut D., Rottiers P. (2006) Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 54, 1856–1866 10.1002/art.21827 [DOI] [PubMed] [Google Scholar]
- 57.Vosjan M. J. W. D., Vercammen J., Kolkman J. A., Stigter-van Walsum M., Revets H., van Dongen G. A. M. S. (2012) Nanobodies targeting the hepatocyte growth factor: potential new drugs for molecular cancer therapy. Mol. Cancer Ther. 11, 1017–1025 10.1158/1535-7163.MCT-11-0891 [DOI] [PubMed] [Google Scholar]
- 58.Maussang D., Mujić-Delić A., Descamps F. J., Stortelers C., Vanlandschoot P., Stigter-van Walsum M., Vischer H. F., van Roy M., Vosjan M., Gonzalez-Pajuelo M., van Dongen G. A., Merchiers P., van Rompaey P., Smit M. J. (2013) Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo. J. Biol. Chem. 288, 29562–29572 10.1074/jbc.M113.498436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Roy D., Di Padova F., Tees R., Lengacher S., Landmann R., Glauser M. P., Calandra T., Heumann D. (1999) Monoclonal antibodies to murine lipopolysaccharide (LPS)-binding protein (LBP) protect mice from lethal endotoxemia by blocking either the binding of LPS to LBP or the presentation of LPS/LBP complexes to CD14. J. Immunol. 162, 7454–7460 [PubMed] [Google Scholar]
- 60.Tilstam P. V., Qi D., Leng L., Young L., Bucala R. (2017) MIF family cytokines in cardiovascular diseases and prospects for precision-based therapeutics. Expert Opin. Ther. Targets 21, 671–683 10.1080/14728222.2017.1336227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pruitt K., Brown G., Tatusova T., Maglott D. (2012) The reference sequence (RefSeq): current status, new features and genome annotation policy. Nucleic Acids Res. 40, D130–D135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun H. W., Bernhagen J., Bucala R., Lolis E. (1996) Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc. Natl. Acad. Sci. USA 93, 5191–5196 10.1073/pnas.93.11.5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orr B. A., Carr L. M., Wittrup K. D., Roy E. J., Kranz D. M. (2003) Rapid method for measuring ScFv thermal stability by yeast surface display. Biotechnol. Prog. 19, 631–638 10.1021/bp0200797 [DOI] [PubMed] [Google Scholar]
- 64.Wörn A., Plückthun A. (2001) Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol. 305, 989–1010 10.1006/jmbi.2000.4265 [DOI] [PubMed] [Google Scholar]
- 65.Leng L., Chen L., Fan J., Greven D., Arjona A., Du X., Austin D., Kashgarian M., Yin Z., Huang X. R., Lan H. Y., Lolis E., Nikolic-Paterson D., Bucala R. (2011) A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J. Immunol. 186, 527–538 10.4049/jimmunol.1001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarasuk M., Poungpair O., Ungsupravate D., Bangphoomi K., Chaicumpa W., Yenchitsomanus P.-T. (2014) Human single-chain variable fragment antibody inhibits macrophage migration inhibitory factor tautomerase activity. Int. J. Mol. Med. 33, 515–522 10.3892/ijmm.2014.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riechmann L., Muyldermans S. (1999) Single domain antibodies: comparison of camel VH and camelised human VH domains. J. Immunol. Methods 231, 25–38 10.1016/S0022-1759(99)00138-6 [DOI] [PubMed] [Google Scholar]
- 68.Meza-Romero R., Benedek G., Leng L., Bucala R., Vandenbark A. A. (2016) Predicted structure of MIF/CD74 and RTL1000/CD74 complexes. Metab. Brain Dis. 31, 249–255 10.1007/s11011-016-9798-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vujicic M., Nikolic I., Krajnovic T., Cheng K.-F., VanPatten S., He M., Stosic-Grujicic S., Stojanovic I., Al-Abed Y., Saksida T. (2014) Novel inhibitors of macrophage migration inhibitory factor prevent cytokine-induced beta cell death. Eur. J. Pharmacol. 740, 683–689 10.1016/j.ejphar.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 70.Tsai T.-L., Lin T.-H. (2014) Virtual screening of some active human macrophage migration inhibitory factor antagonists. J. Biomol. Screen. 19, 1116–1123 10.1177/1087057114523317 [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., Zeng X., Chen S., Zhang Z., Li P., Yi W., Huang H., Yao J., Li S., Hu C. (2011) Characterization, epitope identification and mechanisms of the anti-septic capacity of monoclonal antibodies against macrophage migration inhibitory factor. Int. Immunopharmacol. 11, 1333–1340 10.1016/j.intimp.2011.04.017 [DOI] [PubMed] [Google Scholar]
- 72.Merk M., Zierow S., Leng L., Das R., Du X., Schulte W., Fan J., Lue H., Chen Y., Xiong H., Chagnon F., Bernhagen J., Lolis E., Mor G., Lesur O., Bucala R. (2011) The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF). Proc. Natl. Acad. Sci. USA 108, E577–E585 10.1073/pnas.1102941108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vincke C., Loris R., Saerens D., Martinez-Rodriguez S., Muyldermans S., Conrath K. (2009) General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J. Biol. Chem. 284, 3273–3284 10.1074/jbc.M806889200 [DOI] [PubMed] [Google Scholar]
- 74.Martin A. C. R. (1996) Accessing the Kabat antibody sequence database by computer. Proteins 25, 130–133 10.1002/(SICI)1097-0134(199605)25:1%3c130::AID-PROT11%3e3.3.CO;2-Y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.