Abstract
Cysteine-rich 61 (Cyr61) belongs to the CCN family and mediates cell proliferation, survival, and apoptosis. Our previous studies showed that Cyr61 protected against hyperoxia-induced lung cell death via Akt phosphorylation. Caveolin-1 (cav-1), a 22-kDa transmembrane scaffolding protein, is the principal structural component of caveolae. Emerging data show that cav-1 regulates signal transduction-associated proteins that reside in the caveolae. Numerous integrin-related pathways, including PI3K/Akt-induced cell survival are controlled by cav-1-mediated signaling. Our data showed that recombinant Cyr61 promoted cell proliferation and resistance to hyperoxia-induced cell death in vitro. Neutralizing antibodies reversed the above effects, indicating functional role of secreted Cyr61 in response to hyperoxic stress. While deletion of cav-1 protected cells from hyperoxia-induced cell death, Cyr61-neutralizing antibodies abolished this protective effect. Furthermore, Cyr61 and cav-1 colocalized and physically interacted via integrins in bronchial epithelial cells. Deletion of cav-1 increased extracellular and decreased cytosolic Cyr61, both in vitro and in vivo. Pretreatment with Brefeldin A increased intracellular Cyr61 in cav-1−/− cells, while decreasing extracellular Cyr61. Taken together, Cav-1/Cyr61 interaction via integrins represents a novel pathway of Cyr61 signaling involving cav-1-dependent processes, which play a critical role in regulating hyperoxia-induced cell death.—Jin, Y., Kim, H. P., Cao, J., Zhang, M., Ifedigbo, E., Choi, A. M. K. Caveolin-1 regulates the secretion and cytoprotection of Cyr61 in hyperoxic cell death.
Keywords: CNN, exocytosis, signaling
cysteine-rich61 (Cyr61) was the first cloned protein of the CCN (cysteine-rich 61, connective tissue growth factor, and nephroblastoma) family. To date, a total of six members have been identified. Additional CCN proteins are the wnt-induced secreted proteins (WISPs) 1, 2, and 3 (1⤻2⤻3⤻4⤻5⤻6)⤻. The CCN proteins share a uniform modular structure and exhibit diverse cellular functions (1⤻2⤻3⤻4⤻5⤻6)⤻. Cyr61 (CCN1) mediates cell adhesion, migration, proliferation, cell survival, apoptosis, and angiogenesis (3⤻, 6⤻, 7⤻8⤻9)⤻. It is a secreted heparin-binding protein that integrates into the extracellular matrix (ECM) (9⤻10⤻11⤻12⤻13)⤻. Cyr61 as secreted protein is internalized and degraded through the lysosomal pathway (14)⤻ and functions as an autocrine or paracrine protein through integrins (9⤻10⤻11⤻12⤻13)⤻. It can either induce or suppress apoptosis in a cell type-dependent manner. While Cyr61 promotes survival of endothelial cells, myocytes, and breast cancer cells (9⤻, 13⤻14⤻15⤻16⤻17)⤻, its expression was associated with cell death in neuronal cells (18)⤻.
Our previous studies showed that Cyr61 protected hyperoxia-induced lung cell death via Akt phosphorylation (19)⤻. However, whether and how Cyr61 interacts with integrins and subsequently induces phosphorylation of Akt in lung cells remains unclear. In this study, we identified a novel component, caveolin-1, involved in Cyr61 signaling.
The domain structure characterized by lipid packing in the lipid bilayer of plasma membrane consists of cholesterol, sphingolipids, and certain proteins. These regions are defined as cholesterol-enriched membrane microdomains (CEMMs), also known as “lipid rafts” (20)⤻. Caveolae are one subset of lipid rafts that are distinct cholesterol- and sphingomyelin-rich, omega-shaped invaginations (50–100 nm) of the plasma membrane (21)⤻. These structures are dynamically recycled in the plasma membrane, endosomes, and trans-Golgi systems, and participate in a number of cellular processes, including endocytosis, transcytosis, and intracellular signal transduction (20⤻21⤻22⤻23⤻24⤻25)⤻. Caveolin-1 (cav-1), a 22-kDa transmembrane scaffolding protein, serves as the principle structural component of caveolae (20⤻21⤻22⤻23⤻24⤻25)⤻. Accumulating data indicate that cav-1 regulates signal transduction-associated proteins that reside in the caveolae. Direct inhibition by cav-1 has been reported on many proteins, such as Src, the epidermal growth factor (EGF) receptor, endothelial nitric-oxide synthase (eNOS), G protein α and subunits, and H-Ras, (26⤻27)⤻. Direct activation by cav-1 on insulin receptors has also been found (25)⤻. Moreover, numerous integrin-mediated pathways are dependent on caveolin-1, including ERK, PI3K/Akt, and Rac pathways (29⤻, 30)⤻. Integrins regulate cellular processes by controlling the localization of caveolae at the plasma membrane. Loss of integrin-mediated adhesion results in the internalization of caveolae which, in turn, terminates signaling pathways, including activation of multiple integrin-mediated signaling (28⤻29⤻30⤻31⤻32)⤻. So far, we know that three integrin-dependent pathways (PI3K/Akt, Ras/ERK, and Rac/Pak) for cell proliferation and survival are impaired by caveolae internalization (26⤻27⤻28⤻29⤻30⤻31⤻32)⤻. Integrins prevent down-regulation of ERK, PI3K/Akt, and Rac-dependent pathways by inhibiting cav-1-mediated endocytosis (26⤻27⤻28⤻29⤻30⤻31⤻32)⤻.
Cyr61 is well known to function via integrin-mediated pathways (1⤻, 4⤻, 5)⤻. However, to our best knowledge, there is no report whether caveolin-1 is involved in Cyr61-mediated signaling pathways. Our study, demonstrates that cav-1 is involved in Cyr61-integrin signaling and mediated the protective role of Cyr61 in hyperoxia-induced lung cell and tissue injury.
MATERIALS AND METHODS
Reagents
Cyr61 recombinant protein and neutralizing antibodies were kindly provided by Dr. Lester Lau (University of Illinois, Chicago, IL, USA). A commercially available recombinant Cyr61 with tested biological activity was purchased from Abcam (ab50074; Cambridge, MA, USA). Integrin antibodies were obtained from R&D Laboratories (Minneapolis, MN, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), Cyr61 antibodies were obtained from Santa Cruz Biotechnology and Abcam. Cav-1 antibodies were from Santa Cruz and BD Transduction Laboratories, (Lexington, KY, USA). All other reagents and chemicals were from Sigma (St. Louis, MO, USA).
Cell culture and treatments
Primary mouse lung fibroblasts and pulmonary endothelial cells were cultured as described previously (33)⤻ and used for experiments as subconfluent monolayers at passages 7–12. Cells were cultured from the lungs of wild-type C57BL/6 mice and caveolin-1-null (cav-1−/−) mice, as described previously (33)⤻. Beas-2B lung epithelial cells were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in the defined medium, BEGM (bronchial epithelial growth medium; Cambrex, East Rutherford, NJ). All cells were grown in humidified incubators containing an atmosphere of 5% CO2 and 95% air at 37°C. Cell cultures were exposed to hyperoxia in modular exposure chambers as described (19)⤻, using 95% oxygen with 5% CO2.
Animal exposures
Wild-type C57BL/6 mice and inbred cav-1−/− mice, 8–12 wk, were maintained in laminar flow cages in a pathogen-free facility at the University of Pittsburgh. All procedures were performed in accordance with the Council on Animal Care at the University of Pittsburgh and the National Research Council’s Guide for the Humane Care and Use of Laboratory animals. The cav-1−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The animals were exposed to room air or hyperoxia (95% O2, 5% N2). Mice underwent bronchial-alveolar lavage (BAL) and then were sacrificed at 96 h exposure.
Transfections
Beas-2B cells were transfected with cav-1 siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Transiently transfected cells were incubated for an additional 24 h and exposed to hyperoxia. After 48–72 h, cell viability was determined with cell viability assay (Promega, Madison, WI, USA). The rest of the cells were harvested for the determination of caveolin-1 expression. Small-interfering RNA (siRNA) was designed against the coding sequence of caveolin-1 cDNA by using software by Dharmacon Research (Layfayette, CO, USA). Sequences corresponding to the siRNAs were as follows: human caveolin-1 coding region, (CCAGAAGGGACACACAGTT), GenBank accession no. NM_001753.
Immunoprecipitation, Western blot analysis, and immunocytochemistry
After 16–24 h hyperoxia, proteins were isolated from cell cultures in radioimmunoprecipitation assay buffer [1× PBS, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) sodium dodecyl sulfate, 0.1 mg/ml phenylmethylsulfonyl fluoride, 30 μl/ml aprotinin, and 1 mM sodium orthovanadate]. Protein concentration was determined using the Coomassie plus protein assays (Pierce, Rockford, IL, USA) and was equalized between samples. For immunoprecipitation, 1 μg of antibody was added to 500 μg of total protein in 500 μl, rotated overnight at 4°C, and then incubated with 20–30 μl of beads (protein A–sucrose; Santa Cruz Biotechnology) for 2–4 h, spun down at 500 g, and washed 3 times with radioimmunoprecipitation buffer. Then, 30 μl of loading buffer (100 mM Tris-HCl, 200 mM dithiothreitol, 4% SDS, 0.2% bromphenol blue, and 20% glycerol) was added. For SDS-PAGE, samples containing equal amounts of protein were boiled in the loading buffer and separated on SDS-PAGE, followed by transfer to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk and stained with the primary antibodies for 2 to 12 h at 1:250 to 1:1000 dilution. After 5 washes in phosphate-buffered saline with 0.2% Tween 20, the horseradish peroxidase-conjugated secondary antibody was applied, and the blot was developed with enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ, USA).The following antibodies were used for immunoblotting: monoclonal anticav-1, (BD Transduction Laboratories), polyclonal anticav-1 (Santa Cruz Biotechnology), polyclonal rabbit and goat anti-Cyr61 (Santa Cruz). Immunocytochemistry was performed as described previously by the Center for Biological Imaging at the University of Pittsburgh (33)⤻.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
RNA was extracted from cells using TRIzol method (Invitrogen) and was converted to cDNA using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA, USA). Quantitative RT-PCR analyses were performed as described previously (34)⤻; probes and primers for Cyr61 and TaqMan Master Mix for gene expression assays were obtained from Applied Biosystems. Gene expression was analyzed by the ΔΔCt method, with 18s rRNA and βGUS as the endogenous control.
Cell viability assay
Cell viability assays were performed using the CellTiter-Glo Luminescent Cell Viability Assay according to the manufacturer’s protocol (Promega). Briefly, cells were plated into 96-well plates. After transfection and exposure to hyperoxia, cells were washed twice with cold PBS. One hundred microliters of PBS was added into each well, followed by 100 μl CellTiter-Glo Substrate. Cells were incubated at room temperature for at least 10 min; luminescent signal was then measured using an Lmax luminometer (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
All values are expressed as means ± sd from at least three independent experiments. Differences in measured variables between experimental and control group were assessed by using the Student’s t test (Statview II statistical package; Abacus Concepts, Berkeley, CA, USA). Statistically significant difference was accepted at P < 0.05.
RESULTS
Cyr61 promoted cell proliferation and resistance to hyperoxia-induced cell death
We have previously reported that stably transfected Beas2B cells overexpressing Cyr61 demonstrate increased resistance to hyperoxia (19)⤻. In the current study, recombinant Cyr61 with biological activity was used to treat Beas-2B cells and primary fibroblasts. A dose-dependent effect was observed on cell proliferation, both on epithelial cells (Fig. 1A⤻) and fibroblasts (data not shown). Then we evaluated the effect of recombinant Cyr61 on cell survival after hyperoxia (72 h). Besides its effect on cell proliferation, Cyr61 conferred increased resistance to hyperoxia-induced cell death in a dose-dependent manner (Fig. 1B⤻). Trypan blue exclusion assays were performed to confirm the observation in Fig. 1B⤻, indicating that the resistance to hyperoxia-induced cell death was independent of cell proliferation (data not shown).
Figure 1.
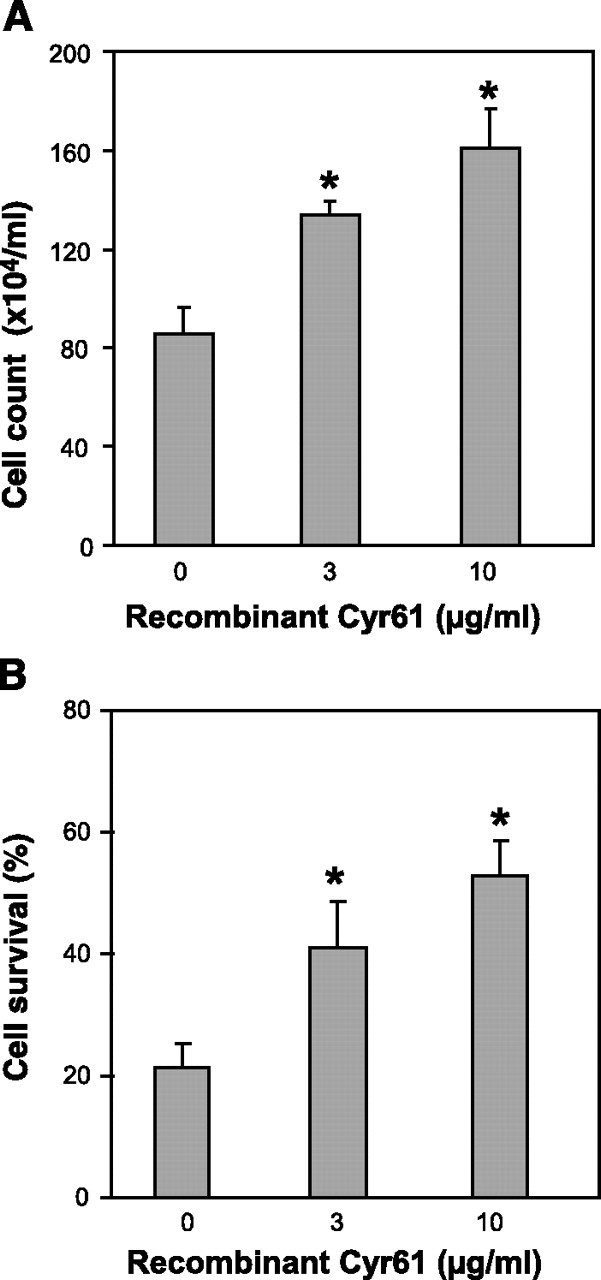
Cyr61 promotes cell proliferation and resists hyperoxia-induced cell death. Beas 2B bronchial epithelial cells were used to determine the effect of recombinant Cyr61 on cell proliferation and cell death after hyperoxia. Cells were treated with Cyr61 (3–10 μg/ml) for 72 h. Albumin (10 μg/ml) was added to the control group. A) Effect of recombinant Cyr61 on cell proliferation. Cells were cultured in serum-free medium overnight, treated with Cyr61 at different dosages. After 72 h, cells were collected in the same amount of culture medium and cell counts were obtained. Albumin (10 μg/ml) was added to the control group (indicated as 0 in figure). B) Effect of recombinant Cyr61 on cell death after hyperoxia. Cells were treated with recombinant Cyr61 as described above and exposed to hyperoxia for 72 h. Results show percentage of viable cells. Each experiment was repeated at least 3 times. *P < 0.05; Student’s t test.
Cyr61-neutralizing antibodies reversed the protective effects
Coating plates with Cyr61-neutralizing antibodies and control immunoglobulin G (IgG), we investigated the cell survival after hyperoxia (72 h) using Beas2B and fibroblasts. After hyperoxia (72 h), cell survival in the neutralizing antibody group was significantly less than that in the control IgG group. These results were similar in both Beas-2B cells and fibroblasts (Fig. 2A⤻ and data not shown).
Figure 2.
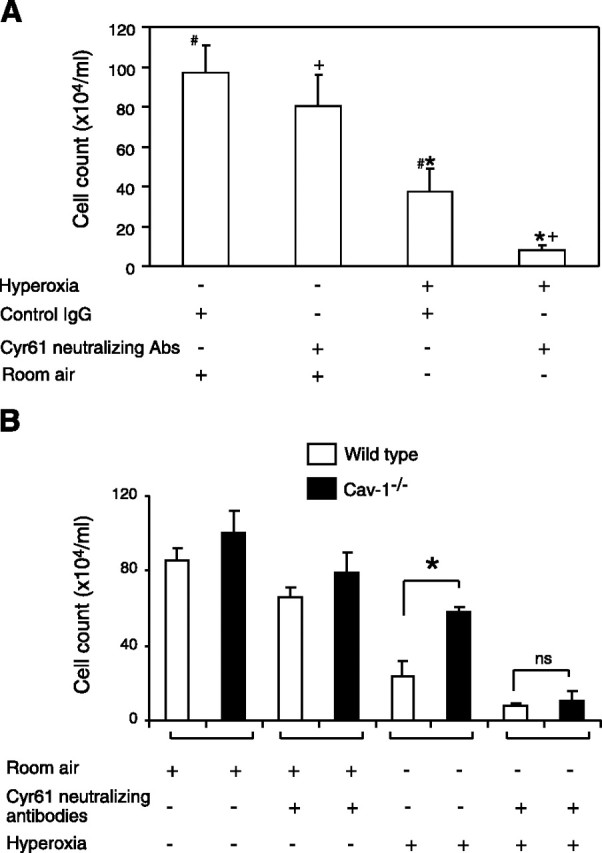
Cyr61-neutralizing antibodies reverse the protective effects of Cyr61-recombinant protein. A) Using a Cyr61-neutralizing antibody, Beas2B cells were placed in anti-Cyr61- and normal IgG-coated plates and treated in the absence or presence of the anti-Cyr61 antibody (20 μg/ml) or a control IgG (20 μg/ml), then exposed to hyperoxia for 72 h. Cells were collected in the same amount of cell culture medium. Results show viable cell counts. B) Using wild-type and cav-1−/− fibroblasts, we repeated the assays described above. Similar results were obtained. Open bars denote wild-type cells. Solid bars denote cav-1−/− cells. Each experiment was repeated at least 3 times. *,#,+P < 0.05; Student’s t test.
Besides Beas2B and wild-type fibroblasts, we further investigated the response to hyperoxia-induced cell death in cav-1−/− fibroblasts. Cav-1−/− fibroblasts were more resistant to hyperoxia than wild-type fibroblasts (Fig. 2B⤻). Interestingly, Cyr61-neutralizing antibodies attenuated the protective effect of cav-1 deletion (Fig. 2B⤻).
Colocalization and interaction between Cyr61 and cav-1
The above findings prompted us to evaluate the crosstalk between cav-1 and Cyr61. First, we used confocal microscopy to test whether Cyr61 and cav-1 colocalized. We found that Cyr61 and cav-1 colocalized at the surface of the cell membrane in room air (Fig. 3A⤻). This colocalization of Cyr61 and cav-1 at the surface attenuated after hyperoxia (data not shown). Furthermore, we confirmed the colocalization between Cyr61 and cav-1 by performing coimmunoprecipitation (co-IP) experiments. Strong interactions between Cyr61 and cav-1 were found in cells in room air. Interestingly, hyperoxia exposure decreased these interactions (Fig. 3B⤻). To further validate our results, we determined the level of cell death in the same conditions under which IP/confocal microscopy was performed. Twenty-four hours after hyperoxia, 80% of Beas2B cells remained alive, and the expression of cav-1 in these viable cells was not altered by hyperoxia (data not shown). Given that we equalized the amount of protein used for co-IP, the attenuated band between cav-1 and Cyr61 after hyperoxia was due to dissociation of this complex.
Figure 3.
Colocalization of Cyr61 and cav-1. A) Beas2B cells stained with cav-1 antibody (red) and Cyr61 antibody (green). Merged image (yellow) is an overlay of the previous two images (red and green). Arrow indicates colocalization of Cyr61 and cav-1 as revealed by yellow staining. B) Co-IP was performed with Cyr61 antibody on Beas2B and mouse lung fibroblast lysates. After precipitation by the rabbit polyclonal Cyr61 antibodies, equal amount of Cyr61 was confirmed using a separated goat polyclonal anti-Cyr61 antibody. Western blot analysis with cav-1 monoclonal antibodies showed that cav-1 interacted with Cyr61 in both cells in room air. Hyperoxia attenuated the interaction of Cyr61 and cav-1 in both cells. Imaging and immunoblots were performed 3 similar times.
Cyr61 and cav-1 colocalization was mediated via integrins
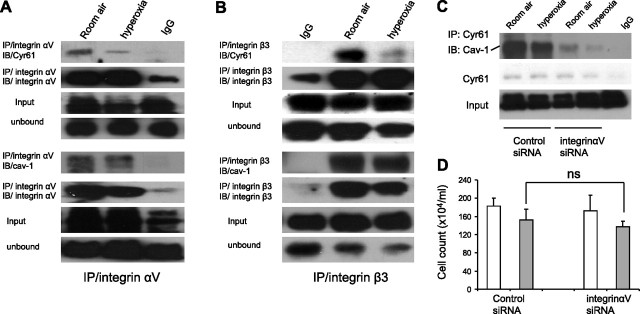
Given that the cav-1-binding motif is not present in Cyr61, we further evaluated whether these two proteins interacted via integrins. We performed co-IP experiments on Cyr61 or cav-1 with integrin αV subunits or integrin β3 subunits, respectively. As shown in Fig. 4⤻, Cyr61 or cav-1 coimmunoprecipitated with integrin αV and integrin β3 subunits. While hyperoxia decreased the interactions between Cyr61 and either integrin αV or integrin β3 subunits, it had no significant effect on cav-1/integrins (Fig. 4⤻).
Figure 4.
Integrins mediate Cyr61 and cav-1 colocalization. A) Co-IP was performed with integrin αV antibody on Beas2B cell lysates. Western blot analysis with Cyr61 and cav-1 antibodies showed that integrin αV coimmunoprecipitates with Cyr61 and cav-1 in room air. Hyperoxia attenuated the interaction of integrin αV and Cyr61; no significant effect on cav-1 was observed. Unbound and precipitated integrin αV were shown to demonstrate equal input. B) Co-IP was performed with integrin β3 antibody on Beas2B cell lysates. Western blot analysis with Cyr61 and cav-1 antibodies showed that integrin β3 coimmunoprecipitated with Cyr61 and cav-1 in room air. Hyperoxia attenuated the interaction of integrin β3 and Cyr61; no significant effect on cav-1 were observed. Unbound and precipitated integrin β3 demonstrated equal input. Immunoblots were performed with least 3 repeats. C) Cyr61/Cav-1 interaction was impaired by knocking down integin αV. Beas2B cells were transfected with integrin αV siRNA and nonspecific control siRNA,respectively. Then cells were exposed to hyperoxia; after 24 h, cell lysates were immunoprecipited with polyclonal anti-Cyr61, fractionated on 12% SDS-PAGE, and blotted with monoclonal anti-av-1. After stripping, original blot was reblotted with anti-Cyr61 to demonstrate equal input. Knocking down integrin αV decreased Cyr61 and cav-1 co-IP with and without hyperoxia. Blot represented at least three similar repeats. D) Effect of integin αV siRNA on cell viability. Cells were pretreated with control siRNA and integin αV siRNA as described previously, followed by 24 h hyperoxia. Open bars denote room air. Solid bars denote hyperoxia. Each experiment was repeated at least 3 times. ns, nonsignificant (P>0.05); Student’s t test.
Integrin αV mediates Cyr61/Cav-1 interaction
To further confirm the critical role of integrins in Cyr61 and cav-1 interaction, we transfected integrin αV siRNA into Beas2B cells. After 24 h of hyperoxia, total cell lysates were collected, immunoprecipitated with polyclonal Cyr61 antibody, and blotted with monoclonal cav-1 antibodies. The same blot was then stripped to check for Cy61 pulldown. The interaction between Cyr61 and cav-1 decreased with hyperoxia, as well as after treatment with integrin αV siRNA (Fig. 4C⤻). To confirm that this observation was not due to the significant cell death caused by integrin siRNA, we transfected cells with integrin αV siRNA. Cells were then evaluated for viability; counts are shown in Fig. 4D⤻. We found that integrin αV siRNA had no significant effect on cell death with or without hyperoxia, compared with the control siRNA in our model.
Cav-1 expression regulates intracellular level of Cyr61
Given that cav-1 and Cyr61 colocalized to the cell surface (Fig. 3⤻), we questioned whether cav-1 mediates the secretion of Cyr61. We tested the intracellular level of Cyr61 in both wild-type and cav-1−/− cells. Cyr61 protein levels significantly decreased in cav-1−/− cells compared with wild type (Fig. 5A⤻). To verify this observation in Beas2B epithelial cells, we performed knockdown assays using cav-1 siRNA. We observed similar effects as in fibroblasts, with decreased Cyr61 levels in cav-1 siRNA-treated Beas2B epithelial cells (Fig. 5B⤻). We further confirmed this result with knock-in assays. Using adeno-cav-1, we overexpressed cav-1 in cav-1−/− cells. With increased levels of cav-1, we observed increased amounts of intracellular Cyr61 (Fig. 5C⤻).
Figure 5.
Altering cav-1 expression affects the cytosolic levels of Cyr61 in Western blot analysis. Lung fibroblasts from wild-type control mice and cav-1−/− mice were cultured as described in Materials and Methods and then exposed to hyperoxia and room air, respectively. After 24 h, cells were collected and cell lysates were analyzed by Western blot analysis. A) Cav-1−/− fibroblasts decreased Cyr61 with and without hyperoxia. Hyperoxia (100% FIO2, 24 h) induced Cyr61 in wild-type cells but not cav-1−/− cells. B) Cav-1 siRNA-treated Beas2B cells decreased Cyr61 with and without hyperoxia. Beas2B cells were treated with cav-1 siRNA and control siRNA, as described in Materials and Methods above. Knocking down cav-1 in Beas2B cells resulted in a decreased level of Cyr61 with and without hyperoxia. C). Increased expression of Cav-1 using ad-cav-1 augmented Cyr61 with and without hyperoxia. Overexpressing cav-1 by adding ad-cav-1 (i.e., knock-in assays) increased the cytosolic level of Cyr61 with and without hyperoxia. All blots represent at least 3 similar repeats.
Deletion of cav-1 (cav-1−/−) increased extracellular level of Cyr61 in vitro and increased the secreted Cyr61 in vivo
Next, we evaluated the extracellular level of Cyr61 in the absence of cav-1 using cav-1−/− cells. Supernatants of cell culture media from wild-type cells and cav-1−/− cells were collected and precipitated with trichloroacetic acid (TCA). Then, using Western blot analysis, we compared the secreted Cyr61 of wild-type cells and cav-1−/− cells with and without hyperoxia. Supernatant from culture medium of cav-1−/− cells exhibited increased Cyr61 protein (Fig. 6A⤻) compared to wild-type control cells. Furthermore, we exposed cav-1−/− mice to room air and hyperoxia. After 72 h, we performed whole lung lavage. BAL fluid was collected, and protein was precipitated using TCA. We found more Cyr61 in the BAL fluid of cav-1−/− mice than of wild-type mice (Fig. 6B⤻).
Figure 6.
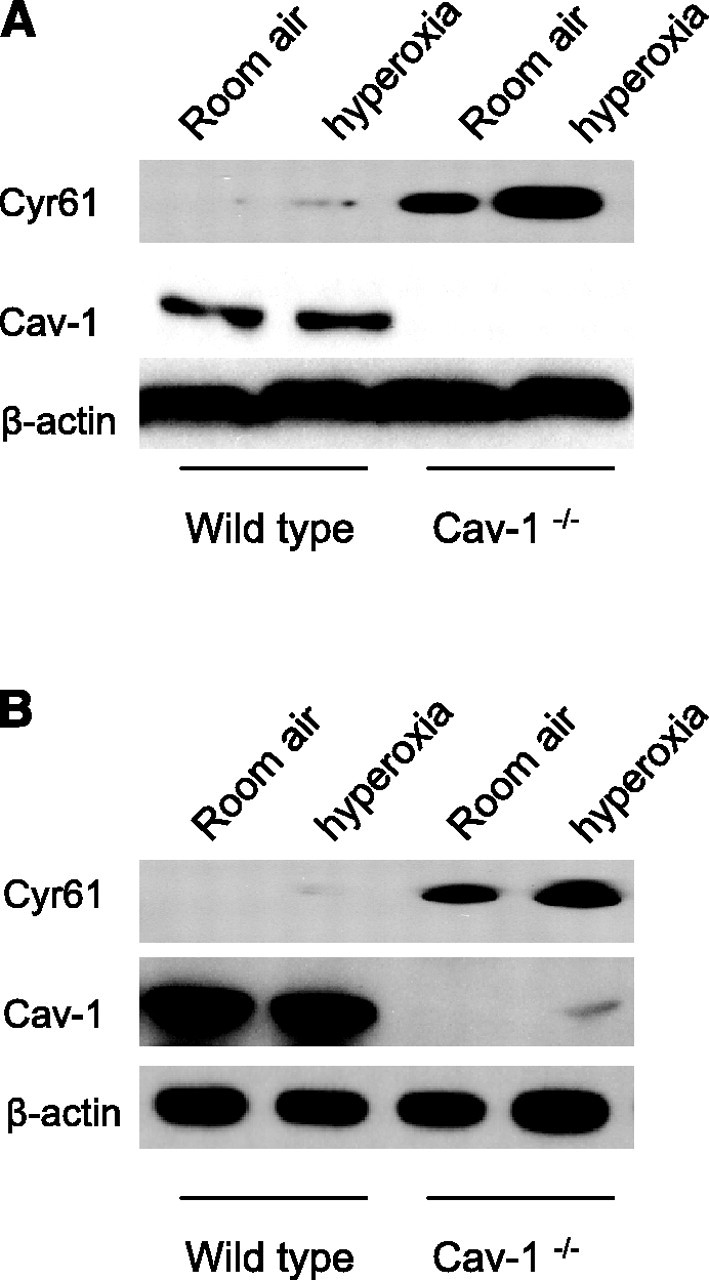
Deletion of cav-1 increases extracellular levels of Cyr61 in vitro and in vivo. A) Wild-type fibroblasts and cav-1−/− fibroblasts were cultured as described above. After hyperoxia (95% FIO2, 24 h), all cell culture media were collected and proteins were precipitated using TCA, as described above. The precipitated protein was analyzed using Western blot analysis. There were elevated Cyr61 levels in cell culture media taken from cav-1−/− cells with and without hyperoxia. Cell lysates were obtained, and β-actin was analyzed to ensure a similar amount of cells in each plate. Cav-1 was analyzed from cell lysates to ensure deletion of cav-1 in cav-1−/− cells. All blots were performed with at least 3 repeats. B) Wild-type and cav-1−/− mice were exposed to room air and hyperoxia, as described in Materials and Methods. Elevated levels of Cyr61 were found in the lavage fluids obtained from cav-1−/− mice, with and without hyperoxia. Cav-1 and β-actin from homogenized lung tissue were analyzed using Western blot analysis to ensure cav-1 deletion. Each blot represents 3 repeats; 3 mice/group.
Hyperoxia exerted no effect on cav-1 expression
Since the amount of caveolae/cav-1 potentially affects the level of Cyr61, we next questioned whether hyperoxia induces cav-1 expression. Using Beas-2B cells, endothelial cells, wild-type fibroblasts, and cav-1−/− fibroblasts, we did not observe significant changes of cav-1 expression (Fig. 5A-C⤻, left columns, and data not shown). Furthermore, we performed Western blot analysis and did not find any difference in cav-1 levels between wild-type mice and cav-1−/− mice (Fig. 6B⤻).
Exocytosis of Cyr61 was enhanced in cav-1−/− cells
To test whether the lack of intracellular Cyr61 was due to enhanced exocytosis of Cyr61, we treated the cav-1−/− fibroblasts with Brefeldin A (BFA), a metabolite of the fungus Eupenicillium brefeldianum, which has a disruptive effect on the putative ER and Golgi vesicular network. Intracellular Cyr61 was significantly higher in BFA-treated cells regardless of treatment (Fig. 7A, B⤻). On the contrary, secreted Cyr61 was less in the supernatant of cell culture medium (Fig. 7B⤻), which was prominent in the absence of cav-1.
Figure 7.
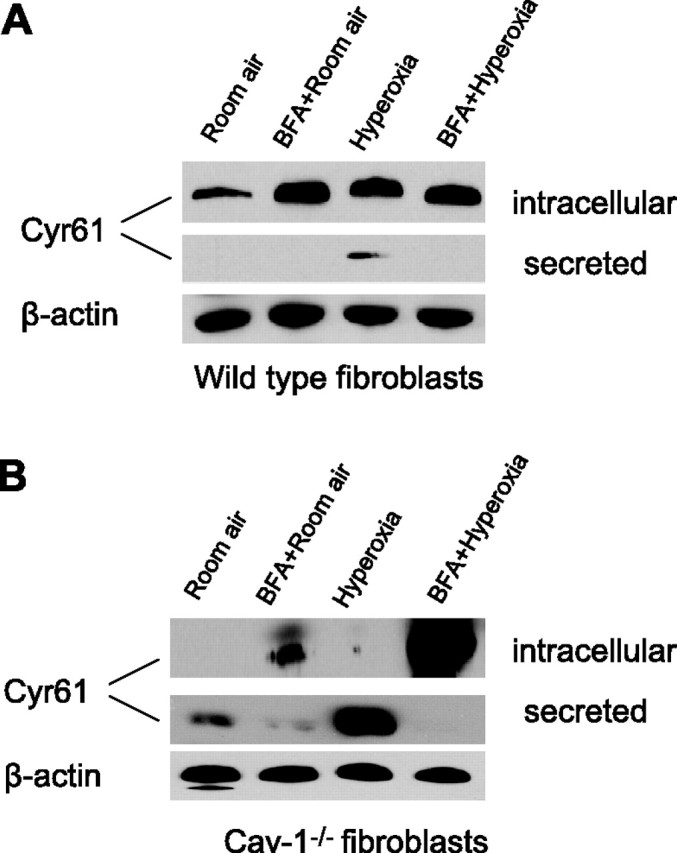
Effect of BFA on exocytosis of Cyr61 in cav-1−/− fibroblasts. Wild-type and cav-1−/− fibroblasts were pretreated with BFA (10 μg/ml), then exposed to room air and hyperoxia, respectively. After 24 h, cell cytosol was collected and Western blot analysis was performed to evaluate intracellular level of Cyr61. The supernatant of cell culture medium was collected at the same time and precipitated with TCA; Western blot analysis was performed to evaluate secreted Cyr61. Blots represent at least 3 repeats. A) Wild-type fibroblasts. B) cav-1−/− fibroblasts. β-Actin from cell lysate was analyzed using Western blot analysis to ensure equal loading. Three independent experiments were performed.
Hyperoxia increased Cyr61 gene expression
One of the remaining questions would be whether hyperoxia modulated Cyr61 gene expression. As shown in Fig. 8A⤻, hyperoxia induced Cyr61 expression after 24 h hyperoxia in Beas-2B cells. This result is consistent with our previous findings (19)⤻. We then performed Taqman assays to evaluate the Cyr61 gene expression after hyperoxia. As shown in Fig. 8B⤻, hyperoxia increased Cyr61 mRNA ∼3- to 4-fold comparing with the room air (Fig. 8B⤻).
Figure 8.

Effect of hyperoxia on Cyr61 gene expression. Beas-2B cells were treated with room air and hyperoxia, respectively. After 24 h, cells were collected, and protein and mRNA were extracted. A) Western blot analysis. B) Taqman real-time PCR. Each experiment was repeated at least 3 times. *P < 0.05; Student’s t test.
DISCUSSION
CCN1/Cyr61 can either induce or suppress apoptosis in a cell type-dependent manner (3⤻, 9⤻, 10⤻, 13⤻14⤻15⤻, 17⤻, 18)⤻. In our previous study, we found that Cyr61 played a protective role in hyperoxia-induced cell death via Akt-related pathways (19)⤻. Cyr61, as the secreted protein, is internalized and degraded through the lysosomal pathway (14)⤻. It can function via an autocrine or paracrine effect (5⤻6⤻7⤻8⤻9⤻10⤻11)⤻. Integrins act as its receptors and regulate its downstream signaling (3⤻4⤻5⤻6⤻7⤻8)⤻. Caveolae, as a subset of lipid rafts, can recruit and exclude signaling proteins (20⤻21⤻22⤻23⤻24⤻25)⤻. The close contact of receptors and signaling proteins in caveolae facilitates efficient signal transduction. It has been shown that caveolin-1, the major component of caveolae, is involved in integrin signaling (23⤻24)⤻.
Many reports have indicated a countereffect on cell survival and proliferation between integrins and caveolin-1. Integrins negatively regulate caveolae internalization, preventing uncoupling of signaling molecules, such as Rac disengagement from its effector, Pak (23⤻24)⤻. Similar mechanisms apply to other pathways, such as ERK and PI3K/Akt. At least three integrin-dependent growth pathways (Ras/ERK, PI3K/Akt, and Rac/Pak) are impaired by Cav-1-mediated caveolae internalization (25⤻26⤻27⤻28⤻29⤻30⤻31⤻32)⤻. Cav-1 links integrin signaling to growth-limit pathways; deletion of cav-1 will dissociate integrins from growth-regulatory pathways and result in tumor-like, anchorage-independent growth (25⤻26⤻27⤻28⤻29⤻30⤻31⤻32)⤻ (Fig. 9⤻).
Figure 9.
Proposed role of cav-1 in Cyr61 signaling in cell proliferation and hyperoxia-induced cell death. This cartoon illustrates Cyr61 signaling involving cav-1/integrin in wild-type and cav-1−/− cells. Without cav-1/caveolae, Cyr61 is secreted into the cell matrix without any barriers. BFA can block the exocytosis of Cyr61 in cav-1−/− cells. In wild-type cells, cav-1/caveolae blocks the exocytosis of Cyr61 and mediates the internalization of Cyr61. In cav-1−/− cells, with elevated secretion, Cyr61 accumulates in extracellular matrix, subsequently functioning via an autocrine or paracrine manner. Increased extracellular Cyr61 continues to stimulate cell proliferation and survival, probably via PI3K/Akt pathways.
Previous studies indicated Cyr61 promotes chemotaxis, proliferation, migration, and intravasation via integrins/Src/PI3K/Akt-dependent pathways (1⤻, 4⤻, 13⤻, 15⤻16⤻17⤻18⤻19)⤻. Although integrins are involved in both Cyr61 and Cav-1 on different occasions, to the best of our knowledge, Cyr61 and cav-1 colocalization and interaction have not been studied. Also, whether cav-1 and caveolae play a role in Cyr61 signaling remains unexplored.
Here, we report for the first time that Cyr61 can signal through caveolae in connection with integrin-mediated pathways. Moreover, our studies showed that cav-1/Cyr61 interaction played a role in regulating Cyr61 exocytosis. Secretion of Cyr61 was enhanced in cav-1−/− cells (Figs. 5⤻6⤻7⤻ and 9⤻). Treatment with BFA, a metabolite of fungus that disrupts Golgi apparatus and protein exocytosis, elevated the intracellular level of Cyr61 in cav-1−/− cells. Without disruption of exocytosis, naturally elevated extracellular Cyr61 in cav-1−/− cells indicated an augmented autocrine/paracrine effect by secreted Cyr61 in matrix (Figs. 7⤻ and 9⤻). In our model, cav-1−/− cells had more rapid proliferation and higher resistance to hyperoxia. This result supports our hypothesis. The elevated secretion of Cyr61 from cav-1−/− cells stimulates cell proliferation and protects cells from hyperoxic cell death via integrin-mediated pathways. The protective effect of Cyr61 after hyperoxia was not counterbalanced by cav-1 in this case. In other words, without cav-1, cav-1−/− cells increased Cyr61 secretion (Fig. 7⤻). Extracellular Cyr61 and its persistent engagement with integrin complexes (in our case, integrin αVβ3) will result in cell survival and uncontrolled proliferation, probably via a PI3K/Akt–dependent pathway (Fig. 9⤻). Indeed, in our previous work, knocking down phosphor–Akt significantly reversed the protective effects of Cyr61 in hyperoxia-induced cell death (19)⤻. Interestingly, studies from the other groups also showed that the absence of cav-1 and caveolae results in activation of an integrin-dependent PI3K/Akt pathway (25⤻26⤻27⤻28⤻29⤻30⤻31⤻32)⤻, which is consistent with our results. Whether this is due to the effect of Cyr61 requires further investigation.
As expected, blocking the circulating Cyr61 in extracellular matrix by Cyr61-neutralizing antibodies attenuated the proliferation and protective effects of cav-1−/− (Fig. 5⤻). Therefore, we suspect that the increased proliferation and resistance to hyperoxia-induced cell death in cav-1−/− cells were due to the following factors. 1) Absence of cav-1 resulted in increased secretion of Cyr61. Given its autocrine and paracrine features, elevated Cyr61 in matrix will lead to increased cell proliferation and decreased cell death after hyperoxia in our model. 2) The deletion of caveolin-1 from integrin /cyr61 complex may result in decreased integrin/Cyr61 internalization, subsequently leading to unbalanced Cyr61 signaling and leading to proliferation/resistance to cell death. This is possibly mediated via a PI3K/Akt-dependent pathway. However, our current study did not focus on the internalization of Cyr61-integrin complex. Whether cav-1 affects the internalization of this complex requires further study. Previous reports had demonstrated that CCN1/Cyr61 inhibited apoptosis and induced proliferations via Akt pathways (13⤻, 15⤻16⤻17⤻18⤻19)⤻. Cyr61 has been shown to stimulate two common cell survival pathways, including PI3K/Akt and ERK1/2 mitogen-activated protein kinase (MAPK) (13⤻, 15⤻16⤻17⤻18⤻19)⤻. Recent data show that these pathways are integrin mediated (1⤻, 4⤻, 13⤻, 15⤻16⤻17⤻18⤻19)⤻. Yoshida et al. (15)⤻ had demonstrated that in cardiac myocytes, β1 integrin mediated the phosphorylation of Akt by Cyr61-promoted cardiac myocyte survival under oxidative stress. Our data here provide a novel mechanism of Cyr61’s effect on proliferations and its protective role in apoptosis. Cav-1 induced cell death via an integrin-mediated pathway. Deletion of cav-1 in the Cyr61/integrin complex promoted cell proliferation and prevented cell death under stress, such as hyperoxia.
While it increased the gene expression of Cyr61 (Fig. 8⤻), hyperoxia decreased the interactions of Cyr61 and cav-1, as well as integrins (Figs. 3⤻ and 4⤻). Hyperoxia had no significant effects on cav-1/integrin complex (Fig. 4⤻). This result indicated that Cyr61 may have a potential countereffect of cav-1. After stress, such as hyperoxia, the Cyr61/integrin complexes were dissociated, leading to less survival signaling via Akt pathways. On the other hand, cav-1 remained to bind with integrins and terminated survival signaling, further limiting the survival signaling or promoting death signaling. The decreased interactions between Cyr61 and cav-1 might be secondary to the lack of interactions of Cyr61/integrins, given that integrins act as a “bridge” between Cyr61 and cav-1. Cells responded to the hyperoxia-induced Cyr61 dissociation from Cyr61/integin complex by increased endogenous expression of Cyr61, as reported in our previous work (19)⤻. We further evaluated the potential involvement of mTOR pathway in our studies, given that hyperoxia is well known as a potent translation inhibitor through inhibition of the mTOR pathway. Hyperoxia down-regulated the phosphor-p70 S6 kinase (data not shown). However, between wild-type and cav-1−/− cells, there is no significant difference on p-P70 S6K after hyperoxia (data not shown).
A few questions arose from this work. We have shown that deletion of cav-1 enhanced the secretion of Cyr61, whereas overexpressing cav-1 increased the intracellular level of Cyr61 (Fig. 5⤻). Whether cav-1 has both an effect on decreasing Cyr61 secretion and enhancing Cyr61/integrin internalization remains unclear. Furthermore, whether Cyr61 prevents cav-1-mediated internalization or only limits the cav-1/integrin signaling by competitive inhibition requires further investigation. We have performed co-IP between cav-1 and Cyr61 in the cytoplasm fraction. However, there is no interaction. Indeed, there is a negligible level of cav-1 in the cytoplasm fraction (data not shown). Previous studies have suggested that Cyr61 secretion utilizes an ER-Golgi apparatus (35)⤻, but the internalization after secretion may use endocytosis-lysosomal degradation routes (14)⤻. So the Cyr61 transport from synthesis probably uses the same routes as cav-1 (ER-Golgi), which may explain the cytoplasm colocalization of cyr61 and cav-1. This hypothesis will be one of our future focuses.
Moreover, the mechanisms by which cav-1, integrin αvβ3, and Cyr61 interact to inhibit exocytosis of Cyr61 remain unclear. Cyr61 comprises four major modules, one of which is a cysteine knot, heparin-binding domain (module IV) (36)⤻. Stress, including hyperoxia, presumably oxidizes these cysteine domains and subsequently changes the conformation of the Cyr61 molecule. Cav-1 is a palmitoylated protein involved in assembly of signaling molecules in caveolae and in intracellular cholesterol transport. Protein palmitoylation is a post-translational modification and is reversible. Three cysteine residues in the C terminus of caveolin-1 are subject to palmitoylation (37⤻, 38)⤻. The palmitoylation of the cysteine residue of cav-1 may be regulated by stress, such as hyperoxia, which, in turn, may regulate the conformation, membrane association, protein-protein interactions, and intracellular localization of the cav-1. However, further investigation is required to confirm this hypothesis.
After hyperoxia, expression of integrins and cav-1 did not change in whole-cell extracts, as shown in Fig. 5⤻. In viable cells, hyperoxia did not alter the amount of cav-1 in membrane-bound protein, as indicated by cell fractionation analyses (data not shown).
Last, another potential focus of our future studies will be evaluation of whether other integrins, such as α6β1, are involved in Cyr61/integrin/cav-1 complex signaling.
In summary, our data for the first time showed that cav-1 is involved in Cyr61/integrin signaling during cell proliferation and cell survival after hyperoxia. Its effect might be mediated by interaction with integrins.
Acknowledgments
We sincerely thank Dr. Lester Lau (University of Illinois, Chicago, IL, USA) for providing us the recombinant Cyr61 and Cyr61 neutralizing antibodies. We thank the Center for Biological Imaging (CBI) at the University of Pittsburgh for their help on confocal microscopy and electromicroscopy.
References
- 1.Kireeva, M. L., Lam, S. C., Lau, L. F. (1998) Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J. Biol. Chem. ,3090-3096 [DOI] [PubMed] [Google Scholar]
- 2.Kolesnikova, T. V., Lau, L. F. (1998) Human CYR61-mediated enhancement of bFGF-induced DNA synthesis in human umbilical vein endothelial cells. Oncogene ,747-754 [DOI] [PubMed] [Google Scholar]
- 3.Babic, A. M., Kireeva, M. L., Kolesnikova, T. V., Lau, L. F. (1998) CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. U. S. A. ,6355-6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau, L. F., Lam, S. C. (1999) The CCN family of angiogenic regulators: the integrin connection. Exp. Cell Res. ,44-57 [DOI] [PubMed] [Google Scholar]
- 5.Leu, S. J., Lam, S. C., Lau, L. F. (2002) Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J. Biol. Chem. ,46248-46255 [DOI] [PubMed] [Google Scholar]
- 6.Schütze, N., Kunzi-Rapp, K., Wagemanns, R., Nöth, U., Jatzke, S., Jakob, F. (2005) Expression, purification, and functional testing of recombinant CYR61/CCN1. Protein Expr. Purif. ,219-225 [DOI] [PubMed] [Google Scholar]
- 7.Brigstock, D. R. (2003) The CCN family: a new stimulus package. J. Endocrinol. ,169-175 [DOI] [PubMed] [Google Scholar]
- 8.Leask, A., Abraham, D. J. (2006) All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. ,4803-4810 [DOI] [PubMed] [Google Scholar]
- 9.Menéndez, J. A., Mehmi, I., Griggs, D. W., Lupu, R. (2003) The angiogenic factor CYR61 in breast cancer: molecular pathology and therapeutic perspectives. Endocr. Relat. Cancer ,141-152 [DOI] [PubMed] [Google Scholar]
- 10.Menéndez, J. A., Mehmi, I., Griggs, D. W., Lupu, R. (2003) The angiogenic factor CYR61 in breast cancer: molecular pathology and therapeutic perspectives. Endocr. Related Cancer ,141-152 [DOI] [PubMed] [Google Scholar]
- 11.Perbal, B. (2001) NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol. Pathol. ,57-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planque, N., Perbal, B. (2003) A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell. Int. ,15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampath, D., Winneker, R. C., Zhang, Z. (2001) Cyr61, a member of the CCN family, is required for MCF-7 cell proliferation: regulation by 17beta-estradiol and overexpression in human breast cancer. Endocrinology ,2540-2548 [DOI] [PubMed] [Google Scholar]
- 14.Kireeva, M. L., Latinkić, B. V., Kolesnikova, T. V., Chen, C. C., Yang, G. P., Abler, A. S., Lau, L. F. (1997) Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp. Cell. Res. ,63-77 [DOI] [PubMed] [Google Scholar]
- 15.Yoshida, Y., Togi, K., Matsumae, H., Nakashima, Y., Kojima, Y., Yamamoto, H., Ono, K., Nakamura, T., Kita, T., Tanaka, M. (2007) CCN1 protects cardiac myocytes from oxidative stress via beta1 integrin-Akt pathway. Biochem. Biophys. Res. Commun. ,611-618 [DOI] [PubMed] [Google Scholar]
- 16.Lin, B. R., Chang, C. C., Chen, L. R., Wu, M. H., Wang, M. Y., Kuo, I. H., Chu, C. Y., Chang, K. J., Lee, P. H., Chen, W. J., Kuo, M. L., Lin, M. T. (2007) Cysteine-rich 61 (CCN1) enhances chemotactic migration, transendothelial cell migration, and intravasation by concomitantly up-regulating chemokine receptor 1 and 2. Mol. Cancer Res. ,1111-1123 [DOI] [PubMed] [Google Scholar]
- 17.Menendez, J. A., Vellon, L., Mehmi, I., Teng, P. K., Griggs, D. W., Lupu, R. (2005) A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene ,761-779 [DOI] [PubMed] [Google Scholar]
- 18.Kim, S. M., Park, J. H., Chung, S. K., Kim, J. Y., Hwang, H. Y., Chung, K. C., Jo, I., Park, S. I., Nam, J. H. (2004) Coxsackievirus B3 infection induces cyr61 activation via JNK to mediate cell death. J. Virol. ,13479-13488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, Y., Kim, H. P., Ifedigbo, E., Lau, L. F., Choi, A. M. (2005) Cyr61 protects against hyperoxia-induced cell death via Akt pathway in pulmonary epithelial cells. Am. J. Respir. Cell Mol. Biol. ,297-302 [DOI] [PubMed] [Google Scholar]
- 20.Lisanti, M. P., Tang, Z., Scherer, P. E., Kübler, E., Koleske, A. J., Sargiacomo, M. (1995) Caveolae, transmembrane signalling and cellular transformation. Mol. Membr. Biol. ,121-124 [DOI] [PubMed] [Google Scholar]
- 21.Okamoto, T., Schlegel, A., Scherer, P. E., Lisanti, M. P. (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. ,5419-5422 [DOI] [PubMed] [Google Scholar]
- 22.Shaul, P. W., Anderson, R. G. (1998) Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. Lung Physiol. ,L843-L851 [DOI] [PubMed] [Google Scholar]
- 23.Echarri, A., Del Pozo, M. A. (2006) Caveolae internalization regulates integrin-dependent signaling pathways. Cell Cycle ,2179-2182 [DOI] [PubMed] [Google Scholar]
- 24.Salanueva, I. J., Cerezo, A., Guadamillas, M. C., del Pozo, M. A. (2007) Integrin regulation of caveolin function. J. Cell Mol. Med. ,969-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto, M., Toya, Y., Schwencke, C., Lisanti, M. P., Myers, M. G., Jr, Ishikawa, Y. (1998) Caveolin is an activator of insulin receptor signaling. J. Biol. Chem. ,26962-26968 [DOI] [PubMed] [Google Scholar]
- 26.Head, B. P., Patel, H. H., Tsutsumi, Y. M., Hu, Y., Mejia, T., Mora, R. C., Insel, P. A., Roth, D. M., Drummond, J. C., Patel, P. M. (2008) Caveolin-1 expression is essential for N-methyl-d-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. ,828-840 [DOI] [PubMed] [Google Scholar]
- 27.Okamoto, T., Schlegel, A., Scherer, P. E., Lisanti, M. P. (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. ,5419-5422 [DOI] [PubMed] [Google Scholar]
- 28.Del Pozo, M. A., Balasubramanian, N., Alderson, N. B., Kiosses, W. B., Grande-Garcia, A., Anderson, R. G., Schwartz, M. A. (2005) Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat. Cell Biol. ,901-908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Pozo, M. A., Schwartz, M. A. (2007) Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. ,246-250 [DOI] [PubMed] [Google Scholar]
- 30.Wary, K. K., Maneiro, F., Isakoff, S. J., Marcantonio, E. E., Giancotti, F. G. (1996) The adapter protein Shc couples a class of integrins to the control of cell cycle progression. Cell ,733-743 [DOI] [PubMed] [Google Scholar]
- 31.Wary, K. K., Mariotti, A., Zurzolo, C., Giancotti, F. G. (1998) A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell ,625-634 [DOI] [PubMed] [Google Scholar]
- 32.Wei, Y., Yang, X., Liu, Q., Wilkins, J. A., Chapman, H. A. (1999) A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. ,1285-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, X. M., Zhang, Y., Kim, H. P., Zhou, Z., Feghali-Bostwick, C. A., Liu, F., Ifedigbo, E., Xu, X., Oury, T. D., Kaminski, N., Choi, A. M. (2006) Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J. Exp. Med. ,2895-2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning, W., Li, C. J., Kaminski, N., Feghali-Bostwick, C. A., Alber, S. M., Di, Y. P., Otterbein, S. L., Song, R., Hayashi, S., Zhou, Z., Pinsky, D. J., Watkins, S. C., Pilewski, J. M., Sciurba, F. C., Peters, D. G., Hogg, J. C., Choi, A. M. (2004) Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. U. S. A. ,14895-14900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechner, A., Schütze, N., Siggelkow, H., Seufert, J., Jakob, F. (2000) The immediate early gene product hCYR61 localizes to the secretory pathway in human osteoblasts. Bone ,53-60erratum in Bone 27, 569 [DOI] [PubMed] [Google Scholar]
- 36.Desnoyers, L. (2004) Structural basis and therapeutic implication of the interaction of CCN proteins with glycoconjugates. Curr. Pharm. Des. ,3913-3928, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Dietzen, D. J., Hastings, W. R., Lublin, D. M. (1995) Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J. Biol. Chem. ,6838-6842 [DOI] [PubMed] [Google Scholar]
- 38.Spisni, E., Tomasi, V., Cestaro, A., Tosatto, S. C. (2005) Structural insights into the function of human caveolin 1. Biochem. Biophys. Res. Commun. ,1383-1390 [DOI] [PubMed] [Google Scholar]