Graphical abstract
Keywords: Anti-diarrhoeal, Gastro-intestinal transit time, Castor oil, Terminalia avicennioides, Rabbit, Guinea pig
Abstract
Due to the shortcomings associated with modern synthetic antidiarrhoeal drugs, it is important to find newer, safer and cheaper antidiarrhoeal agents from natural sources. The study was conducted to evaluate the anti-diarrhoeal activity of the fractions of the stem-bark of Terminalia avicennioides in laboratory animal models. The effect of different concentrations (1.0 × 10−3, 2.0 × 10−3, 4.0 × 10−3 and 8.0 × 10−3 mg/mL) of the aqueous methanol (AMF), ethyl acetate (EAF) and hexane (HXF) fractions of T. avicennioides were tested against spontaneous and acetylcholine-induced contractions of rabbit jejunum as well as on histamine-induced contraction of guinea pig ileum. Similarly, the effects of the AMF on gastro-intestinal transit time, castor oil-induced diarrhoea and castor oil-induced enteropooling were evaluated. The AMF, EAF and HXF at concentrations of 1.0 × 10−3, 2.0 × 10−3, 4.0 × 10−3 and 8.0 × 10−3 mg/mL attenuated the contractile effects of both the spontaneous and acetylcholine-induced contractions of rabbit jejunum and that of histamine-induced contraction of guinea pig ileum in a concentration-dependent manner. The AMF at doses of 200, 300 and 500 mg/kg produced significant (p < 0.05) reductions in gastrointestinal transit time of charcoal and incidence of castor oil-induced diarrhoea in mice relative to the untreated control. Similarly, at doses of 300 and 500 mg/kg, AMF significantly (p < 0.05) reduced the weight and volume of intestinal fluid in the treated mice when compared to the untreated animals. The results of this study showed that the stem-bark of T. avicennioides possesses spasmolytic effect and could be a potential antidiarrhoeal agent. However, detailed pharmacological trials are required to justify the clinical use of the plant for treating diarrhoea.
1. Introduction
Diarrhoea is characterised by increased frequency of bowel, wet stool and abdominal pains [1]. It is usually a symptom of an infection in the intestinal tract, which can be caused by a variety of bacterial, viral, parasitic organisms and other non-infectious causes. [2]. The World Health Organisation (WHO) estimated that 3–5 billion cases of diarrhoea occur each year (1 billion in children less than 5 years of age) [3]. Similarly, Ahmed et al. [4] reported that diarrhoea is the foremost fatal outcome among children in Nigeria under the age of five. Despite the availability of different approaches for diarrhoeal management, vast majority of the people in developing countries rely on herbal drugs for the management of diarrhoea [5]. The use of modern drugs for treating diarrhoea are usually associated with unwanted side effects (e.g. dry mouth and urinary retention often observed with the use of antimuscarinic drugs as atropine and headache, and nausea with calcium channel blockers). The synthetic opioid drugs (diphenoxylate and loperamide) cause severe constipation and may significantly slow gastrointestinal transit and increase the absorption of bacterial toxins in infectious diarrhoea [6]. Due to these shortcomings associated with modern antidiarrhoeal drugs, it is important to find newer, safer and cheaper antidiarrhoeal agents from natural sources [7].
Terminalia avicennioides (Combretaceae) is found in the savannah region of West Africa [8]. In Nigeria, the plant is locally called “baushe”, “Idi”, “kpace”, “kpayi” and “Edo” in Hausa, Yoruba, Nupe, Gwari and Igbo languages, respectively [9], [10]. Different parts of the plant have been used traditionally to manage conditions such as gastric ulcer, gastro-intestinal disorders (diarrhoea), bloody sputum, cough, and gastro-intestinal helminth parasites [11], [12], [13]. Evaluation of the antidiarrhoeal effects of this plant is an attempt not only to validate the traditional claim but that is also hoped to provide an alternative source for an effective treatment against diarrhoea.
2. Materials and methods
2.1. Plant material
The stem-bark of T. avicennioides was obtained from the wild, around Zaria, Nigeria. Samples of the flowers, leaves and seeds of the plant were sent to the Herbarium, Department of Biological Sciences, Ahmadu Bello University, Zaria, for identification. A voucher specimen with number 900239 was deposited at the Herbarium for reference purposes.
2.2. Experimental animals
Five adult New Zealand white rabbits weighing between 2.0 and 2.5 kg and 5 adult guinea pigs of 300 and 400 g weight were purchased from a local market in Zaria. One hundred adult swiss albino mice of both sexes weighing between 22 and 23 g were obtained from the Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Zaria. The animals were acclimatized to laboratory conditions for two weeks and were fed on commercial rodent diet. In addition, water was provided ad libitum. All animal experiments were done according to the ethical guidelines on laboratory animal use and care policy, which is in line with Ahmadu Bello University Research Policy (revised in 2010).
2.3. Equipment and laboratory materials used
Locally made cages; Wooden mortar and pestle; Whatman filter paper size 1; Conical flask; Macerating bottle; Measuring cylinder, Test tubes (Pyrex, France); Syringes (1 mL and 5 mL); Weighing balance (Lab tech. BL 20001 and Mettler P162, USA); Microdynamometer (Ugo Basile, Italy); Water bath (HH-S Digital thermostatic water bath, China); Dissecting kit (Gold Cross Dissecting Set, Malaysia); Plastic ruler and Stop watch.
2.4. Drugs and chemicals
Acetylcholine (Ach) and histamine (H) were purchased from Sigma-Aldrich Inc., 3050 Spruce Street, St. Louis, USA. Castor oil (Bell, Sons and Co Ltd, Southport PR9 9 AL, England); Loperamide (Imodium®– Janssen Pharmaceutical, Pakistan); Medicinal charcoal (Ultracarbon® tablets–Merck KGaA, Darmstadt, Germany); Carboxymethylcellulose.
2.5. Plant extraction and partitioning
The stem-bark of T. avicennioides was air-dried at room temperature to a constant weight. The dried plant part was made into powdered form using wooden mortar and pestle. One thousand five hundred grams of the powdered stem-bark of the plant were extracted by maceration in a macerating bottle using 4.5 litres of methanol (98%) as solvent at room temperature. The process was repeated twice and the extracts were pooled together. The liquid extract was concentrated in vacuo using rotary evaporator at 40 °C. The crude methanol extract was dissolved in water and serially partitioned with n-hexane and ethyl acetate in a separating funnel. Similarly, the fractions obtained were concentrated in vacuo at 40 °C. All the fractions were weighed, labeled and stored in an air tight container at 4 °C until required.
2.6. Phytochemical screening
The fractions were screened to detect the presence of alkaloids, anthraquinones, carbohydrates, cardiac glycosides, flavonoids, saponins, steroids/triterpenes and tannins using standard test methods [14].
2.7. In vitro studies
2.7.1. Effect of fractions of T. Avicennioides on isolated rabbit jejunum
The rabbits were deprived of food but not water for 18 h before the study. Each rabbit was sacrificed by cervical dislocation and exsanguinated. Their abdomens were cut open and segments of the jejunum (2 cm) were cut and dissected from the adhering mesentery. Each tissue was suspended in a 25 mL organ bath containing Tyrode’s solution, aerated with air and allowed to stabilize for 30 min to acclimatize. The effect of different concentrations of acetylcholine (Ach) (1.0 × 10−6 mg/mL–8.0 × 10−6 mg/mL) and the fractions of T. avicennioides (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) were tested on both spontaneous and Ach-induced contractions of the isolated tissue. The contact time for each tested fraction on the tissue was 30 s, which was followed by washing the tissue three times with Tyrode’s solution. The tissue was allowed to rest for a period of 15 min before the next addition of drug or extract. Changes in tension produced by the test agent were recorded with a microdynamometer (sensitivity of 3.0 mV and speed of 24 mm/min coupled to an isotonic transducer [12]
2.7.2. Effect of fractions of T. avicennioides on isolated guinea pig ileum
Similarly, the method described above for the effect of stem-bark extracts of T. avicennioides on isolated rabbit jejunum was used. The effects of histamine (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) and fractions of T. avicennioides (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) against histamine-induced contraction of the tissue were also tested.
2.8. Acute toxicity test
The median lethal dose (LD50) as an indication of the acute toxicity of the extract was determined by the method described by Lorke [15]. The test was carried out in two phases. All the animals were fasted for 12 h prior to oral administration of the AMF. In phase one, nine apparently healthy swiss albino mice were randomly divided into three groups of three mice. Mice in groups one, two and three received AMF orally at 10, 100 and 1000 mg/kg, respectively. The mice were observed over a period of 48 h for signs of toxicity and mortality. In the second phase, three mice were randomly assigned into three groups of one mouse each. Animals in groups one, two and three were treated with AMF orally at 1600, 2900 and 5000 mg/kg, respectively. Similarly, the animals were observed for 48 h for any signs of toxicity or mortality. The obtained results were recorded accordingly.
2.9. In vivo studies
2.9.1. Effect of the aqueous methanol fraction on gastro-intestinal transit time in mice
The method described by Vogel and Vogel [16] was used. Twenty-five mice were deprived of feed for 12 h and randomly allotted into five groups of 5 mice each. Animals in groups one and two were administered with distilled water (5 mL/kg) and loperamide (5 mg/kg) and served as untreated and treated control groups, respectively. Similarly, mice in groups three, four and five were given AMF at 200, 300 and 500 mg/kg, respectively. All treatments were given orally. A solution of charcoal meal was prepared by adding grinded charcoal tablets (250 mg) to a boiled solution of 2% carboxymethycellulose in a 20 mL beaker. Distilled water was added gradually to give a slurry aqueous suspension. The suspension was allowed to cool before administering it to the animals. One hour post-treatment, 0.3 mL of the charcoal meal was administered orally to each mouse. One hour after the charcoal meal was given, all the mice were sacrificed by cervical dislocation, their abdomen were cut open, and their intestines were carefully removed from the cardia to the anus. The intestines were immediately immersed in formalin to arrest peristalsis. Thereafter, the intestines were washed in clean tap water. The distance transverse by the meal through the intestine of each mouse was shown by the charcoal meal front, which was then measured using a graduated ruler and expressed as a percentage of total distance from pylorus to caecum. Charcoal movement was expressed as peristaltic index (PI). The peristaltic index and percentage of inhibition were calculated using the following:
where
Dc: Mean distance travelled by the charcoal in the control group
Dt: Mean distance travelled by the charcoal in the test group.
2.9.2. Effect of the aqueous methanol fraction on castor oil-induced diarrhoea in mice
The method described by Vogel and Vogel [16] was used in this study. Twenty-five mice were allocated at random into five groups of five mice each and were deprived of feed for 12 h. Animals in group one (untreated control) and two (treated control) were given distilled water (5 mL/kg) and loperamide (5 mg/kg), respectively. Mice in groups three, four and five were given AMF at 200, 300 and 500 mg/kg, respectively. All treatments were given through the oral route. One hour after drug treatment, all mice were orally given 5 mL/kg of castor oil and placed individually in cages over white, clean filter paper. Faeces were collected on filter papers, which was changed every 30 min for 6 h. Severity of the diarrhoea was graded as follows:
-
•
Grade 0 = normal pelleted faeces
-
•
Grade 1 = discrete soft-formed faeces
-
•
Grade 2 = soft-formed faeces
-
•
Grade 3 = soft watery stool
-
•
Grade 4 = watery stool with little solid matter
Diarrhoeal index for each group was calculated by multiplying the number of mice in each grade by the number of grade divided by the number of mice in each group.
2.9.3. Effect of the aqueous methanol extract on castor oil-induced enteropooling in mice
The effect of AMF on castor oil-induced enteropooling was done according to the method described by Igboeli et al. [17]. Twenty-five mice were allocated into five groups of five mice each and were deprived of food and water for 12 h before the experiment. Mice in group one (untreated control) were given distilled water (5 mL/kg), while those in group two (treated control) received loperamide (5 mg/kg). Animals in groups three, four and five were given AMF at 200, 300 and 500 mg/kg, respectively. All treatments were administered orally an hour before the oral administration of castor oil (2 mL/kg). One hour after castor oil administration, the mice were sacrificed by cervical dislocation and the small intestine of each mouse was removed after tying the ends with threads and weighed. The intestinal content of each mouse was collected in a graduated cylinder and the volume measured. Thereafter, it was weighed again and the difference between the initial and final weights of the intestines was recorded. The percentage reduction of intestinal secretion (volume and weight) was calculated relative to the negative control using the formula:
2.10. Statistical analysis
Data were expressed as mean ± SEM and were subjected to one way analysis of variance (ANOVA). Tukey post hoc test was used to test for differences between treatment groups. For the data of ordinal type, a non-parametric test was used. Kruskal-Wallis one-way ANOVA was computed for overall significance and for observing significant difference. All the results obtained in the in vivo study were compared with the untreated control group. Values of p < 0.05 were considered statistically significant.
3. Results
A yield of 77.37 g (5.16%) of the crude methanol extract was obtained when 1.5 kg of the dried powder of the stem-bark of T. avicennioides was completely extracted with methanol. Similarly, the aqueous methanol extract after partitioning with ethyl acetate and hexane gave yields of 31.89 g (41.22%), 1.76 g (2.27%) and 0.4 g (0.52%) of AMF, EAF and HXF, respectively.
Qualitative phytochemical tests on the extracts of the stem-bark of T. avicennioides revealed the presence of saponins and steroids/triterpenes in AMF, EAF and HXF. In addition, AMF and EAF contain carbohydrate, tannins and cardiac glycosides. Flavonoids and alkaloids were also detected in the AMF (Table 1).
Table 1.
Phytochemical constituents investigated in the extracts of the stem-bark of Terminalia avicennioides.
| Phytochemical constituent | Phytochemical test | Inference |
||
|---|---|---|---|---|
| AMF | EAF | HXF | ||
| Alkaloids | Mayers | + | − | − |
| Dragendoff | + | − | − | |
| Anthraquinones | Bontragers | − | − | − |
| Modified Bontragers | − | − | − | |
| Carbohydrates | Fehlings | + | + | − |
| Molisch | ||||
| Cardiac glycosides | Kadde | + | + | − |
| Kelle-Kiliani | + | + | − | |
| Flavonoids | Shinoda | + | − | − |
| Sodium hydroxide | + | − | − | |
| Saponins | Frothing | + | + | + |
| Steroids/triterpenes | Liberman Buchard | + | + | + |
| Tannins | Ferric chloride | + | + | − |
| Lead acetate | + | + | − | |
Key: + = present; − = not present; AMF = Aqueous methanol fraction; EAF = Ethyl acetate fraction; HXF = Hexane fraction.
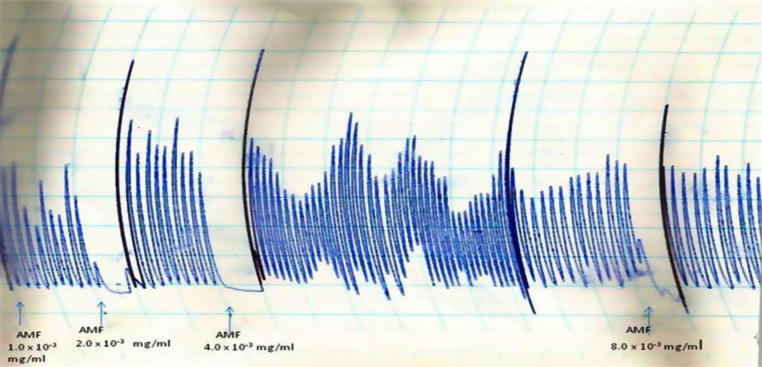
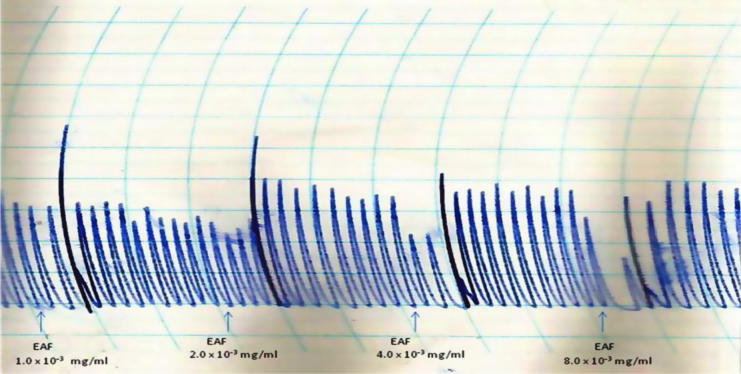
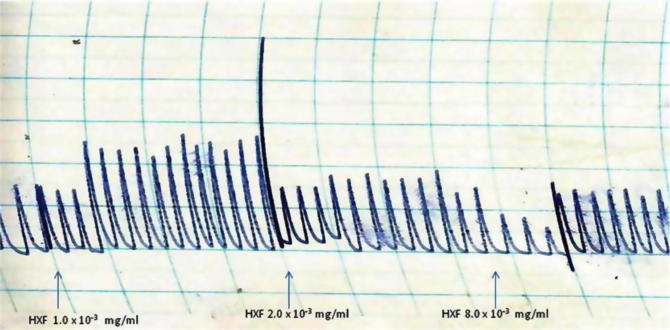
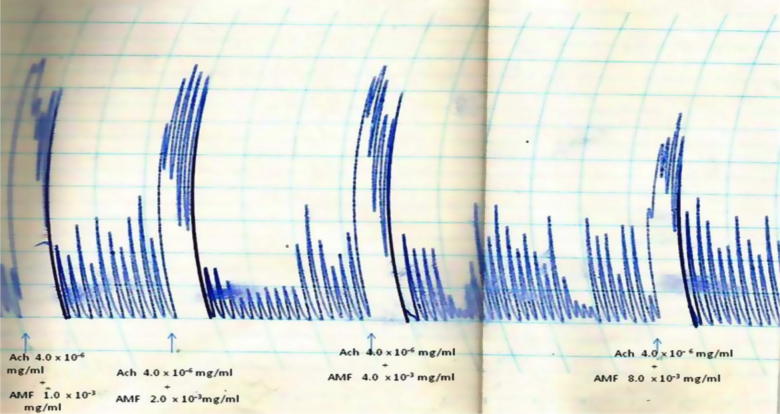
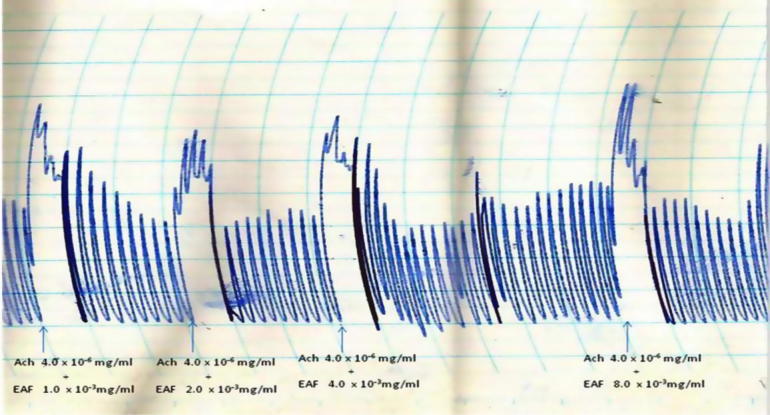
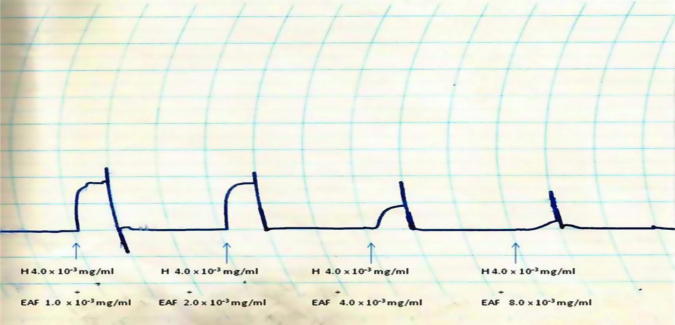
The AMF, EAF and HXF inhibited the spontaneous contractions of the isolated rabbit jejunum in a concentration-dependent fashion (Fig. 1, Fig. 2, Fig. 3). In addition, the AMF and EAF produced a concentration–dependent inhibition of acetylcholine (Ach)-induced contraction of isolated rabbit jejunum (Fig. 4, Fig. 5).
Fig. 1.
Effects of different concentrations (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) of aqueous methanol fraction (AMF) of the stem-bark of Terminalia avicennioides on the spontaneous contraction of isolated rabbit jejunum.
Fig. 2.
Effects of different concentrations (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) of the ethyl acetate fraction (EAF) of the stem-bark of Terminalia avicennioides on spontaneous contraction of isolated rabbit jejunum.
Fig. 3.
Effects of different concentrations (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) of the hexane fraction (HXF) of the stem-bark of Terminalia avicennioides on spontaneous contraction of isolated rabbit jejunum.
Fig. 4.
Effects of different concentrations (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) of the aqueous methanol fraction (AMF) of the stem-bark of Terminalia avicennioides on acetylcholine (Ach)-induced contraction of rabbit jejunum.
Fig. 5.
Effects of different concentrations (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) of the ethyl acetate fraction (EAF) of the stem-bark of Terminalia avicennioides on acetylcholine (Ach)-induced contraction of isolated rabbit jejunum.
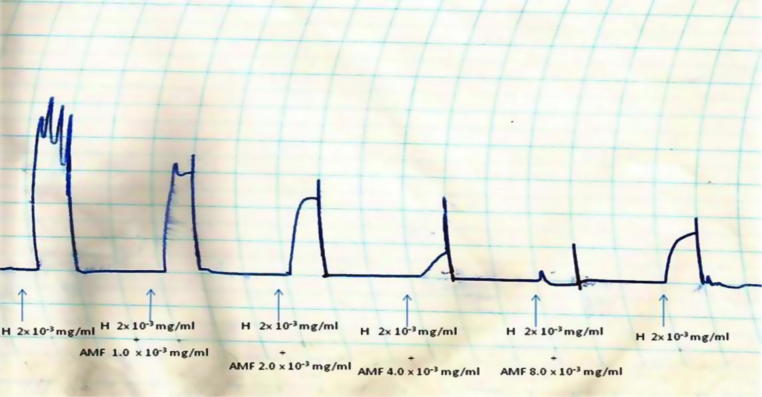
The AMF and EAF inhibited histamine (H)-induced contraction of isolated guinea pig ileum in a concentration-dependent fashion (Fig. 6, Fig. 7).
Fig. 6.
Effects of different concentrations (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) of the aqueous methanol fraction (AMF) of the stem-bark of Terminalia avicennioides on histamine-induced contraction of guinea pig ileum.
Fig. 7.
Effects of different concentrations (1.0 × 10−3 mg/mL–8.0 × 10−3 mg/mL) of the ethyl acetate fraction (EAF) of the stem-bark of Terminalia avicennioides on histamine (H)-induced contraction of guinea pig ileum.
Oral administration of the extract at a dose of up to 5000 mg/kg did not produce mortality or any toxic effect in the tested animals. The median lethal dose (LD50) was therefore considered to be >5000 mg/kg in mice.
The extract at doses of 300 and 500 mg/kg significantly (p < 0.05) reduced both the length transversed by the charcoal meal and the peristaltic index when compared with the untreated control (distilled water) group. Loperamide (5 mg/kg), which is a standard antidiarrhoeal drug, showed a greater inhibitory effect (51.61%) than the extract, as shown in Table 2.
Table 2.
Effect of the aqueous methanol fraction (AMF) of Terminalia avicennioides on gastro-intestinal transit time in mice. (n = 5).
| Treatment (mg/kg) | Mean length of small intestine ± SEM (cm) | Mean distance of charcoal meal ± SEM (cm) | Peristaltic index | % of inhibition |
|---|---|---|---|---|
| Distilled Water (5 mL/kg) | 46.12 ± 1.80 | 37.2 ± 2.43a | 80.34 ± 2.36a | – |
| Loperamide (5) | 43.90 ± 1.11 | 17.12 ± 0.97b | 38.88 ± 1.37b | 51.61 |
| AMF (200) | 47.84 ± 1.44 | 35.66 ± 1.36a | 74.49 ± 0.91a | 7.28 |
| AMF (300) | 45.10 ± 2.26 | 24.90 ± 2.20b | 54.81 ± 2.23b | 31.78 |
| AMF (500) | 49.0 ± 1.64 | 22.44 ± 0.75b | 45.86 ± 1.21b | 42.92 |
Means on the same column with different superscript letters are significantly (p < 0.05) different.
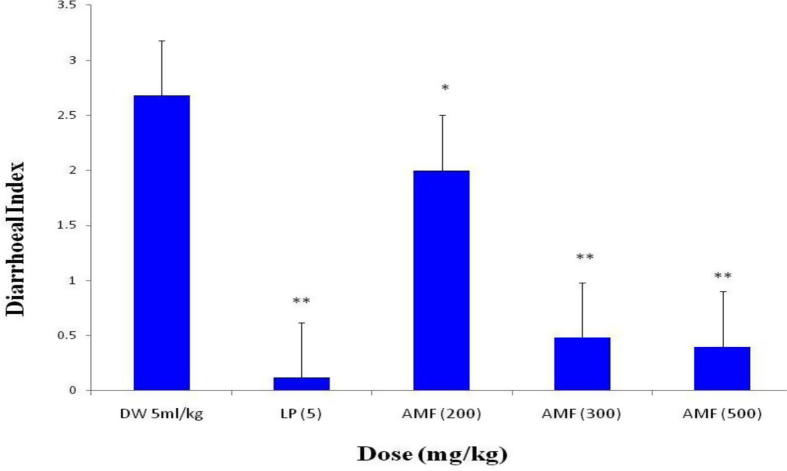
The extract at doses of 200, 300 and 500 mg/kg significantly (p < 0.05) afforded protection against diarrhoea in mice induced by castor oil in a dose-dependent manner when compared with the distilled water (untreated) control group (Fig. 8).
Fig. 8.
Effect of the aqueous methanol fraction (AMF) of the stem-bark of Terminalia avicennioides on castor oil-induced diarrhoea in mice. Untreated control group was dosed with distilled water (DW) while treated control group was dosed with loperamide (LP). Data are expressed as mean ± SEM. *p < 0.05; **p < 0.01 compared to the untreated control group; (Kruskal-Wallis one-way ANOVA test).
The extract at 200, 300 and 500 mg/kg significantly (p < 0.05) inhibited castor oil-induced enteropooling in mice. At the same doses, it also inhibited the weight of intestinal fluid content of treated mice by 21.44%, 32.53% and 43.62%, respectively. The potent antidiarrhoeal drug; loperamide (5 mg/kg), also significantly (p < 0.05) inhibited the weight of intestinal fluid content (54.71%) relative to the untreated control. Similarly, the volume of the intestinal fluid in mice was significantly (p < 0.05) reduced at all the tested doses of the extract, as shown in Table 3.
Table 3.
Effects of the aqueous methanol fraction (AMF) of T. avicennioides on castor oil-induced enteropooling in mice (n = 5).
| Treatment (mg/kg) | Mean weight of small intestine (g) | % inhibition | Mean volume of small intestinal contents (ml) | % inhibition |
|---|---|---|---|---|
| Distilled water (5 ml/kg) | 1.123 ± 0.07 | – | 0.888 ± 0.03a | – |
| Loperamide (5) | 0.486 ± 0.01b | 54.71 | 0.374 ± 0.02c | 61.80 |
| AMF (200) | 0.846 ± 0.02a | 21.44 | 0.734 ± 0.02a | 17.98 |
| AMF (300) | 0.730 ± 0.01a | 32.53 | 0.566 ± 0.03b | 35.96 |
| AMF (500) | 0.630 ± 0.01b | 43.62 | 0.520 ± 0.02b | 43.82 |
Means on the same column with different superscript letters are significantly (p < 0.05) different.
4. Discussion
This study was carried out to evaluate and validate the traditional claims on the antidiarrhoeal effect of the stem-bark of Terminalia avicennioides, which is a plant used widely among the Fulanis (major cattle rearing people of West Africa) for treating diarrhoea in both animals and humans.
The in vitro effect of the different fractions (AMF, EAF and HXF) of the plant obtained from the crude methanol extract was tested against contractions of isolated rabbit jejunum (spontaneous and acetylcholine-induced) and guinea pig ileum (histamine-induced) in an attempt to find the fraction with the most promising effect against diarrhoea.
Diarrhoea is usually the frequent passage of liquid stools. It involves increased gastrointestinal motility and secretion, a decrease in absorption of fluid, and loss of electrolytes and water [18]. In some types of diarrhoea, the secretory component predominates, while other types of diarrhoea are characterized by hypermotility [19].
The results of the phytochemical screening of the stem-bark detected metabolites that concur with that earlier reported by Mann and Yusuf [20]. Garba et al. [21] reported the presence of alkaloids, saponins, cardiac glycosides, steroids, tannins and phenols in the roots of T. avicennioides.
The fractions of the crude methanol extract (AMF, EAF and HXF) of T. avicennioides concentration-dependently inhibited both the spontaneous and acetylcholine-induced contractions of isolated rabbit jejunum. Similarly, the fractions attenuated the contraction of guinea pig ileum caused by histamine in a concentration-dependent manner. Acetylcholine and histamine are agonists that cause intestinal smooth muscle contractions by activating the muscarinic M3 and histamine H1 receptors, respectively. Acetylcholine induces smooth muscle contraction via inositol phosphate (IP3) pathway, which mediates Ca2+ release from sarcoplasmic reticulum [22]. The role of calcium ions (Ca2+) in producing smooth muscle tension has been well established [23]. Ca2+ serves as a second messenger in smooth muscle cells. An increase in cytoplasmic Ca2+ concentration and binding to calmodulin, and the activation of myosin light chain kinase, is the primary stimulus for contraction. The Ca2+ used in the activation of the contractile apparatus enters the Cytoplasmic compartment during periods of membrane depolarisation, mechanical distortion, or stimulation by agonists. Release of Ca2+ from intracellular stores is a second means of increasing calcium ion concentration [24], [25].
In the mammalian tissues, histamine is distributed widely. The enterochromaffin-like cells (ECF) that are found mainly in the fundic mucosa of the stomach produce histamine by decarboxylation of the amino acid, histidine. Histidine is converted to histamine by a specific enzyme, histidine decarboxylase. Histamine is known to contract several types of smooth muscle, including those of the bronchi, gut and large blood vessels [26]. Histamine-induced contraction of the smooth muscle of guinea pig ileum is mediated by H1 receptors found throughout the gastrointestinal tract. [27]. Moreover, the contractile effect of histamine on both vascular smooth muscle and endothelial cells cause increase in vascular permeability [28].
In a similar study, the extract of the roots of T. avicennioides was shown to attenuate acetylcholine-induced contraction of isolated rabbit jejunum [12]. Saponins have been shown to inhibit the in vitro release of histamine [29]. Tannins, flavonoids, saponins, sterols/triterpenes, present in some medicinal plants, were demonstrated to possess spasmolytic effect [30]. It was shown also that flavonoids inhibit intestinal secretion of autacoids and prostaglandins [31] and inhibit intestinal contractions induced by spasmogens [32]. Flavonoids were shown to have the ability to attenuate the contraction of guinea pig ileum induced by some spasmogens such as acetylcholine [33]; they also have the ability to inhibit small intestinal transit [34]. In this study, phytochemicals such as alkaloids, flavonoids, saponins, tannins and terpenoids detected in the fractions of T. avicennioides are reported to be responsible for the observed spasmolytic effect of the plant [35], [36].
It is of interest to note that the AMF produced more spasmolytic effect on both the rabbit and guinea pig ileum during the in vitro tests. This finding clearly suggested that flavonoids which were detected only in the AMF largely are responsible for the spasmolytic effect of the plant.
The median lethal dose (LD50) as an indication of acute toxicity of AMF, which showed the most promising effect during the in vitro trial was calculated to be ≥5000 mg/kg. According to Lorke [15], any substance with LD50 of ≥5000 mg/kg should be considered relatively safe. Determination of median lethal dose of a chemical is usually the first step taken to ascertain the safety of that chemical with the sole intent of using it as a drug. Acute toxicity test also allows further toxicological and pharmacological testing of candidate drugs to justify their use in clinical situations.
Diarrhoea is usually considered a result of altered motility and fluid accumulation within the intestinal tract. Many antidiarrhoeal agents act by reducing gastro-intestinal motility and/or the secretions [37]. The extract significantly reduced intestinal transit time in mice fed charcoal meal. Drugs that display such properties are known to be good candidates as antidiarrheal agents [38]. Loperamide (an opioid derivative), has been shown to slow intestinal motility by its action on mu (µ) receptors on neurons in the submucosal neural plexus of the intestinal wall and by its antimuscarinic activity in the gastrointestinal tract [39]. Antimuscarinic drug possess their antidiarrhoeal action by inhibiting both the gastro-intestinal secretions and motility [18]. The antidiarrhoeal activity of some plant extracts had been ascribed to their antagonistic action on muscarinic receptors found in the gastro-intestinal tract [40]. Studies on the functional role of tannins against diarrhoea, revealed that they inhibit gastro-intestinal movement by reducing the intracellular Ca2+ inward current or by activation of the calcium pumping system [41] as well as forming protein tannates, which make the intestinal mucosa more resistant and hence, reduce peristaltic movement [42]. The extract also inhibited the intestinal propulsion as shown by its inhibitory action against charcoal meal motility. Agents with such activity were known to have antidiarrhoeal effect [43].
Similarly, the study showed that the extract inhibit castor oil-induced diarrhoea in mice in a dose-dependent manner. Castor oil, an irritant or stimulant laxative, is hydrolysed in the upper small intestine to ricinoleic acid, a local irritant that irritates the mucosa of the gastrointestinal tract resulting in increase in intestinal motility [44]. Castor oil has been shown to cause diarrhoea due to release of the active ricinoleic acid that induces fluid hypersecretion in the intestine [45], prevents water and electrolyte absorption, reduces active Na+ and K+ absorption and decreases the level of Na+, K+-ATPase in the small intestine and colon. The sum effect of these processes could lead to increase colon stimulation and peristaltic activity resulting in diarrhoea [46]. Perhaps, the antidiarrhoeal action of AMF was mediated through inhibition of the effect produced by castor oil. Loperamide (a standard antidiarrhoeal agent) is known to inhibit castor oil-induced diarrhoea [47], [48]. The effect of the extract at 500 mg/kg was similar to loperamide, which at present one of the widely employed antidiarrhoeal drug. The AMF produced significant effect against all the diarrhoeic animal models in this study. This perhaps could be due to increased absorption of water and electrolyte from the gastrointestinal tract induced by the fraction (AMF) of the extract.
AMF was found to significantly (p < 0.05) inhibit the weight and the volume of intestinal fluid in mice when compared to animals in the untreated control group. This effect can be attributed to either a decrease in mucosal secretion or increase in mucosal absorption. Nwafor et al. [49] showed that the suppression of intestinal fluid accumulation by plant extracts produced antidiarrhoeal activity via inhibition of the gastrointestinal function. In this study, the extract significantly reduced both the weight and volume of intestinal content which may have promoted reabsorption of fluids due to decrease propulsion in the intestinal of mice. This may possibly explain the mechanism of the extract anti-enteropooling action. Longanga et al. [29] screened a number of medicinal plants and showed that the antidiarrhoeal activity of those plants were due to tannins, alkaloids, saponins, flavonoids, sterols, triterpenes and reducing sugars contained in them. In a similar study, the antidiarrhoeal activities of some medicinal plants were found to be due to tannins, alkaloids, saponins and flavonoids contained in them [50]. Hence, the antidiarrhoeal activity exhibited by the aqueous methanol fraction of the stem-bark of T. avicennioides could be due to presence of one or more of these metabolites.
5. Conclusions
The present study showed that the fractions (AMF, EAF and HXF) of the crude methanol extract of T. avicennioides possess spasmolytic effect in an in vitro study. In addition, the AMF possesses antidiarrhoeal effects which could be due to the metabolites present in the fraction. This study therefore, supports the traditional use of the plant as an antidiarrhoeal agent. Further studies are ongoing to isolate, characterize and elucidate the mechanism(s) of compounds with antidiarrhoeal action in the plant.
Funding
We did not receive any grant for this research.
Acknowledgments
The authors would like to express their sincere appreciation and gratitude to all the technical staff of the Departments of Pharmacology and Therapeutics and Pharmacology and Toxicology, Faculties of Pharmaceutical Sciences and Veterinary Medicine, respectively, Ahmadu Bello University, Zaria, Nigeria for their technical assistance.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Ezekwesili C.N., Obiara K.A., Ugwu O.P. Evaluation of anti-diarrhoeal properties of crude aqueous extract of Ocimum gratissimum L. (Labiatae) in rats. Biochem. 2004;16:122–131. [Google Scholar]
- 2.Mzungu I., Inabo H.I., Olonitola S.O., Aminu M. Antibiotic susceptibilities of Salmonella species prevalent among children of 0–5 years with diarrhoea in Katsina state, Nigeria. Arch Med Biomed Res. 2016;3:39–51. [Google Scholar]
- 3.Heinrich M., Heneka B., Ankli A., Rimpler H., Sticher O., Kostiza T. Spasmolytic and antidiarrhoeal properties of the Yutec Mayan medicinal plant Castmtroa tetrameria. J Pharm Pharmacol. 2005;57:1081–1085. doi: 10.1211/jpp.57.9.0002. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A.A., Zezi A.U., Yaro A.H. Antidiarrhoeal activity of the leaf extracts of Daniella oliveri, Hutch and Dalz (Fabaceae) and Ficus sycomorus, MIQ (Moraceae) Afr J Tradit Complemen Altern Med. 2007;4:524–528. [PMC free article] [PubMed] [Google Scholar]
- 5.Atta A.H., Mouneir S.M. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J Ethnopharmacol. 2004;92:303–309. doi: 10.1016/j.jep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Papich M.G. Drugs affecting gastrointestinal function. In: Riviere J.E., Papich M.G., editors. Veterinary pharmacology and therapeutics. 9th ed. Wiley-Blackwell; Ames, Iowa, USA: 2009. pp. 1247–1272. [Google Scholar]
- 7.Benoit N., Guessan B.B., Dosso K., Gnangoran B.N., Amoateng P., Asiedu-Gyekye I.J. Antibacterial and antispasmodic activities of a dichloromethane fraction of an ethanol extract of stem bark of Pillostigma reticulatum. J Pharm Biol Allied Sci. 2015;7:128–135. doi: 10.4103/0975-7406.154439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann A., Ajiboso O.S.O., Ajeigbe S., Gbate M., Isaiah S. Evaluation of the wound healing activity of ethanol extract of Terminalia avicennioides root bark on two wound models in rat. Int J Med Arom Plants. 2011;1:95–100. [Google Scholar]
- 9.Dalziel J.M. Millbank; London: 1955. The useful plants of west tropical Africa; p. 81. (Crown Agents for Overseas Governments and Administrations). [Google Scholar]
- 10.Salau A.K., Yakubu M.T., Oladiji A.T. Cytotoxic activity of aqueous extracts of Anogeissus leiocarpus and Terminalia avicennioides root barks against Ehrlich ascites carcinoma cells. Ind J Pharm. 2013;83:381–385. doi: 10.4103/0253-7613.115023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerharo J., Adam J.G. Deuxieme inventaire des plantes medicinales et toxiques de la casamace (Senegal) Ann Pharm Fr. 1963;21:853–870. [PubMed] [Google Scholar]
- 12.Abdullahi A.L., Agho M.O., Amos S., Gamaniel K.S., Wambebe C. Antidiarrhoeal activity of the aqueous extract of Terminalia avicennoides roots. Phytother Res. 2001;15:431–434. doi: 10.1002/ptr.860. [DOI] [PubMed] [Google Scholar]
- 13.Mann A. Ayanwola Printing Works; Minna, Niger State, Nigeria: 2007. Survey of ethnomedicine for the treatment of tuberculosis: chemistry perspective; pp. 1–117. [Google Scholar]
- 14.Trease G.E., Evans W.C. Alder Press; Oxford: 1996. Pharmacognosy. p. 213-2. [Google Scholar]
- 15.Lorke D. A new approach to practical acute toxicity testing. Arch Tox. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 16.Vogel H.C., Vogel W.H. Drug discovery and evaluation, pharmacological assays. In: Vogel H.C., Vogel W.H., editors. Methods in clinical pharmacology. Springer; Berlin: 1997. p. 757. [Google Scholar]
- 17.Igboeli N., Onyeto C.A., Okorie A.N. Antidiarrhoeal activity of methanol leaf extract of Lophira lanceolata Tiegh (Ochnaeceae) Mer Res J Environ Sci Tox. 2015;3:59–64. [Google Scholar]
- 18.Hardman J.G., Limberd L.E. Goodman and Gilman's the pharmacological basis of therapeutics. 10th ed. McGraw-Hill; New York: 1992. Drugs affecting gastrointestinal function; pp. 914–931. [Google Scholar]
- 19.Mann A., Yusuf I. Antibacterial activity of methanolic extracts of Terminalia avicennioides against fish pathogenic bacteria. Am J Res Commun. 2014;3:133–147. [Google Scholar]
- 20.Garba S., Salihu L., Shoge M. Antidiarrhoeal activities of some medicinal plants. Med Chem. 2015;S2:001. [Google Scholar]
- 21.Gordienko D.V., Harhun M.I., Kustov M.V., Pucovsky V., Bolton T.B. Sub-plasmalemmal [Ca2+]i upstroke in myocytes of the guinea-pig small intestine evoked by muscarinic stimulation: IP3R-mediated Ca2+ release induced by voltage-gated Ca2+ entry. Cell Calcium. 2008;43:122–141. doi: 10.1016/j.ceca.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- 23.Sanders K.M. Invited review: mechanism of calcium handling in smooth muscles. J Appl Physiol. 2001;91:1438–1449. doi: 10.1152/jappl.2001.91.3.1438. [DOI] [PubMed] [Google Scholar]
- 24.Sanders-Bush E., Mayer S.E. 5-Hydroxytryptamine (serotonin): receptor agonist and antagonist. In: Hardman J.G., Limbird L.E., editors. The pharmacological basis of therapeutics. McGraw-Hill; New York: 2001. pp. 269–290. [Google Scholar]
- 25.Adams H.R. Histamine, serotonin, and their antagonists. In: Riviere J.E., Papich M.G., editors. Veterinary pharmacology and therapeutics. 9th ed. Wiley-Blackwell; Ames Iowa USA: 2009. pp. 411–427. [Google Scholar]
- 26.Bertaccini G., Molina E., Zappia L., Zseli J. Histamine receptors in guinea pig ileum. Naunyn-Schmeideberg's Arch Pharmacol. 1979;309:65–68. doi: 10.1007/BF00498757. [DOI] [PubMed] [Google Scholar]
- 27.Coruzzi G., Poli E., Morini G., Bertaccini G. The histamine H3 receptor. In: Gaginella T.S., Guglietta A., editors. Molecular targets for drug development: GI diseases. Humana Press; New Jersey, USA: 2000. pp. 239–267. [Google Scholar]
- 28.Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. [Google Scholar]
- 29.Loganga O.A., Vercruysse A., Foriers A. Contribution to the ethnobotanical, phytochemical and pharmacology studies of traditionally used medicinal plant in the treatment of dysentery and diarrhoeal in Lomela area, Democratic Republic of Congo (DRC) J Ethnopharmacol. 2000;71 doi: 10.1016/s0378-8741(00)00167-7. 41-23. [DOI] [PubMed] [Google Scholar]
- 30.Vimala R., Nagarajan S., Alam M., Susan T., Joy S. Antiinflammatory and antipyretic activity of Michelia champaca Linn. (White variety), Ixora brachiata Roxb. and Rhynchosia cana (Willd.) D.C. flower. Ind J Exp Biol. 1997;35:1310–1314. [PubMed] [Google Scholar]
- 31.Capasso F., Pinto A., Mascolo N., Autore G., Franco M.P. Effects of flavonoids on PGE2 and LTD4-induced contractions of guinea pig isolated ileum. Pharm Res Commun. 1988;20:201–202. [Google Scholar]
- 32.Hejazian S.H., Bagheri S.M., Dashti-R M.H. Relaxant effect of Humulus lupulus extracts on isotonic rat's ileum contractions. Avian J Phytomed. 2013;4:53–58. [PMC free article] [PubMed] [Google Scholar]
- 33.Khoshnazar S.M., Bahaoddini A., Najafipour H. Effect of alcoholic extract of licorice (Glycyrrhiza glabra) rhizome on isolated duodenum motility in male rats and its interference with cholinergic, nitrergic, and adrenergic systems. Bull Environ Pharm Life Sci. 2013;2:173–177. [Google Scholar]
- 34.Naiz A., Wadood A.S. Antispasmodic activity of Teucrium stocksianum Bioss. Pak J Pharm Sci. 2011;24:171–174. [PubMed] [Google Scholar]
- 35.Cortes A.R., Delgadilo A.J., Hurtado M., Dominguez-Ramizez A.M., Medina J.R., Aoki K. The antispasmodic activity of Buddleja scordiodes and Buddleja perfoliata on isolated intestinal preparations. Biol Pharm Bull. 2006;29:1186–1190. doi: 10.1248/bpb.29.1186. [DOI] [PubMed] [Google Scholar]
- 36.Spruill W.J., Wade W.E. Diarrhoea, constipation and irritable bowel syndrome. In: Matzke G.R., Wells B.G., Possey M.L., editors. Pharmacotherapy: a pathophysiologic approach. McGraw-Hill Medical Publishing Division; New York: 2005. pp. 312–316. [Google Scholar]
- 37.Adzua B., Tarfab F., Gamaniel K.S. The efficacy of Sphaeranthus senegalensis Vaill extract against diarrhoea in rats. J Ethnopharmacol. 2004;95:173–176. doi: 10.1016/j.jep.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 38.Waller D.G., Renwick A.G., Hillier K. 2nd ed. Elsevier Saunders; London: 2005. Medical pharmacology and therapeutics; pp. 417–418. [Google Scholar]
- 39.Rang H.P., Dale M.M., Ritter J.M., Moore P.K. 5th ed. Churchill Livingstone; Edinburgh: 2003. The gastrointestinal tract. pharmacology. p. 376–377. [Google Scholar]
- 40.Offiah V.N., Chikwendu U.A. Antidiarrhoeal effects of Ocimum gratissimum leaf extract in experimental animals. J Ethnopharmacol. 1999;68:327–330. doi: 10.1016/s0378-8741(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 41.Belemtougri R.G., Constantin B., Cognard C. Effects of two medicinal plants, Psidium guajava L. (Myrtaceae) and Diospyros mespiliformis L. (Ebenaceae) leaf extracts on rat skeletal muscle cells in primary culture. J Zhejiang Univ Sci. 2006;7:56–63. doi: 10.1631/jzus.2006.B0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashok P.K., Upadhyay K. Tannins are astringent. J Pharmacogn Phytochem. 2012;1:45–48. [Google Scholar]
- 43.Di Carlo G.D., Mascolo N., Izzo A.A., Capasso F. Effects of quercetin on the gastrointestinal tract in rats and mice. Phytother Res. 1994;8:42–45. [Google Scholar]
- 44.Altman D.F. Drugs used in gastrointestinal diseases. In: Katzung B.G., editor. Basic and clinical pharmacology. 8th ed. McGraw-Hill; San Francisco: 2001. pp. 1070–1071. [Google Scholar]
- 45.Ammon H.V., Thomas P.J., Phillips S.F. Effect of the oleic acid and ricinoleic acid net jejunal water and electrolyte movement. J Clin Invest. 1974;53:374–379. doi: 10.1172/JCI107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zavala M.A., Pérez S., Pérez Z.C., Vergas B., Pérez R.M. Antidiarrhoeal activity of Waltheria americana, Commelina coelestis and Alternanthera repens. J Ethnopharmacol. 1998;61:41–47. doi: 10.1016/s0378-8741(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 47.Niemegeers C.L.E., Lenaerts F.M., Janseen P.A.J. Loperamide (R-18553), a novel type of antidiarrhoeal agent. Part 1: in vitro oral pharmacology and acute toxicity. Comparison with morphine, codeine, diphenoxylate and difenoxine. Arzeimittelforsc. 1974;24:1633–1666. [PubMed] [Google Scholar]
- 48.Tanko Y., Kadiri O.T., Mohammed A., Mahdi M.A., Musa K.Y. Preliminary antidiarrhoeal activity of methanol extract of Cissus cornifolia (bak) planch on experimental animals. Ann Biol Res. 2011;2:229–237. [Google Scholar]
- 49.Nwafor P.A., Okwuasaba F.K., Binda L.G. Antidiarrhoeal and antiulcerogenic effects of methanol extract of Asparagus pubescens root in rats. J Ethnopharmacol. 2002;72:421–427. doi: 10.1016/s0378-8741(00)00261-0. [DOI] [PubMed] [Google Scholar]
- 50.Kubacey T.M., Haggag E.G., El-Toumy S.A., Ahmed A.A., El-Ashmawy I.M., Youns M.M. Biological activity and flavonoids from Centaurea alexanderina leaf extract. J Pharm Res. 2012;5:33–52. [Google Scholar]