Abstract
The factors enhancing mucocoele development in dogs remain poorly understood. A 7-year-old female spayed Miniature Schnauzer was presented to the Massey University Veterinary Teaching Hospital for progressive lethargy, inappetance and abdominal discomfort. Initial physical examination findings revealed a moderate degree of cranial abdominal pain, with subsequent diagnostic tests confirming the patient as having diabetes mellitus, with a concurrent marked hypertriglyceridaemia. In an attempt to localise the source of pain, an ultrasound examination of the abdomen was performed, revealing a marked degree of gallbladder sludge. With appropriate medical management including ursodeoxycholic acid and insulin therapy, the patient stabilised and was discharged. With persistence of clinical signs three months later, progression of the gallbladder sludge towards mucocoele development was suspected. Exploratory laparotomy was instigated, and an emergency cholecystectomy was performed. This case report therefore entails a suspected gallbladder mucocoele that developed in a diabetic patient with previously diagnosed biliary sludge. A unique feature of this case report is the presence of diabetes mellitus, which has been suggested to be a causative factor in the development of gallbladder mucocoeles. It is also hypothesised that gallbladder sludge and mucocoeles are associated, however it is yet to be ascertained whether this association is causal or contributory. The authors examined the possible relationship between this endocrinopathy and biliary sludge, and their possible effects on mucocoele development. Specific associated factors to sludge formation are also examined. The medical and surgical management of gallbladder mucocoeles is discussed.
Keywords: Gallbladder mucocoele, Biliary sludge, Hypertriglyceridaemia, Diabetes mellitus
1. Introduction
Biliary sludge has been defined as a mixture of precipitated crystals, glycoproteins, cellular debris and mucin [1]. Although its composition varies, cholesterol monohydrate crystals, calcium bilirubinate and other calcium salts are the main constituents [2]. Gallbladder sludge has often been considered an incidental finding by radiologists [3], and can occur secondarily to stagnant biliary flow and gallbladder dysmotility. During such cases of stasis, water reabsorption thickens the bile, predisposing to obstruction. The factors enhancing mucocoele development in dogs remain poorly understood. Some authors have previously attributed obstruction of the common bile or cystic duct to be an inciting factor [4], [5]. It is the build-up of intraluminal mucous and/or inspissated bile within the gallbladder, in conjunction with the presence of pathognomonic histopathological findings of cystic mucinous hyperplasia, that characterises it as being a mucocoele [6].
This case report describes a patient with gallbladder sludge suggesting the presence of gallbladder dysmotility and/or a change in the biliary composition. The exact pathogenesis of gallbladder mucocoele formation has not been formally identified, and instead only hypothesised. It is however associated with cystic mucinous hyperplasia and gallbladder dysmotility suggesting a possible association with biliary sludge which arises via a similar dysfunction of gallbladder motility. The association between sludge and mucocoele formation has been minimally described in the literature, and this case report outlines initial information into the possibility that sludge may contribute towards progression to mucocoele formation, however further studies would be necessary to clarify this linkage. The specific underlying associated factors of this case are described, and medical and surgical management is discussed.
2. Case presentation
A 7-year-old female spayed Miniature Schnauzer was presented to the referring veterinarian following development of polydipsia and polyuria after a protracted disease course of lethargy noticed for roughly one month’s duration. As part of an initial database, a urinalysis was performed and revealed a marked glucosuria, and consequently, a fasting serum glucose was assessed. This measured 28.2 mmol/L (reference range (RR) 3.9–6.1 mmol/L) and was significantly elevated. Stress-associated hyperglycaemia was considered, along with diabetes mellitus, with the latter diagnosis subsequently confirmed by examination of serum fructosamine, which showed marked elevation (690 μmol/L; RR 225–365 μmol/L). A day after diagnosis, the owners noted significant abdominal discomfort, and the patient was referred to the Massey University Veterinary Teaching Hospital with concerns of acute pancreatitis owing to this abdominal discomfort and recent diabetes mellitus diagnosis. However, no gastrointestinal signs of vomiting or anorexia were noted.
Physical examination findings included cranial abdominal pain characterised by groaning and shaking during palpation. The patient was of a body condition score of 6/9, and weighed 10 kg. She appeared considerably lethargic and was correspondingly hospitalised. A complete blood count (CBC), biochemistry profile including serum triglycerides, and urinalysis were undertaken. Significant abnormalities included a moderate increase in serum glucose (26.4 mmol/L; RR 3.9–6.1 mmol/L), a moderate increase in alkaline phosphatase (688 IU/L; RR 0–185 IU/L) and alanine aminotransferase (137 IU/L; RR 0–75 IU/L). A marked hypercholesterolaemia was also detected (28.0 mmol/L; RR 3.0–9.0 mmol/L) in conjunction with a marked hypertriglyceridaemia (40.74 mmol/L; RR 0.6–1.20 mmol/L). Also of note was a mild hyponatraemia (132 mmol/L; RR 139–153 mmol/L) and a moderate hypochloraemia (92 mmol/L; RR 105–121 mmol/L). CBC examination revealed a mild mature neutrophilia (17.4 × 109/L; RR 6.0–17.0 × 109/L) and a mild non-regenerative anaemia (0.34L/L; RR 0.37–0.55L/L). Urinalysis was unremarkable other than the presence of a marked glucosuria, and the occasional lipid droplet visualised on sediment examination.
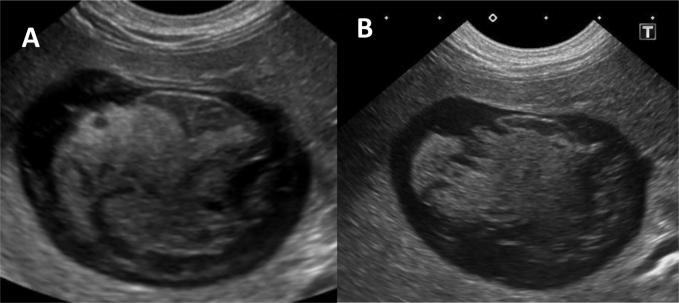
A radiographic study of the abdomen was requested, with ventrodorsal and lateral views obtained. The liver was moderately enlarged, having blunted borders with protrusion beyond the costal arches. The rest of the abdominal viscera were unremarkable, except for the presence of 5–10 variably sized mineral-opaque calculi in the urinary bladder. Ultrasound examination of the abdomen revealed a moderately distended gallbladder (Fig. 1) with heterogeneous hyperechoic bile settling along the dependent wall. The wall was irregularly and inconsistently thickened, with randomly located swellings suspected to be mucosal nodules. Also, a focal area of homogeneous hyperechoic material adhered to the upper aspect of the lumen was detected. This was suspected to be mucous. There was no evidence of bile duct obstruction or occlusion.
Fig. 1.
Ultrasonographic study of the gallbladder. A: Transverse view displaying gallbladder distension with irregular appearance of the wall. Measurements taken reveal a wall thickness of 1.3 mm at point A and 4.3 mm at point B. B: Transverse view with suspected adhered, hyperechoic mucous plug (1). C: Sagittal section displaying heterogeneous, hyperechoic bile (2). D: Highlighted view of heterogeneous bile.
The irregularity of the gallbladder wall was attributed to biliary mucosal hyperplasia, and with the inspissated bile, is suggestive of biliary sludge formation. Percutaneous ultrasound-assisted cholecystocentesis was performed however difficulty was encountered during aspiration owing to the thickness of the gallbladder contents. The patient was placed in dorsal recumbency and the abdominal skin along the ventral midline was aseptically prepared. A 22 gauge needle attached to a 5 mL syringe was guided into the gallbladder using the US transducer to locate and subsequently guide the needle into the correct location. The patient was rather lethargic and no sedation or anaesthesia was necessary. The aspirated contents were smeared for cytological examination and submitted for biliary culture.
Cytological examination of obtained samples revealed an acellular substance, with thick, proteinaceous and mucinous blebs. Culture of the aspirated samples revealed scant growth of Bacillus spp., with the presence of this bacterium suggestive of sample contamination due to the presence of minimal growth of this organism following two-day culture. The absence of any microbial organisms visualised on cytological examination of Romanowsky stained specimens also provided support of this likely conclusion. However it must be noted that healthy dogs also periodically harbour bacteria within the gallbladder, and this is often not clinically significant [7].
A thyroid panel was also performed due to the presence of the protracted period of lethargy and concurrent high-body condition score, as well as markedly elevated serum cholesterol concentrations, which could also be elevated for a number of reasons, including primary hyperlipidaemia in Miniature Schnauzers, or a result of diabetes mellitus. A serum TT4 (total T4) was assessed and was markedly low (2 nmol/L; RR 20–66 nmol/L) which was initially suggestive of concurrent hypothyroidism. A TSH assay was subsequently performed, and measured 0.03 ng/mL (RR 0.03–0.6 ng/mL), which was within normal limits. The marked reduction in TT4 was therefore attributed to euthyroid sick syndrome as a result of the ongoing disease process. Included in the thyroid panel was a measurement of basal serum cortisol levels, which were noted to be at the low end of the reference range (24 nmol/L; RR 20–230 nmol/L). Hyperadrenocorticism was unlikely in this case, owing to the normal basal cortisol levels; however if poor control of diabetes was suspected then further investigation of adrenal function was to be performed. The absence of any abnormalities with regards to the adrenal glands on abdominal ultrasound also yielded favour against the presence of hyperadrenocorticism, which should always be considered in cases of possible mucocoele formation with previous studies indicating a significant association between this endocrinopathy and this gallbladder condition [8].
A significant presenting finding was the presence of acute cranial abdominal pain, which is often attributable to acute pancreatitis. It is well known that the presence of an inflamed pancreas can disturb biliary flow through the common bile duct, and therefore contribute to biliary stasis and possible mucocoele development. Examination of serum levels of pancreatic enzymes revealed no elevations of serum lipase (151 IU/L; RR 13–200 IU/L) or amylase (450 IU/L; RR 30–1020 IU/L). Ultrasonographic examination of the pancreas revealed no areas of hypoechogenicity of the parenchyma, and the surrounding mesentery was homogenous and lacked hyperechogenicity, suggesting no evidence of any concurrent pancreatitis. As a result, a canine pancreatic-specific lipase (cPL) assessment was not indicated. The abdominal pain in this case was therefore suspected to be visceral in nature as a result of the direct effects of distension of the gallbladder and the stimulation of peritoneal stretch receptors.
The results of the above investigations allowed the presence of gallbladder sludge to be identified, suggesting dysmotility and abnormal gallbladder function. In conjunction with the presence of mucous in the gallbladder, this was suggestive of early signs of mucocoele development. The hepatomegaly detected was consistent with endocrine, metabolic and inflammatory causes, all of which were suspected to be occurring simultaneously. Due to the recent diabetes mellitus diagnosis and marked hypertriglyceridaemia, initial therapy was aimed at these endocrine and metabolic concerns. The patient was placed on a low fat diet (Gastro/Low-fat; Royal Canin, Missouri, USA) in an attempt to alleviate the hypertriglyceridaemia, as well as allowing appropriate titration of the newly prescribed insulin therapy (Caninsulin; MSD Animal Health, Upper Hutt, NZ). An initial dose of 5 IU was prescribed, to be administered subcutaneously twenty minutes following a meal, BID (twice-daily). Serial blood glucose measurements were performed, with serum glucose reading 28.5 mmol/L at four hours, and 22.6 mmol/L at six hours (RR 3.9–6.1 mmol/L) post administration. Medical management of the biliary sludge was instigated through the use of the choleretic agent ursodeoxycholic acid (Ursosan; ABM Pharma Ltd, Auckland, NZ). An initial dose of 125 mg PO SID (once-daily) was prescribed. The patient was monitored closely, during which time significant improvement occurred, and was discharged three days following admission. Monthly check-ups were advised, to monitor progression of current therapy, and allow for repeat abdominal ultrasonography.
Three months following initial discharge, the patient suffered vomiting episodes, with tremors and lethargy necessitating an emergency revisit. Once more, cranial abdominal pain was detected during palpation, with moderate dehydration (∼5%) was also noted. Repeat haematological examination was requested, with a CBC and serum biochemistry performed. The previous abnormalities detected continued to be present, with a significant increase in the levels of serum alkaline phosphatase (1604 IU/L) and alanine aminotransferase (466 IU/L). This suggested the progression of severe cholestasis. The patient’s serum triglyceride levels had reduced over the prior course of medical therapy, but were still moderately elevated at 14.27 mmol/L (RR 0.6–1.2 mmol/L). Repeat abdominal ultrasound revealed progression of biliary sludge towards gallbladder mucocoele formation (Fig. 2). With the absence of any other significant findings, the abdominal pain was attributed to the gallbladder disease, and an exploratory laparotomy was requested.
Fig. 2.
Progression of gallbladder disease on the repeated abdominal ultrasound. A: Transverse view one month following the first scan. B: Further progression towards mucocoele development three months following initial presentation.
Stabilisation was achieved through correcting dehydration after placing a 20 g catheter into the right cephalic vein, with compound sodium lactate administered intravenously. The patient was premedicated with methadone (0.5 mg/kg IV), before induction achieved with alfaxalone (2 mg/kg IV). Cefazolin (22 mg/kg IV) was used as an intra-operative antibiotic, with isoflurane maintaining anaesthesia as administered via a rebreathing-circle circuit.
Exploratory laparotomy revealed a markedly distended gallbladder (Fig. 3) and a cholecystectomy was performed. An initial incision through the visceral peritoneum using Metzenbaum scissors was made. Traction was applied to the gallbladder as blunt dissection was undertaken to free it from surrounding hepatic parenchyma. The cystic duct and artery were identified, and were clamped and double-ligated using circumferential ligatures of 3/0 non-absorbable suture (Ethilon; Ethicon, New Jersey, USA), before being incised distally to allow for gallbladder removal. The excised tissue was submitted for histological examination, along with duodenal and hepatic biopsies.
Fig. 3.
Intraoperative view of the gallbladder during exploratory laparotomy, prior to cholecystectomy.
Postoperatively, the patient was maintained on a remifentanil constant rate infusion, before being transitioned to methadone for the provision of analgesia. As pain scores reduced, opioid analgesia was withdrawn in favour of the provision of meloxicam, a non-steroidal anti-inflammatory drug (Metacam; Boehringer Ingelheim, Ingelheim, Germany). Antibiotic therapy was also instigated, with clavulanic acid/amoxicillin given PO at a dose rate of 12.5 mg/kg BID (Clavulox; Zoetis, Auckland, NZ). Previous therapy of ursodeoxycholic acid and insulin was continued, with a marked clinical improvement seen following surgery. The patient was discharged three days later.
Following discharge, the patient was reported to be doing well. Previous abdominal discomfort had resolved, and diabetes was controlled clinically at higher doses of insulin (8IU BID), with the absence of polyuria, polydipsia and polyphagia. Repeated hepatic enzyme assays were requested in the following weeks, to assess for necessity to continue ursodeoxycholic acid therapy.
Histological examination of duodenal biopsies revealed no significant abnormalities. Examination of the hepatic parenchyma revealed diffuse, severe biliary hyperplasia, with infiltration by small to moderate numbers of neutrophils, lymphocytes and plasma cells. The gallbladder propria submucosa contained multifocal collections of lymphocytes, with occasional plasma cells. These findings reflected chronic extra-hepatic functional cholestasis, likely as a result of elevated pressure in the cystic and common bile ducts due to sludge and mucous present within the gallbladder. Pathognomonic histological findings include cystic mucinous hyperplasia, but this was absent in this case. Instead, gross and ultrasonographic findings were suggestive of mucocoele formation however they were not a classical presentation due to the absence of mucosal hyperplasia. In conclusion, this differential remained the most likely cause of the presenting complaint in this case due to the absence of any other significant abnormalities.
3. Discussion
A unique feature of this case is the presence of concurrent diabetes mellitus with gallbladder mucocoele development. Diabetes mellitus is an endocrinopathy that has been previously suggested to be a cause of gallbladder mucocoeles in dogs [8], [9]. In the retrospective case-control study by Mesich et al. [8], no significant association was noted between the presence of concurrent diabetes mellitus and gallbladder mucocoeles. In this case report, however, the presence of diabetes mellitus is thought to be a rather significant finding, and it is hypothesised that this endocrinopathy may directly induce gallbladder mucocoele development, or has an indirect effect through inducing hyperlipidaemia and gallbladder dysmotility. In order to ascertain the possibility of diabetes mellitus predisposing to mucocoele formation, further investigation is warranted to ascertain whether the metabolic and functional effects on the gallbladder and biliary composition induced by this endocrinopathy are significant enough to result in mucocoele development. This case report therefore provides an initial indication to suggest that these two conditions may in fact be associated.
The factors that promote gallbladder mucocoele formation in dogs are poorly understood [10]. Of the suggested causes, it is thought that obstruction of the common bile duct, as well as gallbladder hypomotility, contribute to the condition. Mucocoele development may also result from an abnormality of the mucosa characterised by hyperplasia of mucin-secreting cells that predisposes to excessive mucous production [10]. It has not been ascertained whether this is a primary or secondary lesion, however the reported incidence of this histopathological change following diagnosis is roughly 80% [11]. In vitro studies have suggested that exposure to hydrophobic bile acids results in an increase in mucin production [6]. This combination of factors slows bile progression through the gallbladder, leading to inspissation, mucosal irritation and subsequent mucocoele development.
Biliary salts are well described cytotoxic agents [12], and chronic exposure is hypothesised to result in injury to the epithelial lining of the gallbladder. Cystic mucinous hyperplasia is a universal sequela of epithelial injury, and the absence of this histological change in this case may be due to an insufficient time frame for this to have occurred.
The aetiopathogenesis of biliary sludge formation has been widely reported in human literature. It is suspected that gallbladder dysmotility and hypomotility are the main predisposing factors [13]. The lack of normal biliary flow through the gallbladder is reported to result in sedimentation of microcholecystoliths, crystals, mucin and cellular debris. Once formed, biliary sludge is difficult to clear, with reabsorption of water further inspissating the material [1]. If allowed to progress, obstruction of the cystic duct results, through preventing the normal movement of bile, in mucocoele formation. This mechanism is the suspected pathogenesis in this case.
There are a number of associated factors hypothesised to be contributing to gallbladder disease in this patient. Primary breed-specific hyperlipidaemia has been reported in Miniature Schnauzers [14] and the marked hypertriglyceridaemia and hypercholesterolaemia noted are supportive of this condition. Also the presence of diabetes mellitus, could equally be contributing to these findings due to cellular inability to metabolise and utilise glucose. These biochemical states have a number of effects on the consistency of bile, namely through increasing the levels of biliary cholesterol leading to the formation of a more viscous material [15]. The corresponding biliary stasis leads to mucosal irritation and subsequent increased mucin secretion. This exacerbates the increasing biliary viscosity, and predisposes to mucocoele formation.
Cholecystokinin (CCK) is the primary hormone involved in promoting gallbladder contraction and bile secretion. It is released by enterocytes when fat from a meal enters the duodenum [10]. It is proposed that long-standing exposure of enterocytes to a hyperlipaemic state reduces CCK release. This induces gallbladder hypomotility, and could be a contributing factor to mucocoele development. Insulin resistance has also shown to have an effect on CCK secretion in humans [16], with reduced CCK release seen postprandially. Dogs are generally affected by type I (insulin-dependent) diabetes mellitus characterised by a reduction in the synthesis and secretion of insulin by β cells in the pancreas. Insulin resistance is therefore uncommon in dogs, and it is not yet known if the reduced insulin secretion seen in diabetic dogs has any effects on CCK release in this species. Therefore, the presence of diabetes mellitus in this case could result in lowered CCK release, reducing gallbladder contraction and inducing hypomotility. This predisposes to mucocoele formation, however without specific studies of CCK release in dogs in response to reduced levels of insulin; it is difficult to ascertain whether this CCK secretion pattern seen in humans also pertains to this case.
Medical therapy instigated was the use of the choleretic agent ursodeoxycholic acid. This synthetic bile acid functions to displace hydrophobic bile acids from the circulating pool [6]. The hydrophilic activity draws water into the bile, increasing its fluidity, and normalising biliary secretion. It also conveys hepatoprotectant effects through its anti-oxidative properties [17], which not only aids in relieving the gallbladder congestion, but also protects the hepatic parenchyma. However this medical therapy does have contraindications, particularly for this case. The evidence of biliary hyperplasia suggests extra-hepatic functional cholestasis was present, and this has implications for the use of this drug. Through its choleretic effects, the volume of the bile is increased and in turn, places greater pressure on a compromised gallbladder wall, increasing the risk of gallbladder rupture and subsequent bile peritonitis [3].
Despite these contraindications, the use of ursodeoxycholic acid was warranted as an initial therapy in this case as there was no evidence of complete biliary obstruction on ultrasound and so the risk of gallbladder rupture was concluded to be low. In addition, ursodeoxycholic acid has been shown to reduce bile acid production by limiting gastrointestinal absorption of cholesterol [6], further providing a choleretic effect and reducing serum cholesterol concentrations with appropriate management of the concurrent endocrine disease present. The choleretic, anti-oxidative and secretion-normalising effects of this drug were considered to outweigh its possible complications, particularly for initial management as its biliary thinning effects theoretically allow sludgy bile to exit the gallbladder [10]. It is still unknown whether medical management will allow resolution of the ultrasonographic changes seen in the gallbladder in cases of biliary sludge and mucocoele formation, however with close monitoring including repeating biochemical parameters and follow-up ultrasound examination every 6 weeks, this medical therapy can be used to provide time for the owners to consider cholecystectomy.
Surgical excision of the gallbladder was ultimately necessary to confer a complete cure. Dogs with gallbladder mucoceles that undergo cholecystectomy and survive the immediate post-operative period have an excellent long-term prognosis [18]. Intra-operative complications are severe, with bile leakage reported in 7% of cases. Bile peritonitis is a common sequel to this, with a peri-operative mortality rate of 22–40% described [9]. Therefore, cholecystectomy is not performed without risks; however the mind-set towards this condition has to change. The current trend is to delay removal until medical therapy failure as in this case. However, further emphasis should be placed on removal of the gallbladder at initial presentation. Cholecystectomy at this stage would be performed on a gallbladder with a greater degree of integrity, with minimal compromise of the wall. Correspondingly, an excellent outcome and rapid return to function has been described [9].
Competing interests
No conflicts of interest have been declared.
Acknowledgements
The authors would like to acknowledge and thank Dr M Owen, Dr B Bahr and Dr R Green for their help in ultrasonographic studies and advice, along with Dr A Gal for his help in pathological examination and findings. The authors would also like to thank Dr A Worth and Dr M Jack for their surgical expertise and assistance with obtaining intraoperative photographs.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Secchi P., Pöppl A.G., Ilha A., Kunert Filho H.C., Lima F.E.S., García A.B. Prevalence, risk factors, and biochemical markers in dogs with ultrasound-diagnosed biliary sludge. Res Vet Sci. 2012;93:1185–1189. doi: 10.1016/j.rvsc.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Ko C.W., Sekijima J.H., Lee S.P. Biliary sludge. Ann Intern Med. 1999;130:301–311. doi: 10.7326/0003-4819-130-4-199902160-00016. [DOI] [PubMed] [Google Scholar]
- 3.Berent A.C. Acute biliary diseases of the dog and cat. In: Silverstein D., Hopper K., editors. Small animal critical care medicine. Saunders Elsevier; Missouri: 2008. pp. 542–546. [Google Scholar]
- 4.Newell S.M., Selcer B.A., Mahaffey M.B. Gallbladder mucocele causing biliary obstruction in two dogs: ultrasonographic, scintigraphic, and pathologic findings. J Am Anim Hosp Assoc. 1995;31:467–472. doi: 10.5326/15473317-31-6-467. [DOI] [PubMed] [Google Scholar]
- 5.North D.C. Sudden death in a dog associated with cholelithiasis. Vet Rec. 1977;101:203. doi: 10.1136/vr.101.11.203-a. [DOI] [PubMed] [Google Scholar]
- 6.Cook A.K. Gallbladder mucocele: the endocrine connection. Adv Small Anim Med Surg. 2013;26:1–3. [Google Scholar]
- 7.Kook P.H., Schellenberg S., Grest P., Reusch C.E., Corboz L., Glaus T.M. Microbiologic evaluation of gallbladder bile of healthy dogs and dogs with iatrogenic hypercortisolism: a pilot study. J Vet Intern Med. 2010;24:224–228. doi: 10.1111/j.1939-1676.2009.0413.x. [DOI] [PubMed] [Google Scholar]
- 8.Mesich M.L., Mayhew P.D., Paek M., Holt D.E., Brown D.C. Gall bladder mucoceles and their association with endocrinopathies in dogs: a retrospective case-control study. J Small Anim Pract. 2009;50:630–635. doi: 10.1111/j.1748-5827.2009.00811.x. [DOI] [PubMed] [Google Scholar]
- 9.Pike F.S., Berg J., King N.W. Gallbladder mucocele in dogs: 30 cases (2000–2002) J Am Vet Med Assoc. 2004;224:1615–1622. doi: 10.2460/javma.2004.224.1615. [DOI] [PubMed] [Google Scholar]
- 10.Cornejo L., Webster C.R.L. Canine gallbladder mucoceles. Compend Contin Educ Vet. 2005;27:912–930. [Google Scholar]
- 11.Uno T., Okamoto K., Onaka T., Fujita K., Yamamura H., Sakai T. Correlation between ultrasonographic imaging of the gallbladder and gallbladder content in eleven cholecystectomised dogs and their prognoses. J Vet Med Sci. 2009;71:1295–1300. doi: 10.1292/jvms.001295. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz N.S., Hua J., Powell M.A., Gibb R.K., Mutch D.G., Herzog T.J. Novel cytotoxic agents from an unexpected source: bile acids and ovarian tumor apoptosis. Gynecol Oncol. 2007;107:344–349. doi: 10.1016/j.ygyno.2007.07.072. [DOI] [PubMed] [Google Scholar]
- 13.Pazzi P., Gamberini S., Buldrini P., Gullini S. Biliary sludge: the sluggish gallbladder. Dig Liver Dis. 2003;35:39–45. doi: 10.1016/s1590-8658(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 14.Kutsunai M., Kanemoto H., Fukushima K., Fujino Y., Ohno K., Tsujimoto H. The association between gall bladder mucoceles and hyperlipidaemia in dogs: a retrospective case control study. Vet J. 2014;199:76–79. doi: 10.1016/j.tvjl.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Ridgway M.D., Graves T.K. Gallbladder mucocele in dogs. NAVC Clin Brief. 2012:65–70. [Google Scholar]
- 16.Rushakoff R.A., Goldfine I.D., Beccaria L.J., Mathur A., Brand R.J., Liddle R.A. Reduced postprandial cholecystokinin (CCK) secretion in patients with noninsulin-dependent diabetes mellitus: evidence for a role for CCK in regulating postprandial hyperglycemia. J Clin Endocrinol Metab. 1993;76:489–493. doi: 10.1210/jcem.76.2.8432795. [DOI] [PubMed] [Google Scholar]
- 17.Leveille-Webster C.R. Ursodeoxycholic acid therapy. In: Bonagura J.D., editor. Kirk’s current veterinary therapy xiii. WB Saunders; Philadelphia: 2000. pp. 691–692. [Google Scholar]
- 18.Malek S., Sinclair E., Hosgood G., Moens N.M.M., Baily T., Boston S.E. Clinical findings and prognostic factors for dogs undergoing cholecystectomy for gall bladder mucocele. Vet Surg. 2013;42:418–426. doi: 10.1111/j.1532-950X.2012.01072.x. [DOI] [PubMed] [Google Scholar]