Abstract
Haemonchus contortus is one of the prevalent and pathogenic ruminant parasites that has grown resistance to common anthelmintic treatment. This study evaluated the anthelmintic potential of Moringa oleifera seed ethanolic and aqueous extracts against H. contortus eggs and infective stage larvae (L3s). The efficacy of five extract concentrations (0.95, 1.95, 3.9, 7.8, and 15.6 mg/mL) were tested through egg hatch assay and larval motility test. Phytochemical tests were conducted to detect the different plant secondary metabolites in the extracts. In the ovicidal assay, the ethanolic and aqueous extracts showed 95.89% and 81.72% egg hatch inhibition at 15.6 mg/mL, respectively. The ovicidal activity of 15.6 mg/mL ethanolic extract was comparable with that of albendazole (p > 0.05). The LC50 against the eggs was recorded at 2.91 and 3.83 mg/mL for ethanolic and aqueous extracts, respectively. In the larvicidal assay, the ethanolic and aqueous extracts exhibited 56.94% and 92.50% efficacy at 7.8 mg/mL, respectively. The larvicidal activity of 7.8 mg/mL aqueous extract was similar statistically with that of ivermectin (p > 0.05). The LC50 against L3s was recorded at 6.96 and 4.12 mg/mL for ethanolic and aqueous extracts, respectively. The secondary metabolites detected were tannins in ethanolic extract and saponins in aqueous extract. Both extracts inhibited larvae formation inside the eggs and rendered the L3s immobile. Therefore, M. oleifera seed extracts contained plant bioactive compounds with anthelmintic property against the eggs and L3s of H. contortus.
Keywords: Anthelmintic, Goat, Haemonchus contortus, Moringa oleifera
1. Introduction
Gastrointestinal nematodes (GINs) remain one of the most common ruminant production constraints in many developing countries. Among the GINs, Haemonchus contortus is the most pathogenic species in small ruminant [1]. This type of nematode sucks blood voraciously inside the goat’s abomasum and causes severe anemia and anasarca. Hence, the parasite’s pathogenic effect is attributed to severe blood loss that overwhelms the hematopoietic capacity of the animal thereby causing acute mortality [2]. Aside from its pathogenicity, H. contortus remains prevalent in countries with a tropical climate, such as the Philippines [3]. Therefore, haemonchosis suppressed the ultimate development of small ruminant subsector by contributing to production losses that are attributed to high neonatal mortality, high all-age morbidity, and high cost of treatment [4].
The treatment of haemonchosis has relied deeply on the repeated application of commercial and synthetic anthelmintics, for example, albendazole and ivermectin [5]. The term ‘global worming’ was thus coined describing the era of our dependency to and intensive use of such anthelmintics [6]. Regrettably, this act has resulted in the tremendous upsurge of anthelmintic resistant GINs, especially H. contortus. Resistant parasites have the resistant gene(s) of specific anthelmintic(s) which allow them to persist and cause havoc inside the animal’s body. The worsening anthelmintic resistance has commonly been reported in goat farms and aggravated by the slow commercialization of novel anthelmintic compounds [6], [7]. In the Philippines, benzimidazole resistance was considered widespread in many provinces of Luzon island [8].
The problem with commercial and synthetic anthelmintics is not confined to anthelmintic resistance alone. The indiscriminate application of such anthelmintics also threatens the public health by the increasing reports of drug residues in milk and meat. Moreover, the expensive product poses a question about its sustainability in developing countries where ruminant farming is common [4], [9]. These drawbacks consequently give birth to new approaches in fighting GINs for sustainable livestock farming. One of these approaches is the exploration and screening of various plants for novel anthelmintic compounds [10].
Anthelmintics derived from indigenous plants have pragmatic features for small hold farmers who cannot afford the commercial product, and for large commercial farmers who are shifting to organic farming [11], [12]. Furthermore, herbal anthelmintics as a natural product are implicated to least likely bioaccumulate in the tissues of animal and the environment [13]. They are likewise considered eco-friendly and biodegradable [13], [14]. Above all, multiple bioactive compounds present in herbal anthelmintics may translate to multiple mechanisms in killing the parasites, which then limit the likelihood of developing anthelmintic resistance [15].
Moringa oleifera is one of the important plants in the Philippines utilized for food and traditional medicine. Previous studies revealed the array of its bioactive compounds with nutritive and medicinal values present in the leaves, seeds, roots, barks, and flowers [16]. In addition, this plant was utilized in the treatment of malaria, leishmaniasis, trypanosomiasis, schistosomiasis, dracunculiasis, and filariasis thus suggesting its inherent antiparasitic property [16], [17]. This property was further supported by few studies utilizing the leaf and seed extracts against the gastrointestinal parasites of the ruminant. Tayo et al. [18] concluded that the infused and macerated aqueous and ethanolic leaf extracts exhibited varying level of ovicidal and larvicidal activities against the eggs (fresh and embryonated) and larvae (L1 and L2) of H. contortus. A field study further demonstrated that supplementation of leaves may suppress the burden of strongyle worms in naturally infected cross-bred Xhosa lop eared goats [19] and West African Dwarf goats [20]. Aside from the leaves, the seed extract of M. oleifera was also evaluated against H. contortus [20]. Salles et al. [21] showed that the seed fraction of low molecular weight (<12 kDa) contained potential molecules responsible for over 90% egg hatch inhibition.
To the best of our knowledge, the study of M. oleifera seeds against H. contortus is very limited and has no data pertaining to its larvicidal activity. The current study utilized a different plant extraction approach and evaluated the ethanolic and aqueous extract of M. oleifera seeds against the eggs and L3s of H. contortus. We further tested the extracts for secondary metabolites that might be responsible for its anthelmintic activity.
2. Materials and methods
2.1. Collection and extraction of M. oleifera seeds
Young pods containing the seeds of M. oleifera were collected from Brgy. Sto. Rosario, Baybay City, Leyte. The freshly collected seeds were air-dried for three days to remove 60% of its moisture content. The dried seeds were pulverized and extracted following the protocol of Fernandez et al. [22]. In brief, the powdered seeds were soaked in 99.5% ethanol at the ratio of 1:3 (w/v) and allowed to stand protected from light at 27 °C for 48 h. The ethanol extract was sieved and filtered using a muslin cloth and Whatman™ Grade 54 filter paper, respectively. The ethanol solvent was evaporated at 40 °C in a vacuum rotary evaporator until the remaining volume of the extract was 3% of the original. The product of the ethanol extraction was called M. oleifera seed ethanolic extract (MSEE). A 1% dimethyl sulfoxide (DMSO) was added to enhance the emulsification of MSEE. Following the previous procedure, the seed residue was re-extracted using distilled water as a solvent and concentrated the extract at 60 °C in a vacuum rotary evaporator to obtain the M. oleifera seed aqueous extract (MSAE).
2.2. Phytochemical screening
The ethanolic and aqueous extracts were tested for the presence of alkaloids (Dragendorff’s reagent), flavonoids (Bate-Smith and Metcalf test), saponins (froth test), terpenoids (Salkowski test), and tannins (ferric chloride reaction and gelatin test) [4], [22].
2.3. Egg hatch test
All adult H. contortus were recovered from the abomasum of goat slaughtered in Baybay City Abattoir. For the preparation of egg suspension, all female H. contortus were morphologically selected and macerated to liberate the eggs. The eggs were successively sieved and finally diluted with phosphate-buffered saline (PBS). Every treatment was triplicated in which a single replicate contained 10–15 random sample of eggs in 10 µL PBS. These eggs were exposed to M. oleifera seed extracts (0.95, 1.9, 3.9, 7.8, and 15.6 mg/mL) and control groups (0.5% DMSO, 0.5% ethanol, PBS, and 0.5% albendazole). These were humidly incubated in a room at 27 °C for 72 h. We stopped the development of eggs by adding a drop of Lugol’s iodine and counted the number of eggs that remained morulated, showed ‘larva failing eclosion’, and turned into free L1 [23] (Fig. 1). The ovicidal activity was expressed based on the percentage of eggs that failed to develop and hatch [23].
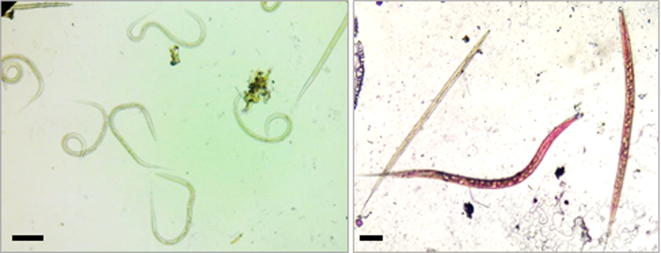
Fig. 1.
Egg hatch assay of H. contortus eggs exposed to M. oleifera seed extracts after 72 h showing an egg that (a) remained morulated (40× magnification), (b) showed ‘larva failing eclosion’ (40× magnification), and (c) free L1 (10× magnification). Scale bar = 50 µm.
2.4. Larval motility test
The procedure for the culture of L3s was based on the technique described by Rupa and Portugaliza [3], while the in vitro larvicidal assay was adopted from Fernandez et al. [22]. There were six replications per treatment and for each replicate an amount of 10 µL PBS with 10–15 alive L3s were randomly pipetted. The L3s were exposed to M. oleifera seed extracts (0.95, 1.9, 3.9, 7.8, and 15.6 mg/mL) and control groups (0.5% DMSO, 0.5% ethanol, PBS, and 0.5% ivermectin). These were humidly incubated at 27 °C for 3 h. After 3 h, we recorded the motility of L3s by gentle prodding. Afterward, we added a drop of 1% Delafield’s Hematoxylin stain and re-incubated the set-up for 24 h. The L3s were re-examined under the microscope for the uptake of stain. We categorized immobile larvae based on motility upon prodding and identified the larvae with cuticle damage based on the uptake of the red stain (Fig. 2). The immobile larvae were either dead or weakened; hence, the larvicidal activity was expressed on the percentage of immobile L3s after exposure [22].
Fig. 2.
Haemonchus contortus L3s before (left) and after (right) treatment. Alive L3s are motile while weak and dead L3s are immobile upon prodding and/or stained pink with 1% Delafield’s Hematoxylin stain. Scale bar = 50 µm.
2.5. Statistical analysis
We calculated the lethal concentrations (LC50) and 95% fiducial limits (95% FL) of M. oleifera seed extracts using the probit analysis and analyzed the difference between treatment groups using one-way ANOVA and Tukey’s HSD in IBM SPSS version 21. All results were considered statistically significant when p < 0.05.
3. Results
Table 1 shows the in vitro anthelmintic activity of M. oleifera seed ethanolic (MSEE) and aqueous (MSAE) extracts against the eggs and L3s of H. contortus. Except for the larvicidal activity of MSAE, the rest of the M. oleifera seed extracts displayed its anthelmintic activity in a dose-dependent manner (Fig. 3). Furthermore, the secondary metabolites detected in the ethanolic and aqueous extract were only tannins and saponins, respectively. Alkaloids, flavonoids and terpenoids were undetected from MSEE and MSAE.
Table 1.
The ovicidal and larvicidal activities of M. oleifera seed ethanol (MSEE) and aqueous extracts (MSAE) at varying concentrations against H. contortus.
| Treatment Group | % Ovicidal Activity (SD) of MSEE | % Ovicidal Activity (SD) of MSAE | % Larvicidal Activity (SD) of MSEE | % Larvicidal Activity (SD) of MSAE |
|---|---|---|---|---|
| M. oleifera Seed Extract | ||||
| 0.95 mg/mL | 26.75 (1.79)bc | 24.45 (3.85)b | 31.48 (10.19)b | 35.28 (6.00)b |
| 1.95 mg/mL | 34.92 (1.37)c | 28.24 (2.21)b | 36.81 (11.31)b | 44.44 (8.61)b |
| 3.9 mg/mL | 56.82 (3.35)d | 47.61 (2.01)c | 42.14 (4.02)b | 32.78 (6.72)b |
| 7.8 mg/mL | 71.11 (1.9)e | 68.63 (1.74)d | 56.94 (11.08)c | 92.50 (11.73)d |
| 15.6 mg/mL | 95.89 (7.22)f | 81.72 (1.67)e | 56.11 (7.12)c | 71.67 (9.31)c |
| Positive control* | 100 (0.00)f | 100 (0.00)f | 100 (0.00)d | 100 (0.00)d |
| 0.5% DMSO | 7.03 (0.58)a | NT | 0.00 (0.00)a | NT |
| 0.5% Ethanol | 0.00 (0.00)a | NT | 0.00 (0.00)a | NT |
| PBS | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a | 0.00 (0.00)a |
DMSO: Dimethyl sulfoxide; PBS: phosphate-buffered saline; MSEE: M. oleifera Seed Ethanolic Extract; MSAE: M. oleifera Seed Aqueous Extract; NT: Not tested; SD: Standard deviation.
Albendazole (0.5%) or Ivermectin (0.5%).
Different superscript letters indicate significant differences at p < 0.05.
Different superscript letters indicate significant differences at p < 0.05.
Different superscript letters indicate significant differences at p < 0.05.
Different superscript letters indicate significant differences at p < 0.05.
Different superscript letters indicate significant differences at p < 0.05.
Different superscript letters indicate significant differences at p < 0.05.
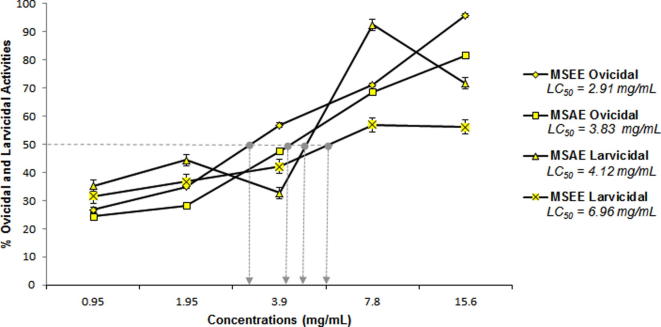
Fig. 3.
Dose-response relationship and the LC50 of M. oleifera seed ethanolic (MSEE) and aqueous (MSAE) extracts against H. contortus eggs and larvae.
In the ovicidal assay, the ethanolic extract exhibited the highest or 95.89% efficacy at 15.6 mg/mL. This result was statistically comparable to the 100% efficacy of 0.5% albendazole (p > 0.05). Interestingly, at least 80% of the inhibited eggs failed to develop into larva and approximately 20% contained larva that failed to hatch (data not shown in the table). For example, out of 95.83% eggs that were inhibited at 15.6 mg/mL MSEE, 79.50% remained morulated while 16.33% showed ‘larva failing eclosion’. On the other hand, the aqueous extract of M. oleifera seeds effectively inhibited egg hatching by 81.72% at 15.6 mg/mL, in which 73.67% of the inhibited eggs remained morulated. At least 70% of the unhatched eggs in all aqueous extracts failed to form larva or remained morulated. The positive control or albendazole showed 100% ovicidal activity by inhibiting the formation of larva inside the egg.
In the larvicidal assay, the highest activity of the ethanolic extract was observed at 7.8 and 15.6 mg/mL with 56.94% and 56.11% efficacy, respectively. Both concentrations, however, were similar statistically in terms of efficacy (p > 0.05). Conversely, the aqueous extract rendered larval mortality by 92.50% at 7.8 mg/mL. This result was statistically comparable to the 100% efficacy of 0.5% ivermectin (p > 0.05). All dead L3s treated with M. oleifera seed extracts were immobile upon prodding but remained unstained with 1% Delafield Hematoxylin. On the contrary, the L3s treated with 0.5% ivermectin were all immobile and stained (Fig. 2).
Fig. 3 shows the LC50 of M. oleifera seed extracts against H. contortus eggs and L3s. The ethanolic and aqueous extracts induced 50% lethality against the eggs at 2.91 mg/mL (2.14–3.93 mg/mL) and 3.83 mg/mL (2.78–5.35 mg/mL), respectively. The LC50 against L3s was observed at 6.96 mg/mL (3.22–77.73 mg/mL) for MSEE and 4.12 mg/mL (95% FL undetermined) for MSAE. Our data further showed that the ovicidal activities of both ethanolic and aqueous extracts were statistically similar (p > 0.05) but the larvicidal activity of the aqueous extract outperformed significantly the ethanolic extract (p < 0.05).
4. Discussion
The emergence of anthelmintic resistant H. contortus spurred the search for plant-based anthelmintics. M. oleifera is reported to contain various bioactive compounds with pharmacological activities such as anthelmintic property [17]. This study evaluated the anthelmintic activity of M. oleifera seeds against H. contortus eggs and L3s. We also determined the different secondary metabolites that are possibly responsible for the anthelmintic activity of M. oleifera seed extracts.
Our phytochemical screening detected tannins in the ethanolic extract and saponins in the aqueous extract as the primary secondary metabolites in M. oleifera seeds. These bioactive substances are implicated in some plants for ovicidal and larvicidal activities against helminths [24], [25].
Our ovicidal study demonstrated that M. oleifera seed extracts inhibited significantly the development of H. contortus eggs at 15.6 mg/mL concentration. Based on the categorization of anthelmintic efficacy [4], [26], the in vitro ovicidal activity of M. oleifera seeds is moderately effective (80–89% efficacy) using the aqueous extract and effective (90–98% efficacy) using the ethanolic extract. In addition, both extracts rendered most of the H. contortus eggs undeveloped suggesting that the bioactive compounds are lethal to the blastomeres. Tannins or saponins possibly penetrated the different layers of the egg and inhibited the formation of larva by affecting the morula [27], [28]. Several studies had demonstrated that saponins and tannins could stop larval formation and hatching process of H. contortus eggs [23], [29]. Although not quite exact on how M. oleifera inhibit egg embryonation, Sreelatha et al. [30] explained that the leaf extract could induce cellular apoptosis, morphological change, and DNA fragmentation in a type of cancer cell.
Aside from the ability to stop larval formation, we also observed a small percentage of the affected eggs with formed larvae that failed to hatch. Vargas-Magaña et al. [23] outlined the plausible mechanisms on how plant extract inhibited hatching of the egg with full-grown larva. They speculated three mechanisms: first, the extract affects the permeability of the eggshell; second, the extract inhibits some enzymes for egg hatching; and third, the extract affects the hatching receptors found in eggshells.
In a related study, Salles et al. [21] observed that the crude aqueous extract of M. oleifera seeds produced greater than 90% egg hatch inhibition against H. contortus. After conducting a membrane dialysis, they further found higher ovicidal activity in low molecular weight (<12 kDa) fraction compared with high molecular weight (>12 kDa) fraction. The fraction of M. oleifera with low molecular weight was reported to contain proteins, peptides, tannins, terpenes and alkaloids. Interestingly, they observed more larvated eggs that failed to hatch than eggs that remained morulated. This result is in contrary to our findings and is probably explained by the difference in extraction procedure. In fact, the different solvent extractions and extract fractionations will yield different type and quantity of bioactive compounds from the plant [10]. Various plant secondary metabolites have solubility specific to the polarity of the solvent used during extraction. In our case, the seeds from the young pods of M. oleifera contained saponins that are polar (water soluble) and tannins that are mid-polar (ethanol soluble) [4], [22].
The in vitro testing of L3s is considered among the best methods to screen the anthelmintic activity of plant compounds [10]. The highest larvicidal activity of M. oleifera was observed at 7.8 mg/mL producing the efficacy of 56.94% for ethanolic extract and 92.50% for aqueous extract. Hence, only the aqueous extract is considered effective in vitro according to Kassai [26]. M. oleifera treated L3s were immobile upon continuous prodding but remained unstained. This result may suggest that the phytochemicals present severely weaken the larvae but did not significantly cause immediate damage to the protective cuticle of L3s thus restricting the stain from entering the body. The stainability of the affected larvae would therefore be pronounced to certain plant extracts with substances (e.g. cysteine proteinases) that can severely damage the protective cuticle [31], [32]. This is one of the reasons why we introduced the staining of L3s. In ivermectin, we observed that the L3s were not motile after 3 h and absorbed the stain after 24 h. This potent anthelmintic paralyzes the nematode which results in death [33]. Immediate death means autolysis of the larva thus the stain penetrates inside the body. Nevertheless, the scope of our larvicidal assay is limited to motility and staining, not to the mechanisms of action or pathologic changes that are produced by the extracts.
In this study, the M. oleifera seed extracts contained either tannins (ethanolic extract) or saponins (aqueous extract). Tannins are found to reduce the motility of the L3s, which may indicate paralysis and interference to neuromuscular coordination of the larvae [27], [34]. Saponins are reported to destabilize the cell membrane and cuticle collagen of the parasite. These mechanisms however depend on the concentrations of saponins and the time of parasite’s exposure [35]. In general, the mechanisms of plant extracts may differ according to the type of parasite, the parasite’s stage of development, and the biochemical characteristics of the plant [27]. Multiple bioactive substances present in plant extracts may target the early developing stages of the parasite, such as the eggs and larvae, preventing further development and perpetuation thus a potential source of a new anthelmintic drug [4].
5. Conclusions
M. oleifera seed aqueous and ethanolic extracts possess plant bioactive compounds, including tannins and saponins, with in vitro anthelmintic activity against H. contortus eggs and L3s. While the in vitro efficacies are comparable to a commercial anthelmintic, it is necessary to conduct a toxicological test and an in vivo assay to understand better the potential of M. oleifera seed extracts in treating subclinical and clinical haemonchosis.
Competing interests
The authors declare that they have no conflict of interest.
Acknowledgement
We express our appreciation to the Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (PCAARRD) Project 4.1 team and the Diagnostic Laboratory personnel of the College of Veterinary Medicine, Visayas State University for partly sharing the needed materials and equipment. We also thank Dr. Hilary Niña O. Vitor for taking the photos and assisting us during the experiment.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Mortensen L.L., Williamson L.H., Terrill T.H., Kircher R.A., Larsen M., Kaplan R.M. Evaluation of prevalence and clinical implications of anthelmintic resistance in gastrointestinal nematodes in goats. J Am Vet Med Assoc. 2003;223(4):495–500. doi: 10.2460/javma.2003.223.495. [DOI] [PubMed] [Google Scholar]
- 2.Bowman DD. Helminths. In: Georgis’ parasitology for veterinarians. 9th ed. St. Louis, Missouri: Saunders Elsevier; 2009. pp. 161–162
- 3.Rupa A.P.M., Portugaliza H.P. Prevalence and risk factors associated with gastrointestinal nematode infection in goats raised in Baybay city, Leyte, Philippines. Vet World. 2016;9(7):728–734. doi: 10.14202/vetworld.2016.728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez T.J., Jr., Portugaliza H.P., Braga F.B., Vasquez E.A., Acabal A.D., Divina B.P. Effective dose (ED) and quality control studies of the crude ethanolic extract (CEE) mixture of makabuhay, caimito and makahiya (MCM) as dewormer for goats against Haemonchus contortus. Asian J Exp Biol Sci. 2013;4(1):28–35. [Google Scholar]
- 5.Lalchhandama K. Anthelmintic resistance: the song remains the same. Sci Vis. 2010;10:111–122. [Google Scholar]
- 6.Kaplan R.M. Prescription-only anthelmintic drugs: the time is now. BioScience. 2013;63(11):852–853. [Google Scholar]
- 7.Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol. 2012;186(1):70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Ancheta P.B., Dumilon R.A. Benzimidazole resistance of some nematodes in small ruminants. Philipp J Vet Med Animal Sci. 2000;26:147–152. [Google Scholar]
- 9.Shalaby H.A. Anthelmintics resistance; how to overcome it? Iran J Parasitol. 2013;8(1):18–32. [PMC free article] [PubMed] [Google Scholar]
- 10.Hernández-Villegas M.M., Borges-Argáez R., Rodriguez-Vivas R.I., Torres-Acosta J.F.J., Méndez-González M., Caceres-Farfan M. Ovicidal and larvicidal activity of the crude extracts from Phytolacca icosandra against Haemonchus contortus. Vet Parasitol. 2011;179(1):100–106. doi: 10.1016/j.vetpar.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Gradé J.T., Arble B.L., Weladji R.B., Van Damme P. Anthelmintic efficacy and dose determination of Albizia anthelmintica against gastrointestinal nematodes in naturally infected Ugandan sheep. Vet Parasitol. 2008;157(3):267–274. doi: 10.1016/j.vetpar.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Taylor J.L.S., Rabe T., McGaw L.J., Jäger A.K., Van Staden J. Towards the scientific validation of traditional medicinal plants. Plant Growth Regul. 2001;34(1):23–37. [Google Scholar]
- 13.Hammond J.A., Fielding D., Bishop S.C. Prospects for plant anthelmintics in tropical veterinary medicine. Vet Res Commun. 1997;21(3):213–228. doi: 10.1023/a:1005884429253. [DOI] [PubMed] [Google Scholar]
- 14.McCorkle C.M. Back to the future: lessons from ethnoveterinary RD&E for studying and applying local knowledge. Agric Hum Values. 1995;12(2):52–80. [Google Scholar]
- 15.Chagas A.C.S. Medicinal plant extracts and nematode control. CAB Rev. 2015;10(8):1–8. [Google Scholar]
- 16.Fahey J.W. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Trees Life J. 2005;1(5):1–15. [Google Scholar]
- 17.Wang L., Chen X., Wu A. Mini review on Antimicrobial Activity and Bioactive Compounds of Moringa oleifera. Med Chem (Los Angeles) 2016;6(9):578–582. [Google Scholar]
- 18.Tayo G.M., Poné J.W., Komtangi M.C., Yondo J., Ngangout A.M., Mbida M. Anthelminthic activity of Moringa oleifera leaf extracts evaluated in vitro on four developmental stages of Haemonchus contortus from goats. Am J Plant Sci. 2014;5:1702–1710. [Google Scholar]
- 19.Moyo B., Masika P.J., Muchenje V. Effects of supplementing cross-bred Xhosa lop eared goats with Moringa oleifera Lam. on helminth load and corresponding body condition score, packed cell volume. Afr J Agric. 2013;8(43):5327–5335. [Google Scholar]
- 20.Asaolu V., Odeyinka S., Akinbamijo O. Evaluation of anthelmintic attributes of moringa and bamboo leaves in gastrointestinal nematode-infested west african dwarf goats. J Nat Sci Res. 2012;2(9):45–53. [Google Scholar]
- 21.Salles H.O., Braga A.C.L., do Nascimento M.T.D.S., Sousa A.M.P., Lima A.R., Vieira L.D.S. Lectin, hemolysin and protease inhibitors in seed fractions with ovicidal activity against Haemonchus contortus. Braz J Vet Parasitol. 2014;23(2):136–143. doi: 10.1590/s1984-29612014050. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez T.J., Jr., Landerito E.O., Acabal A.M. PCAARRD; Los Baños Laguna: 2009. Development of the herbal drugs for the management of common stronglye worm infection in goats. [Google Scholar]
- 23.Vargas-Magaña J.J., Torres-Acosta J.F.J., Aguilar-Caballero A.J., Sandoval-Castro C.A., Hoste H., Chan-Pérez J.I. Anthelmintic activity of acetone–water extracts against Haemonchus contortus eggs: interactions between tannins and other plant secondary compounds. Vet Parasitol. 2014;206(3):322–327. doi: 10.1016/j.vetpar.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Anthony J.P., Fyfe L., Smith H. Plant active components–a resource for antiparasitic agents? Trends Parasitol. 2005;21(10):462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Akhtar M.S., Iqbal Z., Khan M.N., Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Rum Res. 2000;38(2):99–107. [Google Scholar]
- 26.Kassai T. 1st ed. Reed Educational and Professional Publishing Ltd., Oxford; London: 1999. Veterinary Helminthology; p. 150. [Google Scholar]
- 27.Molan AL, Alexander RA, Brookes IM, McNabb WC, Peterson SW. Effects of an extract from sulla (Hedysarum coronarium) containing condensed tannins on the migration of three sheep gastrointestinal nematodes in vitro. In: Proceedings of the New Zealand Society of Animal Production. 2000;60:21–25.
- 28.Tava A., Avato P. Chemical and biological activity of triterpene saponins from Medicago species. Nat Prod Comm. 2006;1(12):1159–1180. [Google Scholar]
- 29.Marie-Magdeleine C., Hoste H., Mahieu M., Varo H., Archimede H. In vitro effects of Cucurbita moschata seed extracts on Haemonchus contortus. Vet Parasitol. 2009;161(1):99–105. doi: 10.1016/j.vetpar.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Sreelatha S., Jeyachitra A., Padma P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011;49(6):1270–1275. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Stepek G., Behnke J.M., Buttle D.J., Duce I.R. Natural plant cysteine proteinases as anthelmintics? Trends Parasitol. 2004;20(7):322–327. doi: 10.1016/j.pt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Cormanes J.M.Y., Portugaliza H.P. Quilicot AMM. In vivo anthelmintic activity of pineapple (Ananas comosus Merr.) fruit peeling juice in semi-scavenging Philippine native chicken naturally co-infected with Ascaridia galli and Heterakis gallinarum. Livest Res Rural Dev. 2016;28(5):1–12. www.lrrd.org/lrrd28/5/corm28082.htm. [Google Scholar]
- 33.Ballesteros C., Tritten L., O’Neill M., Burkman E., Zaky W.I., Xia J. The effects of ivermectin on Brugia malayi females in vitro: a transcriptomic approach. PLOS Negl Trop Dis. 2016;10:e0004929. doi: 10.1371/journal.pntd.0004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoste H., Jackson F., Athanasiadou S., Thamsborg S.M., Hoskin S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006;22(6):253–261. doi: 10.1016/j.pt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 35.D’Addabbo T., Carbonara T., Leonetti P., Radicci V., Tava A., Avato P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa. Phytochem Rev. 2010;10(4):503–519. [Google Scholar]