Abstract
Objectives. To assess changes in human papillomavirus (HPV) vaccine initiation for adolescent girls and boys in Rhode Island compared with all other states.
Methods. We estimated the gender-specific effects of Rhode Island’s school-entry HPV vaccination policy on self-reported HPV vaccination initiation by using a difference-in-differences design with the National Immunization Survey–Teen from 2010 through 2016.
Results. Compared with boys in other states, boys in Rhode Island increased their HPV vaccine initiation rate by 11% (b = 0.11; 95% confidence interval [CI] = 0.05, 0.18) after enactment of the requirement. No difference was seen in the probability of HPV vaccine initiation among girls in Rhode Island compared with girls in the multistate control (b = −0.01; 95% CI = −0.08, 0.05).
Conclusions. Our analysis identified an 11% increase in HPV vaccine initiation rate among boys in Rhode Island after the school-entry requirement was enacted, whereas no significant change was observed for girls.
Public Health Implications. Given suboptimal vaccine uptake rates in the United States, continued pursuit of state-level public policy to improve HPV vaccination is needed. School-entry requirements for HPV vaccination may be a strategy for closing the gap in HPV vaccine uptake for boys and girls.
The human papillomavirus (HPV) vaccine is approved for the prevention of HPV-attributable cancers (i.e., cervical, vaginal, vulvar, anal, and penile) and genital warts.1 Despite the well-established safety and effectiveness of this vaccine, uptake has been suboptimal in the United States. In 2016, uptake (≥ 1 dose) among US adolescents was 65.1% for girls and 56.0% for boys.2 Differences in male and female vaccine uptake are attributed primarily to a delay in HPV vaccine approval among males.3
Few states have enacted policies for HPV vaccine school-entry requirements. Virginia and the District of Columbia were early adopters of HPV vaccine school requirements in 2008 and 2009, respectively. However, in both cases, the requirements focus on only girls and involve broadly defined, easily obtained exemptions, particularly in the District of Columbia. In 2015, Rhode Island enacted an HPV vaccine school-entry requirement; it requires receipt of the first dose of the vaccine before entering seventh grade and completion of the series before entering ninth grade.4 Moreover, it uses a narrow definition of exemption—making it more difficult to opt out of vaccination.5
Previous studies evaluated the implementation of school-entry requirements and other HPV vaccine state policies. However, these studies did not take into account the vaccination rates before implementation of a policy, and the studies were carried out before the Rhode Island requirement was implemented and therefore focused on only the policies in Virginia and the District of Columbia.6,7 A recent qualitative analysis of policies found that a multifactorial approach to policy may be most effective at promoting HPV vaccination but also recommended complementary statistical analyses to evaluate the effects of HPV vaccine policy.8 Given the recent enactment of Rhode Island’s HPV vaccine school-entry requirement, we assessed changes in HPV vaccine initiation for adolescent girls and boys in Rhode Island compared with all other states.
METHODS
We used parental report of HPV vaccination initiation from the National Immunization Survey–Teen (NIS-Teen) from 2010 through 2016 to estimate the gender-specific effects of Rhode Island’s school-entry HPV vaccination policy with a difference-in-differences design. The difference-in-differences design treats Rhode Island’s policy change as a natural experiment, comparing the change in parental reporting of HPV vaccination among adolescents in Rhode Island with the change in HPV vaccination in a control group of all other states before and after Rhode Island’s policy change. To further strengthen our quasi-experimental approach, we allowed for differential trends in HPV vaccine initiation over time by gender and controlled for individual-level demographic covariates.
Data
We examined individual data from the NIS-Teen, an annual repeated cross-sectional study assessing vaccination initiation at the national and state level for 13- to 17-year-old persons. HPV vaccination was determined by a parental report of “yes” to whether the adolescent had ever received any immunization for HPV. Gender was coded as male or female. Age was coded in years. Race/ethnicity was coded as non-Hispanic White, non-Hispanic Black, Hispanic, or other. Income was coded as greater than $75 000, from the poverty line to $75 000, and below the poverty line. Mother’s education was coded as fewer than 12 years, 12 years, more than 12 years but non–college graduate, and college graduate. Number of health care visits in the past 12 months was treated as a continuous variable.
Rhode Island’s HPV vaccination school-entry requirement was officially enacted in August 2015, whereas NIS-Teen collects data throughout the calendar year. Consequently, adolescents whose parents were surveyed in the first half of 2015 were not subject to the mandate, but those whose parents were surveyed in the second half of 2015 were subject to the mandate. To be conservative, our primary coding of the policy indicator for Rhode Island’s HPV requirement included only adolescents surveyed in 2016 as being subject to the mandate. We used a secondary indicator including adolescents from both 2015 and 2016 during sensitivity analyses.
Statistical Analysis
To evaluate the gender-specific effects of Rhode Island’s HPV vaccination requirement, we estimated the following linear probability model:
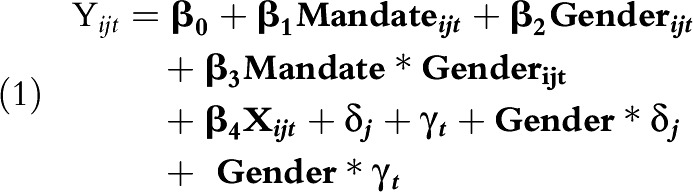 |
Yijt is an indicator for HPV vaccination for person i in state j in year t. Mandateijt is the policy indicator for Rhode Island’s HPV vaccination mandate. Genderijt is the participant’s self-reported gender. Xijt is a vector of age, race/ethnicity, income, mother’s education, and number of health care visits. δj, γt, Gender∗δj, and Gender∗γt are gender-specific state and year fixed effects. These state and year fixed effects allow for differential baseline HPV vaccination rates for boys and girls in each state, as well as gender-specific nonlinear trends over time.
All analyses accounted for the complex survey design of NIS-Teen by using PROC SURVEYREG in SAS version 9.4 (SAS Institute, Cary, NC). We used linear probability models to aid in the interpretation of the interaction term, although we found similar results with multiplicative models. In addition to determining model estimates, we estimated the predicted probability of an HPV vaccination in Rhode Island and the control group of all other states by gender and year.
RESULTS
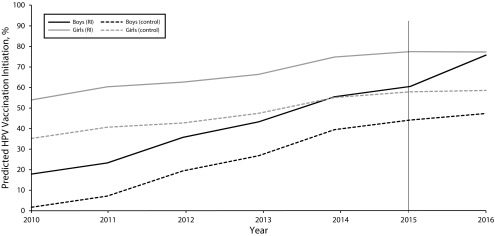
Our analysis showed a statistically significant difference in the effect of Rhode Island’s HPV vaccination requirement by gender (F1 = 7.38; P = .007). Compared with boys in other states, boys in Rhode Island increased their predicted HPV vaccination initiation rate by 11% (b = 0.11; 95% confidence interval [CI] = 0.05, 0.18) after enactment of the requirement. No difference was seen in the predicted probability of HPV vaccination among girls in Rhode Island compared with girls in the multistate control (b = –0.01; 95% CI = −0.08, 0.05). However, girls in Rhode Island maintained the highest HPV vaccination rates throughout the study period, with boys in Rhode Island achieving similar levels of HPV vaccination after enactment of the requirement (Figure 1).
FIGURE 1—
Predicted Percentage of Human Papillomavirus (HPV) Vaccine Initiation Stratified by Policy Enactment and Gender: National Immunization Survey–Teen, Rhode Island and United States, 2010–2016
We found similar results when we used the policy indicator including 2015 and when we excluded jurisdictions that enacted a school-entry requirement for HPV vaccination before the start of the study (Virginia, District of Columbia) from the multistate control (data not shown).
DISCUSSION
Our analysis identified an 11% increase in HPV vaccine initiation among boys in Rhode Island after the school-entry requirement was enacted, whereas no significant change was observed for girls. This set of findings indicates that school-entry requirements may reduce gender disparities and close the gap in HPV vaccine uptake.9 We probably did not observe significant differences in HPV vaccine initiation among girls because of their already high HPV vaccination rate (87.9%) in 2015.10 Roberts et al.8 found that multiple policies may be needed to improve HPV vaccination, but this study found that a policy focused solely on an HPV vaccination school-entry requirement may have an effect on HPV vaccine initiation, especially among boys.
This study should be considered in the context of its limitations. We could not assess other school-entry requirements for HPV vaccination (i.e., Virginia, District of Columbia) because data before these policies were implemented were not available. Additionally, our reliance on self-reported vaccination status is a limitation; however, self-report has been shown to be reasonably accurate in the NIS-Teen.11 Finally, this analysis captured the early implementation of the school-entry requirement, and future research should continue to examine this policy transition.
PUBLIC HEALTH IMPLICATIONS
Given suboptimal vaccine uptake rates in the United States, continued pursuit of state-level public policy to improve HPV vaccination is needed, especially to achieve the Healthy People 2020 objective of 80% coverage for HPV vaccination completion.12 In particular, school-entry requirements with narrow opt-out policies, such as Rhode Island’s, may be more successful; however, these policies face more resistance for implementation.
ACKNOWLEDGMENTS
These data were derived from public use data sets on the 2010–2016 National Immunization Survey–Teen, available on the Centers for Disease Control and Prevention Web site.
HUMAN PARTICIPANT PROTECTION
This study was considered exempt by the North Texas Regional institutional review board as this work did not involve human participant research.
REFERENCES
- 1.Petrosky E, Bocchini JA, Jr, Hariri S et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 2.Walker TY, Elam-Evans LD, Singleton JA et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882. doi: 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 4.Barraza L, Weidenaar K, Campos-Outcalt D, Yang YT. Human papillomavirus and mandatory immunization laws: what can we learn from early mandates? Public Health Rep. 2016;131(5):728–731. doi: 10.1177/0033354916663184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barraza L, Campos-Outcalt D. More research needed to increase policies for HPV vaccine uptake. Am J Public Health. 2018;108(4):430–431. doi: 10.2105/AJPH.2018.304323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss JL, Reiter PL, Truong YK, Rimer BK, Brewer NT. School entry requirements and coverage of nontargeted adolescent vaccines. Pediatrics. 2016;138(6):pii: e20161414. doi: 10.1542/peds.2016-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins RB, Lin M, Wallington SF, Hanchate AD. Impact of school-entry and education mandates by states on HPV vaccination coverage: analysis of the 2009-2013 National Immunization Survey-Teen. Hum Vaccin Immunother. 2016;12(6):1615–1622. doi: 10.1080/21645515.2016.1150394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts MC, Murphy T, Moss JL, Wheldon CW, Psek W. A qualitative comparative analysis of combined state health policies related to human papillomavirus vaccine uptake in the United States. Am J Public Health. 2018;108(4):493–499. doi: 10.2105/AJPH.2017.304263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daley EM, Vamos CA, Zimet GD, Rosberger Z, Thompson EL, Merrell L. The feminization of HPV: reversing gender biases in US human papillomavirus vaccine policy. Am J Public Health. 2016;106(6):983–984. doi: 10.2105/AJPH.2016.303122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reagan-Steiner S, Yankey D, Jeyarajah J et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 11.Ojha RP, Tota JE, Offutt-Powell TN, Klosky JL, Ashokkumar R, Gurney JG. The accuracy of human papillomavirus vaccination status based on adult proxy recall or household immunization records for adolescent females in the United States: results from the National Immunization Survey-Teen. Ann Epidemiol. 2013;23(5):281–285. doi: 10.1016/j.annepidem.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 12. Healthy People 2020. Immunization and infectious diseases. 2015. Available at: http://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed April 6, 2016.