Abstract
Objectives. To estimate health outcomes of policies to mitigate the opioid epidemic.
Methods. We used dynamic compartmental modeling of US adults, in various pain, opioid use, and opioid addiction health states, to project addiction-related deaths, life years, and quality-adjusted life years from 2016 to 2025 for 11 policy responses to the opioid epidemic.
Results. Over 5 years, increasing naloxone availability, promoting needle exchange, expanding medication-assisted addiction treatment, and increasing psychosocial treatment increased life years and quality-adjusted life years and reduced deaths. Other policies reduced opioid prescription supply and related deaths but led some addicted prescription users to switch to heroin use, which increased heroin-related deaths. Over a longer horizon, some such policies may avert enough new addiction to outweigh the harms. No single policy is likely to substantially reduce deaths over 5 to 10 years.
Conclusions. Policies focused on services for addicted people improve population health without harming any groups. Policies that reduce the prescription opioid supply may increase heroin use and reduce quality of life in the short term, but in the long term could generate positive health benefits. A portfolio of interventions will be needed for eventual mitigation.
An estimated 64 000 persons in the United States died from drug overdose in 2016, mostly from opioids.1 Once focused on postsurgery, traumatic injury, and terminal illness, opioid prescribing in recent decades broadened to treatment of chronic noncancer pain2,3 including for conditions for which opioids have no evidence of benefit.4 Increased prescribing has produced iatrogenic opioid use disorder (addiction) in some patients and large-scale diversion of opioids to others for whom they were not intended.5,6 People addicted to prescription opioids may overdose from them or may transition to cheaper illicit opioids—notably heroin—as tolerance increases and users’ need for opioids exceeds what can be garnered from the health care system.7
Efforts are under way to stem the opioid epidemic.8 Many initiatives curb prescribing rates, thereby reducing the risk of iatrogenic addiction and decreasing the likelihood that individuals can acquire opioid pain relievers through diversion or falsely acquired prescriptions. These policies include prescription drug monitoring programs (PMPs) and practice guidelines recommending more judicious prescribing. Other policies focus on reducing the potential for misuse of, or harm from, prescription opioids, including tamper-resistant reformulations, expanded access to the overdose rescue medication naloxone, and medication-assisted treatment (MAT).
Limiting the supply of prescription opioids is likely to generate both positive and negative health effects. Reduced opioid prescribing could simultaneously reduce opioid addiction incidence while decreasing the quality of pain management for patients with legitimate need.9 Experts debate whether the rise in heroin deaths that began in 2010 was driven in part by the imposition of greater controls on prescription opioids.7,10,11 Reduction in the prescription opioid pill supply could spur some currently addicted individuals to seek addiction treatment but incite others to transition to illicit opioids, including heroin. Such a reduction could generate net harm in the short term by diverting some currently addicted individuals to heroin, but net benefit in the long term by reducing the number of people who become opioid addicted (i.e., addiction that would occur if the supply of opioids is not reduced).
Modeling is a powerful tool to estimate the effects of public policy options. This study models the short- and long-term impact of a range of potential responses to the opioid epidemic.
METHODS
We aimed to project the impact of policies that affect the opioid supply and sequelae of addiction. We developed a dynamic compartmental model, dividing the population into compartments that individuals flow between according to parameters that describe the dynamics of opioid prescribing and addiction. This is common for evaluating the spread of contagious disease12,13 and appropriate for modeling the opioid epidemic because it allows for dynamic modeling of addiction incidence to reflect the changing number of prescription holders.
Figure A (available as a supplement to the online version of this article at http://www.ajph.org) illustrates our model schematic. Arrows indicate possible transitions between compartments. For example, a “chronic pain, nonuser [of opioids]” may be prescribed opioids and transition to the “chronic pain with Rx” compartment. He may become addicted and transition to the “chronic pain SOUD [severe prescription opioid use disorder] with Rx” compartment. If he is later unable to continue getting opioids prescribed, he transitions to the “SOUD without Rx” compartment. Compartments representing addiction incur elevated mortality risk, so individuals therein transition to “dead” at higher rates. However, implementing a policy that, for example, increases naloxone availability, would dampen this effect, slowing such a transition.
We estimated model parameter values (e.g., prescribing rates, mortality rates) dictating rates of flow between compartments based on published literature, expert opinion, and model calibration (Table A, available as a supplement to the online version of this article at http://www.ajph.org). Because many values are highly uncertain, we created 10 base case models, each with a different set of parameter values that produce plausible status quo results (Supplemental Methods S1.6, Table C, available as a supplement to the online version of this article at http://www.ajph.org). We simulated the period 2016 to 2025 with calculations in monthly increments. We measured life years (LYs), quality-adjusted life years (QALYs), prescription opioid and heroin addiction–related deaths, and addiction prevalence and incidence.
We segmented the US population aged 12 years and older according to pain status (acute pain, chronic pain, and pain-free), opioid use status (no use, use with a prescription, and use without a prescription), and addiction status. We defined individuals with addiction as those meeting Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for severe substance use disorder.14,15 The addiction statuses included SOUD; severe heroin-use disorder (SHUD), including simultaneous use of pills; enrollment in MAT for SOUD or SHUD; and nonaddicted.
We estimated acute pain prevalence from inpatient16 and outpatient surgery17 rates and emergency department visits for trauma,18,19 adjusted to account only for moderate to severe pain, based on a postoperative pain survey.20 We assumed acute pain lasts for up to 1 month, after which the pain either becomes chronic or the individual heals and returns to the pain-free state. We estimated chronic pain prevalence using published data,21–23 adjusted to account only for moderate to severe pain24 and tuned our estimated rates of chronic pain incidence and resolution to achieve constant prevalence over the time horizon.
Some individuals with acute pain receive a short-term prescription for opioid analgesics25,26; if the pain becomes chronic, some may begin opioid treatment of chronic pain. We assumed a 4.6% annual decline in opioid prescribing from 2012 to 2015,27 and a constant prescribing level from 2016 to 2025. We accounted for the possibility that individuals with SOUD may also be prescribed opioids.
We used RAND Corporation’s estimate of chronic heroin users as a proxy for SHUD prevalence28 and assumed that 80% of individuals with SHUD first misused prescription opioids.29 We included only these heroin users in our model. We estimated SOUD prevalence on the basis of reported death rates,30 adjusted for underreporting,31 and our approximation for overdose risk.
We modeled SOUD incidence among the pained population prescribed opioids (iatrogenic addiction) as a constant proportion of users. We modeled SOUD incidence caused by diversion of pills to “pain-free nonusers” with a susceptible–infected epidemic model in which “pain-free nonusers” are the “susceptible” population and prescription holders are the “infected” population. Interaction between these groups leads to drug diversion and results in some “pain-free nonusers” becoming addicted to opioids. Pills may also be diverted to the “SOUD without prescription” population. We assumed these individuals must acquire pills through diversion to sustain their addiction; if too few pills are available for diversion, some of these individuals will escalate to SHUD, enter MAT, or cease opioid use through other means (e.g., psychosocial treatment). In addition, we assumed some individuals with SOUD escalate to SHUD regardless of the pill supply.
We did not explicitly model the complex and often relapsing nature of recovery from substance use disorder,32 but instead assumed that each month, some fraction of addicted individuals cease opioid use and that individuals with SHUD are less likely to desist than those with SOUD. It has been shown that MAT is the most effective available treatment (more than purely psychosocial treatment) for reducing illicit opioid use and mortality.33 We modeled the potential for individuals with SOUD and SHUD to enroll in MAT; for those not enrolled in MAT, we assumed psychosocial addiction treatment increases their rate of desistance, but to a lesser extent.
We assumed higher overdose mortality risk for individuals with SHUD than with SOUD, and that MAT lowers mortality risk.33 Individuals with SHUD incur additional mortality risk because of infection from injection drug use (primarily HIV and hepatitis C virus).34
To calculate QALYs, we associated acute and chronic pain with quality-of-life decrements.35 We assumed that, for acute pain only, opioid analgesics mitigate that decrement.36 No clear evidence supports an average utility benefit for the treatment of chronic pain with opioids37–39; we tested other assumptions in sensitivity analysis. We assigned utility decrements for SOUD and SHUD,40–42 and assumed that MAT mitigates these losses.43
Interventions
We considered 11 interventions (Table B, Supplemental Methods S1.9, available as a supplement to the online version of this article at http://www.ajph.org). Some are directed mainly at preventing new instances of SOUD: reduced opioid prescribing rates for acute pain, transitioning pain, and chronic pain; rescheduling opioids under the Controlled Substances Act, causing reduced likelihood of prescriptions getting refilled; expanding excess opioid disposal programs to reduce drug diversion; and tamper-resistant and abuse-deterrent opioid reformulation. Other interventions aim to treat or mitigate the impact of current cases of addiction: expanding MAT availability to increase enrollment, increasing psychosocial treatment availability to increase desistance rates, increasing naloxone availability to reduce overdose death rates, and expanding needle exchange programs to reduce infection mortality among heroin users. A final policy, enhancing PMPs (e.g., improving provider access, mandating use) supports appropriate prescribing for all patients and helps identify patients who are misusing drugs, reducing the likelihood of refills and prescribing to SOUD individuals and slightly reducing prescribing in general. Table B (available as a supplement to the online version of this article at http://www.ajph.org) describes the assumed magnitude and effect of each intervention on model parameters in our base analyses. These values are hypothetical but designed to be sufficiently plausible to help develop intuitive understanding of the consequences of different interventions.
Analyses
We evaluated the 5- and 10-year impact of interventions on each outcome measure (relative to the status quo with no incremental interventions) for each base case. At the end of the modeled time horizon, additional Markov models captured the future LYs and QALYs accruing to individuals still alive in the model. Threshold analysis determined the effect magnitude required to reduce opioid-related deaths by 10% over the modeled time horizons. We performed sensitivity analyses on key model parameters.
RESULTS
Without intervention, we would expect 235 000 opioid-related deaths (85 000 from prescription opioids and 150 000 from heroin) from 2016 to 2020; and 510 000 opioid-related deaths (170 000 from prescription opioids and 340 000 from heroin) from 2016 to 2025 (Tables D and E, Figures B–K, available as supplements to the online version of this article at http://www.ajph.org). This is the “status quo.” Our model projects how these trajectories would change under various interventions.
Five-Year Analysis
Table 1 and Appendix Table F (available as a supplement to the online version of this article at http://www.ajph.org) show projected outcomes of each intervention over 5 years, compared with the status quo. Notably, none of the policies substantially reduces opioid-related deaths. Increasing naloxone availability resulted in the greatest number of addiction deaths averted among the 11 interventions, representing a 4% reduction.
TABLE 1—
Estimated Effects of Individual Interventions Over 5 Years: United States, 2016–2020
| Intervention | Mean Changea Compared With the Status Quo | ||||
| Discounted Net Present LYs,b No. in Thousands (%) | Discounted Net Present QALYs,b No. in Thousands (%) | Pill Deaths, No. (%) | Heroin Deaths, No. (%) | Total Opioid Deaths, No. (%) | |
| Acute pain prescribing | 240 (0.004) | −770 (−0.013) | −2 000 (−2.3) | 100 (0.0) | −1 900 (−0.8) |
| Prescribing for transitioning pain | 30 (0.001) | 90 (0.002) | −800 (−1.0) | 700 (0.4) | −200 (−0.1) |
| Chronic pain prescribing | −90 (−0.002) | 320 (0.005) | −8 300 (−9.7) | 10 600 (6.9) | 2 300 (1.0) |
| Drug rescheduling | −1 330 (−0.021) | 110 (0.002) | −45 500 (−53.3) | 70 000 (45.6) | 24 500 (10.3) |
| PMP | −1 260 (−0.020) | −880 (–0.014) | −19 100 (−22.4) | 34 400 (22.4) | 15 300 (6.4) |
| Drug reformulation | 150 (0.002) | 1 060 (0.017) | −15 600 (−18.3) | 16 900 (11.0) | 1 300 (0.5) |
| Excess opioid disposal | 80 (0.001) | 270 (0.004) | −2 800 (−3.3) | 2 500 (1.7) | −300 (−0.1) |
| Naloxone availability | 640 (0.010) | 530 (0.009) | −4 200 (−4.9) | −6 000 (−3.9) | −10 200 (-4.3) |
| Needle exchange | 160 (0.003) | 130 (0.002) | 0 (0.0) | −2 700 (−1.8) | −2 700 (−1.1) |
| MAT | 390 (0.006) | 670 (0.011) | −900 (−1.1) | −4 000 (−2.6) | −4 900 (−2.1) |
| Psychosocial treatment | 220 (0.004) | 340 (0.005) | −600 (−0.7) | −1 300 (−0.9) | −1 900 (−0.8) |
Note. LY = life year; MAT = medication-assisted treatment; PMP = prescription monitoring program; QALY = quality-adjusted life year.
Ranges over the 10 base cases are shown Table F (available as a supplement to the online version of this article at http://www.ajph.org).
Discounted to 2016.
Naloxone availability, needle exchange, MAT, and psychosocial treatment policies generate gains in LYs and QALYs and reduce deaths, without harming any group. Reduced prescribing for transitioning pain and excess opioid disposal increase LYs and QALYs and decrease total addiction deaths; however, the mitigation in deaths from prescription opioids is partially offset by increased heroin-related deaths. Tamper-resistant drug reformulation increased total addiction deaths because heroin-related deaths increased to a greater extent than prescription opioid deaths decreased. However, LYs and QALYs increased because many individuals avoided addiction.
Reduced acute pain prescribing has effects similar to reduced transitioning pain prescribing; however, undertreatment of pain causes a QALY loss. Reduced chronic pain prescribing, drug rescheduling, and PMPs reduce deaths from prescription opioid use, but increase heroin deaths, yielding a net increase in addiction-related deaths. Drug rescheduling and reduced prescribing for chronic pain reduce total LYs but the net impact on QALYs, paradoxically, is positive: enough people avert SOUD and ultimately live in substantially higher-utility health states (e.g., “pain-free nonuser”) to counterbalance the much larger number who transition to SHUD (a health state only slightly worse than SOUD) and the relatively small number of people who die.
Ten-Year Analysis
We projected the effect of each policy over 10 years (Table 2, Table H, available as a supplement to the online version of this article at http://www.ajph.org). Addiction deaths avoided under the naloxone availability and needle-exchange policies grow approximately proportionally to the time horizon. However, some policies—reduced prescribing for acute pain, reduced prescribing for transitioning pain, excess opioid disposal, psychosocial treatment, and MAT—avert significantly more deaths over 10 years than would be proportionally expected compared with 5 years. Furthermore, over 10 years, reduced acute pain prescribing decreases deaths from heroin use and drug reformulation decreases total addiction deaths, despite increasing deaths over 5 years. Reduced chronic pain prescribing and drug rescheduling still increase total addiction deaths and the PMP policy results in disproportionally more deaths over 10 years compared with 5 years. However, total LYs increase under reduced chronic pain prescribing, despite decreasing relative to the status quo over a 5-year horizon.
TABLE 2—
Estimated Effects of Individual Interventions Over 10 Years: United States, 2016–2025
| Intervention | Mean Changea Compared With the Status Quo | ||||
| Discounted Net Present LYs,b No. in Thousands (%) | Discounted Net Present QALYs,b No. in Thousands (%) | Pill Deaths, No. (%) | Heroin Deaths, No. (%) | Total Opioid Deaths, No. (%) | |
| Acute pain prescribing | 500 (0.007) | −450 (−0.007) | −6 100 (−3.6) | −1 900 (−0.6) | −8 000 (−1.6) |
| Prescribing for transitioning pain | 80 (0.001) | 180 (0.003) | −2 600 (−1.5) | 1 500 (0.5) | −1 000 (−0.2) |
| Chronic pain prescribing | 40 (0.001) | 670 (0.010) | −24 400 (−14.2) | 28 200 (8.2) | 3 800 (0.7) |
| Drug rescheduling | −920 (−0.014) | 990 (0.015) | −103 800 (−60.7) | 146 600 (42.8) | 42 800 (8.3) |
| PMP | −1 780 (−0.027) | −1 450 (−0.022) | −47 800 (−28.0) | 90 200 (26.3) | 42 300 (8.2) |
| Drug reformulation | 650 (0.010) | 2 000 (0.030) | −43 300 (−25.3) | 39 400 (11.5) | −3 900 (−0.8) |
| Excess opioid disposal | 210 (0.003) | 510 (0.008) | −7 900 (−4.6) | 5 500 (1.6) | −2 400 (−0.5) |
| Naloxone availability | 790 (0.012) | 670 (0.010) | −8 400 (−4.9) | −12 700 (−3.7) | −21 200 (−4.1) |
| Needle exchange | 210 (0.003) | 180 (0.003) | 0 (0.0) | −5 900 (−1.7) | −5 900 (−1.1) |
| MAT | 560 (0.008) | 940 (0.014) | −2 900 (−1.7) | −9 600 (−2.8) | −12 500 (−2.4) |
| Psychosocial treatment | 440 (0.007) | 650 (0.010) | −1 600 (−0.9) | −6 000 (−1.7) | −7 500 (−1.5) |
Note. LY = life year; MAT = medication-assisted treatment; PMP = prescription monitoring program; QALY = quality-adjusted life year.
Ranges over the 10 base cases are shown in Table H (available as supplement to the online version of this article at http://www.ajph.org).
Discounted to 2016.
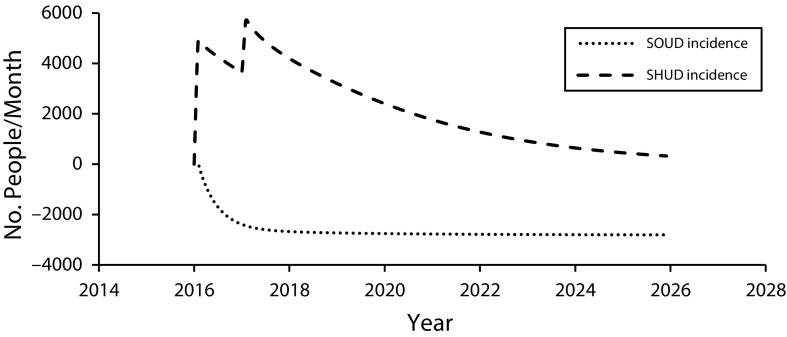
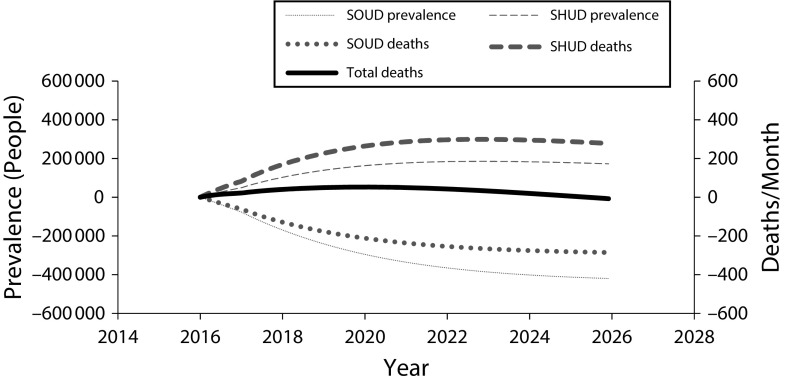
Figure 1 shows monthly SOUD and SHUD incidence over 10 years for the reduced chronic pain prescribing policy and Figure 2 shows SOUD and SHUD prevalence and related deaths for that policy. (Figures L and M, available as supplements to the online version of this article at http://www.ajph.org, show results for all policies and base cases.) This policy reduces iatrogenic addiction incidence, which reduces SOUD prevalence over time. Heroin use immediately increases because some “doctor shoppers” are no longer able to obtain a prescription and, additionally, spikes 1 year after policy initiation when some addicted individuals who can no longer obtain diverted pills switch to heroin (we assumed a 1-year lag before the reduction in pills prescribed would reduce the quantity being diverted), but then declines gradually as iatrogenic addiction to prescription opioids decreases. By 2023, SHUD prevalence under this policy is projected to decline. The SOUD and SHUD deaths mirror the prevalence trajectories, and, by 2026, monthly opioid addiction-related deaths are less than under the status quo.
FIGURE 1—
Estimated Effects of Reduced Prescribing for Chronic Pain on Addiction Incidence: United States, 2016–2025
Note. SOUD = severe prescription opioid use disorder; SHUD = severe heroin use disorder. Mean effects of the reduced prescribing for chronic pain policy on monthly incidence, relative to without intervention, of SOUD and SHUD.
FIGURE 2—
Estimated Effects of Reduced Prescribing for Chronic Pain on Addiction Prevalence and Deaths: United States, 2016–2025
Note. SOUD = severe prescription opioid use disorder; SHUD = severe heroin use disorder. Mean effects of the reduced prescribing for chronic pain policy on prevalence and deaths, relative to without intervention, of or from SOUD and SHUD.
Threshold Analysis
We determined intervention magnitudes needed to reduce addiction-related deaths by 10% over 5 years (Table J, available as a supplement to the online version of this article at http://www.ajph.org). Only the naloxone availability, needle exchange, MAT, and psychosocial treatment policies had the potential to do so. Naloxone availability would need to reduce overdose death risk by 12% compared with the status quo, a possibly achievable level. Needle exchange would need to reduce infection mortality associated with drug injection by 88%, a level that is likely unrealistic.44 Expansion of MAT would need to increase likelihood of enrollment in MAT by 144% compared with the status quo (approximately 10% of individuals with SOUD and SHUD would have to enter MAT each month)—a level that is probably not achievable. Psychosocial treatment would need to increase likelihood of desistance (outside of MAT) by 134%, which is equivalent to approximately 1% of individuals who receive such treatment desisting from opioid use each month. Under these alternative policy implementations, LYs gained ranged from 0.02% (for increased naloxone availability and needle exchange) to 0.04% (for psychosocial treatment) and QALYs gained ranged from 0.02% (for increased naloxone availability and needle exchange) to 0.07% (for psychosocial treatment).
Over 10 years (Table K, available as a supplement to the online version of this article at http://www.ajph.org), drug reformulation could also reduce opioid deaths by 10%, but only under unrealistic effect levels. Notably, our model suggests that, over 5 years, even the mildest implementation of the drug rescheduling policy would increase addiction deaths, but, over 10 years, the policy would reduce addiction deaths if aggressively implemented (Figure N, available as a supplement to the online version of this article at http://www.ajph.org).
Portfolios of Interventions
We examined the effects of combining policies (Supplemental Results S2.4, Tables G and I, available as supplements to the online version of this article at http://www.ajph.org), focusing on pairing interventions that prevent the spread of SOUD with those that treat or mitigate its effects. The results of these combinations were nearly additive. For example, over 5 years, we expect 13 800 addiction deaths relative to the status quo if drug rescheduling and increased naloxone availability are implemented together, compared with 24 500 additional deaths and 10 200 fewer deaths if each intervention were implemented alone. Combining interventions can increase health benefits. For example, our model projects that combining the reduced acute, transitioning, and chronic pain prescribing, excess opioid disposal, MAT, needle exchange, naloxone availability, and psychosocial treatment interventions would reduce 10-year addiction deaths by 59 000 (11%) and increase LYs and QALYs by 0.05% and 0.07%, respectively.
Sensitivity Analyses
We examined the impact of several policies under alternative parameter assumptions and found substantial variation in effects (Figures O–T, available as supplements to the online version of this article at http://www.ajph.org). A key parameter influencing the effects of policies that reduce the opioid pill supply is the likelihood of escalation from SOUD to SHUD: for example, if the likelihood of escalation is 25% lower than we assumed, then by 2022 annual addiction deaths for the drug rescheduling policy (Figure O, part d) would be lower than the status quo; and if the likelihood is 50% lower than we assumed, an immediate and sustained reduction in total opioid deaths would occur. If the overdose mortality risk for individuals with SOUD is 25% greater than we assumed, the drug reformulation policy would reduce total opioid deaths over 5 years (Figure Q, part f).
DISCUSSION
We modeled the projected impact of a range of policies aimed at curbing opioid addiction and reducing addiction deaths. We found that policies that expand addiction treatment or directly mitigate harmful effects of addiction (e.g., overdose, infection) are immediately and uniformly beneficial, with no negative impact on LYs, QALYs, or addiction deaths. Policies that reduce the prescription opioid supply may generate both benefits and harms (at least in the short term); such policies decrease addiction-related deaths from prescription pill use but may increase heroin-related deaths as some people with SOUD turn to cheaper, more dangerous heroin. For some policies and time horizons, the increase in heroin-related deaths may exceed the reduction in opioid pill–related addiction deaths, despite overall gains in quality of life.
Although PMPs reduce prescription opioid deaths,45 our model suggests that the detrimental effect on heroin use and resulting deaths may outweigh this benefit for the period of time modeled here. For other prescription-focused policies, such as reduced opioid prescribing for acute pain, addiction-related deaths are averted, but total QALYs decrease because of undertreatment of pain for individuals who can benefit from opioid analgesics. In cases where an intervention averts deaths for some individuals but reduces QALYs for the general population, or increases overall QALYs while increasing deaths in a subpopulation, policymakers face difficult value judgments on the best course of action, reflecting the reality that policymaking cannot be driven solely by research evidence.46
Though there is substantial uncertainty regarding the likely magnitude of various policies, our threshold analysis suggests that no single policy is likely to have a large enough impact to substantially reduce addiction-related deaths over 5 or even 10 years. Moreover, strategies that focus solely on mitigating immediate impacts of addiction will not address the root of the problem. Instead, to effectively combat the epidemic, a portfolio of interventions is likely needed to prevent iatrogenic addiction, prevent addiction from drug diversion, treat addiction, and mitigate its effects. A key example is policies that result in an addicted person being offered treatment when denied an inappropriate opioid prescription (e.g., because a PMP flagged them), rather than the individual being cast out of the clinical relationship, which could lead them to transition to heroin use.
Furthermore, although some policies provide more benefit than others in the short term, a longer-term perspective is useful: as policies gradually reduce prescription opioid use, incidence of heroin initiation stemming from opioid pill use and addiction will eventually decline. Thus, for example, although our base case analysis found that reduced chronic pain prescribing did not reduce addiction-related deaths over 5 or 10 years, it reduces incidence of opioid addiction and would eventually reduce deaths.
Limitations
Our analysis has several limitations. First, the drivers behind the opioid epidemic are dynamic, nonlinear, and uncertain. Although we tested the impact of each policy on multiple potential models of the current state, the epidemic continues to change and may be substantially different in just 5 years. For example, the increasing prevalence of fentanyl makes heroin use far more deadly.47 Furthermore, given limited published studies of opioid use disorder, we had to make many assumptions about transition probabilities and policy effect sizes and solicit expert opinions from scientists and clinicians.
Second, substance use disorder is a complex disease with varying degrees of severity and high relapse and recurrence rates.32 Our model is a simplification of the phenomenon, intended to capture only enough detail to inform key high-level policy questions.
Third, our model only accounts for the portion of the opioid epidemic that directly results from prescription pill use; we did not account for the approximately 20% of heroin users whose addiction did not originate with opioid prescription pills.29 Thus, for example, we did not account for possible increased heroin initiation when heroin markets emerge in areas with significant levels of prescription opioid use and addiction.48 Interventions that prevent heroin use would appear more attractive if this second-order effect were included.
Fourth, our model does not capture every benefit of interventions. For example, we did not model the benefit of PMPs in helping identify potentially dangerous combinations of medications prescribed to patients, reduced transmission of HIV and hepatitis C stemming from reduced intravenous heroin use, nor deaths from drug-related homicide or suicide.
Finally, though we modeled the US population on average to gain high-level policy insights, different geographical regions, age groups, races, and genders will experience different severities and drivers of opioid-related problems.
Public Health Implications
Our results suggest that some policy responses to the opioid epidemic may reduce prescription opioid misuse but increase heroin use, blunting or even eliminating any public health benefit in the short term (e.g., the next 5 years) but yielding net positive health benefits in the longer term. Policies that focus on services for currently addicted people provide health benefits immediately without causing harm. However, no epidemic has ever been averted solely by treating single affected cases. Instead, portfolios of policies will likely be required, including those that prevent addiction, treat addiction, and mitigate its effects. Our analysis provides insights into important questions about the potential impact of targeted efforts to combat the opioid crisis. Before investment is made, further data will be needed to tailor a model and parameters to specific settings and behavior patterns.
ACKNOWLEDGMENTS
This study was supported by grant R01-DA15612 from the National Institute on Drug Abuse. K. Humphreys was supported by a Senior Career Research Scientist award from the VA Health Services Research and Development Service.
Note. Opinions expressed in this article are not necessarily those of the funders.
HUMAN PARTICIPANT PROTECTION
Institutional review board approval was not needed because there were no human participants involved in this study.
Footnotes
See also Goodin, p. 1286.
REFERENCES
- 1.Ahmad FB, Rossen LM, Spencer MR, Warner M, Sutton P. Provisional drug overdose death counts. Atlanta, GA: National Center for Health Statistics; 2017. [Google Scholar]
- 2.Krashin D, Sullivan M, Ballantyne J. What are we treating with chronic opioid therapy? Curr Rheumatol Rep. 2013;15(3):311. doi: 10.1007/s11926-012-0311-1. [DOI] [PubMed] [Google Scholar]
- 3.Meldrum ML. The ongoing opioid prescription epidemic: historical context. Am J Public Health. 2016;106(8):1365–1366. doi: 10.2105/AJPH.2016.303297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lembke A, Humphreys K, Newmark J. Weighing the risks and benefits of chronic opioid therapy. Am Fam Physician. 2016;93(12):982–990. [PubMed] [Google Scholar]
- 5.Beauchamp GA, Winstanley EL, Ryan SA, Lyons MS. Moving beyond misuse and diversion: the urgent need to consider the role of iatrogenic addiction in the current opioid epidemic. Am J Public Health. 2014;104(11):2023–2029. doi: 10.2105/AJPH.2014.302147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manchikanti L, Helm S, 2nd, Fellows B et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3, suppl):ES9–ES38. [PubMed] [Google Scholar]
- 7.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services. Addressing Prescription Drug Abuse in the United States: Current Activities and Future Opportunities. Washington, DC: US Department of Health and Human Services; 2013. [Google Scholar]
- 9.Glod SA. The other victims of the opioid epidemic. N Engl J Med. 2017;376(22):2101–2102. doi: 10.1056/NEJMp1702188. [DOI] [PubMed] [Google Scholar]
- 10.Cicero TJ, Kurtz SP, Surratt HL et al. Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues. 2011;41(2):283–304. doi: 10.1177/002204261104100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dart RC, Surratt HL, Cicero TJ et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 12.Long EF, Brandeau ML, Galvin CM et al. Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS. 2006;20(17):2207–2215. doi: 10.1097/QAD.0b013e328010c7d0. [DOI] [PubMed] [Google Scholar]
- 13.Zaric GS, Brandeau ML. Dynamic resource allocation for epidemic control in multiple populations. IMA J Math Appl Med Biol. 2002;19(4):235–255. [PubMed] [Google Scholar]
- 14.Hasin DS, O’Brien CP, Auriacombe M et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170(8):834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 16.Cullen KA, Hall MJ, Golosinskiy A. 2009. Ambulatory surgery in the United States, 2006. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 17.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5:1–20. [PubMed] [Google Scholar]
- 18.Sullivan D, Lyons M, Montgomery R, Quinlan-Colwell A. Exploring opioid-sparing multimodal analgesia options in trauma: a nursing perspective. J Trauma Nurs. 2016;23(6):361–375. doi: 10.1097/JTN.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Injury Prevention and Control. CDC Injury Fact Book. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 20.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 22.Tsang A, Von Korff M, Lee S et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Vetter TR. The Epidemiology of Pediatric Chronic Pain. Handbook of Pediatric Chronic Pain. New York, NY: Springer Publishers; 2011. pp. 1–14. [Google Scholar]
- 24.Huguet A, Miró J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9(3):226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Calcaterra SL, Yamashita TE, Min S-J, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478–485. doi: 10.1007/s11606-015-3539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mudumbai SC, Oliva EM, Lewis ET et al. Time-to-cessation of postoperative opioids: a population-level analysis of the Veterans Affairs Health Care System. Pain Med. 2016;17(9):1732–1743. doi: 10.1093/pm/pnw015. [DOI] [PubMed] [Google Scholar]
- 27.Goodnough A, Tavernise S. Opioid prescriptions drop for first time in two decades. New York Times. May 20, 2016. Available at: https://www.nytimes.com/2016/05/21/health/opioid-prescriptions-drop-for-first-time-in-two-decades.html. Accessed July 27, 2018. [Google Scholar]
- 28.RAND Corporation. Washington, DC: 2014. What America’s users spend on illegal drugs: 2000–2010. Office of National Drug Control Policy, Office of Research and Data Analysis. [Google Scholar]
- 29.Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. Center for Behavioral Health Statistics and Quality Data Review. 2013 Available at: http://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Accessed June 17, 2017. [Google Scholar]
- 30.Centers for Disease Control and Prevention. Wide-ranging online data for epidemiologic research (WONDER). 2016. Available at: https://wonder.cdc.gov. Accessed June 23, 2017.
- 31.Ruhm CJ. Geographic variation in opioid and heroin involved drug poisoning mortality rates. Am J Prev Med. 2017;53(6):745–753. doi: 10.1016/j.amepre.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 32.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 33.Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357–368. doi: 10.1056/NEJMra1604339. [DOI] [PubMed] [Google Scholar]
- 34.Evans JL, Tsui JI, Hahn JA, Davidson PJ, Lum PJ, Page K. Mortality among young injection drug users in San Francisco: a 10-year follow-up of the UFO Study. Am J Epidemiol. 2012;175(4):302–308. doi: 10.1093/aje/kwr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nosyk B, Sun H, Guh DP et al. The quality of eight health status measures were compared for chronic opioid dependence. J Clin Epidemiol. 2010;63(10):1132–1144. doi: 10.1016/j.jclinepi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Birke H, Ekholm O, Sjøgren P, Kurita GP, Højsted J. Long-term opioid therapy in Denmark: a disappointing journey. Eur J Pain. 2017;21(9):1516–1527. doi: 10.1002/ejp.1053. [DOI] [PubMed] [Google Scholar]
- 38.Chou R, Turner JA, Devine EB et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 39.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96(9):1267–1278. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- 41.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1–9. doi: 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- 42.Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health. 2000;90(7):1100–1111. doi: 10.2105/ajph.90.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhawan A, Chopra A. Does buprenorphine maintenance improve the quality of life of opioid users? Indian J Med Res. 2013;137(1):130–135. [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan EH, O’Keefe E. Let the needles do the talking! Evaluating the New Haven needle exchange. Interfaces. 1993;23(1):7–26. [Google Scholar]
- 45.Pardo B. Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction. 2017;112(10):1773–1783. doi: 10.1111/add.13741. [DOI] [PubMed] [Google Scholar]
- 46.Humphreys K, Piot P. Scientific evidence alone is not sufficient basis for health policy. BMJ. 2012;344:e1316. doi: 10.1136/bmj.e1316. [DOI] [PubMed] [Google Scholar]
- 47.Lewis N, Ockerman E, Achenbach J, Lowery W. Fentanyl linked to thousands of urban overdose deaths. Washington Post. August 15, 2017. Available at: https://www.washingtonpost.com/graphics/2017/national/fentanyl-overdoses/?noredirect=on&utm_term=.d189cdb6734a. Accessed July 27, 2018. [Google Scholar]
- 48.Quinones S. Dreamland: The True Tale of America’s Opiate Epidemic. New York, NY: Bloomsbury Publishing; 2015. [Google Scholar]