Abstract
Data System. The Pregnancy Risk Assessment Monitoring System (PRAMS) is an ongoing state-based surveillance system of maternal behaviors, attitudes, and experiences before, during, and shortly after pregnancy. PRAMS is conducted by the Centers for Disease Control and Prevention’s Division of Reproductive Health in collaboration with state health departments.
Data Collection/Processing. Birth certificate records are used in each participating jurisdiction to select a sample representative of all women who delivered a live-born infant. PRAMS is a mixed-mode mail and telephone survey. Annual state sample sizes range from approximately 1000 to 3000 women. States stratify their sample by characteristics of public health interest such as maternal age, race/ethnicity, geographic area of residence, and infant birth weight.
Data Analysis/Dissemination. States meeting established response rate thresholds are included in multistate analytic data sets available to researchers through a proposal submission process. In addition, estimates from selected indicators are available online.
Public Health Implications. PRAMS provides state-based data for key maternal and child health indicators that can be tracked over time. Stratification by maternal characteristics allows for examinations of disparities over a wide range of health indicators.
The Pregnancy Risk Assessment Monitoring System (PRAMS) is part of the Centers for Disease Control and Prevention (CDC) initiative to reduce infant mortality and low birth weight and promote safe motherhood. PRAMS was implemented in 1987 because infant mortality rates were no longer declining as rapidly as they had been in prior years.1 Although the US infant mortality rate has dropped 15% over the past decade, the United States continues to have one of the highest infant mortality rates among developed countries, at 5.8 per 1000 live births in 2015.2 Despite recent declines, preterm birth rates remain high (9.9% in 2016),3 and sudden infant death syndrome is the leading cause of death among infants 1 to 12 months old (approximately 1600 deaths in 2015).4
Maternal mortality and morbidity rates have also been increasing. The number of reported pregnancy-related deaths in the United States rose from 7.2 per 100 000 live births in 1987 to 17.3 per 100 000 live births in 2013.5,6 Moreover, the number of women presenting at delivery with 1 or more chronic conditions rose from 66.9 per 1000 delivery hospitalizations in 2005–2006 to 91.8 per 1000 delivery hospitalizations in 2013–2014.7
DATA PROGRAM
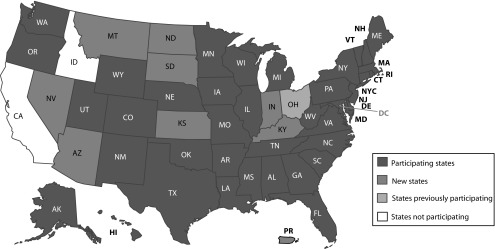
PRAMS is an ongoing state-level, population-based surveillance system of selected maternal behaviors and experiences that occur before, during, and shortly after pregnancy. It is conducted by participating state, territorial, tribal, or local health departments in partnership with CDC’s Division of Reproductive Health. CDC provides annual funding to participating sites through a cooperative agreement, with supplemental funding contributed by recipients. Since the system’s inception, the number of participating states and areas (referred to hereafter as states) has increased from 6 to 51, including 47 states, the District of Columbia, New York City, Puerto Rico, and the Great Plains Tribal Chairman’s Health Board (Figure 1). PRAMS surveillance currently covers approximately 83% of all US births.
FIGURE 1—
Participating States: Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 2017
Note. PRAMS represents approximately 83% of all US live births.
Purpose
The main purposes of PRAMS are to promote the collection, analysis, and dissemination of population-based data of high scientific quality and to support the use of data to develop policies and programs that aim to decrease maternal and infant morbidity and mortality. PRAMS data are used by academic researchers, nonprofit health organizations, state health departments, and federal agencies to guide development of new programs and policies, evaluate existing programs and policies, develop educational materials for health care providers and the public, and contribute to general health knowledge.
Public Health Significance
PRAMS provides state-specific data used to monitor health behaviors, access to care, and receipt of services among recently pregnant women. For example, PRAMS data have been used to monitor targets in Healthy People 2020,8 Title V National Performance Measures for safe sleep and preventive dental visits,9 National Quality Forum performance measures for postpartum contraception,10 preconception health and health care indicators,11 and selected performance measures for various public health programs and initiatives.12,13
PRAMS also provides estimates on a variety of maternal and infant health indicators that are not available from any other data source. For example, PRAMS data have been used to monitor progress over time in terms of infant sleep position,14 unintended births,15 and patterns of health insurance coverage before, during, and after pregnancy.16 By collecting data about the preconception and postpartum periods, in addition to the time during pregnancy, PRAMS has information on a wide range of risk factors that influence maternal and infant health. PRAMS data can be used to examine the associations between risk factors and outcomes, explore disparities by subpopulations, and compare health indicators across states. (Many state examples describing the impact of PRAMS data can be found at https://www.cdc.gov/prams/dta-successstories.html.)
DATA COLLECTION/PROCESSING
States are responsible for their PRAMS data collection activities. All states follow a standard data collection protocol with built-in flexibility to tailor projects to their individual needs.
Data Sources and Collection Mode
PRAMS is a mixed-mode (mail and telephone) surveillance system, based on Dillman and colleagues’ tailored design method, that incorporates many techniques developed to enhance response.17 These techniques include personalized mailing packages, use of response incentives and rewards, and repeated but varied contact attempts. The primary data collection mode is mail, with telephone follow-up for mail nonrespondents.
Multiple attempts to contact sampled women are made within each mode. PRAMS states make up to 5 contact attempts via mail, including an invitation to participate in the survey, first survey mailing, tickler (thank you or reminder note), second survey mailing, and third survey mailing. Telephone contact begins within a week after the last survey mailing. Up to 15 call attempts, staggered over different times of the day and different days of the week, are made to each working telephone number.
A variety of sources are used to obtain 1 or more valid telephone numbers for a mother. Increasingly, telephone numbers are captured on birth certificate files. Approximately half of participating states (48%) have access to telephone numbers from these files. Many states make use of databases of other health department programs (e.g., newborn screening and immunization programs and the Special Supplemental Nutrition Program for Women, Infants, and Children) to locate contact information for mothers who may also be participating in or tracked by those programs. Publicly available phone sources are consulted as well, usually after health department sources have been exhausted.
The processes just described are used by most states according to the CDC model protocol.18 The flexibility of the PRAMS methodology allows each state to tailor various data collection strategies to meet its unique needs, including scheduling of mailings, appearance of mailing materials, and use of response incentives and rewards.
All states use either response incentives (sent before the survey is completed) or rewards (sent after the survey has been completed) to increase participation (Table 1). A wide variety of items are used, with the most popular being prepaid gift cards, cash, complimentary birth certificates, and baby items such as diapers, bibs, music CDs, books, picture frames, and thermometers. In recent years, many PRAMS states have enhanced their incentives and rewards to encourage response. Some states have implemented targeted rewards offered to certain groups that traditionally have lower response rates (e.g., phone respondents, adolescents, and mothers of deceased infants).
TABLE 1—
Stratification Variables, Annual Sample Sizes, Response Rates, and Incentives and Rewards: Pregnancy Risk Assessment Monitoring System, United States, 2014
| State | Stratification Variable(s) | Annual Sample Size | Weighted Response Rate, % | Incentivea | Rewarda |
| Alabama | Medicaid status | 1456 | 60.7 | Baby CD | Choice of baby items: T-shirt, sippy cup, tote bag, toothbrush |
| Alaska | Birth weight, maternal race | 1993 | 65.1 | Choice of $10 gift card or baby CD | |
| Arkansasb | Birth weight, geographic area | 951 | 58.1 | Baby picture magnet | $10 gift card, $20 gift card for phone respondents |
| Colorado | Birth weight, geographic area | 2747 | 59.1 | Pen | Quarterly drawing for $200 gift card, $10 gift card for phone respondents |
| Connecticut | Maternal race/ethnicity | 2206 | 59.6 | Pen, magnetic picture frame | $10 Walmart gift card |
| Delaware | Birth weight | 1451 | 64.5 | $30 gift cards before second mail, $20 gift cards for later respondents | |
| Florida | Birth weight, maternal race | 2589 | 47.5 | Baby bib | $10 gift card |
| Georgia | Geographic area | 2506 | 47.1 | $10 Walmart gift card | |
| Hawaii | Geographic area, birth weight | 2139 | 63.5 | $10 gift card | |
| Illinois | Birth weight | 2175 | 66.3 | Immunization card | Stationary, magnetic notepad, or bookmark |
| Iowa | Maternal race/ethnicity | 1977 | 63.6 | Baby book | $10 diaper gift certificate |
| Louisianac | Birth weight, maternal race, geographic area | 2833 | 58.5 | Baby CD | $20 Walmart gift card |
| Maine | Birth weight | 1515 | 62.1 | Birth certificate | |
| Maryland | Birth weight | 2300 | 66.4 | Manicure file | Monthly drawing for a $100 gift card |
| Massachusetts | Maternal race/ethnicity | 2847 | 60.0 | Pen | 120-minute phone card or $10 CVS gift card |
| Michiganc | Birth weight, maternal race, geographic area | 3233 | 57.4 | $10 Walmart gift card | |
| Minnesota | Maternal race | 2632 | 54.4 | Music CD or $9 birth certificate coupon | |
| Mississippid | Birth weight | … | … | Nylon cinch bag | $10 Walmart gift card |
| Missouri | Birth weight | 1723 | 68.8 | $10 gift card for mail respondents, $20 gift card for phone respondents | |
| Nebraska | Maternal race | 2669 | 60.4 | Baby bib, music CD | $5 gift card |
| New Hampshire | Birth weight | 979 | 64.0 | Pens and notepad | Birth certificate |
| New Jersey | Maternal race/ethnicity, smoking status | 1920 | 71.6 | $10 bill | $25 gift card to smokers who respond by phone |
| New Mexico | Maternal race/ethnicity, geographic area, Medicaid/WIC | 2178 | 65.7 | $10 gift card for mail respondents, $20 gift card for phone respondents | |
| New Yorke | Birth weight | 1543 | 60.9 | Baby CD | $15 CVS gift card for mail respondents, $25 CVS gift card for phone respondents |
| New York City | Birth weight | 1838 | 72.3 | $20 cash | |
| North Carolina | Birth weight | 1808 | 54.5 | Baby CD | |
| Ohio | Geographic area | 2597 | 60.1 | $10 Family Dollar gift card | |
| Oklahoma | Birth weight | 2971 | 61.8 | Baby bib | Book, music CD, lunch bag, or notepad |
| Oregon | Maternal race/ethnicity | 2713 | 57.3 | Magnetic photo frame | Drawing for a $200 gift certificate |
| Pennsylvania | Birth weight | 1674 | 68.6 | $10 bill | |
| Rhode Island | Birth weight | 2000 | 62.3 | $5 Walmart gift card | $10 Walmart gift card for mail and phone respondents |
| South Carolina | Birth weight | 1834 | 50.4 | Nylon cinch bags | $10 Walmart gift card |
| Tennessee | Birth weight | 1312 | 60.3 | Baby bib, refrigerator magnet | |
| Texas | Birth weight, maternal race/ethnicity | 2428 | 52.8 | Baby forehead thermometer | $10 gift card |
| Utah | Birth weight, maternal education | 2339 | 69.1 | Insulated lunch bag and gel pen | |
| Vermont | Birth weight | 1389 | 74.3 | Notepad and pen | Music CD |
| Virginia | Birth weight | 1139 | 49.2 | Bookmark | |
| Washington | Maternal race/ethnicity | 2201 | 60.3 | Two $50 gift certificate raffles every month | |
| West Virginia | Birth weight | 2039 | 63.4 | Birth certificate | |
| Wisconsin | Maternal race/ethnicity, geographic area | 2997 | 60.2 | $10 cash (Black women only) | Baby CD |
| Wyoming | Birth weight, maternal race | 1121 | 62.5 | Bath thermometer and pen | Music CD for mail respondents |
Note. WIC = Special Supplemental Nutrition Program for Women, Infants, and Children.
Baby items are not sent to women whose babies have died.
Arkansas 2014 data are available only for mid-April through December.
Louisiana and Michigan included an extra evaluation component in their samples, resulting in larger than normal sample sizes.
2014 data are not available for Mississippi.
New York excludes New York City.
Most states use health department staff to conduct mail survey operations; however, many states contract out the telephone portion to professional survey research organizations. Recently, there has been an increase in the number of states contracting out all data collection activities. In 2016, 12% of states contracted out all data collection activities, 51% contracted out telephone follow-up activities only, and the remaining 37% conducted all activities at the health department.
Ethical Procedures
The general PRAMS methodology and protocol have been reviewed and approved by the CDC institutional review board. In addition, state PRAMS projects undergo review by the local institutional review board of record for the health department. Any deviations from the PRAMS protocol must be approved by both the local and CDC institutional review boards before implementation.
An informed consent document is included within each survey packet explaining the participant’s rights. No written consent is required; rather, consent is implied if the survey is completed. Similarly, the informed consent document is read verbally on phone interviews, and the participant verbally agrees to proceed with the survey. Minors younger than 18 years who have given birth are considered emancipated for the sake of decisions about their children and do not require consent from their parent or guardian to participate. Some states have mandatory reporting laws regarding physical abuse of minors. PRAMS includes questions regarding physical abuse; however, because participants are told the survey is confidential, it would not be ethical to report any disclosed abuse. In states with mandatory reporting laws, a separate version of the survey is used that excludes physical abuse questions.
Population and Geographic Coverage
The population of interest for each PRAMS state is resident women who recently gave birth within their state to a live-born infant during the surveillance year. Women are sampled between 2 and 6 months after giving birth. A state’s birth certificate file serves as the sampling frame for identifying new mothers. As a means of ensuring that women with multiple births are sampled at the same rate as women with singleton births, only 1 infant from a multiple gestation is randomly selected to be included in the sample frame. Mothers whose infants die after a live birth are actively followed up with the same survey but receive letters and materials acknowledging and expressing sensitivity to their loss.
The PRAMS sample is stratified so that subpopulations of particular public health interest can be oversampled, such as mothers of low-birth-weight infants, those living in high-risk geographic areas, and racial/ethnic minority groups. States choose a stratification plan according to their own priorities. Statistical weighting schemes account for the different sampling rates in different strata, allowing estimates from these groups to be combined to obtain state-level estimates that ultimately reflect the actual proportions of births attributed to these subpopulations.
Unit of Data Collection and Sample Size
Annual sample sizes per state range from about 1000 to 3000 women (Table 1). Sample sizes are determined according to stratification plan, number of births, and available budgets.
In 2014, PRAMS weighted response rates ranged from 47% to 74%, with a median of 61% (Table 1). By mode, 80% of respondents participated by mail and 20% by phone. Harder to contact demographic groups, including Hispanics, non-White women, adolescents, and those with less than a high school education, are more likely to respond by phone.
Weighting
The analysis weights include 3 components: the sampling weight, a nonresponse adjustment, and a noncoverage adjustment. Because birth certificate data are available for both responders and nonresponders, the information available on nonresponders can be used to adjust for nonresponse and to understand factors associated with survey nonresponse. The final cumulative birth certificate file from each state is compared with the PRAMS sampling frame to identify eligible records that were missed and compute noncoverage adjustments.
Survey Design and Frequency of Data Collection
The annual sample is processed in monthly batches to balance the workload throughout the year. CDC implemented the PRAMS Integrated Data Collection System (PIDS) in 2012 to support data collection activities. PIDS is a secure, Web-based system housed at and maintained by CDC that assists in tracking all aspects of data collection. States import contact information from the birth certificate file into the tracking software on a monthly basis. PIDS includes components supporting mail and telephone data collection activities, data entry, and development of reports to facilitate daily operations. After completion of the data collection cycle, the information in PIDS is extracted for data processing and weighting.
Key Data Elements and Data Quality
Currently the PRAMS questionnaire is in its eighth version, with revisions occurring approximately every 3 to 5 years. The current version was implemented with the 2016 birth cohort (https://www.cdc.gov/prams/questionnaire.htm). Each state’s questionnaire can consist of 3 types of questions: core questions common to all PRAMS states, standard questions developed by CDC and made available for selection to all states, and state-developed questions. Core questions generally account for 55% to 60% of the questionnaire. In designing their surveys, states can choose from the library of standard questions or develop their own questions to address state priority topics. Standard questions can be inserted among core questions, resulting in a unique survey for each state.
The mail questionnaire is limited to 14 pages and requires approximately 20 minutes to complete. The phone interview requires approximately 25 to 30 minutes. Given the different modes of survey administration, 2 separate surveys are available: a self-administered survey for the mail component and an interviewer-administered survey for the telephone component. CDC supports English and Spanish versions of the survey; New York City also uses a Mandarin version of the survey that the city developed and supports independently.
The box on the next page lists core topics and commonly used standard question topics available on the current PRAMS questionnaire. In addition, many standard questions are available that include more in-depth information on core topics.
BOX 1. Topics Covered in the 2016–2019 Pregnancy Risk Assessment Monitoring System Surveys.
| Core Topica | Standard Topic |
| Preconception health and health care | Fertility treatment |
| Health insurance coverage | Hospital maternity practices related to breastfeeding |
| Pregnancy intention | Work, leave, and child care |
| Contraception | Folic acid awareness |
| Multivitamin use | Participation in WIC and home visitation programs |
| Prenatal counseling | HIV testing |
| Influenza vaccination | Inductions and cesarean sections |
| Oral health | Infections and chronic conditions |
| Health conditions during pregnancy | Tdap vaccination |
| Cigarette smoking and use of other tobacco products | Pregnancy complications |
| Alcohol use | Household characteristics |
| Physical abuse | Stressful life events |
| Breastfeeding | Safety at home and car seat usage |
| Infant sleep position and sleep environment | Well and sick child care |
| Postpartum depressive symptoms | Social support |
| Postpartum checkup | Emotional and sexual abuse |
| Household income | Discrimination |
| Tobacco cessation | |
| Secondhand smoke exposure | |
| Physical activity | |
| Family history of chronic conditions | |
| Reproductive history | |
| Emergency preparedness | |
| Marijuana and illicit drug use | |
| Prescription drug use | |
| Zika virus |
Note. Tdap = tetanus, diphtheria, pertussis; WIC = Special Supplemental Nutrition Program for Women, Infants, and Children.
Each core topic includes additional standard questions that address the topic in more detail.
Many questions in the core section of the survey remain stable across questionnaire versions; however, periodic revisions provide the opportunity to make adjustments based on emerging issues and changing priorities. The PRAMS questionnaire revision process typically begins 2 years in advance. Initially, an evaluation of the current questions is conducted to identify questions that should be modified or removed. CDC also solicits requests for new topics or enhanced questions on existing topics from a wide array of stakeholders. New core and standard questions and questions that have undergone modifications are sent to CDC’s National Center for Health Statistics Questionnaire Design Research Laboratory for cognitive testing. Once revised to incorporate cognitive testing feedback, the questions undergo field testing to evaluate the wording and flow of the survey. After the field testing, all questions are finalized.
About a year prior to going live with a new survey, CDC begins preparing the individual surveys for each participating state. Mail versions and phone hard-copy versions of each state’s survey in English and Spanish (if applicable) are created by CDC. The PIDS software system is also programmed to allow data entry of mail surveys and administration of telephone surveys via computer-assisted telephone interviewing.
PRAMS incorporates a number of quality control measures. Data entry verification is required for a minimum of 10% of mail surveys, although many states perform 100% verification of mail surveys. Supervisors are required to monitor 10% of all telephone calls to make sure the survey is properly administered and responses are properly recorded. Item nonresponse rates are low (1%–2% for most questions) with the exception of the question on household income (6% nonresponse rate). No imputation procedures are used for item nonresponse.
DATA ANALYSIS/DISSEMINATION
PRAMS analysis files consist of 3 parts: birth certificate data, survey data, and operations data. A separate file of qualitative comment data is available for linkage with survey responses. Analysis files are created for each data collection year.
Linkage Ability
Both birth certificate and infant death certificate numbers, when applicable, are included in the data set to facilitate linkage of PRAMS data with birth and death certificate data. Many states have linked PRAMS data to other data sources including Medicaid records,19 office of corrections records, child protective service records,20 and various health department databases.21
Five states (Alaska, Missouri, Oregon, Oklahoma, and Rhode Island) conduct a toddler follow-up study to collect information on early childhood development by recontacting PRAMS participants when their babies are 2 to 3 years old.22 Data from the follow-up surveys can be linked with PRAMS data to assess how risk factors during pregnancy and early infancy affect early childhood development. The follow-up studies are developed and implemented independently by state health departments.
Data Release and Accessibility
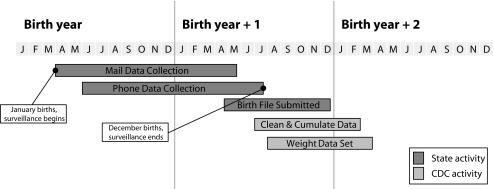
Figure 2 illustrates the PRAMS data collection and data release timeline. Data collection for a given year is completed by July of the following year to allow time to gather postpartum information. Weighting is conducted once CDC receives the final-year birth files from the individual state health departments. Typically, these files are provided 5 to 12 months after the end of the birth year. Most states receive their weighted data 3 to 6 months after the conclusion of data collection. Under the current protocol, the timeframe for making PRAMS data available to all states is approximately 8 to 12 months after the completion of data collection in a given year (e.g., data collection for 2016 births was completed in July 2017 and a cleaned, aggregate data set was released in mid-2018).
FIGURE 2—
Pregnancy Risk Assessment Monitoring System (PRAMS) Data Collection and Data Processing Timeline
Note. CDC = Centers for Disease Control and Prevention.
To further ensure high-quality data and motivate states to adhere to the data collection protocol, CDC currently imposes a response rate threshold for inclusion of information in reports, publications, and data made available to the public. States not meeting the threshold still receive their weighted data for internal health department use, but the information is not included in data released by CDC. The response rate threshold was set at 70% until 2006; subsequent levels were 65% (2007 to 2011), 60% (2012 to 2014), and 55% (2015 to 2016). On average, 75% of states have met or exceeded the threshold since 2007. During this period response rates for most federal health surveys have declined, and the threshold has been lowered accordingly over time.
A public use PRAMS analytic data set is available to researchers upon request from CDC after completion of a short application with a brief research proposal summary. The PRAMS Web site provides information about the years of data available, codebooks, and the application process as well as other information for researchers. Certain birth certificate variables are aggregated or truncated in the analytic data file to protect individual confidentiality. For example, no geographic indicators smaller than the state level are included in the file. Maternal age is aggregated into 5-year groupings, and only months and years are provided for dates of birth. Researchers can directly contact participating states to request access to variables not included in the analytic file. In addition, selected PRAMS indicators are available online and through other sources, such as the March of Dimes PeriStats Web system.23
Key References
Numerous fact sheets, Morbidity and Mortality Weekly Report articles, and peer-reviewed journal articles incorporating PRAMS data have been published. Key sources of information include the following:
PRAMS Web site (http://www.cdc.gov/prams),
PRAMS online indicators (https://www.cdc.gov/prams/prams-data/mch-indicators.html), and
PRAMS data (https://www.cdc.gov/prams/researchers.htm).
In addition, this review updates 3 general PRAMS methods articles published in 1991, 1999, and 2006.24–26
PUBLIC HEALTH IMPLICATIONS
PRAMS state data sets can be analyzed individually to monitor the health of mothers before, during, and shortly after pregnancy within a state or aggregated to provide a multistate analytic data set well suited for cross-sectional studies. PRAMS data are frequently used to evaluate public health programs and policies at both the state and national levels. For example, PRAMS data have been used to evaluate the impact of state prescription contraception insurance mandates on unintended and mistimed births.27
The ongoing nature of PRAMS surveillance makes it especially useful for tracking trends in health indicators over time and monitoring health behaviors and practices. PRAMS and the Maternal and Infant Health Assessment (MIHA) survey from California28 (currently not a PRAMS state) serve as the data sources for tracking 9 Healthy People 2020 objectives. PRAMS and MIHA staff collaborate to ensure that identical survey questions are used to produce the combined estimates.
PRAMS has also proven to be a versatile system to address emerging health issues through the use of questionnaire supplements. Leveraging the existing state-based PRAMS infrastructure allows supplements (short lists of up to 12 questions added to the end of the survey) on an emerging topic to be quickly implemented across some or all participating sites, providing timely data for analysis and dissemination. For example, in 2009 during the H1N1 flu pandemic, a supplement designed to collect information on vaccination uptake among pregnant women was developed and rapidly implemented at 30 of the 38 participating sites. The PRAMS supplement provided timely data on influenza vaccination rates and provider counseling to inform policy and provider practices.29
On the basis of the success of the influenza supplement, supplements have become a standard part of the PRAMS methodology. In the case of emerging issues, supplement data can be extracted and weighted with the most recently available birth certificate file at the time for immediate analysis and dissemination. To date, supplements have been developed for a variety of topics including family history of cancer, Zika virus, marijuana and prescription drug use, and disaster preparedness.30
PRAMS also has been used for evaluation of programs serving women during and after pregnancy. In 2010, PRAMS began a partnership with the W. K. Kellogg Foundation.31 Participating states modified their PRAMS samples to oversample Kellogg Foundation targeted communities in their state. The overarching goal of this partnership was to use PRAMS data to assess the potential impact of the Kellogg Foundation’s interventions in terms of improving maternal and child health outcomes.
The success of the survey supplements and the Kellogg evaluation has led to new opportunities for PRAMS. PRAMS is currently collaborating with the Health Resources and Services Administration to evaluate the Healthy Start program.32 Eleven participating states oversampled Healthy Start clients who gave birth in 2017 and 2018 and will compare them with similar populations not participating in Healthy Start.
Finally, PRAMS is exploring the expansion of its sampling frame beyond women with recent live births. Currently, Utah is piloting a surveillance system to assess the feasibility of using the existing PRAMS methodology to learn more about the behaviors and experiences of women who have experienced a stillbirth.33 Promising formative research has also explored the feasibility of extending the reach of PRAMS to fathers of recent live-born infants to better understand the influence of fathers on maternal and infant health and the experience of transitioning to fatherhood.34
CONCLUSIONS
PRAMS, which recently completed its 30th consecutive year of surveillance, continues to be a comprehensive source of perinatal data and has proven an effective system for addressing emerging issues affecting the health of mothers and babies. The PRAMS methodology is standardized across participating states but is also flexible in allowing states to tailor various aspects of the surveillance system, including survey appearance, survey topics, and sampling and stratification plans, to meet their data needs and populations. The success of PRAMS is largely attributable to the partnership between CDC and participating states that collect, analyze, and disseminate timely data to inform maternal and child health programs and policies.
ACKNOWLEDGMENTS
We thank Katherine Bower Kortsmit for assisting with formatting and compiling the references and the PRAMS (Pregnancy Risk Assessment Monitoring System) Working Group for its role in conducting PRAMS surveillance.
The PRAMS Working Group members include Izza Afgan, MPH (Alabama); Kathy Perham-Hester, MS, MPH (Alaska); Ruby D. Brown (Arkansas); Ashley Juhl, MSPH (Colorado); Jennifer Morin, MPH (Connecticut); George Yocher, MS (Delaware); Elizabeth C. Stewart, MSPH (Florida); Florence A. Kanu, MPH (Georgia); Wendy Nihoa, MPH (Hawaii); Julie Doetsch, MA (Illinois); Jessica Eagan (Iowa); Rosaria Trichilo, MPH (Louisiana); Tom Patenaude, MPH (Maine); Laurie Kettinger, MS (Maryland); Emily Lu, MPH (Massachusetts); Peterson Haak (Michigan); Mira Sheff (Minnesota); Brenda Hughes, MPPA (Mississippi); David McBride, PhD (Missouri); Emily Healy, MS (Montana); Jessica Seberger (Nebraska); Sara Riordan, MEd (New Hampshire); Sharon Smith Cooley, MPH (New Jersey); Eirian Coronado (New Mexico); Anne Radigan (New York State); Pricila Mullachery, MPH (New York City); Kathleen Jones-Vessey, MS (North Carolina); Sandra Anseth (North Dakota); Connie Geidenberger, PhD (Ohio); Ayesha Lampkins, MPH, CHES (Oklahoma); Tina Kent (Oregon); Sara Thuma, MPH (Pennsylvania); Karine Tolentino Monteiro, MPH (Rhode Island); Kristen Simpson (South Carolina); Uvonne Leverett (Tennessee); Tanya Guthrie, PhD (Texas); Barbara Algarin (Utah); Peggy Brozicevic (Vermont); Kenesha Smith (Virginia); Linda Lohdefinck (Washington); Melissa Baker, MA (West Virginia); Christopher Huard (Wisconsin); Lorie Chesnut, PhD (Wyoming); and the Centers for Disease Control and Prevention PRAMS Team, Women’s Health and Fertility Branch, Division of Reproductive Health.
Note. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
HUMAN PARTICIPANT PROTECTION
Ethical approval from an institutional review board was not needed for this review because no human participants were involved.
Footnotes
REFERENCES
- 1.Safe Motherhood Act for Research and Treatment (HR 4602) Available at: https://www.govtrack.us/congress/bills/107/hr4602/text. Accessed July 25, 2018.
- 2.Centers for Disease Control and Prevention. QuickStats: leading causes of neonatal and postneonatal deaths—United States, 2002. MMWR Morb Mortal Wkly Rep. 2005;54(38):966. [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJ et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67(1):1–54. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. About SUID and SIDS. Available at: https://www.cdc.gov/sids/aboutsuidandsids.htm. Accessed July 25, 2018.
- 5.Berg CJ, Atrash HK, Koonin LM, Tucker M. Pregnancy-related mortality in the United States, 1987–1990. Obstet Gynecol. 1996;88(2):161–167. doi: 10.1016/0029-7844(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 6.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366–373. doi: 10.1097/AOG.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Admon LK, Winkelman TNA, Moniz MH et al. Disparities in chronic conditions among women hospitalized for delivery in the United States, 2005–2014. Obstet Gynecol. 2017;130(6):1319–1326. doi: 10.1097/AOG.0000000000002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Office of Disease Prevention and Health Prevention. Healthy People 2020: maternal, infant, and child health. Available at: https://www.healthypeople.gov/2020/data-search/Search-the-Data#source=3564. Accessed July 25, 2018.
- 9.US Department of Health and Human Services. National Performance Measures. Available at: https://mchb.tvisdata.hrsa.gov/PrioritiesAndMeasures/NationalPerformanceMeasures. Accessed July 25, 2018. [DOI] [PubMed]
- 10.National Quality Forum. Measures, reports, and tools. Available at: http://www.qualityforum.org/Measures_Reports_Tools.aspx. Accessed July 25, 2018.
- 11.Robbins CL, Zapata LB, Farr SL et al. Core state preconception health indicators—Pregnancy Risk Assessment Monitoring System and Behavioral Risk Factor Surveillance System. MMWR Surveill Summ. 2014;63(3):1–62. [PubMed] [Google Scholar]
- 12.Connecticut Dental Health Partnership. Connecticut’s Perinatal and Infant Oral Health Quality Improvement Project: pregnancy risk assessment monitoring system data for evaluation. Available at: https://www.ctdhp.com/documents/Using_PRAMS_Data_For_Evaluation_Of_CT_PIOHQIP.pdf. Accessed July 25, 2018.
- 13.National Institute for Children’s Health Quality. Breaking barriers to healthy birth spacing in underserved populations. Available at: http://www.nichq.org/insight/breaking-barriers-healthy-birth-spacing-underserved-populations. Accessed July 25, 2018.
- 14.Bombard JM, Kortsmit K, Warner L et al. Vital signs: trends and disparities in infant safe sleep practices—United States, 2009–2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):39–46. doi: 10.15585/mmwr.mm6701e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Available at: http://www.guttmacher.org/pubs/StateUP10.pdf. Accessed July 25, 2018.
- 16.Adams EK, Johnston EM. Insuring women in the United States before, during, and after pregnancy. Am J Public Health. 2016;106(4):585–586. doi: 10.2105/AJPH.2016.303132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail and Mixed-Mode Surveys: The Tailored Design Method. 4th ed. New York, NY: John Wiley and Sons; 2014. [Google Scholar]
- 18.Centers for Disease Control and Prevention. PRAMS model surveillance protocol, 2015 version. Available at: http://www.cdc.gov/prams/methodology.htm. Accessed July 25, 2018.
- 19.Blackwell S. Linking PRAMS and Medicaid data: preliminary findings. Available at: https://www.dhs.wisconsin.gov/stats/prams/prams-medicaid-oct2015.pdf. Accessed July 25, 2018.
- 20.Parrish JW, Young MB, Perham-Hester K, Gessner BD. Identifying risk factors for child maltreatment in Alaska: a population-based approach. Am J Prev Med. 2011;40(6):666–673. doi: 10.1016/j.amepre.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Mobley SC, Thomas S, Sutherland D, Kosacz N, Ogbuanu C. Using linked PRAMS data to evaluate outcomes of a rural Healthy Start program. Available at: https://dph.georgia.gov/sites/dph.georgia.gov/files/UsingLinkedPRAMSDataEvaluateOutcomesRuralHealthyStart.pdf. Accessed July 25, 2018.
- 22.Rosenberg KD, Hembroff L, Drisko J et al. New options for child health surveillance by state health departments. Matern Child Health J. 2011;15(3):302–309. doi: 10.1007/s10995-010-0589-4. [DOI] [PubMed] [Google Scholar]
- 23. March of Dimes. PeriStats. Available at: http://www.marchofdimes.org/peristats/Peristats.aspx. Accessed July 25, 2018.
- 24.Shulman HB, Colley Gilbert B, Lansky A. The Pregnancy Risk Assessment Monitoring System (PRAMS): current methods and evaluation of 2001 response rates. Public Health Rep. 2006;121(1):74–83. doi: 10.1177/003335490612100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert BC, Shulman HB, Fischer LA, Rogers MM. The Pregnancy Risk Assessment Monitoring System (PRAMS): methods and 1996 response rates from 11 states. Matern Child Health J. 1999;3(4):199–209. doi: 10.1023/a:1022325421844. [DOI] [PubMed] [Google Scholar]
- 26.Adams MM, Shulman HB, Bruce C, Hogue C, Brogan D. The Pregnancy Risk Assessment Monitoring System: design, questionnaire, data collection and response rates. Paediatr Perinat Epidemiol. 1991;5(3):333–346. doi: 10.1111/j.1365-3016.1991.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnston E, Adams K. State prescription contraception insurance mandates: effects on unintended births. Health Serv Res. 2017;52(6):1970–1995. doi: 10.1111/1475-6773.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.California Department of Public Health. Maternal and Infant Health Assessment. Available at: https://www.cdph.ca.gov/Programs/CFH/DMCAH/MIHA/Pages/default.aspx. Accessed July 25, 2018.
- 29.Centers for Disease Control and Prevention. Influenza vaccination coverage among pregnant women—29 states and New York City, 2009–10 season. MMWR Morb Mortal Wkly Rep. 2012;61(7):113–118. [PubMed] [Google Scholar]
- 30.Zotti ME, Williams AM, Wako E. Post-disaster health indicators for pregnant and postpartum women and infants. Matern Child Health J. 2015;19(6):1179–1188. doi: 10.1007/s10995-014-1643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahluwalia IB, Harrison L, Simpson P et al. Pregnancy Risk Assessment Monitoring System and the W.K. Kellogg Foundation joint project to enhance maternal and child health surveillance: focus on collaboration. J Womens Health (Larchmt) 2015;24(4):257–260. doi: 10.1089/jwh.2015.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banks J, Atrash H. Healthy Start national evaluation plan. Paper presented at: Pregnancy Risk Assessment Monitoring System national meeting, September 2015, Atlanta, GA.
- 33.Christiansen-Lindquist L, Sahay K, Hogue C. Improving surveillance for the hidden half of fetal-infant mortality: a pilot study of the expansion of the Pregnancy Risk Assessment Monitoring System to include stillbirth. Ann Epidemiol. 2016;26(6):401–404. doi: 10.1016/j.annepidem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner L. A public health approach to responsible fatherhood. Paper presented at: Fourth Annual Summit on Fatherhood and the Health and Wellness of Boys and Men, March 2018, Washington, DC.