Abstract
Methionine is one of the most limiting amino acids in dairy diets and low feed intake around the time of calving could lead to decreased synthesis of phosphatidylcholine. An alternative pathway for phosphatidylcholine is to have choline as a precursor. The objective of this study was to determine the effects of feeding rumen-protected methionine and choline pre – and postpartum on reproduction of Holstein cows. Seventy-two Holstein cows were randomly assigned to four treatments from 21 days before calving to 30 days in milk (DIM): supplementation with rumen-protected methionine (MET; n = 20, received 0.08% of the dry matter (DM) of the diet/d as methionine, Smartamine M® to a Lys:Met = 2.9:1), rumen-protected choline (CHO; n = 17, received 60 g/d choline, Reassure), both rumen protected methionine and choline (MIX; n = 19, received 0.08% of the DM of the diet/d as methionine to a Lys:Met = 2.9:1 and 60 g/d choline), or no supplementation to serve as control (CON; n = 16, fed total mixed ration with a Lys:Met = 3.5:1). Cows were evaluated at 4, 7, 10, 13, 15, 17, and 30 d after calving for the presence of secretion using the Metricheck® device. On 15, 30, and 72 d after calving, the uterine endometrium of all cows was sampled using a cytological brush and streaked onto slides for analysis of the presence of polymorphonuclear neutrophils (PMN). We hypothesized that cows supplemented with methionine would have lower metricheck smell scores and lower rates of PMN than non-supplemented cows. On d 30, a treatment difference was detected using the metricheck score and smell (P < 0.04), with treatment MIX (score = 0.38) having a lower score than CHO (score = 2.11). Supplementing cows with rumen-protected methionine may have a beneficial effect on cows’ uterine health.
Keywords: Choline, Endometritis, Holstein cows, Methionine, Metritis, Polymorphonuclear neutrophils
1. Introduction
Cows usually experience negative energy balance after calving, which leads to fatty liver and ketosis [1], [2]. Cows that develop ketosis are at risk for other metabolic disorders, impaired reproduction, and endometritis [3], [4]. During this transition period, amino acids (AA) are needed to export fat away from the liver in the form of very low density lipoproteins, formed by phosphatidylcholine, which is made from methionine [5]. Supplementing rumen-protected methionine has been shown to improve milk production and composition, increase dry matter intake (DMI), reduce lipid accumulation postpartum, and promote liver function [6], [7], [8]. A study by Osorio et al. [7] also reported a faster recovery rate from negative energy balance and a tendency for a lower incidence of ketosis when supplementing methionine [7]. However, methionine is one of the most limiting AA in dairy diets [9] and low feed intake around the time of calving could lead to decreased synthesis of phosphatidylcholine. An alternative pathway for phosphatidylcholine is to have choline as a precursor. Choline supplementation before and after calving has been shown to reduce fatty liver and incidence of ketosis and mastitis [10].
Reducing the risk of metabolic disorders by improving liver function and increasing immune function in the transition period is a key to better reproductive health. Retained placenta (RP), metritis, and endometritis are diseases from impaired immune function and can have lasting negative effects on uterine health [11]. In the absence of clinical illness, metritis is defined as purulent uterine discharge within 21 d postpartum and endometritis is defined as either clinical: purulent discharge 21 d or more postpartum and mucopurulent discharge more than 26 d postpartum, or subclinical: endometrial inflammation determined by cytology in the absence of clinical endometritis [12].
The majority of cows develops metritis within the first 14 d after calving with a peak around 5 to 7 DIM, so targeting specific days within this time frame in combination with a physical examination is efficacious in diagnosing cows with metritis [13]. Diagnosing subclinical endometritis has been effectively done by using a cytology brush in both cows and mares and can be superior to other techniques [14], [15], [16]. The objective of this study was to determine the effects of feeding rumen-protected methionine and choline pre – and postpartum on reproduction of Holstein cows through the assessment of vaginal discharge and uterine cytology. We hypothesized that cows supplemented with methionine would have lower metricheck smell scores and lower rates of PMN than cows that were not supplemented.
2. Materials and methods
2.1. Experimental design and dietary treatments
The University of Illinois Institutional Animal Care and Use Committee (IACUC) approved all following experimental procedures. Seventy-two (n = 72) pregnant Holstein cows entering their 2nd or greater lactation (parity 3.1 ± 1.2 and body weight (BW) 773 ± 16 kg) were enrolled in a randomized complete block design. Cows were blocked with regard to lactation number and previous lactation 305-days milk yield to ensure minimal influence of these variables on the experimental outcome. Cows were housed in tie stalls bedded with sand at the University of Illinois Dairy Cattle Research Unit (Urbana, Illinois). All cows were fed the same fresh cow diet during the transition period and a high cow diet from 31 to 72 DIM to meet but not exceed 100% of the energy requirements as outlined by NRC 2001. Cows were milked three times daily. During the experimental period, cows were fed for ad libitum intake.
At −21 ± 2 d relative to calving, cows were randomly assigned to one of four treatments, given to each cow individually as a top-dress on a total mixed ration (TMR): supplementation with rumen-protected methionine (MET; n = 20, received 0.08% of the DM of the diet/d as methionine, Smartamine M®, Adisseo, Alpharetta, GA, USA, to a Lys:Met = 2.9:1), rumen-protected choline (CHO; n = 17, received 60 g/d choline, Reassure, Balchem Corporation, New Hampton, NY), both rumen protected methionine and choline (MIX; n = 19, received 0.08% of the DM of the diet/d as methionine to a Lys:Met = 2.9:1 and 60 g/d choline), or no supplementation to serve as control (CON; n = 16, fed TMR with a Lys:Met = 3.5:1).
After calving from 30 ± 1 DIM to 72 ± 1 DIM, cows were randomly re-assigned to two new treatments: control (CON; n = 36, fed basal diet with a Lys:Met = 3.4:1) and methionine (MET; n = 36, fed basal diet plus methionine to a Lys:Met = 2.9:1). Therefore, after 30 DIM there were 8 treatments: CON-CON (n = 6), CON-MET (n = 10), MET-CON (n = 10), MET-MET (n = 10), CHO-CON (n = 11), CHO-MET (n = 6), MIX-CON (n = 9), and MIX-MET (n = 10). A schematic of the treatment designs after parturition is shown in Fig. 1.
Fig. 1.
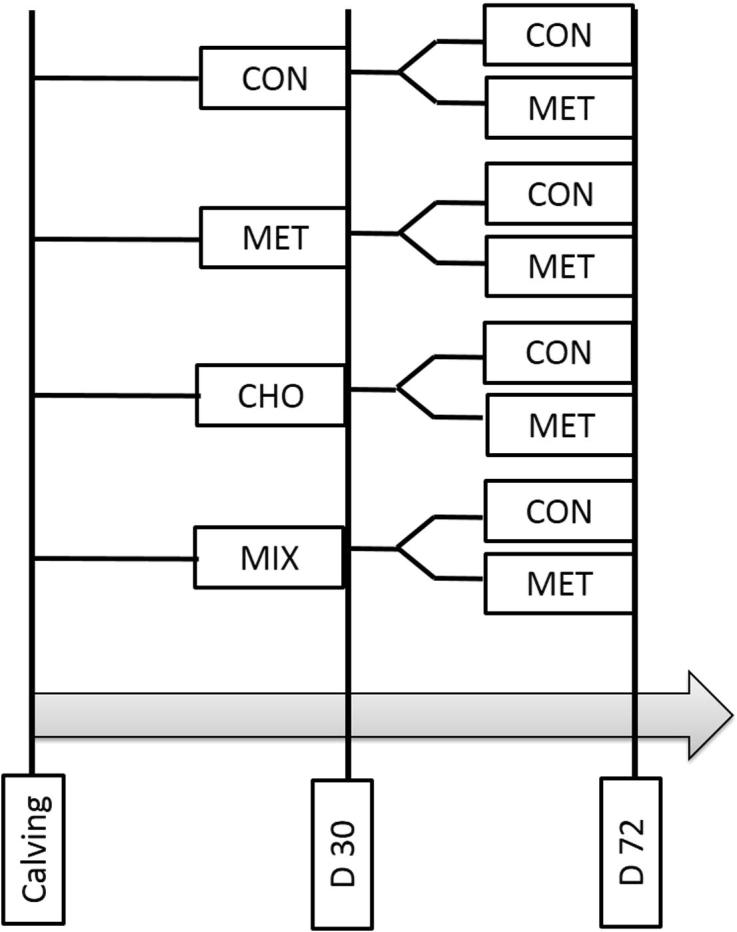
Schematic of treatment design in chronologic order through the experiment.
Dry matter intake was determined daily throughout the dry period and first 72 d post-calving. Refused feed from the previous day was weighed for each cow daily before feeding. Body weight and body condition scores (BCS; scale of 1 = emaciated to 5 = obese [17]) were obtained weekly throughout the study. Body condition score was assigned in quarter-unit increments by two individuals each time and the average of the score was used for that week. Health disorders were recorded for RP, displaced abomasum (DA), clinical ketosis, mastitis, hypocalcemia, hoof problems, and fever. Retained placenta was defined as a placenta that failed to deliver completely longer than 12 h after calving; Displaced abomasum was diagnosed by a veterinarian; ketosis was diagnosed by farm staff or a veterinarian by urinalysis strip (Ketostix, Bayer Corp. Diagnostics Division, IN); mastitis was diagnosed by altered milk composition and confirmed by positive microbiological culture; hypocalcemia was diagnosed by trained farm staff and veterinarians; hoof problems were defined as cows with abnormal hoof disorders that required extra hoof care such as warts, ulcers, punctures or other injury, abscesses, etc.; fever was defined as cows having a temperature of greater than 39.5 °C on d 4, 7, 10, 13, 15, 17, or 30 relative to calving.
2.2. Metricheck exploration of vaginal content
Cows were evaluated at 4, 7, 10, 13, 15, 17, and 30 d after calving for the presence of vaginal secretions by inserting a device into the vagina of the cow (Metricheck®, Simcro, New Zealand). The Metricheck® (MC) device consists of a 50 cm long stainless steel rod with a 4 cm rubber hemisphere tip that is used to collect vaginal contents. The MC was disinfected before each use with chlorhexidine diacetate disinfectant (Nolvasan Solution, Zoetis Animal Health, Florham Park, NJ). To minimize contamination, the tails of cows were held aside and the vulva was cleaned with Nolvasan solution and dried with paper towels. Sterile lubricant (Therio-gel, Agtech, Inc., Manhattan, KS) was applied to the convex part of the rubber tip before insertion. The MC was inserted through vulva and into the cranial portion of the vagina fornix, after which the tool was retracted at a slight upward angle to not lose any contents. The vaginal contents were examined and scored on a scale of 0–3: score 0 = clear or translucent mucus; score 1 = mucus containing small flecks of white or off-white pus; score 2 = discharge containing ≤50% white or off-white mucopurulent material; and score 3 = discharge containing ≥50% purulent material, usually white or yellow, but sometimes sanguineous [12]. Immediately following externalization of the MC, the contents were also smelled and quantified (smell 0 = no odor or smell 3 = fetid odor). The MC was not used if cows had retained placentas or other severe physical injuries to the vulva. Temperature was taken at the time of discharge evaluation.
2.3. Endometrial cytology
Endometrial samples were collected for cytology analysis at 15, 30, and 72 d after calving using a cytology brush (Andwin Scientific, CA). The cytology brush was inserted into a sterile stainless steel rod and then placed into a stainless steel tube for passage through the cervix. The tube was placed in a sanitary plastic sleeve to prevent contamination. The vulva was washed with warm water and dried with paper towels before being sprayed and wiped with ethanol. The instrument was passed into the cervix where the plastic sleeve was punctured, and the instrument was advanced into the body of the uterus. In the uterine body, the stainless steel tube was pulled back to expose the cytology brush. Endometrial samples were collected by rotating the handle of the stylet while in contact with the uterine wall. The cytology brush was then retracted back into the stainless steel tube prior to removal from the cow. The instrument was sanitized with disinfectant or autoclaved between uses.
Slides were prepared immediately following collection by rolling the cytology brush onto clean, glass microscope slides and fixing the sample with cytofixative (Cytoprep; Fisher Scientific, Pittsburgh, PA). Slides were brought to the laboratory and stained with a Giemsa stain (Camco Quik Stain II – Self Buffered Differential Wright-Giemsa Stain, Cambridge Diagnostic Products, FL). Slides were allowed to dry for 24 h before a glass coverslip was added using a mounting medium (Permount, Fisher Scientific, USA). Slides were scanned using whole slide imaging (NanoZoomer Digital Pathology System, Hamamatsu Photonics, Japan) at the Institute for Genomic Biology at the University of Illinois.
A minimum of 5 images were captured from 5 different representative areas on each slide (NDP.view software, Hamamatsu Photonics) using 20× magnification. A minimum of 100 total cells per slide were counted (Image J, National Institutes of Health, MD) and the percentage of epithelial cells and polymorphonuclear neutrophils (PMN) was determined. An example of an epithelial cell and PMN can be seen in Fig. 2. All slides were manually counted by the same technician.
Fig. 2.
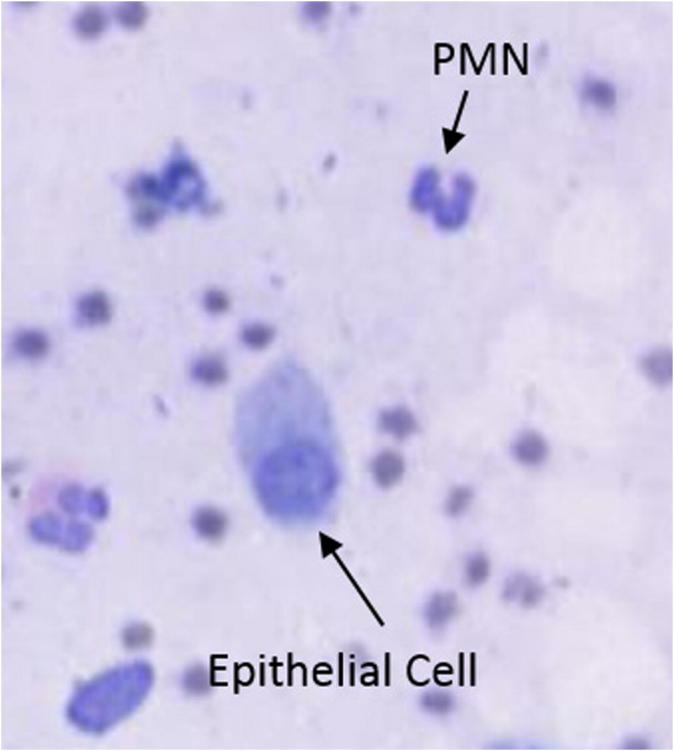
Epithelial cell compared to a polymorphonuclear neutrophil (PMN).
2.4. Statistical analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., NC). Measurements for dry matter intake were reduced to weekly means before statistical analysis. Mixed models were created using the MIXED procedure in SAS to analyze production (BW, BCS, and DMI), MC, and PMN variables. The model for production data contained the fixed effects of Treatment, Week, the interaction of Treatment by Week, and covariates; cow was the experimental unit and was analyzed as a random variable. Week 1 BW and BCS were used as covariates for BW and BCS; and calving season was used in the model for all production variables. Covariates were not included in the model if found to be non-significant (P > 0.05). Week was used as a repeated measure with cow as the subject. Residual distribution was evaluated for normality and homoscedasticity. For variables with treatment differences, letters were assigned so that treatments with the same letter are not different.
The MC and PMN data were analyzed for each day sampled. This analysis used the same model as explained for the production data; however, it excluded the fixed effect of week and the interaction with treatment. The P-values for MC and PMN data were transformed by taking the log of the measurements for better homoscedasticity of the residuals. The P-values are reported as the transformed results; however, all least squares means estimates were back-transformed for the reported results. Letters were also assigned to the MC and PMN results when applicable, so that treatments with the same letter are not different.
Logistic regression was done using the LOGISTIC procedure in SAS considering the binary response variables of metritis and for each health event and odds ratios (OR) were calculated. The likelihood for the response variable of metritis or endometritis was done for MC score, smell, score plus smell, and the percentage of PMN. Cows were considered to have metritis if the MC score was = 3, smell = 3, or the combination of score plus smell was ≥3 on d 4 to 17 and endometritis if the MC score was ≥2, smell = 3, or the combination of score plus smell was ≥2 on d 30 [12]. Using the percentage of PMN, cows were considered to have subclinical endometritis if greater 18% on d 30 [12] and 5% on d 72 [16]. There are no literature cutoff values reported for the percentage of PMN on d 15 so our cutoff (40%) was determined by taking the median of our data from all cows on d 15. For health data, cows were considered positive for the health event if they were diagnosed with the disorder at any time during the experiment or with a fever if they had a temperature of >39.5 °C on d 4, 7, 10, 13, 15, 17, or 30 after calving. For all logistic analyses, the referent treatment is CON for d 0 to 30 and to CON-CON for d 31 to 72. Statistical significance was declared as P-value lower than 0.05, and a tendency declared as P value lower than 0.10.
3. Results
The ingredient composition of the diets is shown in Table 1 and the analyzed chemical composition of the diets is shown in Table 2. Values for milk yield were: wk 1 to 4 = 41.1 kg/day (range: 38.1 to 42.3 kg/day) and wk 5 to 10 = 46.4 (44.4 to 48.3 kg/day). Body weight and DMI were not affected by treatment (P > 0.17) at any time in the experiment. Values for BW were as follows: wk 1 to 4 = 675.8 kg (range: 660.5 to 702.5 kg) and wk 5 to 10 = 640.3 kg (range 612.7 to 655.7). Values for DMI were: wk 1 to 4 = 18.2 kg/day (range: 17.1 to 19.2 kg/day) and wk 5 to 10 = 21.9 kg/day (range: 20.4 to 23.2 kg/day).
Table 1.
Ingredient composition of diets fed to cows throughout the experiment.
| Ingredient, % of DM | Period |
||||
|---|---|---|---|---|---|
| 1a | 2b | 3c | |||
| Alfalfa silage | 8.34 | 5.07 | 6.12 | ||
| Alfalfa hay | 4.29 | 2.98 | 6.94 | ||
| Corn silage | 36.40 | 33.41 | 35.09 | ||
| Wheat straw | 15.63 | 2.98 | – | ||
| Cottonseed | – | 3.58 | 3.26 | ||
| Wet brewers grains | 4.29 | 9.09 | 8.16 | ||
| Ground shelled corn | 12.86 | 23.87 | 25.09 | ||
| Soy hulls | 4.29 | 4.18 | 4.74 | ||
| Soybean meal, 48% CP | 2.57 | 2.39 | 2.45 | ||
| Expeller Soybean meal2 | 2.57 | 5.97 | 1.22 | ||
| Blood meal | 0.86 | 1.50 | 1.43 | ||
| Urea | 0.30 | 0.18 | 0.33 | ||
| Rumen-inert fat1 | – | 1.02 | 1.43 | ||
| Limestone | 1.29 | 1.31 | 1.14 | ||
| Salt | 0.30 | 0.30 | 0.30 | ||
| Dicalcium phosphate | 0.18 | 0.30 | 0.30 | ||
| Magnesium oxide | 0.08 | 0.12 | 0.12 | ||
| Sodium bicarbonate | – | 0.79 | 0.78 | ||
| Potassium carbonate | – | 0.30 | 0.30 | ||
| Calcium sulfate | – | 0.12 | 0.12 | ||
| Mineral and vitamin mix3 | 0.17 | 0.18 | 0.53 | ||
Energy Booster 100 (Milk Specialties Global, Eden Prairie, MN).
SoyPLUS (West Central Cooperative, Ralston IA).
Mineral and vitamin mix was formulated with 5% Mg, 10% S, 7.5% K, 2.0% Fe, 3.0% Zn, 3.0% Mn, 5000 mg/kg of Cu, 250 mg/kg of I, 40 mg/kg of Co, 150 mg/kg of Se, 2200 kIU/kg of vitamin A, 660 kIU/kg of vitamin D3, and 7700 IU/kg of vitamin E.
Period 1; from 21 days before calving until parturition.
Period 2; a fresh cow diet from 0 to 30 DIM.
Period 3; a high cow diet from 31 to 72 DIM
Table 2.
Mean chemical composition and SD of diet fed throughout the experiment.
| Item | Period1 |
SD2 | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| DM, % | 47.1 | 47.9 | 47.1 | 0.57 |
| CP, % of DM | 18.0 | 17.6 | 18.3 | 0.27 |
| ADF, % of DM | 22.7 | 24.4 | 23.2 | 1.05 |
| NDF, % of DM | 35.6 | 37.3 | 36.3 | 1.71 |
| Lignin, % of DM | 4.53 | 4.00 | 3.80 | 0.29 |
| NFC, % of DM | 33.4 | 32.6 | 33.2 | 1.71 |
| Starch, % of DM | 22.3 | 21.4 | 23.6 | 1.73 |
| Crude fat, % of DM | 5.23 | 4.70 | 4.57 | 0.19 |
| Ash, % of DM | 7.79 | 7.77 | 7.59 | 0.25 |
| TDN, % of DM | 70.0 | 69.0 | 70.0 | 0.77 |
| NEL, Mcal/kg of DM3 | 1.63 | 1.63 | 1.65 | 0.03 |
| Ca, % of DM | 0.98 | 1.07 | 1.02 | 0.10 |
| P, % of DM | 0.44 | 0.45 | 0.45 | 0.01 |
| Mg, % of DM | 0.28 | 0.29 | 0.31 | 0.01 |
| K, % of DM | 1.51 | 1.55 | 1.41 | 0.05 |
| Na, % of DM | 0.30 | 0.38 | 0.32 | 0.01 |
| S, % of DM | 0.24 | 0.25 | 0.27 | 0.01 |
| Fe, ppm | 493 | 955 | 480 | 231 |
| Zn, ppm | 156 | 210 | 191 | 18.3 |
| Cu, ppm | 27.0 | 38.0 | 33.3 | 3.01 |
| Mn, ppm | 120 | 138 | 133 | 5.39 |
| Mo, ppm | 0.83 | 0.87 | 0.77 | 0.10 |
Period 1: from 21 days before calving until parturition; period 2: a fresh cow diet from 0 to 30 DIM; and period 3: a high cow diet from 31 to 72 DIM.
Maximum value within periods.
NRC, 2001.
Body condition score was not affected by treatment (P = 0.49) in wk 1 to 4, but there was a difference among treatments (P = 0.004) for wk 5 to 10. The average BCS were: wk 1 to 4 = 3.4 (range 3.36 to 3.44) and of wk 5 to 10 = 3.1 (range 2.80 to 3.20). In wk 5 to 10, CON-CON had the lowest BCS of any treatment (mean = 2.80), and CON-MET had the highest (mean = 3.20; P = 0.004). There were no differences in BCS among the other treatments (P > 0.05). The BCS were: MET-MET (mean = 3.01), MET-CON (mean = 3.09), CHO-CON (mean = 3.07), CHO-MET (mean = 3.12), MIX-CON (mean = 3.03) and MIX-MET (mean = 3.16).
Table 3 shows the incidence of the health events during the experiment for four treatments. Treatment MIX was the only treatment that did not have any prevalence of RP or hoof problems, but was the only treatment that had an incidence of hypocalcemia, and had the highest prevalence of fever (13.89%). Treatment CHO had the highest prevalence of RP (4.17%) and DA (5.56%). Treatments CON, MET, and CHO all had the same percentage of cows with ketosis (4.17%) and MIX had 1.39% prevalence of ketosis. Both CON and MET also had the same prevalence of RP (2.78%), and DA (0%). Logistic analysis was also done for the health events, but no significant differences were found in the OR between treatments for health event. (P > 0.24).
Table 3.
Health events for cows throughout the experiment.
| Variable | Treatmenta % (n/n) |
Total % (n/n) |
|||
|---|---|---|---|---|---|
| CON | MET | CHO | MIX | ||
| Retained placenta | 2.78 (2/72) | 2.78 (2/72) | 4.17 (3/72) | 0 (0/0) | 9.72 (7/72) |
| Displaced abomasum | 0 (0/0) | 0 (0/0) | 5.56 (4/72) | 1.39 (1/72) | 6.94 (5/72) |
| Ketosis | 4.17 (3/72) | 4.17 (3/72) | 4.17 (3/72) | 1.39 (1/72) | 13.89 (10/72) |
| Hypocalcemia (milk fever) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 1.39 (1/72) | 1.39 (1/72) |
| Mastitis | 0 (0/0) | 2.78 (2/72) | 1.39 (1/72) | 0 (0/0) | 4.17 (3/72) |
| Hoof problems | 1.39 (1/72) | 4.17 (3/72) | 1.39 (1/72) | 0 (0/0) | 6.94 (5/72) |
| Feverb | 6.94 (5/72) | 11.11 (8/72) | 9.72 (7/72) | 13.89 (10/72) | 41.67 (30/72) |
Treatment: CON: control, n = 16; MET: methionine, n = 20; CHO: choline, n = 17; MIX: methionine and choline, n = 19.
Temperature of 39.5°C or greater on d 4, 7, 10, 13, 15, 17, or 30.
The least squares means and SEM data from vaginal contents and temperature can be seen in Table 4. The MC score and smell were evaluated separately as well as combined into a single variable. A treatment tendency was observed for MC score on d 4 (P = 0.08) and difference on d 30 (P = 0.03). On d 4, cows that received CHO (2.75) had the lowest score and this was different from treatments MET and MIX (3.00) (P < 0.05). There was no difference between treatment CON and any other treatment. However, on d 30, cows that received CHO (1.36) had the highest score and were different (P = 0.03) from cows that received MET (0.43) and MIX (0.11), which had the lowest scores. Again, no difference was found between CON and the other three treatments, where cows that received CON had the second highest score on d 30.
Table 4.
Treatment least squares means and SEM of each day for metricheck traits.
| Variable | Day | n | Treatmenta |
SEM | P-Valueb,c | |||
|---|---|---|---|---|---|---|---|---|
| CON | MET | CHO | MIX | |||||
| Score | 4 | 48 | 2.92de | 3.00d | 2.75e | 3.00d | 0.06 | 0.08 |
| 7 | 67 | 2.30 | 2.21 | 2.53 | 2.04 | 0.23 | 0.42c | |
| 10 | 60 | 2.93 | 2.88 | 3.00 | 2.81 | 0.07 | 0.19 | |
| 13 | 64 | 2.51 | 2.49 | 2.26 | 2.30 | 0.24 | 0.51c | |
| 15 | 69 | 2.50 | 2.53 | 2.27 | 2.21 | 0.23 | 0.17 | |
| 17 | 70 | 2.06 | 2.37 | 2.38 | 1.74 | 0.23 | 0.29 | |
| 30 | 47 | 0.65de | 0.43d | 1.36e | 0.11d | 0.29 | 0.03c | |
| Smell | 4 | 56 | 0 | 0.75 | 0.75 | 0 | 0.24 | 0.14 |
| 7 | 62 | 0d | 1.38e | 0.94e | 0.47d | 0.27 | 0.005 | |
| 10 | 64 | 0d | 1.17e | 0.94e | 0d | 0.25 | 0.003 | |
| 13 | 64 | 0.60 | 1.06 | 1.00 | 1.06 | 0.35 | 0.77 | |
| 15 | 69 | 0.75 | 0.95 | 1.40 | 0.47 | 0.31 | 0.26 | |
| 17 | 65 | 0d | 0d | 0.75e | 0d | 0.16 | <0.0001 | |
| 30 | 45 | 0d | 0d | 0.67e | 0d | 0.16 | 0.04 | |
| Temperature | 4 | 58 | 38.50 | 38.53 | 38.34 | 38.51 | 0.09 | 0.52 |
| 7 | 66 | 38.59 | 38.62 | 38.59 | 38.59 | 0.10 | 0.97 | |
| 10 | 67 | 38.75 | 38.59 | 38.60 | 38.60 | 0.09 | 0.63 | |
| 13 | 63 | 38.34 | 38.35 | 38.67 | 38.57 | 0.11 | 0.13 | |
| 15 | 68 | 38.35 | 38.41 | 38.40 | 38.54 | 0.10 | 0.63 | |
| 17 | 65 | 38.46 | 38.45 | 38.61 | 38.49 | 0.08 | 0.50 | |
| 30 | 46 | 38.39 | 38.23 | 38.29 | 38.63 | 0.14 | 0.25 | |
| Score + smell | 4 | 55 | 2.57de | 3.50d | 1.67e | 3.25d | 0.32 | 0.07 |
| 7 | 67 | 2.53 | 3.41 | 3.50 | 2.58 | 0.43 | 0.29 | |
| 10 | 69 | 3.13 | 3.94 | 3.50 | 2.79 | 0.40 | 0.30 | |
| 13 | 64 | 3.13 | 3.65 | 3.33 | 3.35 | 0.48 | 0.89 | |
| 15 | 69 | 3.25 | 3.47 | 3.67 | 2.68 | 0.46 | 0.67 | |
| 17 | 70 | 2.06 | 2.84 | 3.13 | 2.05 | 0.37 | 0.15 | |
| 30 | 47 | 1.15de | 1.08de | 2.11d | 0.38e | 0.40 | 0.04 | |
Treatments: CON: control, n = 16; MET: methionine, n = 20; CHO: choline, n = 17; MIX: methionine and choline, n = 19.
P-Values have been log transformed.
Calving season was used as a covariate when significant (P <0.05).
Means within a row with different superscripts differ significantly.
Means within a row with different superscripts differ significantly.
A difference was observed among treatments for smell on d 7 (P = 0.005), d 10 (P = 0.003), d 17 (P < 0.0001) and d 30 (P = 0.04). On d 7 and d 10, no difference was observed between cows that received MET and CHO, and these groups had a higher smell score when compared with CON and MIX. However, on d 17 and d 30, cows that received MET were no longer different from cows received CON and MIX, with all three groups having a smell score of 0 and the CHO group much higher with a score of 0.67 (Table 4). When the MC score and smell was combined into a single score, the results were similar to just score alone, with differences detected on d 4 and d 30 (P < 0.07); however, on d 30 only a difference between treatments CHO and MIX was detected (Table 4).
Logistic analysis for the likelihood of cows having metritis based on MC score, smell, and score + smell was analyzed. No differences were found in the OR for score or score + smell. However, a trend (P = 0.08) was observed in the OR on d 7 for smell, where cows that received MET were more likely to have metritis when compared to group CON (OR = 7.64). No other differences were observed for smell (P > 0.11). In addition, no differences were found among treatments for temperature on any day (P > 0.13).
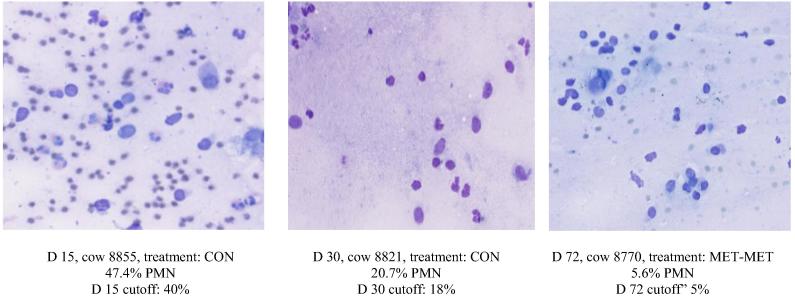
Data for the percentage of PMN are shown in Table 5. Examples of images from cows with percentage of PMN close to these cutoff values can be seen in Fig. 3. For all three days, no difference among treatments was detected (P > 0.22). Logistic analysis was done for the likelihood of cows having endometritis based on the percentage of PMN on d 15, 30, and 72. No differences were found among treatments on any day (P > 0.28) with the exception of treatment MIX significantly more likely (OR = 5.60; P < 0.05) to have endometritis on d 30 when compared with treatment CON.
Table 5.
Treatment least squares means and SEM for percentage of polymorphonuclear neutrophils (PMN) in uterine endometrium cytology samples.
| Day | n | Treatmenta |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | MET | CHO | MIX | SEM | P-valuec,d | ||||||
| 15 | 62 | 41.96 | 37.77 | 45.76 | 48.01 | 7.18 | 0.97 | ||||
| 30 | 70 | 10.15 | 10.30 | 12.61 | 18.31 | 3.93 | 0.22d | ||||
| CON-CON | CON-MET | MET-CON | MET-MET | CHO-CON | CHO-MET | MIX-CON | MIX-MET | ||||
| 72 | 69 | 15.76 | 9.58 | 16.30 | 5.86 | 6.37 | 4.06 | 2.50 | 2.77 | 4.74 | 0.46d |
Treatments: CON: control, n = 16; MET: methionine, n = 20; CHO: choline, n = 17; MIX: methionine and choline, n = 19; CON-CON: control then control, n = 6; CON-MET: control then methionine, n = 10; MET-CON: methionine then control, n = 10; MET-MET: methionine then methionine, n = 10; CHO-CON: choline then control, n = 11; CHO-MET: choline then methionine, n = 6; MIX-CON: methionine and choline then control, n = 9; MIX-MET: methionine and choline then methionine, n = 10.
P-values have been log transformed.
Calving season was used as a covariate when significant (P < 0.03).
Fig. 3.
Examples of the percentage of polymorphonuclear neutrophils (PMN) found from d 15, 30, and 72. D 15, cow 8855, treatment: CON 47.4% PMN D 15 cutoff: 40%. D 30, cow 8821, treatment: CON 20.7% PMN D 30 cutoff: 18%. D 72, cow 8770, treatment: MET-MET 5.6% PMN D 72 cutoff” 5%
4. Discussion
The aim of this study was to determine the effects of rumen-protected methionine and rumen-protected choline on markers of the uterine pro-inflammatory process of Holstein cows. We postulated that cows supplemented with methionine and choline would have lower metricheck smell scores and lower rates of PMN than cows that were not supplemented. The results in this study agree with a previous report, which observed no difference in DMI between cows fed rumen-protected methionine (Smartamine M®) and cows not supplemented (control) [6]. On the other hand, other authors reported increases [7] and decreases [18] in DMI for cows supplemented with rumen-protected methionine. Health results are in contrast with a previous report by Lima et al. [10] who observed a significant decrease in the incidence of ketosis and mastitis in cows supplemented with rumen-protected choline when compared with cows that were not supplemented [10].
The logistic analysis from this study of cows having metritis based on MC score, smell and score + smell supports the hypothesis that a fever does not always accompany fetid and/or purulent discharge and diagnosis of metritis should consist of factors in addition to temperature [19]. The differences between d 4 and d 30 for score and score + smell may be somewhat explained with the incidence of health events. On d 4, cows treated with CHO had the lowest score and score + smell, which might be in part because cows that received CHO had a higher incidence of RP. It has been reported that if RP occurs, the membrane is retained on average for 7 d [20]. Metricheck was not performed if cows still had retained membranes so most likely there were less scores reported for cows in the CHO treatment on d 4, which may account for the difference when compared with other treatments. Likewise, treatment MIX had the lowest score and score + smell by d 30 and did not have any incidence of RP, and treatments CON and MET had similar RP incidence and similar scores and score + smell. The higher occurrence of RP in cows treated with CHO may also account for the change from the lowest score on d 4 to the highest by d 30 and the higher smell score on d 17 and 30, since RP is a risk factor for metritis and endometritis. Cows that received MET had significantly higher smell than cows in both CON and MIX groups and even numerically higher than cows treated with CHO on d 7 and 10, but by d 17 cows treated with MET were the same as cows in both CON and MIX groups, with a smell of 0. Fetid odor has been associated with a greater load of bacteria such as T. pyogenes, which has been associated with purulent discharge, subsequent endometritis, and impaired reproductive performance [11], [12]. Treatment with MET was able to “resolve” the smell by d 30 but CHO was not. Therefore, supplementing methionine early postpartum may be beneficial to cows challenged with bacterial uterine infections.
The function of PMN is associated with the risk of RP, metritis, and endometritis [21], [22]. In the present study, we did not observe cows with RP in the MIX group, meanwhile, significantly higher MC score at d 4 but lower MC scores at d 30, and numerically higher PMN early on but lower PMN on d 72 were recorded in MIX group. It may be possible that a higher percentage of PMN early postpartum may be indicative of a greater immune response. Therefore, supplementation with MIX may enhance the immune response. For all treatments, with the exception of MIX-CON and MIX-MET, the cows that received MET after 30 d had numerically lower percentage of PMN by d 72 (i.e. CON-MET was lower than CON-CON).
In a recent study, Zhou et al. [23] reported that cows supplemented with methionine upon an in vitro pathogen challenge, blood PMN leukocyte (PMNL) phagocytosis capacity and oxidative burst activity were greater in methionine-supplemented cows when compared with control. The authors also reported that cows fed rumen-protected methionine had greater plasma albumin and IL-6 as well as a tendency for lower haptoglobin when compared with cows fed rumen-protected choline. Zhou et al. [24] concluded that the activation of inflammation within PMNL interference with an optimal transition to lactation remains to be determined. Overall, the authors indicated that liver and blood biomarker revealed favorable changes in liver function, inflammation status, and immune response in cows supplemented with rumen-protected methionine. For the present work, continuing to supplement with methionine after d 30 may be beneficial to long-term uterine health.
5. Conclusions
Significant treatment differences were observed in MC score, smell, and a combined score plus smell. Cows that received MIX had no incidence of RP, had significantly lower MC scores at d 30 when compared with CHO, and had numerically lower percentage of PMN on d 72 when compared with all other treatments. Rumen-protected methionine may be beneficial to uterine immune response and long-term reproductive health. This improvement may be even greater when methionine is used in combination with choline supplementation.
Funding statement
Adisseo supplied the rumen-protected methionine used in this study. Adisseo played no role in the study design nor in the collection, analysis and interpretation of data, nor in the decision to submit the manuscript for publication. None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Drackley J.K. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci. 1999;82:2259–2273. doi: 10.3168/jds.s0022-0302(99)75474-3. [DOI] [PubMed] [Google Scholar]
- 2.Komaragiri M.V.S., Erdman R. Factors affecting body tissue mobilization in early lactation dairy cows. 1. Effect of dietary protein on mobilization of body fat and protein. J Dairy Sci. 1997;80:929–937. doi: 10.3168/jds.S0022-0302(97)76016-8. [DOI] [PubMed] [Google Scholar]
- 3.Dubuc J., Duffield T.F., Leslie K.E., Walton J.S., LeBlanc S.J. Risk factors for postpartum uterine diseases in dairy cows. J Dairy Sci. 2010;93:5764–5771. doi: 10.3168/jds.2010-3429. [DOI] [PubMed] [Google Scholar]
- 4.Reist M., Koller A., Busato A., Kupfer U., Blum J.W. First ovulation and ketone body status in the early postpartum period of dairy cows. Theriogenology. 2000;54:685–701. doi: 10.1016/S0093-691X(00)00383-6. [DOI] [PubMed] [Google Scholar]
- 5.Durand D., Chilliard Y., Bauchart D. Effects of lysine and methionine on in vivo hepatic secretion of VLDL in the high yielding dairy cow. J Dairy Sci. 1992;75:279. [Google Scholar]
- 6.Ordway R.S., Boucher S.E., Whitehouse N.L., Schwab C.G., Sloan B.K. Effects of providing two forms of supplemental methionine to periparturient Holstein dairy cows on feed intake and lactational performance. J Dairy Sci. 2009;92:5154–5166. doi: 10.3168/jds.2009-2259. [DOI] [PubMed] [Google Scholar]
- 7.Osorio J.S., Ji P., Drackley J.K., Luchini D., Loor J.J. Supplemental Smartamine M or MetaSmart during the transition period benefit postpartal cow performance and blood neutrophil function. J Dairy Sci. 2013;96:1–16. doi: 10.3168/jds.2012-5790. [DOI] [PubMed] [Google Scholar]
- 8.Pisulewski P.M., Rulquin H., Peyraud J.L., Verite R. Lactational and systemic responses of dairy cows to postruminal infusions of increasing amounts of methionine. J Dairy Sci. 1996;79:1781–1791. doi: 10.3168/jds.S0022-0302(96)76546-3. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council . 7th rev. ed. Natl. Acad. Press; Washington, DC: 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- 10.Lima F.S., Sa Filho M.F., Greco L.F., Santos J.E.P. Effects of feeding rumen-protected choline on incidence of diseases and reproduction of dairy cows. Vet J. 2012;193:140–145. doi: 10.1016/j.tvjl.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 11.LeBlanc S.J. Postpartum uterine disease and dairy herd reproductive performance: a review. Vet J. 2008;176:102–114. doi: 10.1016/j.tvjl.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Sheldon I.M., Lewis G.S., LeBlanc S., Gilbert R.O. Defining postpartum uterine disease in cattle. Theriogenology. 2006;65:1516–1530. doi: 10.1016/j.theriogenology.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Galvão KN. Identifying and treating uterine disease in dairy cows. In: Proceedings 47th Florida dairy production conference; 2011.
- 14.Defontis M., Vaillancourt D., Grand F.X. Comparison of three methods of sampling for endometrial cytology in the mare. Preliminarily study. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2011;39:171–175. [PubMed] [Google Scholar]
- 15.Kasimanickam R., Duffield T.F., Foster R.A., Gartley C.J., Leslie K.E., Walton J.S. A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can Vet J. 2005;46:255–259. [PMC free article] [PubMed] [Google Scholar]
- 16.Oral H., Sozmen M., Serin G., Kaya S. Comparison of the cytobrush technique, vaginoscopy and transrectal ultrasonography methods for the diagnosis of postpartum endometritis in cows. J Anim Vet Adv. 2009:1252–1255. [Google Scholar]
- 17.Ferguson J.D., Galligan D.T., Thomsen N. Principal descriptors of body condition score in Holstein cows. J Dairy Sci. 1994;77:2695–2703. doi: 10.3168/jds.S0022-0302(94)77212-X. [DOI] [PubMed] [Google Scholar]
- 18.Socha M.T., Putnam D.E., Garthwaite B.D., Whitehouse N.L., Kierstead N.A., Schwab C.G. Improving intestinal amino acid supply of pre-and postpartum dairy cows with rumen-protected methionine and lysine. J Dairy Sci. 2005;88:1113–1126. doi: 10.3168/jds.S0022-0302(05)72778-8. [DOI] [PubMed] [Google Scholar]
- 19.Benzaquen M.E., Risco C.A., Archbald L.F., Melendez P., Thatcher M.J., Thatcher W.W. Rectal temperature, calving-related factors, and the incidence of puerperal metritis in postpartum dairy cows. J Dairy Sci. 2007;90:2804–2814. doi: 10.3168/jds.2006-482. [DOI] [PubMed] [Google Scholar]
- 20.Eiler H. Retained placenta. In: Youngquist RS, editor. Current therapy in large animal theriogenology. WB Saunders Co.; Philadelphia, PA: 1997. pp. 340–348. [Google Scholar]
- 21.Hammon DS, Goff JP. Immune function and energy status in Holstein cows with uterine infections. In: Proc of the Mid-South Ruminant Nutr Conf; 2006, p. 30–6.
- 22.Kimura K., Reinhardt T.A., Goff J.P. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J Dairy Sci. 2006;89:2588–2595. doi: 10.3168/jds.S0022-0302(06)72335-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Z., Bulgari O., Vailati-Riboni M., Trevisi E., Ballou M.A., Cardoso F.C. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J Dairy Sci. 2016;99:8956–8969. doi: 10.3168/jds.2016-10986. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z., Bu P., Vailati-Riboni M., Khan M.J., Graugnard D.E., Luo J. Prepartal dietary energy level affects peripartal bovine blood neutrophil metabolic, antioxidant, and inflammatory gene expression. J Dairy Sci. 2015;98:5492–5505. doi: 10.3168/jds.2014-8811. [DOI] [PubMed] [Google Scholar]