Abstract
Purpose
To analyze the surgical outcomes and complication rates in a group of patients with refractory glaucoma who underwent simultaneous pars plana vitrectomy (PPV) and PC7 or PC8 Ahmed glaucoma valve (AGV) implantation.
Methods
Retrospective case series study of 10 eyes in 9 patients with secondary glaucoma, refractory to other treatment, who underwent 23G-PPV and implantation of PC7 or PC8 AGV between 2012 and 2014. Study variables were postoperative BCVA, IOP and the number of glaucoma medications, which were evaluated preoperatively and at 1 day, 1 week and 1, 3, 6, and 12 months after the surgical intervention. Absolute success was defined as IOP less than 21 mmHg in the absence of any medication and qualified success if medication was needed to control IOP under 21 mmHg.
Results
The average follow-up was 10.2 ± 2.89 months. Postoperative IOP levels decreased in all cases in comparison with preoperative values (p < 0.05). Absolute success rate was 60%, reaching 100% in terms of qualified success. Kaplan–Meier survival analysis showed 60% absolute success at 12 months. Changes in postoperative BCVA were not statistically significant in comparison with preoperative data. Early postoperative complications were athalamia, hyphema, and retinal detachment; late complications were pars plana clip extrusion and cystic bleb.
Conclusions
PC7 and PC8 Ahmed valve implantation via pars plana is a safe and useful option in patients with secondary refractory glaucoma who are either candidates for PPV or have been previously vitrectomized.
Keywords: Pars plana clip, Ahmed valve, PC7, Pars plana vitrectomy, Refractory glaucoma
Introduction
Glaucoma is the second leading cause of blindness, and the first cause of irreversible blindness, affecting 66.8 million people worldwide.1 Even though the frequency of glaucoma surgery has been decreasing since the 1990s2, it still offers several benefits such as a lower cost3 and more substantial4 IOP reduction with fewer fluctuations5 compared to pharmacological treatment.
Glaucoma drainage devices (GDDs), first described by Molteno in 19696, are an increasingly popular surgical option in the management of complex glaucoma.7 Due to its relatively low rate of complications, the Ahmed Glaucoma Valve (AGV) is the most frequently used valvular tube shunt.8, 9 GDD’s tube placement can vary, the most frequent being the anterior chamber (AC). However, other locations such as the posterior chamber or vitreous cavity have been employed in order to reduce the associated complications, such as corneal decompensation, graft failure in keratoplasty or cataract formation.10, 11, 12, 13, 14, 15
The PC7 AGV model and its pediatric equivalent, PC8 (New World Medical, Rancho Cucmonga, CA, EEUU), have recently been developed. These devices are modified with a pars plana clip (PPC), which allows the tube to be anchored to the sclera and to be given a suitable angle, thus facilitating its placement in the posterior segment via pars plana.
The aim of this study is to analyze the surgical outcomes and complication rates in a group of glaucoma patients with high risk of surgical failure who underwent simultaneous pars plana vitrectomy (PPV) and PC7 or PC8 AGV implantation.
Methods
This is a retrospective case series study of patients presenting secondary glaucoma refractory to other treatment who underwent 23G pars plana vitrectomy and simultaneous implantation of PC7 or PC8 AGV between 2012 and 2014. The study was conducted in two surgical centers (Hospital Universitario Donostia and Instituto Clínico Quirúrgico de Oftalmología) and all surgeries were performed by the same surgeon (I.R.A). Medical records of all patients were reviewed and the following data were collected and analyzed: demographic information, type of glaucoma, number of glaucoma hypotensive medications, concurrent ocular surgeries, mean follow-up after surgery, best corrected visual acuity (BCVA), and intraocular pressure (IOP) (measured with Goldmann applanation tonometer).
Study variables were postoperative BCVA, IOP and the number of glaucoma medications. Examinations were performed preoperatively and 1 day, 1 week, 1 month, 3 months, 6 months and 1 year after surgery, except for 3 patients who received 6 months’ follow-up.
Statistical analysis
Data are displayed as mean ± standard deviation and 95% confidence intervals. BCVA was calculated using logMAR scale for statistical comparisons; visual acuity scores no better than light perception were excluded from the analysis. Comparisons of preoperative and postoperative values of BCVA, IOP, and number of medications were performed using the General Linear Model (GLM) Repeated Measures procedure. Criteria for success were defined before reviewing the data. Absolute success was achieved if IOP was less than 21 mmHg in the absence of any medication and qualified success if IOP was under 21 mmHg with medication. Kaplan Meyer’s survival curves were used to determine the surgical survival rate. P values of <0.05 were considered statistically significant. All statistics were performed using PASW 18.0 software.
Surgical technique
PC7 is a silicone Ahmed valve model with a 16 mm long and 13 mm wide drainage plate, and a 0.635 mm external diameter and 0.305 mm internal lumen tube. It has a clip that enables a 90° bending of the tube and a proper anchoring to the sclera. The PC8 pediatric model (Fig. 1A) has similar characteristics with reduced plate dimensions (10 × 9.60 mm).16
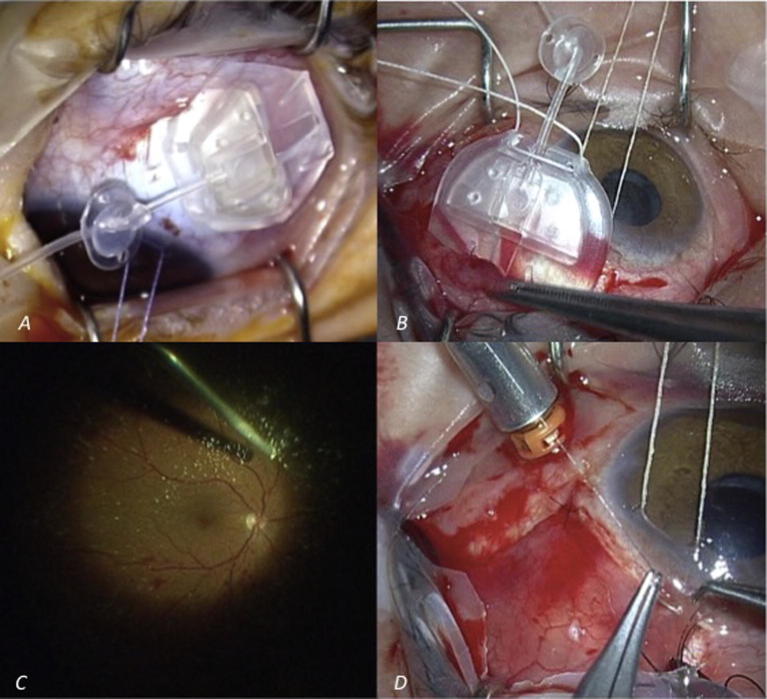
Fig. 1.
(A) Intraoperative image of PC8 Ahmed valve. (B) Placement of the valvular plate in the superior quadrant. (C) Intraoperative image during 23-G pars plana vitrectomy. (D) Conjuntival suture with 10/0 nylon after fascia lata patch or scleral graft placement and prior to trocar removal.
Surgeries and additional procedures performed in each patient are listed in Table 1. All patients were operated by the same experienced surgeon under peribulbar anesthesia, with the exception of one pediatric patient who was operated under general anesthesia. The same procedure for the AGV placement was used in all patients. First, a fornix-base peritomy was performed. The valvular plate was then placed in the superior quadrant (Fig. 1B) and secured with non-absorbable 5/0 nylon sutures while the pars plana clip was sutured with a 10/0 nylon. The AGV was purged with saline solution prior to its placement.
Table 1.
Concurrent surgeries and additional procedures.
| Implanted GDD | 23G PPV | Panretinal photocoagulation | Phacoemulsification of cataract | Silicone oil extraction | Intravitreal anti-VEGF injection | |
|---|---|---|---|---|---|---|
| 1 | Ahmed PC7 | X | X | |||
| 2 | Ahmed PC7 | X | X | X | ||
| 3 | Ahmed PC7 | X | X | |||
| 4 | Ahmed PC7 | X | ||||
| 5 | Ahmed PC7 | X | ||||
| 6 | Ahmed PC7 | X | X | |||
| 7 | Ahmed PC7 | X | ||||
| 8 | Ahmed PC8 | X | ||||
| 9 | Ahmed PC8 | X | ||||
| 10 | Ahmed PC7 | X | X |
GDD: Glaucoma Drainage Device; 23G PPV: 23gauges pars plana vitrectomy; VEGF: Vascular endothelial Growth Factor.
In all cases, a simultaneous 3-port 23-G pars plana vitrectomy was performed (Fig. 1C), using one of the sclerotomies to insert the valve’s tube, which was trimmed to an appropriate length before insertion. Particular attention was paid to the vitreous base shaving in the area where the AGV tube was to be placed. Glycerin-preserved sclera was used to cover the clip in six cases; fascia lata patch graft was used in the remaining four. The conjunctiva was approximated to the limbus using 10/0 nylon sutures (Fig. 1D).
Topical antibiotics (tobramycin 4 times daily), steroids (dexamethasone phosphate 4 times daily) and cycloplegic (3 times daily) were prescribed and tapered during the 4 postoperative weeks. Fig. 2 shows postoperative image of a patient with good visualization of the tube.
Fig. 2.
Postoperative image of a patient showing excellent visualization of the tube in the posterior segment.
Results
Ten patients were included in the study. Demographic characteristics and type of glaucoma are shown in Table 2. The mean age of the patients was 46.6 ± 26.28 years (range 4–80) and neovascular glaucoma was the most common type of glaucoma (4 cases out of 10).
Table 2.
Demographic characteristics and postoperative complications.
| Cases | Glaucoma Diagnosis | Eye | Age | Complications |
|---|---|---|---|---|
| 1 | Neovascular | OD | 68 | Hyphema Cystic bleb |
| 2 | Neovascular | OS | 67 | Cystic bleb Clip extrusion |
| 3 | Secondary to silicon oil injection | OS | 46 | Retinal detachment |
| 4 | Inflammatory | OD | 51 | |
| 5 | Traumatic | OD | 34 | Athalamia Clip extrusion |
| 6 | Neovascular | OD | 67 | |
| 7 | Congenital | OD | 4 | |
| 8 | Congenital | OS | 4 | |
| 9 | Traumatic | OS | 45 | |
| 10 | Neovascular | OS | 80 | Choroidal detachment Tube obstruction Retinal detachment |
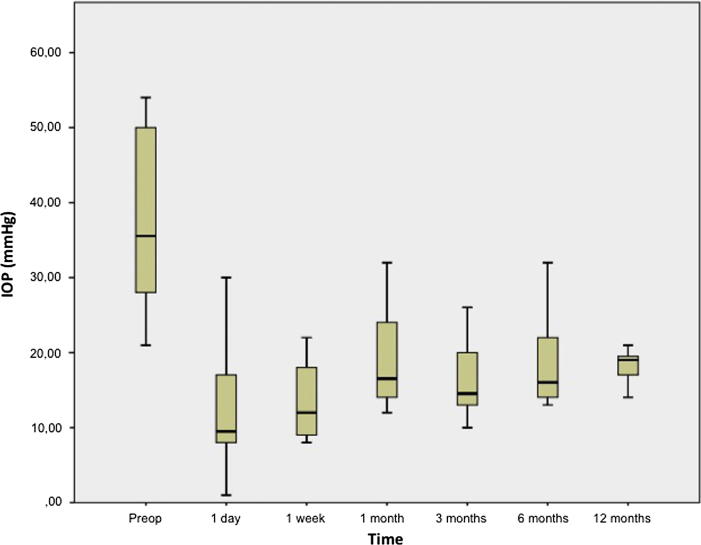
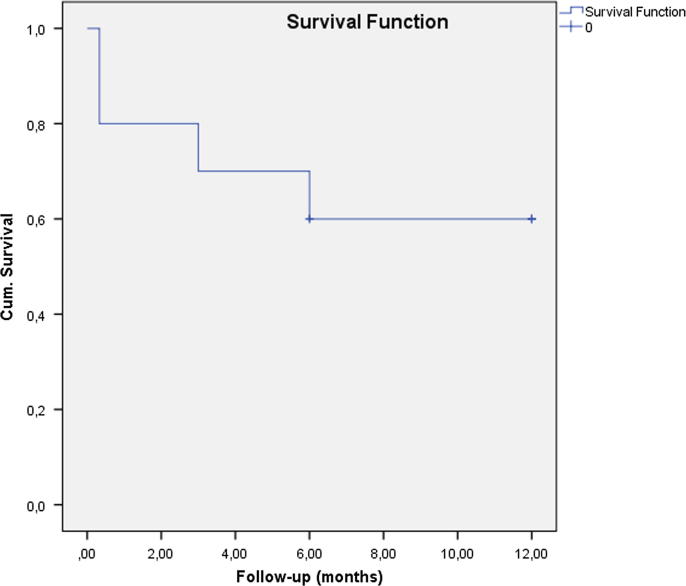
The preoperative and last postoperative data (12 months) are displayed in Table 3 while mean postoperative data at every visit are displayed in Table 4. Follow-up was 12 months in 7 cases, and 6 months for the remaining patients; the average follow-up was 10.2 ± 2.89 months. The mean ± SD preoperative IOP was 37.2 ± 11.3 mmHg and the mean postoperative IOP was 16.3 ± 3.03 mmHg; the average number of medications decreased from 2.2 ± 1.48 preoperatively to 0.87 ± 1.41 on last follow-up (12 months). Postoperative IOP levels decreased in all cases in comparison with preoperative values (p < 0.05), as shown in Fig. 3. Absolute success rate was 60%, reaching 100% in terms of qualified success (glaucoma medication was necessary in 4 cases to achieve normal IOP levels). Fig. 4 represents Kaplan–Meier survival analysis of absolute success versus postoperative time showing 60% absolute success at 12 months.
Table 3.
Preoperative and postoperative data (12-month follow-up).
| Preoperative data |
Postoperative data (12 month follow-up) |
||||
|---|---|---|---|---|---|
| BCVA (Snellen) | IOP (mmHg) | Number of medications | BCVA (Snellen) | IOP (mmHg) | Number of medications |
| 0.7 | 24 | 3 | 0.5 | 21 | 2 |
| 0.05 | 50 | 2 | 0.01 | 20 | 3 |
| 0.001 | 21 | 4 | 0.001 | 19 | 2 |
| 0.001 | 34 | 3 | 0.01 | 19 | 0 |
| 0.01 | 40 | 2 | 0.05 | 13a | 2 |
| LP | 50 | 4 | LP | 19a | 0 |
| 0.1 | 35 | 0 | 0.1 | 18 | 0 |
| 0.1 | 36 | 0 | 0.1 | 16 | 0 |
| 0.01 | 28 | 3 | 0.001 | 14 | 0 |
| 0.16 | 54 | 1 | 0.01 | 17a | 0 |
IOP at 6 month follow-up.
Table 4.
Mean preoperative and postoperative data at every examination.
| Preoperative | 1 day | 1 week | 1 month | 3 months | 6 months | 12 months | |
|---|---|---|---|---|---|---|---|
| IOP (mmHg) | 37.2 ± 11.33 | 12.1 ± 8.53 | 13.1 ± 4.81 | 19 ± 7.21 | 16.2 ± 5.22 | 19 ± 6.93 | 18.14 ± 2.41 |
| BCVA (logMar) | 1.6 ± 0.93 | 1.6 ± 0.79 | 1.3 ± 0.55 | 1.5 ± 0.82 | 1.2 ± 0.62 | 1.6 ± 0.84 | 1.7 ± 1.16 |
| No. of Medication | 2.2 ± 1.48 | 0.0 ± 0.0 | 0.5 ± 1.08 | 0.6 ± 1.35 | 0.9 ± 1.52 | 1 ± 1.49 | 0.87 ± 1.41 |
| Relative Success (%) | 80.00 | 90.00 | 70 | 80.00 | 70.00 | 100 | |
| Absolute Success (%) | 80.00 | 80 | 60 | 60.00 | 50.00 | 60 |
Fig. 3.
Preoperative and postoperative IOP levels (1 year follow-up except for 3 patients).
Fig. 4.
Kaplan–Meier survival analysis of absolute success versus postoperative time (1 year follow-up except for 3 patients).
Changes in postoperative BCVA were not statistically significant in comparison with preoperative BCVA, as shown in Fig. 5. Mean preoperative logMar VA declined from 1.59 ± 0.93 to 1.68 ± 1.16 after the operation. At the last follow-up, 20% of patients improved their VA, 30% stayed the same, and the VA of 50% of patients worsened. We found no postoperative loss of light perception.
Fig. 5.
Preoperative and postoperative BCVA (1 year follow-up except for 3 patients).
Early postoperative complications were athalamia, hyphema, and retinal detachment; the last was observed in two cases (20%). One of the patients had undergone prior retinal detachment surgery and suffered a re-detachment 4 days after the Ahmed placement. The other case was caused by a serous choroidal detachment. Late complications were pars plana clip extrusion (Fig. 6) and cystic bleb (Table 2).
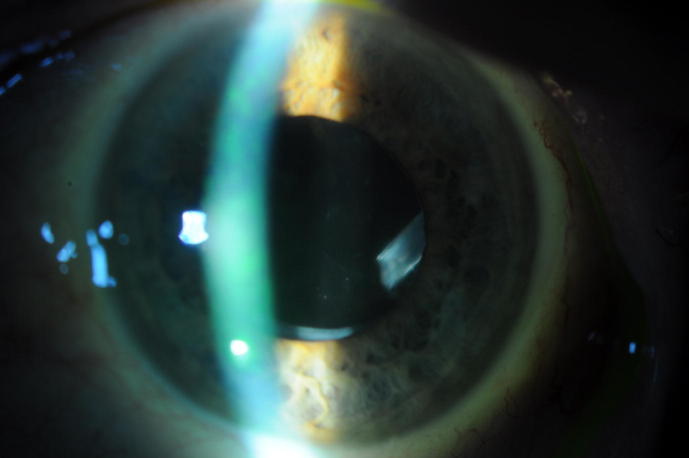
Fig. 6.
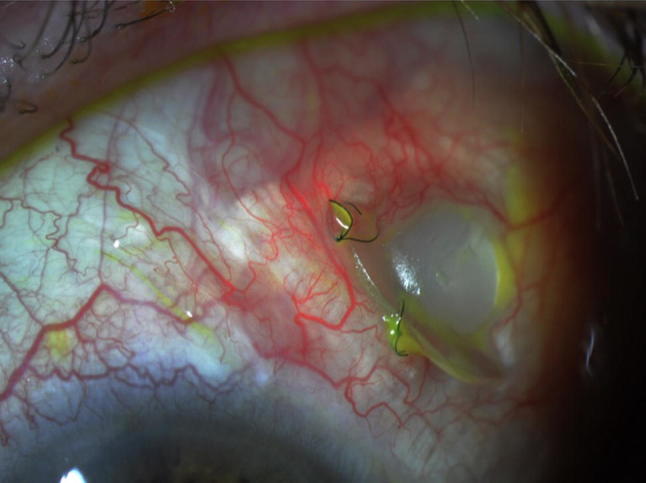
Postoperative image of a patient showing extrusion of the clip.
Discussion
Since its initial description in 1995, the AGV has allowed an improvement in surgical success rates and has decreased the number of complications compared to other surgical techniques previously used in refractory glaucoma.17 This can be due to the equatorial placement of the implant and the trabecular bypass.14, 15 However, the presence of the tube in the AC is associated with a higher risk of complications, such as endothelial decompensation and corneal graft failure.12, 18, 19, 20, 21, 22, 23, 24 Pars plana placement of the tube has considerable advantages. Firstly, it decreases the risk of endothelial cell loss.12, 25, 26, 27, 28 Although the mechanism for endothelial cell loss remains unclear, intermittent tube-corneal contact, low grade inflammation and high IOP seem to be involved.12, 25, 26, 27 Therefore, the pars plana placement of the tube is more suitable in patients with keratoplasties, endothelial disease or shallow ACs. Secondly, it constitutes a better option in eyes where the AC placement of the tube might be challenging, such as eyes with an AC intraocular lens or extensive peripheral anterior synechiae. In addition, it enables the use of the 23G sclerotomy performed for the vitreoretinal surgery for the insertion of the tube. Finally, the clip can be easily removed and the tube placed in the AC if any complication occurs.
The disadvantages of this technique include the difficulty visualizing the tube by anterior segment biomicroscopy, the possible tube obstruction caused by vitreous incarceration and potential posterior segment complications.
Several studies have shown the effectiveness of pars plana placement of Ahmed valve.23, 24, 25, 29, 30, 31, 32, 33, 34, 35, 36, 37 However, only few studies have analyzed the efficacy and safety of the PC7 model.24, 26, 29, 30, 31 In the present study, the absolute success rate was 60% at last follow-up, reaching 100% in terms of qualified success. These results are comparable to those in previous reports. Diaz-Llopis et al.30 achieved absolute success rates of 70% (100% qualified success) and Dada et al.31 reported absolute success rates of 54% (81% qualified success), both at 12 months. There are two studies where the outcomes after PC7 pars plana placement versus FP7 AC placement are compared.24, 29 The absolute and qualified success rates reported by Perihar24 et al. at 24 months were 28% and 72%, respectively. Maris et al.29 have reported 90% and 74,3% success rates at 12 and 24 months, respectively.
Nevertheless, direct comparison between these studies is difficult due to variable success criteria, different follow-up times and heterogeneous patient population. For instance, in the study conducted by Maris29, the success criterion was IOP between 5 and 21 mmHg with or without additional medications, whereas Diaz-Llopis30 considered the intervention successful if IOP was less than 21 mmHg without any glaucoma medication. Success criterion for Dada et al.31 was similar to ours, except for a lower IOP threshold (18 mmHg). Regarding the type of glaucoma, Maris et al.29 reported a 25.8% rate of primary open-angle glaucomas, whereas Parihar et al.24 excluded patients with neovascular glaucoma or retinal diseases. Diaz–Llopis30 and Dada31 analyzed refractory secondary glaucomas, similar to the cases in our study. All studies had a 12 month follow up period except two: one conducted by Parihar24 with a 24 month follow up and another by Maris29 with a mean follow-up of 20.9 months.
Other models of AGV and other GDDs have been implanted in the posterior segment with successful outcomes, mainly Baerveldt implants and less frequently Molteno and Krupin drainage devices.22, 27, 38, 39, 40, 41, 42 In terms of IOP levels, our surgical outcomes are similar to those reported by other authors using pars plana placement of AGV (72.2–100% in terms of qualified success)23, 25, 34, 35, 36, 37 and other GDDs (61.1–100%).3, 12, 32, 33, 38, 40 Finally, we achieved better success rates than those reported in Ahmed versus Baerveldt (AVB)9 and Ahmed Baerveldt Comparison (ABC)8 studies, where the results of two GGDs placed in the anterior chamber are compared.
The effect of the tube position on the IOP reduction remains unclear. The two studies comparing the anterior versus posterior segment placements of the tube have not found statistically significant differences between both groups in terms of IOP control, suggesting that GDDs with the same structure and filtration capacity should give similar results.24, 29 Whether this clip affects the draining capacity by ensuring a stable anchorage at a 90° angle thereby decreasing the tube obstruction rates is still uncertain. Schlote was the first to share the results of using the PPC with PS2 Ahmed Valve (polypropylene PC7 equivalent), achieving 64% absolute success rate without any tube obstruction.36 The tube obstruction rate in our study (1 case – 10%) is comparable to those with AGV including the PPC (12% reported by Parihar24, 12.9% by Maris29, 10% by Diaz-Llopis30, 3% by Chihara26 and 0% by Dada31). Similar devices have been employed previously with this purpose, such as the Hoffman’s elbow with Baerveldt GDD employed by Lutrull et al., with 90–95% success rates and 2% cases of tube incarceration.38 Regarding the studies analyzing surgical outcomes of the pars plana placement of AGV or other GDDs without employing a clip, tube blockage rates vary from 0% to 25%.23, 25, 27, 32, 33, 34, 35, 38, 39, 40, 42 Further studies with bigger samples employing GDDs with or without PPC should be conducted in order to find out whether a firm anchorage of the tube at a 90° angle can improve IOP control and decrease the rates of tube obstruction.
Visual acuity after glaucoma surgery can vary depending on the severity of glaucoma, surgical complications and other ocular comorbidities. In addition, the different preoperative stage of the glaucomas among studies makes difficult the comparison between them. In our study most of the advanced refractory glaucomas with other severe ocular conditions carried a poor VA prognosis. Half of the patients stabilized or improved their VA at last follow-up. Different VA improvement rates have been reported with pars plana placement of AGV: Maris29 (90.3%), Parihar24 (56%), Lieberman23 (52%), Seo25 (100%), Jeong35 (100%), Schlote36 (27.7%), Faghihi37 (77.7% improved or remained unchanged), Wallsh34 (66.7%). VA improvement outcomes with other GDDs range from 22% to 77.8%.12, 18, 22, 27, 38, 39, 40, 42
No intraoperative complications were registered in the present study. The most frequent postoperative complications were retinal detachment (20%), cystic bleb (20%), and pars plana clip extrusion (20%). The two patients with cystic bleb presented an IOP increase that returned to normal levels after needling and 5-fluorouracil injection of the bleb. It has been suggested that the pars plana clip might decrease the incidence of tube extrusion as it provides stable anchorage of the tube to the sclera.30 However, despite covering the clip with fascia lata or scleral patch, conjunctival erosion and clip extrusion can occur as a result of clip-conjuntiva contact, as happened in two of our cases. We report two major posterior segment complications. A patient who had been operated three times for recurrent retinal detachment suffered a re-detachment 4 days after AGV implantation and silicone oil removal surgery. The other case of retinal detachment was caused by a serous choroidal detachment associated with blockage of the tube with vitreous remnants. This patient required two more retinal detachment surgeries and the replacement of the tube in the posterior chamber. Vitreous incarceration might have been avoided by employing triamcinolone during vitrectomy, as suggested by Dada et al.31 It is difficult to elucidate, though, if the retinal detachments observed in our study are related to the PPV and posterior placement of the tube or to the underlying retinal disease. Posterior segment complications after GDD implantation have been reported both with AC and posterior segment placements.8, 43 These complications range from 2.5% to 32% after PP placement of AGV tube.23, 24, 25, 26, 29, 30, 31, 34, 35, 36, 37 The frequency and severity of complications in our study were not superior to other studies of AGV placement in the posterior segment.23, 24, 25, 26, 29, 30, 31, 34, 35, 36, 37
The Ahmed valve and other GDDs have also been proved valid and effective options in the management of pediatric glaucoma, both with AC and posterior segment placement of the tube.44, 45, 46, 47, 48, 49, 50, 51, 52 We are aware that data extrapolation from our two cases of PC8 model implantation is not possible. However, to our knowledge, outcomes after using this pediatric model have not been previously reported.
Limitations of our study include its retrospective, non-comparative and non-randomized design, the small sample size, variable severity of diseases, and the heterogeneous follow-up times. Prospective studies with larger sample size are needed comparing surgical outcomes after Ahmed AC vs posterior segment implantation in order to provide more meaningful long-term follow-up results. Our results in terms of efficacy and safety are consistent with those in previous reports. In conclusion, Ahmed valve implantation via pars plana can be a safe and useful option in patients with refractory glaucoma and high risk of surgical failure.
Acknowledgments
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
M. de Frutos-Lezaun, Email: mdefrutoslezaun@gmail.com.
I. Rodriguez-Agirretxe, Email: ira@icqo.org.
F. Eder Labairu, Email: fabiolaeder@gmail.com.
C. Irigoyen, Email: cirigoy@hotmail.com.
References
- 1.Quigley H.A. The number of persons with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker K.W., Gillow J.T., Cunliffe I.A. Is the role of trabeculectomy in glaucoma management changing? Eye. 2001;15:449–452. doi: 10.1038/eye.2001.153. [DOI] [PubMed] [Google Scholar]
- 3.Lindblom B., Nordmann J.P., Sellem E., Chen E., Gold R., Polland W. A multicentre, retrospective study of resource utilization and costs associated with glaucoma management in France and Sweden. Acta Ophthalmol Scand. 2006;84:74–83. doi: 10.1111/j.1600-0420.2005.00560.x. [DOI] [PubMed] [Google Scholar]
- 4.Lichter P.R., Musch D.C., Gillespie B.W., Guire K.E., Janz N.K., Wren P.A., CIGTS Study Group Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 5.Migdal C., Gregory W., Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101:1651–1656. doi: 10.1016/s0161-6420(94)31120-1. [DOI] [PubMed] [Google Scholar]
- 6.Molteno A.C. New implant for drainage. Animal trial. Br J Ophthalmology. 1969;53:161–168. doi: 10.1136/bjo.53.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramulu P.Y., Corcoran K.J., Corocran S.L., Robin A.L. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114:2265–2270. doi: 10.1016/j.ophtha.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Barton K., Feuer W.J., Budenz D.L., Schiffman J., Costa V.P., Godfrey D.G., Ahmed Baerveldt Comparison Study Group Three-year treatment outcomes in the ahmed baerveldt comparison study. Ophthalmology. 2014;121:1547–1557. doi: 10.1016/j.ophtha.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christakis P.G., Tsai J.C., Kalenak J.W., Zurakowski D., Cantor L.B., Kammer J.A. The Ahmed versus Baerveldt study: three-year treatment outcomes. Ophthalmology. 2013;120:2232–2240. doi: 10.1016/j.ophtha.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Hill R.A., Heuer D.K., Baerveldt G., Minckler D.S., Martone J.S. Molteno implantation for glaucoma in young patients. Ophthalmology. 1991;98:1042–1046. doi: 10.1016/s0161-6420(91)32179-1. [DOI] [PubMed] [Google Scholar]
- 11.Lim K.S., Allan B.D., Lloyd A.W., Muir A., Khaw P.T. Glaucoma drainage devices: past, present and future [review] Br J Ophthalmology. 1998;82:1083–1089. doi: 10.1136/bjo.82.9.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joos K.M., Lavina A.M., Tawansy K.A., Agarwal A. Posterior repositioning of glaucoma implants for anterior segment complications. Ophthalmology. 2001;108:279–284. doi: 10.1016/s0161-6420(00)00521-2. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen Q.H. Avoiding and managing complications of glaucoma drainage implants [review] Curr Opin Ophthalmol. 2004;15:147–150. doi: 10.1097/00055735-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Gedde S.J., Schiffman J.C., Feuer W.J., Herndon L.W., Brandt J.D., Budenz D.L., Tube versus Trabeculectomy Study Group Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gedde S.J., Singh K., Schiffman J.C., Feuer W.J. The Tube Versus Trabeculectomy Study: interpretation of results and application to clinical practice. Curr Opin Ophthalmol. 2012;23:118–126. doi: 10.1097/ICU.0b013e32834ff2d1. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia L.S., Chen T.C. New Ahmed valve designs. Int Ophthalmol Clin. 2004;44:123–138. doi: 10.1097/00004397-200404410-00014. [DOI] [PubMed] [Google Scholar]
- 17.Coleman A.L., Hill R., Wilson M.R., Choplin N., Kotas-Neumann R., Tam M. Initial clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1995;120:23–31. doi: 10.1016/s0002-9394(14)73755-9. [DOI] [PubMed] [Google Scholar]
- 18.Ritterband D.C., Shapiro D., Trubnik V., Marmor M., Meskin S., Seedor J., Cornea Glaucoma Implant Study Group (COGIS) Penetrating keratoplasty with pars plana glaucoma drainage devices. Cornea. 2007;26:1060–1066. doi: 10.1097/ICO.0b013e3181342835. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood M.B., Smith M.F., Driebe W.T., Jr, Stern G.A., Beneke J.A., Zam Z.S. Drainage tube implants in the treatment of glaucoma following penetrating keratoplasty. Ophthalmic Surg. 1993;24:185–189. [PubMed] [Google Scholar]
- 20.Arroyave C.P., Scott I.U., Fantes F.E., Feuer W.J., Murray T.G. Corneal graft survival and intraocular pressure control after penetrating keratoplasty and glaucoma drainage device implantation. Ophthalmology. 2001;108:1978–1985. doi: 10.1016/s0161-6420(01)00803-x. [DOI] [PubMed] [Google Scholar]
- 21.Kwon Y.H., Taylor J.M., Hong S., Honkanen R.A., Zimmerman M.B., Alward W.L. Long-term results of eyes with penetrating keratoplasty and glaucoma drainage tube implant. Ophthalmology. 2001;108:272–278. doi: 10.1016/s0161-6420(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 22.Witmer M.T., Tiedeman J.S., Olsakovsky L.A., Conaway M.R., Prum B.E. Long term intraocular pressure control and corneal graft survival in eyes with a pars plana Baerveldt implant and corneal transplant. J Glaucoma. 2010;19:124–131. doi: 10.1097/IJG.0b013e3181a98cc1. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman R.A., Maris P.J., Jr, Monroe H.M., Al-Aswad L.A., Bansal R., Lopez R. Corneal graft survival and intraocular pressure control in coexisting penetrating keratoplasty and pars plana Ahmed Glaucoma Valves. Cornea. 2012;31:350–358. doi: 10.1097/ICO.0b013e31823cbd29. [DOI] [PubMed] [Google Scholar]
- 24.Parihar J.K., Jain V.K., Kaushik J., Mishra A. Pars plana-modified versus conventional Ahmed glaucoma valve in patients undergoing penetrating keratoplasty: a prospective comparative randomized study. Curr Eye Res. 2016;27:1–7. doi: 10.1080/02713683.2016.1185130. [DOI] [PubMed] [Google Scholar]
- 25.Seo J.W., Lee J.Y., Nam D.H., Lee D.Y. Comparison of the changes in corneal endothelial cells after pars plana and anterior chamber Ahmed valve implant. J Ophthalmol. 2015;2015:486832. doi: 10.1155/2015/486832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chihara E., Umemoto M., Tanito M. Preservation of corneal endothelium after pars plana tube insertion of the Ahmed glaucoma valve. Jpn J Ophthalmol. 2012;56:119–127. doi: 10.1007/s10384-011-0108-1. [DOI] [PubMed] [Google Scholar]
- 27.Sidoti P.A., Mosny A.Y., Ritterband D.C., Seedor J.A. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology. 2001;108:1050–1058. doi: 10.1016/s0161-6420(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 28.Lee E.K., Yun Y.J., Lee J.E., Yim J.H., Kim C.S. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol. 2009;148:361. doi: 10.1016/j.ajo.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Maris P.J., Jr, Tsai J.C., Khatib N., Bansal R., Al-Aswad L.A. Clinical outcomes of Ahmed Glaucoma valve in posterior segment versus anterior chamber. J Glaucoma. 2013;22:183–189. doi: 10.1097/IJG.0b013e318237c115. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Llopis M., Salom D., García-Delpech S., Udaondo P., Millan J.M., Arevalo J.F. Efficacy and safety of the pars plana clip in the Ahmed valve device inserted via the pars plana in patients with refractory glaucoma. Clin Ophthalmol. 2010;4:411–416. doi: 10.2147/opth.s10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dada T., Bhartiya S., Vanathi M., Panda A. Pars plana Ahmed glaucoma valve implantation with triamcinolone-assisted vitrectomy in refractory glaucomas. Indian J Ophthalmol. 2010;58:440–442. doi: 10.4103/0301-4738.67068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suárez-Fernández M.J., Gutiérrez-Díaz E., Julve San Martín A., Fernández-Reyes M.F., Mencía-Gutiérrez E. Simultaneous pars plana vitrectomy and glaucoma drainage device implant. Arch Soc Esp Oftalmol. 2010;85:97–102. [PubMed] [Google Scholar]
- 33.Rososinski A., Wechsler D., Grigg J. Retrospective review of pars plana versus anterior chamber placement of baerveldt glaucoma drainage device. J Glaucoma. 2015;24:95–99. doi: 10.1097/IJG.0b013e31829d9be2. [DOI] [PubMed] [Google Scholar]
- 34.Wallsh J.O., Gallemore R.P., Taban M., Hu C., Sharareh B. Pars plana Ahmed valve and vitrectomy in patients with glaucoma associated with posterior segment disease. Retina. 2013;33:2059–2068. doi: 10.1097/IAE.0b013e31828992c3. [DOI] [PubMed] [Google Scholar]
- 35.Jeong H.S., Nam D.H., Paik H.J., Lee D.Y. Pars plana Ahmed Implantation Combine with 23-gauge vitrectomy for refractory neovascular glaucoma in diabetic retinopathy. Korean. J Ophthalmol. 2012;26:92–96. doi: 10.3341/kjo.2012.26.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlote T., Ziemssen F., Bartz-Schmift K.U. Pars-plana modified Ahmed Galucoma Valve for treatment of refractory glaucoma: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2006;244:336. doi: 10.1007/s00417-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 37.Faghihi H., Hajizadeh F., Mohammadi S.F., Kadkhoda A., Peyman G.A., Riazi-Esfahani M. Pars plana Ahmed valve implant and vitrectomy in the management of neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2007;38:292–300. doi: 10.3928/15428877-20070701-04. [DOI] [PubMed] [Google Scholar]
- 38.Luttrull J.K., Avery R.L., Baerveldt G., Easley K.A. Initial experience with pneumatically stented baerveldt implant modified for pars plana insertion for complicated glaucoma. Ophthalmology. 2000;107:143–149. doi: 10.1016/s0161-6420(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd M.A., Heuer D.K., Baerveldt G., Minckler D.S., Martone J.F., Lean J.S. Combined Molteno implantation and pars plana vitrectomy for neovascular glaucomas. Ophthalmology. 1991;98:1401–1405. doi: 10.1016/s0161-6420(91)32120-1. [DOI] [PubMed] [Google Scholar]
- 40.De Guzman M.H., Valencia A., Farinelli A.C. Pars plana insertion of glaucoma drainage devices for refractory glaucoma. Clin Exp Ophthalmol. 2006;34:102–107. doi: 10.1111/j.1442-9071.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 41.Varma R., Heuer D.K., Lundy D.C., Baerveldt G., Lee P., Minckler D.S. Pars plana Baerveldt tube insertion with vitrectomy in glaucomas associated with pseudophakia and aphakia. Am J Ophthalmol. 1995;119:401–407. doi: 10.1016/s0002-9394(14)71224-3. [DOI] [PubMed] [Google Scholar]
- 42.Kolomeyer A.M., Seery C.W., Emami-Naeimi P., Zarbin M.A., Fechtner R.D., Bhagat N. Combined pars plana vitrectomy and pars plana baerveldt tube placement in eyes with neovascular glaucoma. Retina. 2015;35:17–28. doi: 10.1097/IAE.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 43.Gedde S.J., Herndon L.W., Brandt J.D., Budenz D.L., Feuer W.J., Schiffman J.C., Tube Versus Trabeculectomy Study Group Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen A., Yu F., Law S.K., Giaconi J.A., Coleman A.L., Caprioli J. Valved glaucoma drainage devices in pediatric glaucoma: retrospective long-term outcomes. JAMA Ophthamolo. 2015;133:1030–1035. doi: 10.1001/jamaophthalmol.2015.1856. [DOI] [PubMed] [Google Scholar]
- 45.Balekudaru S., Vadalkar J., George R., Vijaya L. The use of Ahmed glaucoma valve in the management of pediatric glaucoma. J AAPOS. 2014;18:351–356. doi: 10.1016/j.jaapos.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Mandalos A., Tailor R., Parmar T., Sung V. The long-term outcomes of glaucoma drainage device in pediatric glaucoma. J Glaucoma. 2014;14 doi: 10.1097/IJG.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 47.Al-Mobarak F., Khan A.O. Two-year survival of Ahmed valve implantation in the first 2 years of life with and without intraoperative mitomycin-C. Ophthalmology. 2009;116:1862–1865. doi: 10.1016/j.ophtha.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 48.Beck A.D., Freedman S., Kammer J., Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003;136:994–1000. doi: 10.1016/s0002-9394(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 49.Djodeyre M.R., Peralta Calvo J., Abelairas Gomez J. Clinical evaluation and risk factors of time to failure of Ahmed Glaucoma Valve implant in pediatric patients. Ophthalmology. 2001;108:614–620. doi: 10.1016/s0161-6420(00)00603-5. [DOI] [PubMed] [Google Scholar]
- 50.El Sayed Y., Awadein A. Polypropylene vs silicone Ahmed valve with adjunctive mitomycin C in paedriatic age group: a prospective controlled study. Eye (Lond) 2013;27:728–734. doi: 10.1038/eye.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morad Y., Donaldson C.E., Kim Y.M., Abdolell M., Levin A.V. The Ahmed drainage implant in the treatment of pediatric glaucoma. Am J Ophthalmol. 2003;135:821–829. doi: 10.1016/s0002-9394(02)02274-2. [DOI] [PubMed] [Google Scholar]
- 52.Albis-Donado O., Gil-Carrasco F., Romero-Quijada R., Thomas R. Evaluation of Ahmed glaucoma valve implantation through a needle-generated scleral tunnel in Mexican children with glaucoma. Indian J Ophthalmol. 2010;58:365–373. doi: 10.4103/0301-4738.67039. [DOI] [PMC free article] [PubMed] [Google Scholar]