Abstract
Electrosurgery reduces surgical bleeding, and delayed wound healing. This study evaluated comparative incisional wound healing potential of honey in wound created with electroscalpel and cold scalpel. The study used twelve (12) adult male albino rats (130 ± 20 g), randomly grouped into Electro-cautery (n = 6) and Cold scalpel (n = 6). Each rat had three full thickness (6 mm diameter) skin wounds (a, b and c) created on its dorsum with either Electroscalpel (ES) or Cold blade scalpel (CS), and treated topically with Silver sulphadiazine (SSD, wound a), untreated (control, wound b) and Bee honey (H, wound c). The wounds were evaluated for gross (exudation, edema, hyperemia, contraction), histologic (granulation, angiogenesis, fibroplasia, epithelialization) and immunologic healing indices using standard techniques. Data were analyzed with Chi-square, Two-way Analysis of Variance (ANOVA) and Duncan Multiple Range tests (DMRT) at α = 0.05. Wound hyperemia and edge edema were prominent in the ES group from day 4 to 6 (P = .000). Percentage wound contraction was higher in the CS than ES group from days 5 to 7 (P = .006) and in the CS treated with honey than ES treated with honey from days 7 to 14 (P = .000). Granulation tissue reduced in ES group treated with SSD than in honey and control wounds. Fibroelastic tissue increased in SSD and honey treated wounds of ES group, and higher in honey treated wounds of CS group (P < .05). Fibroplasia was sustained in honey and SSD treated wounds than control. Honey can be applied to electroscalpel surgical wound to facilitate rapid healing during surgical management of tumours having vascular network.
Keywords: Electrocautery, Epithelialization, Incisional methods, Silver sulphadiazide, Wound management, Wound contraction
1. Introduction
Surgery is an active skill and equipment based specialty that uses, incisions, excision, manipulation, or other invasive procedures, with the aid of local, regional, or general anaesthesia for patient management [1]. The Cold blade scalpel (CBS) is one of the most commonly used instruments for surgical incisions and excisions [2]. Its’ use is however limited in tumor excision due to tumor tissues hyper-vascularity that make them prone to excessive bleeding and makes surgical hemostasis tedious, with concomitant obscurity of surgical field and risk of hypovolaemia [2]. This has made surgeons to prefer the use of electrosurgical devices, alongside with CBS. Conventional electrosurgery devices are cutting instruments that uses continuous-waveform radiofrequency energy delivered by means of an electrode to “incise” tissue by thermal ablation, which also produces simultaneous hemostasis [3], [4], [5], [6]. Electrosurgical cutting tools date back to the 1920s, and are currently used globally [3], [7], [8]. Although electrosurgical tools hemostatic capabilities aid reduction of surgical bleeding, the device is associated with delayed wound healing due to heat associated tissue damage which results in prolonged debridement process [8], [9], [10]. Despite this apparent disadvantage, their use remains valuable, especially in poor resource settings, necessitating a search for wound healing agents, which could facilitate healing of electro-scalpel wounds.
A wound is a disruption of tissues’ normal anatomic structure and functions with or without tissue loss [11]. Wound healing is a biologic process following injury geared at restoration of lost tissues and tissue integrity [12], [13], [14]. It is characterized by a dynamics of simultaneous, interwoven phases that involve a complex arrays of chemical mediators and the immune cells, that ultimately result in repair processes characterized by angiogenesis, fibroplasia and remodeling [11]. Wound healing is influenced by host factors [12], wound characteristics [15], and applied healing agents [13], [14], [15], [16], [17]. Wound healing effects of various substances including potash-table salt [13], Moringa oliefera [14], aqueous pineapple juice [18], honey [14], [15], to mention a few; have been investigated on animal model wounds created with cold scalpel. Honey, however, has been reported to have a potential healing effect on chemical burn wounds [17]. We hereby hypothesize that honey will positively influence rapid healing of electro-scalpel wound.
Honey is an ancient remedy for the treatment of wounds, particularly where conventional modern therapeutic agents has failed. Honey outstanding potentials in accelerating varying wound types have been described in both clinical and experimental studies [19], [20], [21]. Its healing properties and mechanism of action have also been elucidated in our previous studies [17], [22]. Despite, however, the robust information on honey wound healing potentials; there is a dearth of information on honey application to enhance healing of electro-scalpel created wounds. This study therefore evaluated honey potentials in healing of electro-scalpel wounds of adult Wister albino rat.
2. Materials and methods
2.1. Ethical approval
Approval of the Institutional Animal Use and Care Committee was sought before commencement of the study and all applicable guidelines (international, national, and/or institutional) were followed.
2.2. Honey
Natural, unprocessed, un-boiled multi-flora honey obtained from a bee hive farm in Ibadan, Nigeria was studied.
2.3. Experimental animals and research design
Twelve (12) adult male albino rats weighing 130 ± 20 g were used for the study. The rats were sourced from a local breeding unit, housed at the Experimental Animal Housing Unit of the Faculty of Veterinary Medicine, University of Ibadan, in well aerated cages and exposed to a 12 h light/dark cycle, 21 ± 0.5 °C temperature and 23–30% relative humidity. The rats were provided with rat diet and clean water ad libitum. The rats were randomized into two groups; Electroscalpel (ES) group, (n = 6) and Cold scalpel (CS) group, (n = 6).
2.4. Anaesthesia and wound site preparation
Each rat was anaesthetized with an intramuscular injection of 5% Ketamine (Rottex medica, Germany) (35.0 mg/kg) and 2% Xylazine (VMD, Belgium) (5.0 mg/kg) via the quadriceps group of muscles as earlier described [23]. Following anaesthesia, the skin of dorsum (thoraco-lumber region) of each rat was prepared for aseptic surgery by shaving and sterilization with chlorhexidine and alcohol.
Each rats in the ES group had 3 full thickness incisional skin wounds created on the dorsum (thoraco-lumber region) with an electroscalpel device (Surtron, Italy), set at 30watts as previously described [24]. Also, each rats in the CS group, had 3 full thickness incisional skin wounds created on the dorsum (thoraco-lumber region) with a size 15 scalpel blade fixed on a size 3 Bird Parker blade holder.
2.5. Treatment application
The three wounds on each rat were identified cranio-caudally as: Silver sulphadiazide (SSD) treatment, control (C) (untreated), and honey (H) treatment. The treatment preparations were topically applied to the wounds once daily for 14 days.
2.6. Evaluation of gross wound healing indices
Wounds were daily evaluated for healing indices as previously described [21]. Wound edge edema and hyperemia were graded as severe, mild or absent. Granulation tissue was graded as high, low or absent. The wound size (mm) was determined with a digital venial caliper (Globetronics & Co. ltd, Germany), and the percentage wound contraction was calculated as:
2.7. Evaluation of histologic wound healing indices
Following euthanasia with Ketamine (70 mg/kg) and xylazine (15 mg/kg) at days 5, 7 and 14 as described previously [17], wound tissues consisting of the entire wound region excised in-depth with liberal margins of the surrounding skin including underlying connective tissues above the external fascia of the dorsal muscles, were obtained from the animals per group, at days 5, 7 and 14, and stored in (10%) formalin. The tissues were processed and stained with Haematoxylin and Eosin [25]. Granulation tissue, vascularization, fibro elastic tissue and epithelialization response were evaluated with a semi-quantitative four-point scale scoring system [26], by two histopathologists who worked independently.
Sections were equally stained with Giemsa for quantification of inflammatory cells and fibroblast responses during the healing episodes. Photomicrographs of lesions were taken appropriately from each slide with the aid of a computer enabled digital camera device (MU900, Amscope, England) attached to a microscope (CX21, Olympus, China). The cells were quantified on the open source Fiji software (Image J, Japan).
2.8. Data analysis
The data generated were presented in frequencies, percentages, ‘Mean ± standard deviation’ and analyzed with Chi square, two way analysis of variance (ANOVA) and Duncan Multiple Range Test (DMRT) at 5% confident limit.
3. Results
3.1. Wound exudation
Wounds of rats in the ES and CS groups were wet between days 1 and 4. The level of wound wetness was more in rats in the ES group (P = .029) from day 5 to 9 with the degree of wetness low in the honey treated wound (SSD > control > H), but less in rats in the CS group between day 2 and 4 with lower degree of wetness observed in honey treated wounds compared to Silver sulphadiazide and untreated wounds (SSD > control > H) (Table 1).
Table 1.
Evaluation of wound exudation, edge edema and hyperemia in electro-scalpel and cold scalpel induced wounds in rats.
| Day | Electroscalpel |
Cold Scalpel |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SILVER SULPHADIAZINE |
Control |
Honey |
SILVER SULPHADIAZINE |
Control |
Honey |
|||||||||||||
| WE | WEE | WH | WE | WEE | WH | WE | WEE | WH | WE | WEE | WH | WE | WEE | WH | WE | WEE | WH | |
| 1 | 0 | 6(100%) | 6(100%) | 0 | 6(100%) | 6(100%) | 0 | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) |
| 2 | 0 | 6(100%) | 6(100%) | 0 | 6(100%) | 6(100%) | 0 | 6(100%) | 6(100%) | 3(50%) | 5(83%) | 5(66%) | 4(66%) | 4(66%) | 6(100%) | 3(50%) | 4(66%) | 6(100%) |
| 3 | 0 | 5(83%) | 6(100%) | 0 | 5(83%) | 6(100%) | 0 | 2(33%) | 6(100%) | 3(50%) | 3(50%) | 5(83%) | 4(66%) | 3(50%) | 5(83%) | 3(50%) | 3(50%) | 4(66%) |
| 4 | 0 | 3(50% | 3(50%) | 0 | 5(83%) | 3(50%) | 0 | 2(33%) | 3(50%) | 2(33%) | 2(33%) | 2(33%) | 2(33%) | 3(50%) | 2(33%) | 2(33%) | 2(33%) | 1(16%) |
| 5 | 0 | 3(16%) | 1(16%) | 1(16%) | 4(66%) | 1(16%) | 0 | 2(33%) | 1(16%) | 0 | 0 | 0 | 0 | 0 | 1(16%) | 0 | 0 | 0 |
| 6 | 1(16)* | 3(50%) | 0 | 0 | 4(66%) | 0 | 0 | 2(33%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 1(16)* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1(16%) | 0 | 0 | 0 | 0 | 0 |
| 8 | 1(16)* | 0 | 0 | 1(16%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 1(16)* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10> | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
WE – wound exudation; WEE – wound edge edema; WH – wound hyperemia.
3.2. Wound edge edema
Wound edge edema was severe in all the rats’ wounds in the ES and SC induced wounds from day 1 to 2. It was severe in the ES group between day 4 and 6 (P = .000) (honey < silver<control), but mild in the CS group from day 2 to 4 (p < .05).
3.3. Wound surface hyperemia
The wound surface was hyperemic in all the wounds on day 1–2, but severe on day 4 and 5 in the ES group with more redness observed in SSD treated wounds (SSD ≥ honey ≥ control). The hyperemia reduced from day 3 to 5 in the CS group (honey > SSD ≥ control).
3.4. Wound contraction
Percentage wound contraction was gradual between ES and CS wounds from day 1 to 14 (Fig. 1A–D) but better improvement was observed in CS wounds (Fig. 1D). The Percentage wound contraction was increased in the CS than ES wounds at days 5–7 (P = .006) and days 7–14 (P = .000) (Table 2).
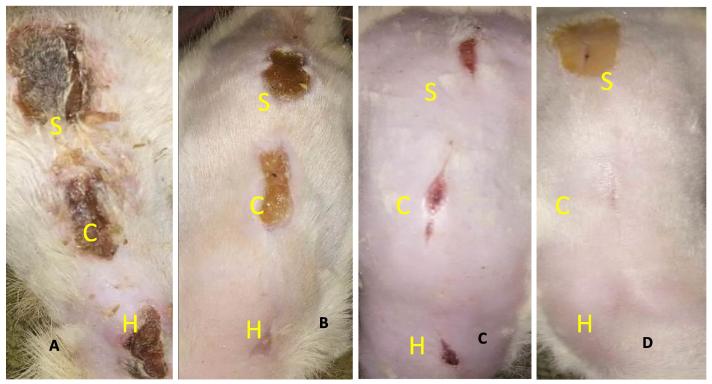
Fig. 1.
Gross examination of wounds: A – wounds created with electroscalpel at day 5 still having exudation and thick granulation tissue. B – Wounds created with cold scalpel at days 5 already healing progress and contraction of wound surface. C – Wounds created with electroscalpel at day 14 showing wound surface contraction. D – Wounds created with cold scalpel at days 14 already contracted. (S = silver sulphadiazine, C = control, H = honey).
Table 2.
Mean daily percentage wound contraction in electroscalpel and cold scalpel induced wounds in rats.
| Day | Electro-scalpel |
Cold Scalpel |
||||
|---|---|---|---|---|---|---|
| SSD | Control | Honey | SSD | Control | Honey | |
| 1. | 6.75 ± 0.86a | 4.99 ± 2.64a | 7.26 ± 1.48a | 16.87 ± 7.39a | 7.60 ± 3.21a | 9.76 ± 3.95a |
| 2. | 10.21 ± 4.31a | 7.91 ± 2.24a | 9.01 ± 4.72a | 31.56 ± 4.51b | 19.82 ± 12.35b | 16.27 ± 4.69a |
| 3. | 11.26 ± 3.89a | 11.40 ± 6.10a | 11.32 ± 4.84a | 7.6 ± 12.788c | 27.87 ± 11.59b | 20.53 ± 5.80b |
| 4. | 16.12 ± 9.19a | 12.90 ± 3.62a | 13.49 ± 5.52a | 47.30 ± 16.05d | 32.74 ± 6.99b | 33.18 ± 6.17b |
| 5. | 21.82 ± 11.35b | 18.91 ± 10.53a | 19.31 ± 6.39b | 66.98 ± 3.32d | 47.44 ± 11.29c | 38.10 ± 3.98b |
| 6. | 24.60 ± 17.53b | 21.48 ± 16.45b | 21.55 ± 7.89b | 78.22 ± 14.58d | 51.57 ± 20.22c | 47.55 ± 11.09c |
| 7. | 19.19 ± 8.46b | 16.58 ± 4.95a | 19.09 ± 1.14b | 88.81 ± 9.67d | 60.69 ± 23.90c | 47.55 ± 11.09c |
| 8. | 20.76 ± 7.31b | 19.28 ± 2.96b | 20.74 ± 4.02b | 97.22 ± 5.56d | 64.56 ± 18.52c | 59.46 ± 16.42c |
| 9. | 24.10 ± 5.41b | 20.75 ± 5.84b | 22.31 ± 4.19b | 97.22 ± 5.56d | 68.31 ± 16.53c | 69.55 ± 17.73c |
| 10. | 25.67 ± 8.29b | 23.70 ± 4.72b | 26.28 ± 2.77b | 99.3 ± 8.30d | 69.70 ± 24.92c | 75.48 ± 15.82c |
| 11. | 26.79 ± 2.52b | 23.61 ± 1.96b | 31.77 ± 11.65b | 101 ± 12.0d | 80.43 ± 27.68d | 87.94 ± 17.06d |
| 12. | 33.48 ± 3.15b | 26.74 ± 6.39b | 39.71 ± 14.56c | 98 ± 12.0d | 80.00 ± 18.1d | 90 ± 14.14d |
| 13. | 40.02 ± 3.57c | 29.86 ± 10.80b | 39.71 ± 14.56c | 110 ± 7.70d | 83.34 ± 23.57d | 96.67 ± 4.72d |
| 14. | 43.75 ± 8.84c | 29.86 ± 10.80b | 44.71 ± 21.63c | 100 ± 9.00d | 103 ± 12.00d | 100 ± 8.00d |
Values with different superscript are significant across rows and columns at α = 0.05.
SSS = Silver sulphadiazine.
3.5. Granulation tissue
Wound granulation tissues on day 7 was high in the untreated wounds as compared to SSD and honey treatment in the ES rats (SSD < honey ≥ control) (Fig. 2A–C). It was less in SSD (Fig. 3A) and honey treated wounds than control (Fig. 3B and C) of the CS induced wounds (P < .05), thus indicating the remarkable progress of healing (Table 3).
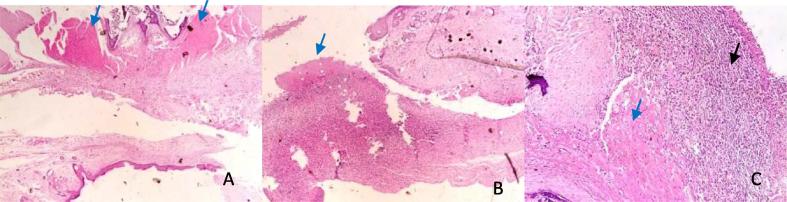
Fig. 2.
Photomicrographs of electroscalpel wounds at day 7: A – (SSD) shows moderate granulation tissue (blue arrow). B – (Control) show granulation tissue (blue arrow). C (Honey) shows presence of marked cellular infiltrates (black arrow) and granulation tissue (blue arrow). HE ×400.
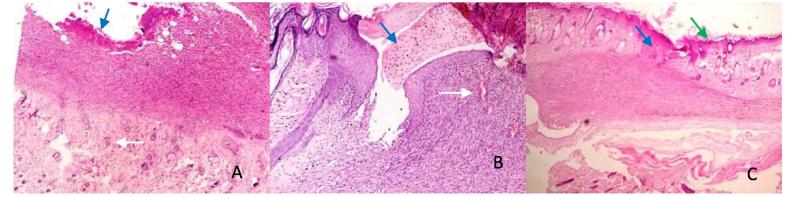
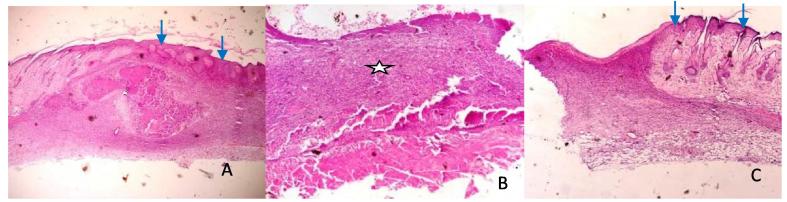
Fig. 3.
Photomicrographs of coldscapel wound at day 7: A – (SSD) shows severe granulation (blue arrow), and moderate angiogenesis (white arrow). B (Control) shows moderate granulation (blue arrow), and moderate angiogenesis (white arrow). C (Honey) shows epithelialization (green arrow), moderate granulation (blue arrow), mild angiogenesis (thin arrow) and moderate fibrosis. HE ×400.
Table 3.
Wound histologic indices and inflammatory cell counts of electroscalpel and cold scalpel induced wounds in rats at day 5, 7 and 14.
| Parameters | Electroscalpel |
Cold scalpel |
||||
|---|---|---|---|---|---|---|
| SSD | CONTROL | HONEY | SSD | CONTROL | HONEY | |
| Day 5 | ||||||
| Granulation tissue | 3.00 ± 0.10a | 3.00 ± 0.20a | 3.00 ± 0.13a | 3.00 ± 0.20a | 3.00 ± 0.30a | 2.00 ± 0.10a |
| Vascularization | 2.00 ± 0.06a | 2.00 ± 0.10a | 2.00 ± 0.11a | 2.00 ± 0.06a | 2.00 ± 0.10a | 1.00 ± 0.07a |
| Fibro-elastic tissue | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Epithelialization | 0.00 ± 0.00a | 0.00 ± 0.00a | 1.00 ± 0.01a | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.00 ± 0.10a |
| Macrophages | 20 ± 8.49a | 43.0 ± 11.31b | 27.50 ± 2.12a | 7.5 ± 3.54a | 15.0 ± 21.21a | 29.5 ± 4.95b |
| Neutrophils | 16.5 ± 0.71a | 28.5 ± 0.71b | 20.50 ± 3.54a | 2.5 ± 3.54a | 8.5 ± 12.02a | 19.0 ± 24.0a |
| Lymphocytes | 15.0 ± 1.41a | 21.0 ± 16.97a | 32.00 ± 4.24b | 10.5 ± 2.12a | 20.0 ± 28.3a | 31.0 ± 7.1a |
| Mast cells | 3.0 ± 4.24a | 0.0 ± 0.00b | 6.00 ± 5.66c | 10.5 ± 6.4c | 0.00 ± 0.00b | 0.0 ± 0.00b |
| Fibroblast | 200 ± 28.5a | 78.0 ± 14.14b | 200 ± 15.80a | 10.0 ± 4.2c | 66.0 ± 77.8b | 200.0 ± 25.0a |
| Day 7 | ||||||
| Granulation tissue | 1.00 ± 0.03a | 3.00 ± 0.30b | 3.00 ± 0.20b | 3.00 ± 0.10b | 2.00 ± 0.10a | 1.00 ± 0.04a |
| Vascularization | 1.00 ± 0.10a | 2.00 ± 0.10a | 1.00 ± 0.10a | 2.00 ± 0.08a | 1.00 ± 0.03a | 1.00 ± 0.08a |
| Fibro-elastic tissue | 2.00 ± 0.10a | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 1.00 ± 0.03a |
| Epithelialization | 1.00 ± 0.05a | 0.00 ± 0.00a | 3.00 ± 0.20b | 1.00 ± 0.06a | 2.00 ± 0.10b | 2.00 ± 0.20b |
| Macrophages | 9.50 ± 4.94a | 18.0 ± 4.24b | 14.50 ± 14.85a | 27.5 ± 7.8b | 23.3 ± 9.89b | 16.5 ± 9.19a |
| Neutrophils | 7.50 ± 7.77a | 7.0 ± 5.65a | 2.50 ± 3.53a | 11.5 ± 7.78a | 9.5 ± 3.54a | 6.5 ± 9.19a |
| Lymphocytes | 20.0 ± 25.5a | 15.0 ± 2.82a | 11.00 ± 11.31a | 19.5 ± 0.71a | 21.0 ± 11.3a | 23.5 ± 23.3b |
| Mast cells | 7.00 ± 4.24a | 0.0 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b |
| Fibroblast | 30.5 ± 20.5a | 37.0 ± 19.8a | 116.5 ± 118.1b | 146.5 ± 76b | 156.0 ± 0.0b | 62.2 ± 21.0b |
| Day 14 | ||||||
| Granulation tissue | 0.00 ± 0.00a | 2.00 ± 0.20b | 1.00 ± 0.01a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Vascularization | 1.00 ± 0.10a | 2.00 ± 0.08b | 1.00 ± 0.10a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Fibro-elastic tissue | 2.00 ± 0.20a | 0.00 ± 0.00b | 2.00 ± 0.10a | 2.00 ± 0.10a | 2.00 ± 0.10a | 3.00 ± 0.20a |
| Epithelialization | 2.00 ± 0.10a | 1.00 ± 0.05b | 3.00 ± 0.20a | 3.00 ± 0.10a | 3.00 ± 0.20a | 3.00 ± 0.10a |
| Macrophages | 21.0 ± 18.4a | 18.0 ± 2.83a | 18.00 ± 2.83a | 36.5 ± 14.9b | 24.5 ± 4.95a | 13.0 ± 11.3a |
| Neutrophils | 10.0 ± 14.1a | 27.0 ± 24.04b | 2.00 ± 2.83a | 8.0 ± 5.7a | 10.5 ± 2.12a | 3.5 ± 4.95a |
| Lymphocytes | 29.0 ± 12.7a | 21.0 ± 1.31a | 37.50 ± 21.9b | 35.5 ± 6.4b | 26.0 ± 4.24a | 16.5 ± 2.1a |
| Mast cells | 3.50 ± 5.0a | 0.0 ± 0.00a | 0.00 ± 0.00a | 5.5 ± 7.8a | 2.5 ± 3.54a | 0.5 ± 0.71a |
| Fibroblast | 113.5 ± 60a | 114.5 ± 108.2a | 147.50 ± 74.3a | 200.0 ± 0.0b | 129.5 ± 99.7a | 94. ± 11.3a |
Values with different superscript are significant across rows at α = 0.05.
SSS = Silver sulphadiazine.
3.6. Epithelialization
Honey treated wounds of ES and SC showed a significantly higher (P < .05) level of epitheliazation on day 5, 7 (honey > SSD > control). All wounds showed complete epithelialization on day 14 (Fig. 4A–C).
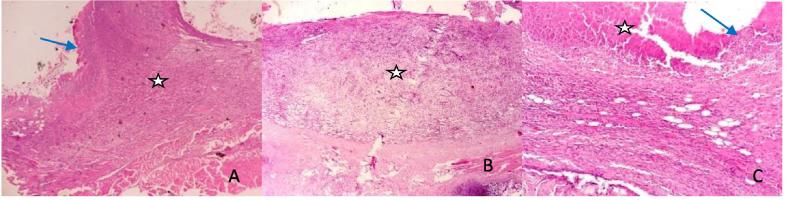
Fig. 4.
Photomicrographs of electroscalpel wounds at day 14: A (SSD) shows granulation (asterick), moderate contraction and epithelialization (blue arrow). B (Control) shows abundant granulation tissue (asterick) with loose connective tissue and no contraction. C (Honey) shows wound contraction (white arrow), moderate granulation (asterick), angiogenesis and epithelialization (blue arrow) HE ×400.
3.7. Vascularization
Blood vessels regressed faster in the wounds treated with SSD and honey than control in the ES group with the level of vascularization significantly higher (P < .05) in control wounds on days 7 and 14. Blood vessels also regressed faster on days 5 and 7 in the wounds treated with honey than in control and SSD with the trend being honey < control < SSD. Blood vessels had regressed in all the wounds by day 14 (Fig. 5A–C).
Fig. 5.
Photomicrographs of coldscapel wound at day 14: A (SSD) shows complete epithelialization (blue arrow) and wound contraction. B (Control) shows granulation tissues (asterick), and moderate angiogenesis. C (Honey) shows complete epithelialization (blue arrow) and loose connective tissue. HE ×400.
3.8. Fibroelastic tissue
No fibroelastic tissue was seen in ES and CS groups wounds on day 5, but significantly higher P < .05) in wounds treated with SSD and honey in ES and CS groups on day 7 and 14 (honey > SSD > control).
3.9. Inflammatory cells and fibroblast response
Inflammatory cells were high in the Honey and SSD treated wounds as compared to the control of the ES group at day 5 (Table 3). Fibroplasia was more and sustained in the Honey and SSD treated groups more than in the control at days 7 and 14. Inflammatory cellular infiltrate (macrophages, neutrophils and lymphocytes) was markedly high in the Honey treated group as compared to the SSD and control of CS group on day 5.
4. Discussion
The result of this study shows honey efficacy in enhancing healing in electroscalpel induced wound of wister rats. Silver sulphadiazine was used as a positive control, for its burn wound healing effects in previous studies [27], [28]. The wound healing indices adopted for this study are conventional assessment parameters in wound healing studies [22], [26], [29].
The rate of inflammatory cellular infiltrate (neutrophils, macrophages and lymphocytes) were markedly high in the honey and SSD treatments of the electroscalpel group, than control at day 5 (p < .05). Honey immunostimulatory potentials must have influenced the observed immune cellular infiltration to tissues for autolytic debridement and growth of cell for wound repairs [30]. This honey immune-stimulatory activity has been attributed to substances present in honey, such as: major royal jelly proteins-1, arabinogalactan and some phytochemicals [30], [31]. The reduction in edema and surface exudation especially in honey treated group from day 3 was as a result of honey’s physical and anti-inflammatory properties which reduces edema and the amount of wound exudation by down-regulating the inflammatory process [30]. Wound edge edema and hyperemia are local signs of acute inflammation from day 0 to 3 of injury in clean wounds, and could be signs of wound infection and evidence of debridement challenges when it progresses beyond day 3 of injury [22]. Honey also drives edema by mobilizing fluid from the surrounding tissue [32], [33], [34]. The reduction in edema and surface hyperemia seen in wounds treated with SSD is in line with the use of Silver sulphadiazine as a drug of choice in treating burn wounds due to its wide spectrum of bactericidal activity against both gram-positive and gram-negative organisms [28]. This observation coupled with the exudations observed between days 5 and 9 may provide a rationale for the prolonged wound surface hyperemia seen in the electroscalpel group beyond day 3. The marked reduction in wound hyperemia observed in honey treated wounds was due to honey associated mechanisms in inflammation reduction by inhibition of nitric acid production by macrophages [35], inhibition of complement factors [20], decrease of thermal injury induced oxidative stress due to apalbumina-1 [36], an anti-inflammatory factor.
Wound contraction was faster in cold blade scalpel than in the electroscalpel group due to thermal injury, compromised tissue blood circulation, and decreased immune potentials of the thermal damaged tissues [37], resulting in prolongation of debridement and repair phases [38]. Wounds treated with honey, however, showed improved contraction and faster epithelialization in both the CBS and ES groups than sulphadiazine and control. Honey’s accelerated wound healing properties have been well reported and its effects linked with its antibacterial and immunostimulatory effects on fibroblasts, epithelial cells growth, and development of new capillaries in damaged tissues [28], [39], [40], [41], [42].
The observed faster blood vessel regression on days 7 and 14, in SSD and honey treated wounds compared with control shows honey’s ability to facilitates wound healing process and conversion of fleshy and well vascular collagen type 11 granulation tissues to the less vascular collagen type 1 [24], [43]. This reflected in the expanse of fibroelastic tissue observed in honey and SSD treated wounds on day 14 across the ES and CBS groups. Fibroblasts play an important role in producing the collagen necessary to restore the tensile strength of wounded skin [44]. Tumor vessels are more permeable than normal vessels; their immature nature means they are poorly invested with smooth muscle cells and may have a discontinuous endothelial cell lining with an abnormal basement membrane [45], [46]. Increased vessel permeability may result in aberrant osmotic forces, accumulation of vascular contents and elevated interstitial fluid pressure leading to excessive bleeding.
5. Conclusions
Honey enhanced the healing process in electroscalpel induced wound. This prospect can facilitate a rapid healing especially in surgical management of tumours with marked vascular network. Thus, knowledge of use of honey in management of electroscalpel induced will allow full exploration of electro-surgery.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
All the authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Eyarefe O.D., Dei D. Retrospective study of prevalence and pattern of surgical conditions presented at the Ashanti regional veterinary clinic, Kumasi, Ghana. Glob Vet. 2014;13:408–413. [Google Scholar]
- 2.Loh S.A., Carlson G.A., Chang E.I., Huang E., Palanker D., Gurtner G.C. Comparative healing of surgical incisions created by the PEAK plasmablade, conventional electrosurgery, and a scalpel. Plast Reconstr Surg. 2009;124:1850–1859. doi: 10.1097/PRS.0b013e3181bcee87. [DOI] [PubMed] [Google Scholar]
- 3.Hainer B.L. Fundamentals of electrosurgery. J Am Board Fam Pract. 1991;4:419–426. [PubMed] [Google Scholar]
- 4.Barry L., Hainer M.D., Richard B., Usatine M.D. Electrosurgery for the Skin. Am Fam Physician. 2002;66:1259–1266. [PubMed] [Google Scholar]
- 5.Brown D.B. Concepts, considerations, and concerns on the cutting edge of radiofrequency ablation. J Vasc Interv Radiol. 2005;16:597–613. doi: 10.1097/01.RVI.0000156097.63027.7B. [DOI] [PubMed] [Google Scholar]
- 6.Massarweh N.N., Cosgriff N., Slakey D.P. Electrosurgery: history, principles, and current and future uses. J Am Coll Surg. 2006;202:520–530. doi: 10.1016/j.jamcollsurg.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Pollinger H.S., Mostafa G., Harold K.L., Austin C.E., Kercher K.W., Matthews B.D. Comparison of wound-healing characteristics with feedback circuit electrosurgical generators in a porcine model. Am Surg. 2003;69:1054–1060. [PubMed] [Google Scholar]
- 8.Vore S.J., Wooden W.A., Bradfield J.F., Aycock E.D., Vore P.L., Lalikos J.F. Comparative healing of surgical incisions created by a standard “bovie”, the Utah Medical Epitome Electrode, and a Bard-Parker cold scalpel blade in a porcine model: a pilot study. Ann Plast Surg. 2002;49:635–645. doi: 10.1097/00000637-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Arashiro D.S., Rapley J.W., Cobb C.M., Killoy W.J. Histologic evaluation of porcine skin incisions produced by CO2 laser, electrosurgery, and scalpel. Int J Periodontics Restorative Dent. 1996;16:479–491. [PubMed] [Google Scholar]
- 10.Hambley R., Hebda P.A., Abell E., Cohen B.A., Jegasothy B.V. Wound healing of skin incisions produced by ultrasonically vibrating knife, scalpel, electrosurgery, and carbon dioxide laser. J Dermatol Surg Oncol. 1988;14:1213–1217. doi: 10.1111/j.1524-4725.1988.tb03478.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper R.A., Molan P.C., Krishnamoorthy L., Harding K.G. Manuka honey used to heal a recalcitrant surgical wound. Euro J Clin Microbiol Infect Dis. 2001;20(10):758–759. doi: 10.1007/s100960100590. [DOI] [PubMed] [Google Scholar]
- 12.Fossum T.W., Cheryl S.H., Johnson A.L., Schulz K.S., Seim H.B., Willard M.D. Surgical infection and antibiotic selection. In: Fossum T.W., editor. Textbook of small animal surgery. 3rd ed. St Louis Missouri Elsevier Science, Morsby Inc.; 2007. pp. 79–89. [Google Scholar]
- 13.Oguwike F.N., Nwozor C.M., Onwurah C.N., Orjiewulu N., Olisah M.C. Comparative study on wound healing using potash-table salt mixture and honey on albino rats. Afrimedic J. 2013;4:29–32. [Google Scholar]
- 14.Eyarefe O.D., Idowu A., Afolabi J.M. Healing potentials of oral moringa oleifera leaves extract and tetracycline on methicillin resistant staphylococcus aureus infected wounds of wistar rats. Niger J Physiol Sci. 2015;30:73–78. [PubMed] [Google Scholar]
- 15.Alizadeh A.M., Sohanaki H., Khaniki M., Mohaghgheghi M.A., Ghmami G., Mosavi M. The effect of Teucrium Polium honey on the wound healing and tensile strength in rat. Iran J Basic Med Sci. 2011;14:499–505. [PMC free article] [PubMed] [Google Scholar]
- 16.Nikpour M., Shirvani M.A., Azadbakht M., Zanjani R., Mousavi E. The effect of honey gel on abdominal wound healing in cesarean section: a triple blind randomized clinical trial. Oman Med J. 2014;29:255–259. doi: 10.5001/omj.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyarefe O.D., Oguntoye C.O. Honey, an unexplored topical wound dressing agent in Nigerian veterinary practice. Sok J Vet Sci. 2016;14:8–17. [Google Scholar]
- 18.Eyarefe O.D., Fabiyi B.O. Wound healing potentials of aqeous pineapple extract – a preliminary study. Global J Pharm. 2016;10:23–30. [Google Scholar]
- 19.Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24:157–161. doi: 10.1016/s0305-4179(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 20.Van den Berg A.J., Van den Worm E., Van Ufford H.C., Halkes S.B., Hoekstra M.J., Beukelman C.J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17:172–178. doi: 10.12968/jowc.2008.17.4.28839. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S.S., Singh O., Bhagel P.S., Moses S., Shukla S., Mathur R.K. Honey dressing versus silver sulfadiazene dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4:183–187. doi: 10.4103/0974-2077.91249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyarefe O.D., Ologunagba F.M., Emikpe B.O. Wound healing potentials of natural honey in diabetic and non diabetic wister rats. Afr J Biomed Res. 2014;17:15–21. [Google Scholar]
- 23.Eyarefe O.D., Amid S.A. Small bowel wall response to enterotomy closure with polypropylene and polyglactin 910 using simple interrupted suture pattern in rats. Int J Anim Vet Adv. 2010;2:72–75. [Google Scholar]
- 24.Cervantes-Sanchez C.R., Cu-Zetina C., Serrano-Rico E., Rojero-Vallejo J., Lazoz-Ochoa M., Gutierrez-Vega R. Incisió n cuté nea: Bisturf vs electrocauterio. Estudio experimental en ratas. Revista Medica del. 2002;65:1–14. [Google Scholar]
- 25.Winsor L. Tissue processing. In: Woods A., Ellis R., editors. Laboratory histopathology. Churchill Livingstone; New York: 1994. 4.2-1-39. [Google Scholar]
- 26.Akriti G., Pramod K. Assessment of the histological state of the healing wound. Plastic Aesth Res. 2015;5:239–242. [Google Scholar]
- 27.Muangman P., Muangman S., Opasanon S., Keorochana K., Chutiasakul C. Benefit of hydrocolloid silver sulphadiazine in the outpatient management of partial thickness burns. J Med Assoc Thai. 2009;92:1300–1305. [PubMed] [Google Scholar]
- 28.Miller A.C., Rashid R.M., Falzon L., Elamin M., Zehtabchi S. Silver sulfadiazine for the treatment of partial-thickness burns and venous stasis ulcers. J Am Acad Dermatol. 2012;66:159–165. doi: 10.1016/j.jaad.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Rosique R.G., Rosique M.J., Farina J.A. Curbing inflammation in skin wound healing: a review. Int J Inflam. 2015;25:73–78. doi: 10.1155/2015/316235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molan P., Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27:141–151. [PubMed] [Google Scholar]
- 31.Majtan J. Honey: an immunostimulator in wound healing. Wound Rep Regen. 2012;22:187–192. doi: 10.1111/wrr.12117. [DOI] [PubMed] [Google Scholar]
- 32.Tonks A., Cooper R.A., Price A.J., Molan P.C., Jones K.P. Stimulation of TNF-alpha release in monocytes by honey. Cytokine. 2001;14:240–242. doi: 10.1006/cyto.2001.0868. [DOI] [PubMed] [Google Scholar]
- 33.Molan P.C. Re-introducing honey in the management of wounds and ulcers – theory and practice. Ostomy Wound Manage. 2002;48:28–40. [PubMed] [Google Scholar]
- 34.Tonks A.J., Cooper R.A., Jones K.P., Blair S., Parton J., Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21:242–247. doi: 10.1016/s1043-4666(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 35.Kassim M., Achoui M., Mustafa M.R., Mohd M.A., Yusoff K.M. Ellagic acid, phenolic acids and flavonoids in Malaysian honey extracts demonstrates in vitro anti-inflammatory activity. Nutr Res. 2010;30:650–659. doi: 10.1016/j.nutres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad A., Khan R.A., Mesaik M.A. Anti inflammatory effect of natural hon ey on bovine thrmobin-induced oxidative burst in phagocytes. Phytother Res. 2009;23:801–808. doi: 10.1002/ptr.2648. [DOI] [PubMed] [Google Scholar]
- 37.Sinha U.K., Gallagher L. Effects of steel scalpel, ultrasonic scalpel, CO2 laser, and monopolar and bipolar electrosurgery on wound healing in guinea pig oral mucosa. Laryngoscope. 2003;113:228–236. doi: 10.1097/00005537-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Church D., Elsayed S., Reid O., Winston B., Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bang L., Buntting C., Molan P. The effect of dilution on the rate of hydrogen peroxide production in honey and its imlications for wound healing. J Altern Complement Med. 2003;9:267–273. doi: 10.1089/10755530360623383. [DOI] [PubMed] [Google Scholar]
- 40.Molan P.C. A brief review of honey as a clinical dressing. Primary Intention. 1998;6:148–158. [Google Scholar]
- 41.Molan P.C. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;1:13–19. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 42.George N.M., Cutting K.F. Antibacterial hony (Medihoney): Invitro activity against clinical isolates of MRSA, VRE and other multirestant gram-negative organisms including Pseudomonas aeruginosa. Wounds. 2007;19:231–236. [PubMed] [Google Scholar]
- 43.Abuharfeil N., Al-Oran R., Abo-Shehada M. The effect of bee honey on proliferative activity of human B- and T-lymphocytes and the activity of phagocytes. Food Agri Immunol. 1999;11:169–177. [Google Scholar]
- 44.Jorgensen P.H., Andreassen T.T. Influence of biosynthetic human growth hormone on biomechanical properties of rat skin incisional wounds. Acta Chir Scand. 1988;154:623–626. [PubMed] [Google Scholar]
- 45.Gee M.S., Procopio W.N., Makonnen S., Feldman M.D., Yeilding N.M., Lee W.M.F. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 2003;162:183–193. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siemann D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]