Abstract
On-farm hygienic practices are important in assuring quality and safety of milk for consumers and for reducing losses at production and at post-harvest. This study investigated the relationship between milking practices, mastitis as well as milk somatic cell counts (SCC) and the effects of high SCC on milk production and post-harvest losses (PHL) in smallholder dairy (n = 64) and pastoral camel (n = 15) herds in Kenya. The collected data included milking practices, mastitis test on udder quarters (n = 1236) and collection of milk samples for laboratory analyses: SCC, detection of Staphylococcus aureus and Streptococcus species. Production losses were computed as a proportion of cows and herds with SCC (>200,000 cells/mL) and PHL as quantity of milk exceeding 4 × 105 cells/mL. Practices associated with production herds included hands, udder washing and drying, and milk let down stimulation with calves suckling or manually (p < 0.001). Udder drying was only applied in peri-urban herds (100%). Herd level prevalence of mastitis was lower in smallholder than in pastoral herds (60.7% vs 93.3%). Mastitis positive samples had higher prevalence of S.aureus than of Streptococcus species in both smallholder (57.9% vs 23.7%) and pastoral (41.6% vs 36.5%) herds. Moreover, SCC was significantly affected by presence of mastitis and S.aureus (p < 0.001). Milk PHL from high SCC was higher in smallholder rural herds (27%) compared to peri-urban (7%) and in pastoral peri-urban (81%) compared to rangelands (76%). Milking practices may have contributed to maintain mastitis pathogens in herds. This has led to substantial pre and postharvest milk losses in smallholder and pastoral herds. Therefore teat dipping, dry cow period and herd level mastitis treatment may complement current practices for lower SCC and milk PHL.
Keywords: Herd level therapy, Milk handling, Mastitis, Teat dipping, Staphylococcus aureus, Streptococcus species
1. Introduction
Milk consumed in Kenya is from cattle, camels and goats reared in smallholder or pastoral herds [1], [2]. On-farm hygienic practices are important in assuring quality and safety of milk for consumers and for reducing losses at production and at post-harvest. Hygiene practices of importance include cleanliness of animals (udder), milking environment, milking person and milk harvesting and storage containers [3].
Mastitis is a complex disease characterized by inflammation of the mammary glands with heavy economic losses related to medication and veterinary services, microbiological diagnostic and additional management inputs, culling and replacement of the infected animals [4]. Significant reduction in milk yield has been associated with intra-mammary infection [5], [4]. The inflammation severity depends on the causative agent and the host response whose somatic cells play an essential role in immediate defense against local infection [6]. Somatic cells are cells of the immune system and are part of the natural defense mechanisms, including lymphocytes, macrophages, polymorphonuclear and some epithelial cells [7]. Somatic cell count (SCC) provides good indications of infected and uninfected quarters, the former identified by increased SCC as a result of the inflammatory response to the infection [6], [7]. The assessment of udder inflammation has therefore been based on detection of elevation of SCC either in individual quarter milk or in bulk milk from farms [8], [9].
The most common mastitic pathogens identified in cow and camel milk and presenting high risk of pathogenicity to humans are Streptococcus agalactiae and Staphylococcus aureus [3], [10]. S. aureus has toxin producing activity which may cause intoxication while S. agalactiae may cause human infections, particularly in newborn children. S. agalactiae isolates from camels seem more closely related to the human than to the bovine biotype and can survive for up to 7 days in souring camel milk and at pH of 4.5 [3].
This study determined the: relationship between milking practices, intra mammary infections and milk somatic cell counts (SCC) as well as effects of high SCC on milk production and post-harvest losses in a sample of smallholder dairy and pastoral camel herds.
2. Materials and methods
2.1. Sampling procedure
Milk samples were obtained from lactating cows and camels in smallholder dairy and pastoral herds respectively in Nakuru and Isiolo Counties in Kenya. Smallholder dairy herds were categorized into two groups: rural and peri-urban herds. Farmers in Olenguruone Division in the highlands of Nakuru County represented rural herds because of high concentration of dairy farmers practicing free grazing system, long distance (80 km) to Nakuru town center and limited access to diversified market outlets.Peri-urban dairy farmers were sampled in Bahati and Dundori Divisions within the vicinity of Nakuru town center (Fig. 1). This was because of low concentration of dairy farmers, milk production in intensified systems (semi-zero and zero grazing systems), short distance to town center and access to diversified market outlets in Nakuru town. In these two production herds, sampling was done randomly. Pastoral camel herds, however, could only be sampled from those willing to participate in the study and ease of accessing grazing fields where the herds had been moved to. Local Country livestock and veterinary offices aided identification and access to the herds.
Fig. 1.
Study sites.
2.2. Data collection
Milk samples were collected from 32 smallholder rural and peri-urban herds each, and 15 pastoral camel herds. In the pastoral system, two distinct categories of herds were observed: rangeland browsing herds (n = 11 herds) and peri-urban herds feeding on Euphorbia tirucalli (n = 4 herds). Udder quarters (n = 1236) of all milking animals (n = 94 and 222 in smallholder and pastoral herds respectively) were tested for mastitis using California Mastitis Test (CMT) (KENOTEST, Belgium). Individual quarter milk samples were collected when found positive for mastitis; otherwise a composite milk sample of the four quarters was collected in a sterile sampling bottle for further analysis. Milk yield per animal was weighed and information on animals’ characteristics was recorded for each sample herd.
All collected milk samples taken to the laboratory were subjected to direct microscopic somatic cell count in accordance with Sarikaya, [11].
Milk samples were further subjected to microbiological identification of S. aureus and Streptococcus, being the major contagious pathogenic mastitis causing organisms. Milk was diluted in peptone water to 10−1 then streaked using an inoculating loop on Baird Parker agar and KF streptococcal agar (HIMEDIA).
Production (pre-harvest) losses in smallholder herds were estimated as a proportion (percentage) of sample herds with high somatic cell count in milk based on the procedure of Tyler et al. [4] to quantify milk yield losses (Table 1). Milk post-harvest losses (PHL) were estimated as quantity of milk exceeding 4 × 105 cells/mL corresponding to the level of clinical mastitis. However, most of the milk with high SCC reached the market since both milks failing and passing the tests were pooled, collected by transporters and delivered to the targeted market outlet (collection centers or informal outlets). In pastoral camel herds an assumption was made that all milk positive for mastitis was a postharvest loss because of the insufficient good postharvest handling practices to making ‘suusa’ (traditionally fermented camel milk) on the basis of observations of Mwangi [12]. Therefore, the proportion of milk from camels positive for mastitis was used to estimate the PHL.
Table 1.
Production losses estimation in relation to SCC.
| SCC (×1000 cells/mL) | Production loss (%) |
|---|---|
| 0–20 | 00 |
| 21–30 | 0.47 |
| 31–55 | 0.99 |
| 56–90 | 0.05 |
| 91–148 | −0.88 |
| 149–245 | −4.47 |
| 246–403 | −6.78 |
| 404–665 | −7.82 |
| 667–1097 | −7.85 |
| 1098–1808 | −7.12 |
| 1809–2981 | −12.44 |
| > 2981 | −15.22 |
Source: Tyler et al. (1989).
2.3. Statistical analysis
Chi-square test of dependence was used to determine association of milking routine and handling practices with the herds. Logistic regression (PROC LOGISTIC) was used to determine the relative risk of mastitis presence over total cases at quarter, cow/camel and herds type (smallholder vs pastoral camel) level [13]. Herd type was treated as factor with smallholder (peri-urban vs rural) and pastoral camel (rangelands vsperi-urban).
Regression models were run using GENMOD procedure of SAS [14] to assess relationship between practices and log transformed SCC. From this analysis, regression models with P ≤ 0.05 were selected for subsequent analysis using the GLM procedure of SAS [14] to determine differences in SCC between practices in smallholder (rural vsperi-urban) and pastoral (rangelands vs peri-urban). Finally, milks scored according to the SCC threshold of less than 2 × 105; 2 × 105–4 × 105 and above 4 × 105 cells/mL were used to estimate production and post-harvest losses. Milk yield per cow within a herd was related to its somatic cell count score and yield loss correction factor to calculate the cow and herd milk production losses.
3. Results
The herd size was variable, from 2 cows in the smallholder herds to 17 camels in pastoral herds with parities from 2.8 to 3.5. The average number of days in lactation was 230 and 239 days in smallholder peri-urban and rural, respectively, but 12% and 37% of cows were milked beyond 305-days. Pastoral herds had an average of 399 days in milk with 37% of camels milked beyond the 305-days. The herd average milk production per day in smallholder farms was 9.1 kg and 12.3 kg for rural and peri-urban herds respectively, and 26.2 kg/day for camel herds, but cow productivity was highest in the rural herds (6.0 kg/day) and lowest in the pastoral herds (Table 2).
Table 2.
Herd characteristics.
| Variable | Smallholder peri-urban (n = 32) | Smallholder rural (n = 32) | Pastoral (n = 15) |
|---|---|---|---|
| Milking cows (n) | 2.3 ± 1.3 | 2.3 ± 1.4 | 17.1 ± 2.1 |
| Milk production (kg/herd) | 9.1 ± 8.5 | 12.3 ± 11.9 | 26.2 ± 5.0 |
| Milk yield (kg/animal/day) | 4.9 ± 3.6 | 6.0 ± 3.9 | 1.5 ± 1.8 |
| Age of cows (years) | 8.8 ± 8.8 | 5.9 ± 3.8 | 9.5 ± 4.2 |
| Parity of cows (n) | 2.8 ± 1.7 | 3.2 ± 2.1 | 3.5 ± 1.8 |
| Lactation stage (days) | 230.5 ± 157.8 | 239.6 ± 119.9 | 399.1 ± 278.6 |
| Cows with lactation > 305 d (%) | 11.9 | 36.7 | 37 |
| Log10 SCC (cells/ml) | 5.4 ± 0.1 | 5.3 ± 0.1 | 7.4 ± 0.1 |
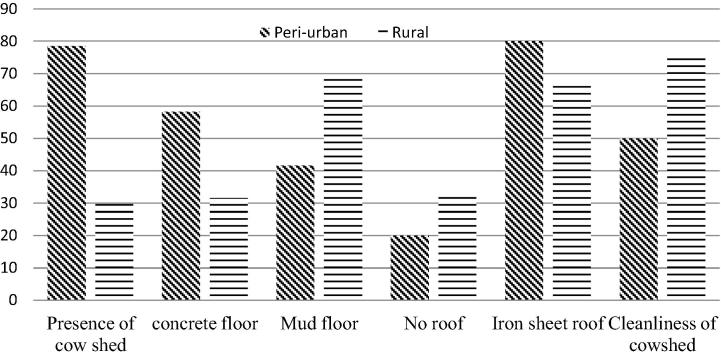
The majority of smallholder farms in the peri-urban had a cow shed with concrete floor (58.3%) and covered with iron sheets (80%). In rural farms, cows were mostly kept in open grazing areas (69.2%) and a few with a cow-shed (29.8%) of which those with majority had mud floors (68.4%) and iron sheet roofing (66.7%). Cleanliness of cow-shed in peri-urban farms was average and generally high in rural farms (Fig. 2).
Fig. 2.
Cowsheds characteristics in smallholder peri-urban and rural farms.
Milking was manual and routinely twice a day (morning and evening) in both smallholder and pastoral herds, but milking in pastoral herds were all in the morning (5:00–6:00 am and 9:00–10:00 am) without a practice of pre milking hygiene of hands and udder washing, unlike in smallholder herds.
In both rural and peri-urban smallholder herds, hand washing was a practice in the pre milking hygiene routine (93.8–100%) and udder washing a practice too (95–98.8%). In contrast, hand and udder washing before milking was not a practice in pastoral herds. Teat drying before milking was a common practice in herds in the peri-urban but not in rural and pastoral herds. Pre-milking palpation to stimulate milk let down by allowing calves to suckle prior to milking (Table 3) was a common practice in herds grazed in the smallholder rural (68.8%) and in pastoral rangelands (93.3%).
Table 3.
Milking routine and milk handling practices (% herds) in the sample herds.
| Variable | Smallholder peri-urban herds (n = 32) | Smallholder rural herds (n = 82) | Pastoral herds (n = 15) | Chi-Sq significance | |
|---|---|---|---|---|---|
| Hand washing | Yes | 100.0 | 93.8 | 0.0 | 67.9*** |
| No | 0.0 | 6.3 | 100.0 | ||
| Udder washing | Use cold water | 0.0 | 12.5 | 0.0 | 73.5*** |
| Use warm water | 100.0 | 81.3 | 0.0 | – | |
| No water used | 0.0 | 6.3 | 100.0 | ||
| Pre-milking palpation | Yes | 100.0 | 100.0 | 100.0 | – |
| No | 0.0 | 0.0 | 0.0 | ||
| Calves suckling prior to milking | Yes | 0.0 | 68.8 | 93.3 | 113.7*** |
| No | 100.0 | 31.3 | 6.7 | ||
| Dry udder prior to milking | Yes | 100.0 | 0.0 | 0.0 | 79.0*** |
| No | 0.0 | 100.0 | 100.0 | ||
| Post-milking treatment | Yes | 0.0 | 0.0 | 0.0 | – |
| No | 100.0 | 100.0 | 100.0 | ||
| Type of milking containers | Aluminum | 0.0 | 31.3 | 0.0 | 16.8*** |
| Plastic | 100.0 | 68.8 | 100.0 | ||
| Bulking container | Aluminum | 50.0 | 62.5 | 0.0 | 21.3*** |
| Plastic | 50.0 | 37.5 | 100.0 | ||
| Sample (n) | 32 | 82 | 15 |
P < 0.0001.
Milking was shorter in pastoral herds than in smallholder herds (5 vs. 10 min). In all the sample herds, post milking hygiene was not a practice and mostly used plastic containers for milking. Pooling of milk in pastoral herds was in plastic containers, while aluminum containers were common (62.5%) in peri-urban. Either of the containers was used in rural farms (50%) (Table 3). Among pastoral camel herds, milk from first and second milking were in some cases mixed in the same container and kept under a shade from where it was later transported using motorbikes to urban collection centers owned by women groups.
Udder quarters tested in smallholder peri-urban herds had a high mastitis prevalence (11.1%) corresponding to 36.2% of cows tested with at least one quarter infected compared to samples from smallholder rural herds where mastitis prevalence was 7.0% of infected quarters corresponding to 23.5% of cows with at least one quarter mastitis infected. Odds of mastitis in cows in smallholder rural herds were 1.68 times lower than in smallholder peri-urban herds. The odds of finding a cow positive for mastitis were 1.18 times higher in pastoral rangeland camel than in peri-urban camels (Table 4).
Table 4.
Prevalence risk of mastitis in smallholder dairy and pastoral herds.
| Herds | Mastitis positive cases* (%) | Mastitis negative cases (%) | Odds ratio | 95% C.I. | P-value |
|---|---|---|---|---|---|
| Udder quarters by herds | |||||
| Smallholder herds | |||||
| Peri-urban (n = 172) | 11.1 | 88.9 | 1.68 | 1.4–2.0 | 0.0001 |
| Rural (n = 204) | 6.9 | 93.1 | Ref | ||
| Total smallholder herds (n = 376) | 8.8 | 91.2 | 1.00 | 1.006–1.007 | 0.0001 |
| Pastoral herds | |||||
| Rangelands (n = 666) | 31.1 | 68.9 | 1.18 | 1.1 – 1.2 | 0.0001 |
| Peri-urban (n = 184) | 34.8 | 65.2 | Ref | ||
| Total pastoral herds (n = 850) | 31.5 | 68.5 | Ref | ||
| Cows camel with ≥ 1 quarter infected | |||||
| Smallholder herds | |||||
| Peri-urban (n = 43) | 32.6 | 67.4 | 1.75 | 1.36–2.28 | 0.0001 |
| Rural (n = 51) | 23.5 | 76.5 | Ref | ||
| Total smallholder herds (n = 94) | 27.7 | 72.3 | 0.65 | 0.58–0.71 | 0.0001 |
| Pastoral herds | |||||
| Rangelands (n = 175) | 34.3 | 65.7 | 1.09 | 0.94–1.26 | 0.2799 |
| Peri-urban (n = 47) | 36.2 | 63.8 | Ref | ||
| Total pastoral herds (n = 222) | 34.7 | 65.3 | Ref | ||
Mastitis positive = CMT ≥ +1; Prevalence risk computed for the odds of finding mastitis positive cases over the total cases (positive + negative).
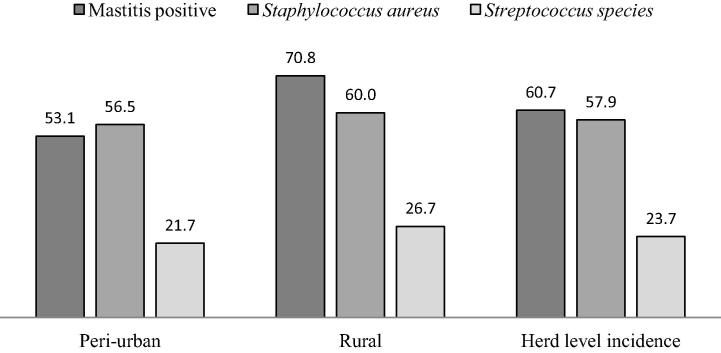
At the herd level, the prevalence of mastitis was higher in rural (70.8%) compared to peri-urban (53.1%) smallholder herds. Fig. 3 illustrates a higher prevalence of mastitis causing pathogens (S. aureus and Streptococcus species) in smallholder rural compared to peri-urban herds. S. aureus was more prevalent in mastitis positive samples from rural (60%) than from Peri-urban (56.5%) herds. Regardless of the smallholder dairy herd, mastitis positive milk samples had lower cases Streptococcus species (23.7%) than S. aureus (57.9%).
Fig. 3.
Prevalence of mastitis, Staphylococcus aureus and Streptococcus species in smallholder dairy herds.
As shown in Fig. 4 the prevalence of mastitis (CMT ≥ +1) in the pastoral herds was 93.3% of the sample herds with at least one camel positive. It was higher in peri-urban herds (100%) than in the rangelands (90.9%). Prevalence of Staphylococcus in mastitis positive milk samples was higher (40.6%) than of Streptococcus species (34.6%). Positive mastitic camel milk samples in rangelands had a prevalence of 40% of Staphylococcus which was lower than peri-urban camels (48.4%). Prevalence of Streptococcus species was higher in milk from rangeland camels (41.5%) than in peri-urban (12.9%).
Fig. 4.
Prevalence of mastitis, Staphylococcus aureus and Streptococcus species in pastoral camel herds.
Results in Table 5 are somatic cell scores according to the threshold. Herd type, presence of mastitis and S. aureus had a significant influence (P < 0.0001) on somatic cell count. Presence of cowshed (P = 0.116) and farm practices did not affect SCC in the herds. Herd effect was also found not significant (P = 0.056). Presence of Streptococcus species did not influence SCC (P = 0.922).
Table 5.
Final model for outcome variable Log10 SCC, parameter β, standard error and probability for characteristics and practices.
| Variable | β | S.E. (β) | P |
|---|---|---|---|
| Intercept | 1.758 | 0.030 | 0.0001*** |
| Herd (pastoral vs smallholder) | 0.324 | 0.056 | 0.0001*** |
| Hand washing (No vs Yes) | −0.006 | 0.051 | 0.901NS |
| Dry udder (No vs yes) | −0.001 | 0.029 | 0.959NS |
| Milking container (aluminum vs plastic) | −0.014 | 0.043 | 0.740NS |
| Pooling container (aluminum vs plastic) | −0.007 | 0.025 | 0.762NS |
| Calves suckling before milking (No vs yes) | −0.008 | 0.022 | 0.721NS |
| Presence of cowshed (No vs yes) | 0.005 | 0.026 | 0.840NS |
| Lactation stage (early/mid vs late) | 0.012 | 0.025 | 0.642NS |
| Parity (1 to 2 vs 3 and above) | −0.017 | 0.047 | 0.708NS |
| CMT test (Positive vs Negative) | −0.065 | 0.012 | <0.0001*** |
| S. aureus presence (Positive vs Negative) | −0.021 | 0.011 | 0.045** |
| Strep spp presence (Positive vs Negative) | −0.025 | 0.014 | 0.076NS |
P ≤ 0.01.
P ≤ 0.05.
P > 0.05.
There was no significant difference in log10 SCC of cows’ milk between smallholder rural and peri-urban herds. The difference was significant between infected and uninfected quarters tested with 4.9 vs 5.8 cells/mL for mastitis negative and positive respectively in peri-urban herds and 5.0 vs 5.6 cells/mL in rural herds. Camels’ positive for mastitis in rangelands herds had higher log10 SCC (7.5 cells/mL) than those found negative (7.2 cells/mL). In peri-urban herds there was no difference in SCC between camels’ positive and negative for mastitis (Table 6).
Table 6.
Effects of animal characteristics on SCC in smallholder dairy and pastoral herds.
| Variable | Smallholder herds |
Pastoral herds |
||
|---|---|---|---|---|
| Peri-urban | Rural | Peri-urban | Rangeland | |
| Herd group | 5.4 ± 0.1 | 5.3 ± 0.1 | 7.4 ± 0.0 | 7.4 ± 0.1 |
| Lactation stage | ||||
| Early/mid | 5.3 ± 0.2 | 5.2 ± 0.1 | 7.4 ± 0.1 | 7.5 ± 0.1 |
| Late | 5.1 ± 0.1 | 5.5 ± 0.2 | 7.3 ± 0.4 | 7.5 ± 0.1 |
| Udder inflammation | ||||
| Mastitis negative | 4.9 ± 0.1a | 5.0 ± 0.1a | 7.4 ± 0.2 | 7.2 ± 0.1a |
| Mastitis positive | 5.8 ± 0.1b | 5.6 ± 0.1b | 7.4 ± 0.2 | 7.5 ± 0.0b |
| Presence of S. aureus | ||||
| Absent | 5.1 ± 0.1a | 5.2 ± 0.1a | 7.3 ± 0.3 | 7.7 ± 0.1 |
| Present | 5.6 ± 0.1b | 5.7 ± 0.1b | 7.2 ± 0.4 | 7.5 ± 0.1 |
Within each production herd, means followed by different letters for one variable are different at 5%.
For smallholder herds, estimated milk production losses within the threshold of 200 × 103–400 × 103 cells/mL are higher in rural (11.4%/cow/day) than in peri-urban (10.6%/cow/day). In rural and peri urban farms respectively, this loss corresponds to 145.8 kg and 273.9 kg of milk yield losses per cow during a 305 d lactation period. Cows with milk SCC > 400 × 103 had an estimated production loss of 10.6% and 11.6%/day in rural and peri-urban herds respectively (Table 7).
Table 7.
Estimated production losses (%) in smallholder dairy herds.
| Herds | SCC threshold (1000 cells/mL) | Herds within the threshold (%) | Average milk yield (cow/day) | Production losses (%) |
|---|---|---|---|---|
| Rural (n = 32) | <200 | 36.4 | 5.7 | 1.8 |
| 200–400 | 22.7 | 4.4 | 11.4 | |
| >400 | 40.9 | 8.5 | 10.6 | |
| Peri urban (n = 32) | <200 | 41.2 | 6.0 | 1.7 |
| 200–400 | 23.5 | 8.5 | 10.6 | |
| >400 | 35.3 | 4.3 | 11.6 | |
Cows’ milk samples with SCC scoring > 400 × 103 cells/mL were considered a postharvest loss. Results of milk PHL are presented in Table 8. They show overall milk PHL of 18.96% of the milk produced per day. Rural herds were found with higher daily milk PHL (27.3%) than peri-urban herds (7.4%).
Table 8.
Estimated milk PHL based on SCC levels in smallholder and pastoral herds.
| Smallholder herds | N | SCC ≥ 4 × 105 (%) | Milk PHL (kg/day) | Total milk (kg/day/herd) | Milk PHL (%) |
|---|---|---|---|---|---|
| Rural | 32 | 21.6 | 43.5 | 159.5 | 27.3 |
| Peri-urban | 32 | 35.3 | 8.5 | 114.7 | 7.4 |
| All smallholder herds | 64 | 27.0 | 52.0 | 274.2 | 19.0 |
| Pastoral herds | |||||
| Pastoral rangelands | 185 | 75.7 | 210.3 | 277.7 | 75.7 |
| Peri-urban | 53 | 80.6 | 121.8 | 151.1 | 80.6 |
| All pastoral herds | 222 | 58.8 | 311.6 | 529.9 | 58.8 |
Estimation of PHL in the pastoral camel system is based on mastitis, all sample camel milk had SCC beyond 400,000 cells/ml pathogenic microorganisms causing mastitis.
4. Discussion
In smallholder herds, higher mastitis risk (O.R. = 1.68 at quarter and 1.75 at cow level) of peri-urban herds than in rural was reflected in slightly higher log10 SCC (5.4 vs 5.3 cells/mL). Constraints of maintaining hygiene of zero grazing units may partly explain the difference since overall cleanliness of cow-shed in smallholder peri-urban (78.6%) was average. Indeed Barnouin et al. [15] and Chassagne et al. [16] have highlighted the importance of clean cow housing for higher milk quality. However the overall SCC levels in both herds were high probably because of inadequate hygienic milking practices which may have contributed to the mastitis prevalence of 71% in rural and 53% peri-urban.
Although most farmers practiced hand washing and udder washing with warm water prior to milking, there was no pre or post milking teat dipping. The use of warm water (55–60 °C) and drying udder with individual towels prior to milking has been demonstrated to reduce microbial loads in milk [17]. However, drying of teats prior to milking was only practiced in Peri-urban herds and individual towels for drying teats were only used in farms with one milking cow. Also, no farmer used cleaning agent for udder washing, yet it has proven more efficient in reducing microbial loads on teat surface than warm water only [18]. Though in free grazing systems such as in the majority of rural farms, use of cleaning agents for udder washing may not be economical in dry seasons when the cows’ udders are relatively clean and dry. They are of more importance in rainy seasons in rural herds and throughout the year in zero grazed herds, such as peri-urban herds where teats are heavily soiled (mud floors in 40% of peri-urban farms’ cowsheds) and risk of mastitis is high [19].
There was no significant difference in SCC in milk from pastoral rangelands and peri-urban herds and the level of SCC in camels was high (7.4 cells/mL) in both of these pastoral herds. Hygienic milking practices may have contributed to the increase of SCC in camel milk because pastoralists did not apply any hygienic practice due to lack of water.
S. aureus and Streptococcus species had a high prevalence of 40.6% vs 34.6% in pastoral and 57.9% vs 23.7% in smallholder herds, confirming findings from Matofari et al. [10] and Younan, [3]. They reported relationships between occurrences of mastitis pathogen hygienic milking practices. The spread of mastitis pathogens in camel herds might even be higher because only one of the 15 sample herds did not have a case of mastitis. Since unidentified infected animals constitute the reservoir for S. aureus and Streptococcus species, transmission may be done from an animal to the other during milking through the milker’s hands [6]. Additionally, inefficient personnel hygiene and udder cleaning in pastoral herds, non-application of udder drying with individual towels in smallholder rural and unclean cow-sheds in peri-urban herds may have contributed to spreading and maintaining the pathogens in the herds. Practices such as teat dipping and herd level therapy can reduce infections [20].
A relatively high proportion of camels in pastoral (37%) and cows in rural (36.7%) herds were milked continuously without a dry off period. The dry off period is important because it contributes to cell turnover in the mammary gland and optimization of milk production in the next lactation [21], [22], [23]. It is also used to eliminate existing and preventing new intra-mammary infections with appropriate antibiotic treatment at the end of lactation [24]. However, chronic infection of S. aureus can be lingering lifelong and success in curing chronic Streptococcus species infection is low [20].
Public health concerns may be raised if the milk is not properly handled along the marketing chain. Also, improper handling with heat treatment may not limit the action of spoilage and pathogenic microorganisms in milk [25], [26]. Risks are higher with milk from pastoral camel herds because a reasonable amount is fermented into ‘suusa’ along the marketing channel without boiling [12].
Herds in smallholder dairy farms and pastoral herds had low daily milk production of 5–6 kg and 1.5 kg respectively. Feeding practices [27] and hygienic situation of these herds may have contributed to this low production and postharvest losses may further reduce. High somatic cell counts, an indicator of milk quality, have been reported to affect milk yield in dairy cows [4]. In smallholder herds, high SCC contributed substantially to milk yield losses of 145.8 kg and 273.9 kg per cow in 305 d lactation in rural and peri-urban respectively. Milk yield losses constituted therefore a critical loss to farmers whose low average daily milk yield already limits bargaining power and the ability to reach better outlets for their milk [1], [28]. Daily milk PHL was estimated at 19.0% and 58.8% in smallholder and pastoral herds respectively. These losses in smallholder were higher than previously reported levels of 6% at the farm level [1], [29]. While previous researchers reported losses based on survey data, the current research used actual production measurements, farm tests and laboratory analyses to estimate postharvest losses which may explain the difference.
5. Conclusions
Somatic cell counts were not affected by milking practices, but by intra-mammary infections. Milking practices such as hand washing, udder washing and udder drying were strongly associated with production herds and may have contributed to the high mastitis prevalence in herds. There was high prevalence of S.aureus and Streptococcus species in smallholder and pastoral herds. Since these pathogens are hardly eradicated from the herds, pre and post milking teat dipping, using of cleaning agents to wash the udder, keeping cowsheds cleaner and a dry off cow period (35–60 days) were recommended. Milk postharvest losses were high in both smallholder and pastoral herds.
Acknowledgement
Authors wish to thank the German Federal Ministry of Education and Research (BMBF) through RELOAD project for funding this research, Egerton University through Departments of Animal Sciences and Dairy, Food Science and Technology for availing facilities to conduct laboratory analyses and extension staff and farmers’ group for cooperation in data collection.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Muriuki H.G., Thorpe W. Smallholder dairy production and marketing in eastern and southern Africa: regional synthesis. In: FAO, editor. Milk and dairy products, post-harvest losses and food safety in sub-Saharan Africa and the near east. ILRI (International Livestock Research Institute); 2003. [Google Scholar]
- 2.Noor I.M., Guliye A., Tariq M., Bebe B. Assessment of camel and camel milk marketing practices in an emerging peri-urban production system in Isiolo County, Kenya. Pastoralism. 2013;3:1–8. [Google Scholar]
- 3.Younan M. Milk hygiene and udder health. In: Farah Z., Fischer A., editors. Milk and meat from the camel. Handbook on products and processing. 2004. pp. 67–76. [Google Scholar]
- 4.Tyler J.W., Thurmond M.C., Lasslo L. Relationship between test-day measures of somatic cell count and milk production in California dairy cows. Can J Vet Res. 1989;53:182–187. [PMC free article] [PubMed] [Google Scholar]
- 5.Hortet P., Seegers H. Calculated milk production losses associated with elevated somatic cell counts in dairy cows: review and critical discussion. Vet Res. 1998;29:497–510. [PubMed] [Google Scholar]
- 6.Carrillo-Casas E.M., Miranda-Morales R.E. Milk production – an up-to-date overview of animal nutrition, management and health. InTech; 2012. Bovine mastitis pathogens: prevalence and effects on somatic cell count. ISBN: 978-953-51-0765-1. [Google Scholar]
- 7.Ruegg P.L., Pantoja J.C.F. Understanding and using somatic cell counts to improve milk quality. Irish J Agric Food Res. 2013;52:101–117. [Google Scholar]
- 8.Cervinkova D., Vlkova H., Borodacova I., Makovcova J., Babak V., Lorencova A., Vrtkova I., Marosevic D., Jaglic Z. Prevalence of mastitis pathogens in milk from clinically healthy cows. Vet Med. 2013;58:567–575. [Google Scholar]
- 9.Pantoja J.C.F., Reinemann D.J., Ruegg P.L. Associations among milk quality indicators in raw bulk milk. J Dairy Sci. 2009;92:4978–4987. doi: 10.3168/jds.2009-2329. [DOI] [PubMed] [Google Scholar]
- 10.Matofari J.W., Younan M., Nanua N., Mwatha E.W., Okemo P.O. Microorganisms associated with sub-Clinical mastitis and their impact on milk production in camels (CamelusDromedarius) in semi-arid lands of Northern Kenya. Int J Agric Rural Dev. 2005;6:182–187. [Google Scholar]
- 11.Sarikaya H., Schlamberger G., Meyer H.H.D., Bruckmaier R.M. Leukocyte populations and mRNA expression of inflammatory factors in quarter milk fractions at different somatic cell score levels in dairy cows. J Dairy Sci. 2006;89:2479–2486. doi: 10.3168/jds.S0022-0302(06)72322-0. [DOI] [PubMed] [Google Scholar]
- 12.Mwangi L.W. Egerton University; Kenya: 2015. Safety and nutritional quality of pastoral traditional fermented camel milk (suusa) (Master thesis) [Google Scholar]
- 13.Diaz-Quijano A simple method for estimating relative risk using logistic regression. BMC Med Res Method. 2012;12:1471–2288. doi: 10.1186/1471-2288-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SAS Institute Inc . SAS Institute Inc; Cary, NC: 2008. SAS® Component Language 9.2: Reference. [Google Scholar]
- 15.Barnouin J., Bord S., Bazin S., Chassagne M. Dairy management practices associated with incidence rate of clinical mastitis in low somatic cell score herds in France. J Dairy Sci. 2005;88:3700–3709. doi: 10.3168/jds.S0022-0302(05)73056-3. [DOI] [PubMed] [Google Scholar]
- 16.Chassagne M., Barnouin J., Le Guenic M. Expert assessment study of milking and hygiene practices characterizing very low somatic cell score herds in France. J Dairy Sci. 2005;88:1909–1916. doi: 10.3168/jds.S0022-0302(05)72867-8. [DOI] [PubMed] [Google Scholar]
- 17.AL-Hubaety A.K., Awad R.H., Thanon R.I., Ez-Aldin F.A. Udder preparation and its effect on udder cleanliness and milk quality. AL-Qadisiya J Vet Med Sci. 2013;2(1):128–138. [Google Scholar]
- 18.Gleeson D., O'Brien B., Flynn J., O' Callaghan E., Galli F. Effect of pre-milking teat preparation procedures on the microbial count on teats prior to cluster application. Irish Vet J. 2009;62:461–467. doi: 10.1186/2046-0481-62-7-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton J.M., Penry J., Malmo J., Mein G.A. Pre-milking teat disinfection: Is it worthwhile in pasture-grazed dairy herds. J Dairy Sci. 2014;97:7525–7537. doi: 10.3168/jds.2014-8185. [DOI] [PubMed] [Google Scholar]
- 20.Keefe G.P. Streptococcus agalactiae mastitis: a review. Can Vet J. 1997;38:429. [PMC free article] [PubMed] [Google Scholar]
- 21.Hou X., Lin L., Xing W., Yang Y., Duan X., Li Q., Lin Y. Spleen tyrosine kinase regulates mammary epithelial cell proliferation in mammary glands of dairy cows. J Dairy Sci. 2016;99:3858–3868. doi: 10.3168/jds.2015-10118. [DOI] [PubMed] [Google Scholar]
- 22.Steeneveld W., Schukken Y.H., Van Knegsel T.M., Hogoveen H. Effects of different dry period lengths on milk production and somatic cell count in subsequent lactations in commercial Dutch dairy herds. J Dairy Sci. 2013;96:2988–3001. doi: 10.3168/jds.2012-6297. [DOI] [PubMed] [Google Scholar]
- 23.Pezeshki A., Mehrzad J., Ghorbani G.R., De Spiegeleer B., Collier R.J., Burvenich C. The effect of dry period length reduction to 28 days on the performance of multiparous dairy cows in the subsequent lactation. Can J Anim Sci. 2008;88:449–456. [Google Scholar]
- 24.Leelahapongsathon K., Piroon T., Chaisri W., Suriyasathaporn W. Factors in dry period associated with intra-mammary infection and subsequent clinical mastitis in early postpartum cows. Asian-Australas J Anim Sci. 2016;29:580–585. doi: 10.5713/ajas.15.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebelein T. University of Munchen; 2010. The effect of heat treatment on microbiological qualities of bovine colostrum, passive immune transfer of neonatal calves, and future animal performance. (Thesis) [Google Scholar]
- 26.Hassan N.B.A., Abdalla M.O.M., Nour A.A.A.M. Microbiological quality of heat-treated milk during storage. Pakistan J Nutr. 2009;8:1845–1848. [Google Scholar]
- 27.Njarui D.M.G., Gatheru M., Wambua J.M., Nguluu S.N., Mwangi D.M., Keya G.A. Feeding management for dairy cattle in smallholder farming systems of semi-arid tropical Kenya. LRRD. 2011;23:111. [Google Scholar]
- 28.Bebe B.O. Herd dynamics of Smallholder dairy in the Kenya highlands. In: FAO, editor. Milk and dairy products, post-harvest losses and food safety in sub-Saharan Africa and the near east. 2003. PhD thesis, Wageningen University, The Netherlands. ISBN: 90-5808-788-3. [Google Scholar]
- 29.Lore T., Omore A., Staal S. ILRI (International Livestock Research Institute); Nairobi, Kenya: 2005. Types, levels and causes of post-harvest milk and dairy losses in sub-Saharan Africa and the Near East: Phase two synthesis report; pp. 7–10. [Google Scholar]