Abstract
Heat shock protein 70 (Hsp70) is a highly conserved and ubiquitous protein that is reported to provide cytoprotection in various cell types and tissues. However, the importance of Hsp70 expression during skeletal muscle atrophy, when Hsp70 levels are significantly decreased, is not known. The current study aimed to determine whether plasmid-mediated overexpression of Hsp70, in the soleus muscle of rats, was sufficient to regulate specific atrophy signaling pathways and attenuate skeletal muscle disuse atrophy. We found that Hsp70 overexpression prevented disuse muscle fiber atrophy and inhibited the increased promoter activities of atrogin-1 and MuRF1. Importantly, the transcriptional activities of Foxo3a and NF-κB, which are implicated in the regulation of atrogin-1 and MuRF1, were abolished by Hsp70. These data suggest that Hsp70 may regulate key atrophy genes through inhibiting Foxo3a and NF-κB activities during disuse. Indeed, we show that specific inhibition of Foxo3a prevented the increases in both atrogin-1 and MuRF1 promoter activities during disuse. However, inhibition of NF-κB did not affect the activation of either promoter, suggesting its requirement for disuse atrophy is through its regulation of other atrophy genes. We conclude that overexpression of Hsp70 is sufficient to inhibit key atrophy signaling pathways and prevent skeletal muscle atrophy.—Senf, S. M., Dodd, S. L., McClung, J. M., Judge, A. R. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy.
Keywords: atrogin-1/MAFbx, MuRF1, immobilization
heat shock protein 70 (Hsp70) is one of the most prominent members of the heat shock family of proteins, a family that also includes small Hsps, Hsp60, and the Hsp90 family. Although Hsp70 is constitutively expressed in skeletal muscle, its expression levels are increased rapidly, and several-fold, in response to cellular stress (1)⤻ , which provides protection to the cell. While the function of Hsp70 as a molecular chaperone becomes especially important during cellular stress, the mechanisms by which increased levels of Hsp70 provides cytoprotection are increasingly being linked to the direct regulation of specific cell signaling pathways by Hsp70 (2⤻ 3⤻ 4)⤻ . It is therefore speculated that a decrease in Hsp70 may contribute to the pathogenicity that occurs in response to stressful stimuli, as occurs during skeletal muscle disuse. In fact, Hsp70 is significantly down-regulated in multiple models of skeletal muscle atrophy (5⤻ 6⤻ 7⤻ 8⤻ 9⤻ 10)⤻ , and during disuse this appears to be sustained for up to 9 wk (10)⤻ . Given this down-regulation, we previously used whole-body hyperthermia to increase Hsp70 prior to (6)⤻ , and during (11)⤻ , skeletal muscle disuse, which, in both experiments, significantly attenuated the muscle atrophy. However, although effective, whole-body hyperthermia likely differentially regulates the expression of multiple cellular proteins, which may ultimately protect against or promote muscle atrophy. Therefore, no direct evidence suggests the involvement of Hsp70 in the maintenance of skeletal muscle mass during an atrophy condition nor its regulation of specific atrophy signaling pathways in muscle, in vitro or in vivo.
Skeletal muscle atrophy is a consequence of both reduced muscle use (e.g., immobilization, unloading, bed rest, denervation, and space flight) and several disease states (e.g., cancer, sepsis, diabetes, and uremia). The muscle atrophy in these conditions is a result of both reduced protein synthesis and increased protein degradation, but it appears that the latter plays the larger role (12)⤻ . Protein degradation is primarily mediated through the ubiquitin proteasome pathway (13⤻ , 14)⤻ , and two commonly used markers of this pathway are the muscle-specific E3 ligases, atrogin-1/muscle atrophy F-box (MAFbx), and muscle-specific RING finger 1 (MuRF1). These ligases are significantly up-regulated in multiple models of muscle atrophy (15)⤻ . In addition, atrogin-1−/− mice and MuRF1−/− mice show attenuation of muscle atrophy following denervation (16)⤻ , demonstrating their requirement for disuse muscle atrophy.
The expression of atrogin-1/MAFbx and MuRF1 may be regulated by the two transcription factors, forkhead box O (Foxo) and NF-κB (17⤻ 18⤻ 19)⤻ . The Foxo family consists of 4 members in mammalian cells—Foxo1, Foxo3a, Foxo4, and Foxo6 (20)⤻ . Foxos 1 and 3 receive the most attention with regard to skeletal muscle atrophy because they are up-regulated during various atrophy conditions (9⤻ , 21⤻ , 22)⤻ . Furthermore, muscle-specific overexpression of Foxo1 is sufficient to cause skeletal muscle atrophy in vivo (23)⤻ and is required for atrogin-1/MAFbx induction during fasting (19)⤻ . In addition, overexpression of Foxo3a is sufficient to up-regulate atrogin-1/MAFbx mRNA (19)⤻ , activate an atrogin-1/MAFbx reporter gene (18)⤻ , and cause skeletal muscle fiber atrophy (19)⤻ . Moreover, expression of a dominant negative (d.n.) Foxo3a attenuates dexamethasone-induced atrogin-1 expression and prevents myotube atrophy (19)⤻ .
NF-κB activation is both sufficient to cause skeletal muscle atrophy in vivo (17)⤻ and required for skeletal muscle atrophy in vivo caused by either cachexia (17⤻ , 24)⤻ or disuse (17⤻ , 25⤻ , 26)⤻ . This NF-κB-mediated muscle atrophy may be due to its transcriptional regulation of MuRF1 because mice overexpressing a muscle-specific constitutively active (c.a.) IκB kinase β (IKKβ, (referred to as MIKK mice), which activates NF-κB, demonstrated a >3-fold increase in MuRF1 mRNA expression but no change in atrogin-1/MAFbx (17)⤻ . Furthermore, when MIKK mice were crossed with mice overexpressing the IκBα super repressor (IκBα SR) (referred to as MISR mice), NF-κB activity and MuRF1 mRNA expression were reduced to baseline. These findings demonstrate that an increase in NF-κB activity is required for the IKKβ-induced increase in MuRF1 transcription.
Thus, the purpose of this study was to determine the role of Hsp70 in the regulation of muscle atrophy signaling pathways (Foxo and NF-κB) and, therefore, the regulation of skeletal muscle mass during disuse. To do this, we injected, and electrotransfered, an Hsp70 expression plasmid into the soleus muscle of rats prior to hind-limb immobilization. This use of plasmid-mediated overexpression of Hsp70 provides the advantages of causing muscle-specific overexpression in adult muscle only and the capacity to dictate the level of overexpression. We show here that overexpression of Hsp70 completely abolished muscle fiber atrophy caused by immobilization. Further, we demonstrate that Hsp70 overexpression inhibited the promoter activation of the E3 ligases, atrogin-1/MAFbx, and MuRF1 and inhibited both Foxo3a and NF-κB activities during disuse. In addition, we demonstrate that the transcriptional activity of Foxo3a, but not NF-κB, is necessary for the increase in atrogin-1/MAFbx and MuRF1 promoter activities during disuse. We conclude that elevated levels of Hsp70 are sufficient to prevent skeletal muscle disuse atrophy, at least in part through inhibition of atrogin-1/MAFbx and MuRF1, which are regulated by Foxo3a. To our knowledge, this work is the first to report that Hsp70 can regulate Foxo3a signaling and draws further attention to the physiological importance of the decrease in Hsp70 that occurs during skeletal muscle atrophy.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (200 g) purchased from Charles River Laboratories (Wilmington, MA, USA) were used for all animal experiments, and all animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Expression and reporter plasmids
The pCMV5 expression vector containing Hsp70 cDNA was obtained from Dr. Bianca Brundel (University of Groningen, The Netherlands). To create the Hsp70-EGFP plasmid, we used polymerase chain reaction (PCR) to amplify the Hsp70 insert out of pCMV5 and subcloned it, in frame, into the HindIII and SalI sites on the COOH terminus of an EGFP plasmid (EGFP-c1 from Clontech, Palo Alto, CA, USA). Verification that Hsp70 was in frame was confirmed by DNA sequencing (DNA Sequencing Core, University of Florida, Gainesville, FL, USA). The NF-κB-GL3 reporter plasmid was obtained from Dr. Steffan Ho and the Foxo3a-GL3 reporter from Dr. Alex Toker (Beth Israel Deaconess Medical Center, Boston, MA, USA), and both have been previously used and described (26⤻ 27⤻ 28)⤻ . The atrogin-1-GL2 and MuRF1-GL3 reporter plasmids, both of which have been previously described (17⤻ , 18)⤻ , were generous gifts of Dr. William Mitch (Baylor College of Medicine, Houston, TX, USA) and Dr. Steve Shoelson (Joslin Diabetes Center and Harvard Medical School, Boston, MA, USA), respectively. The IκBα SR plasmid was a kind gift of Dr. Jean-Francois Peyron (INSERM, France), and the d.n. Foxo3a (Forkhead (Drosophila) homologue (rhabdomyosarcoma) like 1-DNA binding domain, FKHRL1-DBD) a kind gift of Dr. Paul Coffer (University Medical Center, Utrecht, The Netherlands), and again both have previously been described (29⤻ , 30)⤻ . Plasmid DNA was prepared and isolated using an Endotoxin-Free Maxi or Mega Prep Kit (Qiagen, Valencia, CA, USA).
Plasmid injection and electroporation
Plasmid injection and sequential transfection of skeletal muscle has been detailed previously (31)⤻ . Briefly, a small incision was made on the lateral side of the lower leg and the soleus muscle isolated. Using a 0.3 ml insulin syringe, 50 μl of 1× PBS, containing the plasmid or plasmid combinations, was injected evenly along the longitudinal axis of the soleus muscle. Following injection, electric pulses were delivered using an electric pulse generator (Electro Square porator ECM 830; BTX, Hawthorne, NY, USA), by placing two paddle-like electrodes on each side of the muscle. Five pulses were delivered in 200 ms interpulse intervals, each with an effective intensity of 125 V/cm and 20 ms duration. Electrotransfer of a mixture containing two vectors in skeletal muscle shows cotransduction of a given fiber 75–95% of the time (32⤻ , 33)⤻ ; thus, a fiber that takes up one vector will most likely also take up the other. The plasmid amounts injected were 10 μg of the expression plasmids (Hsp70, Hsp70-EGFP, IκBαS32/36, or FKHRL1-DBD) and/or 40 μg of the reporter plasmids (NF-κB-GL3, Foxo3a-GL3, atrogin-1-GL2, or MuRF1-GL3).
Immobilization
Four days following plasmid injection, animals were immobilized bilaterally with the ankle joint in the plantarflexed position to induce maximal atrophy of the soleus muscle, as described previously (11⤻ , 34)⤻ . Surfaces to be casted were first wrapped in a thin layer of protective padding (Medipore Dress-it; 3M, St. Paul, MN, USA) to prevent abrasions due to the plaster cast. The padding began just below the ribs and continued down to encompass the lower abdomen and hind limbs, leaving adequate space so that urination would not be affected. A layer of plaster was then applied and allowed to dry (Specialist; Johnson & Johnson, New Brunswick, NJ, USA). To prevent the rats from chewing through the cast, a strip of Plexiglas wrap was applied (Scotchcast Plus; 3M). Muscles of immobilized and weight-bearing animals were extracted 7 days later (11 days following plasmid injection) except in the case in which NF-κB activity and gene expression were measured at 3 days of immobilization (7 days after plasmid injection).
Muscle preparation and analysis
Soleus muscles were removed and either rapidly frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analyses, processed immediately for RNA isolation, or fixed in tissue-freezing medium and frozen for fiber sectioning and subsequent immunohistochemical analysis.
NF-κB and Foxo3a reporter activity
Following homogenization in a passive lysis buffer (Promega, Madison, WI, USA) and centrifugation for 20 min at 5000 g, 20 μl of the supernatant was added to 100 μl of luciferase reagent (Promega) for determination of total muscle luciferase activity, using an LMax II microplate luminometer (Molecular Devices Corp., Sunnyvale, CA, USA).
Immunohistochemistry
Cross-sections (10 μm) from the midbelly of the soleus muscle were cut with a cryostat microtome (Microm HM 550; Microm International, Walldorf, Germany) and fixed in 4% paraformaldehyde. For visualization of muscle fibers under fluorescence microscopy muscle sections were incubated with wheat germ agglutinin Texas Red-X conjugate (Invitrogen, Carlsbad, CA, USA). Images were captured with an Olympus IX50 camera and the muscle fiber area of ∼200 fibers from each muscle was traced and measured using Image Pro Discovery software (Media Cybernetics, Bethesda, MD, USA).
In vivo images
Prior to euthanasia, in rats whose soleus muscles were injected with either EGFP or Hsp70-EGFP, the skin on both hind limbs was retracted, and rats were placed in a MousePOD (LI-COR Biosciences, Lincoln, NE, USA) on top of an Odyssey infrared imaging system (LI-COR Biosciences) for in vivo imaging.
Western blot
A detergent-compatible assay (Bio-Rad, Hercules, CA, USA) was used to determine protein concentration of muscle homogenates. Samples were diluted in loading buffer (Bio-Rad) containing 5% β-mercaptoethanol to achieve a protein concentration of 2 mg/ml and were heat denatured. Equal amounts of protein were loaded onto 4–15% linear gradient gels and separated using SDS-polyacrylamide gel electrophoresis. Proteins were transferred for 90 min at 100 V onto an Immobilon-FL polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA), blocked in PBS containing 5% milk and 0.05% Tween for 1 h, and incubated overnight with primary antibody diluted in blocking buffer. The following primary antibodies were used: anti-ubiquitin (U-5379; Sigma-Aldrich, St. Louis, MO, USA); anti-Hsp70 (ab6535; Abcam, Cambridge, MA, USA); anti-GFP (sc-8334; Santa Cruz Biotechnology, Santa Cruz, CA, USA); and anti-IκBα (sc-371; Santa Cruz Biotechnology). Following a series of washes, the membranes were incubated with Alexa Fluor 680 or IRDye800 (LI-COR Biosciences) fluorescent-dye conjugated secondary antibodies and visualized using the Odyssey infrared imaging system (LI-COR Biosciences). Relative quantification of proteins was determined by measuring the fluorescence of each lane at the appropriate molecular weight, or in the case of total ubiquitinated proteins, total fluorescence in each lane.
RNA isolation, cDNA synthesis, and RT-PCR
Following 3 days of immobilization or weight-bearing activity, the soleus muscles were removed and total RNA was isolated using the Trizol-based method previously described (35)⤻ . The RNA quality was subsequently improved by using RNeasy columns (Qiagen). Total RNA (1 μg) and oligo dT primers were used to synthesize cDNA using the RETROscript First Strand Synthesis Kit (Ambion, Austin, TX, USA), according to the manufacturers instructions. cDNA (5 μl) was then used as a template for real-time qRT-PCR using primer sets (Hspa1a, GenBank accession no. NM_031971; Hspa2, GenBank NM_021863; atrogin-1, GenBank NM_133521; MuRF1, GenBank NM_080903; Nedd4, GenBank U50842; 18S, GenBank X03205.1) from Applied Biosystems (Austin, TX, USA) and a 7300 real-time PCR system (Applied Biosystems). TaqMan probe-based chemistry was used to allow detection of PCR products, and quantitation of gene expression was performed using the relative standard curve method.
Statistical analysis
Hspa1a and Hspa2 mRNA expression and Hsp70 protein expression were analyzed using a 1-tailed Student’s t test, with all other data analyzed using a 2-way ANOVA followed by Bonferroni corrections for multiple comparisons when appropriate (GraphPad Software, San Diego, CA). All data are expressed as means ± se, and significance was established at the P < 0.05 level.
RESULTS
Hsp70 expression
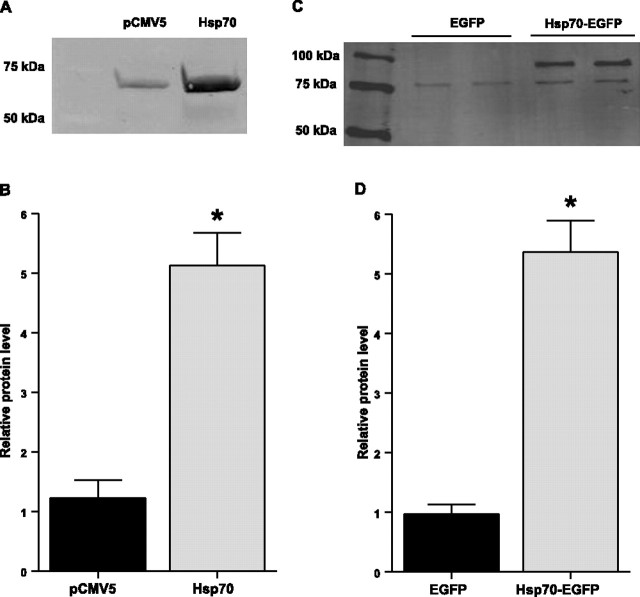
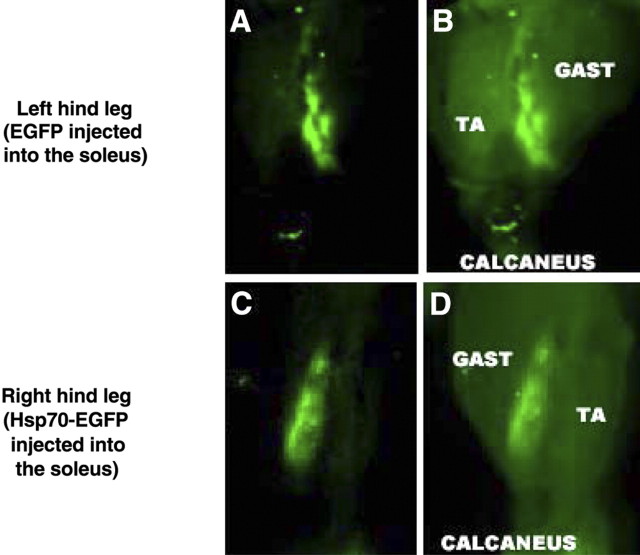
Hsp70 levels are consistently down-regulated during skeletal muscle disuse (5⤻ , 7⤻ , 10)⤻ . In this study the mRNA expression of Hspa1a (Hsp70–1) and Hspa2 (Hsp70–2), which individually code for Hsp70, were both decreased by ∼70% after 3 days of immobilization (Fig. 1⤻ A). Furthermore, although Hsp70 protein expression was unchanged following 3 days of hind-limb immobilization, it was decreased by 28% following 7 days of immobilization (Fig. 1B⤻ ). To overexpress Hsp70 we injected and electrotransfered an Hsp70 expression plasmid (pCMV5-Hsp70) or a control plasmid (pCMV5) into the soleus muscle of rats. Injection of Hsp70 resulted in a 5-fold increase in Hsp70 protein expression over endogenous Hsp70 (Fig. 2⤻ B). In addition, so that we could visualize those fibers expressing the Hsp70 plasmid, we used an Hsp70-EGFP plasmid or an EGFP plasmid as control. Overexpression of the Hsp70-EGFP fusion protein was confirmed via Western blot analysis (Fig. 2C⤻ ) and also resulted in a 5-fold increase in Hsp70-EGFP protein expression over endogenous Hsp70 (Fig. 2D⤻ ). Furthermore, localization of the Hsp70-EGFP fusion protein to the soleus muscle was confirmed by imaging the hind limb, in vivo, using a MousePOD and the Odyssey imaging system(LI-COR) (Fig. 3⤻ ).
Figure 1.
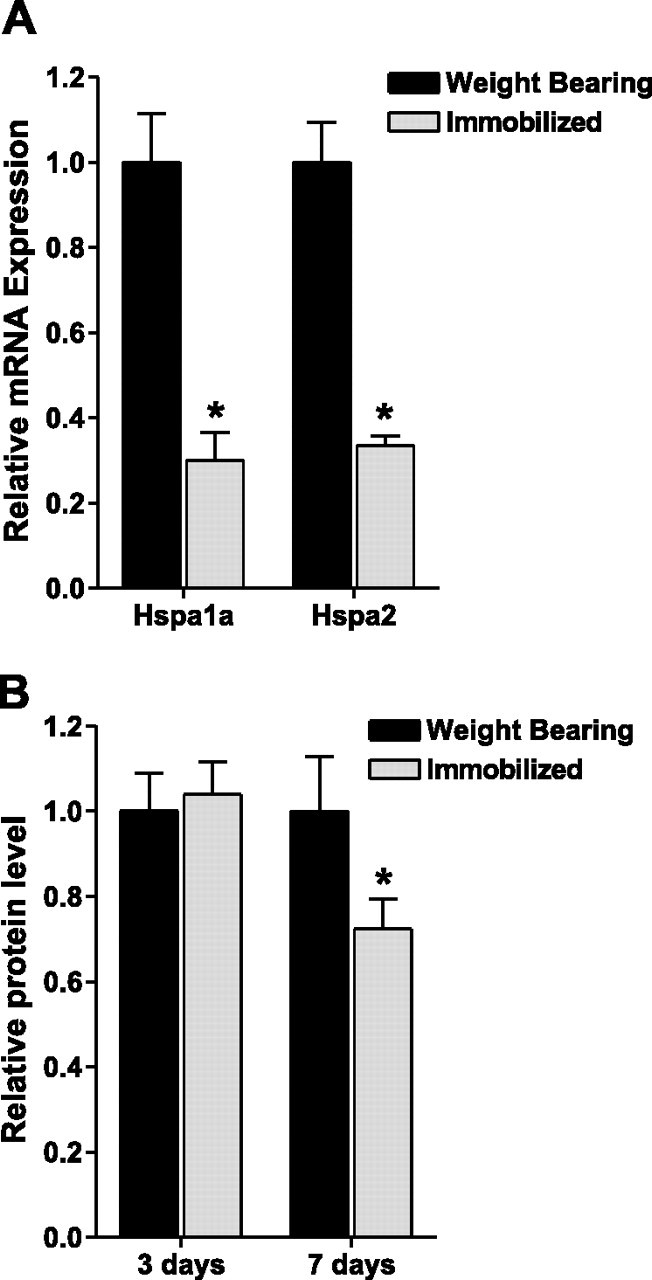
Hind-limb immobilization decreases Hsp70 mRNA and protein levels. A) Hspa1a and Hspa2 mRNA expression from weight-bearing and immobilized soleus muscles after 3 days. B) Hsp70 protein expression from weight-bearing and immobilized soleus muscles after 3 days and 7 days. Bars represent means ± se from 6 muscles. *P < 0.05 vs. weight bearing.
Figure 2.
Overexpression of Hsp70. A) Representative Western blot of whole-cell lysates from solei injected with either a control or Hsp70 expression plasmid and blotted for Hsp70. B) Quantification of Hsp70 levels from muscle extracts described in A. C) Representative Western blot of whole-cell lysates from solei injected with either EGFP or Hsp70-EGFP and blotted for Hsp70. EGFP is 27 kDa; therefore, Hsp70-EGFP is ∼97 kDa. D) Quantification of Hsp70 levels from muscle extracts described in C. Bars represent means ± se from ≥6 muscles. *P < 0.05 vs. control.
Figure 3.
Localization of the overexpressed Hsp70-EGFP to the soleus muscle. Representative in vivo images (MousePOD; LI-COR) of a left and right rat hind leg injected with EGFP or Hsp70-EGFP, respectively. A, C) Brightness low to demonstrate localization of fluorescent signal to one area. B, D) Brightness increased to show the outline of the hind leg and, therefore, to identify the soleus as the muscle of localized fluorescence. TA, tibialis anterior; GAST, gastrocnemius.
Hsp70 overexpression prevents immobilization-induced muscle fiber atrophy
To determine whether overexpression of Hsp70 attenuates skeletal muscle fiber atrophy, we injected the Hsp70-EGFP plasmid into the soleus muscle of both hind limbs of weight-bearing and 7-day immobilized rats. The cross-sectional area of green fluorescent fibers (fibers expressing the Hsp70-EGFP fusion protein) was measured and compared to the cross-sectional area of nonfluorescing fibers (fibers not expressing Hsp70-EGFP) (Fig. 4⤻ A, B). The transfection efficiency in these experiments, measured by calculating the number of GFP-expressing fibers compared to the total number of fibers, was 59%. Muscle fiber cross-sectional area was reduced with 7 days of immobilization by 28% in fibers not expressing Hsp70-EGFP (weight bearing, 2373±85.5 μm2; immobilized, 1719±160.6 μm2). However, immobilization did not cause significant muscle fiber atrophy in those fibers expressing Hsp70-EGFP (weight bearing, 2321±52.4 μm2; immobilized, 2147±32.7 μm2), demonstrating a key role for Hsp70 in disuse muscle atrophy (Fig. 4C⤻ ). Verification that EGFP has no effect on muscle fiber cross-sectional area has previously been confirmed (26)⤻ .
Figure 4.
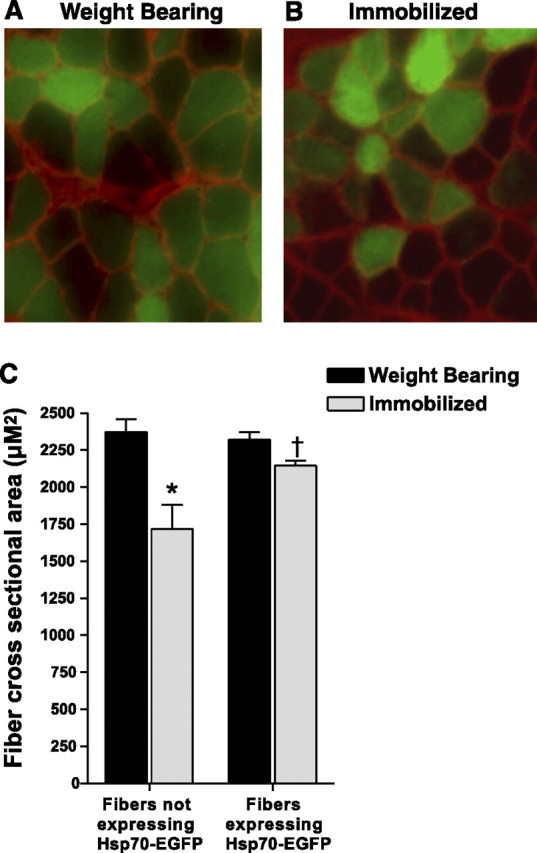
Hsp70 overexpression prevents skeletal muscle fiber atrophy. A, B) Representative cross sections taken from the soleus muscle of weight bearing (A) and 7 day immobilized rats (B) injected with Hsp70-EGFP. The cross-sectional area of green fluorescent fibers (fibers expressing Hsp70-EGFP) were compared to the cross-sectional area of nonfluorescent fibers (fibers not expressing Hsp70-EGFP) within the same muscle. C) Muscle fiber cross-sectional area of ∼250 fibers/muscle, from 6 muscles/group. *P < 0.05 vs. nonexpressing weight-bearing fibers; †P < 0.05 vs. nonexpressing immobilized fibers.
Overexpression of Hsp70 attenuates the increased expression of ubiquitin ligases
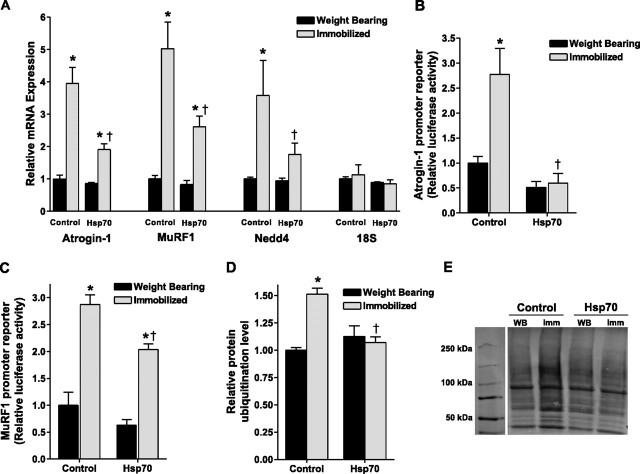
Because the ubiquitin proteasome pathway is the predominant pathway of protein degradation in atrophying skeletal muscle, we determined whether Hsp70 overexpression attenuated components of this pathway. Specifically, we determined whether Hsp70 overexpression could attenuate the widely reported increase in the mRNA expression of the E3 ubiquitin ligase genes, atrogin-1/MAFbx, MuRF1, and neuronal precursor cell-expressed developmentally down-regulated 4 (Nedd4) (8⤻ , 16⤻ , 26⤻ , 36⤻ , 37)⤻ . Three days of hind-limb immobilization increased the mRNA expression of atrogin-1/MAFbx, MuRF1, and Nedd4 by approximately 4-fold, 5-fold, and 3-fold, respectively, which were attenuated by 65, 56, and 68%, respectively, in muscles injected with Hsp70 (Fig. 5⤻ A). The unchanged 18S rRNA illustrates the standardization of loading for these genes and the Hspa1a and Hspa2 genes.
Figure 5.
Hsp70 inhibits the immobilization-induced increase in ubiquitin ligases. A) mRNA expression of MAFbx/atrogin-1, MuRF1, and Nedd4 in weight-bearing and 3 day immobilized soleus muscles injected with either a control or Hsp70 plasmid. The standardization of loading is illustrated by the unchanged 18S rRNA. B, C) Atrogin-1 (B) and MuRF1 (C) promoter reporter activity from the soleus muscles of weight-bearing and 3-day immobilized rats, injected with a control or Hsp70 plasmid plus the respective promoter reporter plasmid. D) Total ubiquitinated proteins from weight-bearing and 7 day immobilized soleus muscles injected with either a control or Hsp70 plasmid. E) Representative Western blot of whole-cell lysates from weight-bearing or immobilized muscles injected with either a control or Hsp70 expression plasmid and blotted for ubiquitin. The sum fluorescence of each lane was used to quantify total ubiquitinated protein of that sample. Bars represent means ± se from ≥6 muscles. *P < 0.05 vs. weight-bearing control; †P < 0.05 vs. immobilized control.
Because the transfection efficiency was 50–70%, yet we isolated mRNA from whole muscle for the measurement of mRNA expression, the attenuated increase in atrogin-1/MAFbx, MuRF1 and Nedd4 mRNA could be dilutions of what would be seen with increased transfection efficiency. Therefore, we coinjected Hsp70 with either an atrogin-1 promoter-luciferase reporter plasmid or a MuRF1 promoter-luciferase reporter plasmid to further determine the specific effect of Hsp70 on the transcriptional activity of these genes. The advantage of coinjecting two plasmids is that cotransduction of a given fiber occurs 75–95% of the time (32⤻ , 33)⤻ . Thus, the atrogin-1 and MuRF1 promoter reporter plasmids only report from those fibers that also took up the Hsp70 plasmid. The results are, therefore, a true representation of the Hsp70 effect on these genes. Atrogin-1 and MuRF1 promoter activities were increased >2.5-fold following 3 days of immobilization. However, in muscles injected with Hsp70, atrogin-1 transcriptional activity was completely abolished and MuRF1 transcriptional activity was significantly attenuated following 3 days of immobilization, confirming that Hsp70 can inhibit both atrogin-1 and MuRF1 promoter activities (Fig. 5B, C⤻ ). In addition, we measured total ubiquitinated proteins in whole muscle cell lysates from weight-bearing and 7-day immobilized rats and found a 50% increase in control-injected muscles that was abolished in muscles injected with Hsp70 (Fig. 5D, E⤻ ).
Hsp70 overexpression abolishes NF-κB-dependent reporter activity
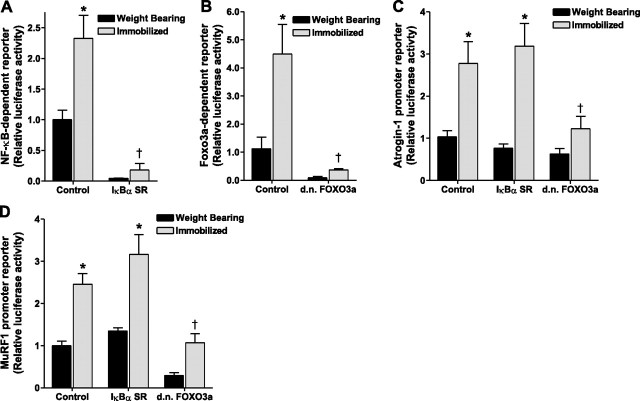
Because NF-κB is required for disuse muscle atrophy (25⤻ , 26)⤻ , sufficient to increase MuRF1 expression (17)⤻ , and is inhibited by Hsp70 in specific nonmuscle cells (3⤻ , 38⤻ , 39)⤻ , we next sought to determine whether Hsp70 could inhibit the reported increase in NF-κB activity during disuse. NF-κB-dependent reporter activity was increased by ∼2-fold and 5-fold in immobilized muscles injected with a control plasmid plus an NF-κB reporter plasmid following 3 and 7 days of hind-limb immobilization, respectively, compared to weight bearing. However, in muscles injected with Hsp70 plus an NF-κB reporter plasmid, NF-κB-dependent reporter activity was completely abolished at both time points in weight-bearing and immobilized muscles (Fig. 6⤻ A). These findings demonstrate that an increase in Hsp70 expression is sufficient to inhibit NF-κB activation in skeletal muscle. This inhibition of NF-κB by Hsp70 is not due to changes in Rel A (p65) or NF-κB1 (p105/p50) transcription, because their levels were unaffected by Hsp70 overexpression (data not shown). To ensure that the Hsp70-EGFP fusion protein also inhibited NF-κB reporter activity, we coinjected the NF-κB reporter plasmid with either pCMV5, EGFP, Hsp70, or Hsp70-EGFP into the solei of rats and immobilized the hind limbs of all rats. Immobilization-induced NF-κB reporter activity was inhibited the same with Hsp70-EGFP as with Hsp70, confirming that the Hsp70-EGFP fusion protein functions the same as Hsp70 (data not shown).
Figure 6.
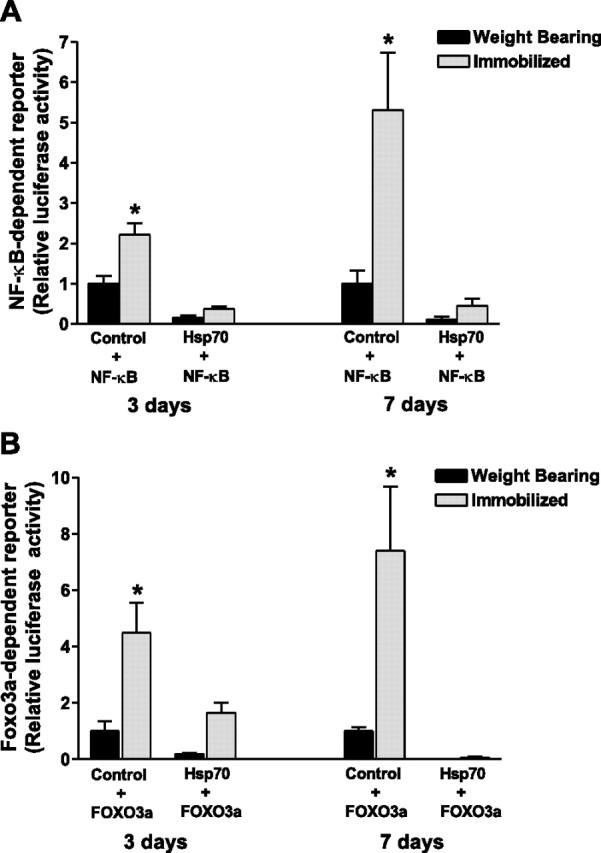
Hsp70 abolishes immobilization-induced NF-κB and FOXO3a transcriptional activation. NF-κB (A) and Foxo3a (B) reporter activity from the soleus muscle of weight-bearing and 3 and 7 day immobilized rats, injected with either a control or Hsp70 plasmid plus the respective reporter plasmid. Bars represent means ± se from 6 or 8 muscles (3 and 7 day study, respectively). *P < 0.05 vs. weight-bearing control.
Hsp70 overexpression abolishes Foxo3a-dependent reporter activity
Given that specific inhibition of NF-κB activity during disuse attenuates skeletal muscle fiber atrophy by 40–45% (17⤻ , 26)⤻ and that overexpression of Hsp70 completely abolished muscle fiber atrophy, we determined whether Hsp70 could also inhibit the other major signaling pathway known to regulate skeletal muscle mass, Foxo. Foxo3a transcriptional activity was increased by 5-fold and 7-fold following 3 and 7 days of hind-limb immobilization in control-injected muscles and was completely abolished in muscles injected with Hsp70 (Fig. 6B⤻ ). Therefore, increased levels of Hsp70 are sufficient to inhibit Foxo3a activation. Similar to NF-κB, the inhibition of Foxo3a activation, by Hsp70, is not a transcriptional inhibition since Foxo3a mRNA expression was unaffected by Hsp70 overexpression (data not shown). Again we tested the Hsp70-EGFP fusion protein and found a similar magnitude of Foxo inhibition (data not shown).
NF-κB is not required for atrogin-1 or MuRF1 transcription during disuse
To determine whether the inhibition of atrogin-1 and MuRF1 promoter activities by Hsp70 could be due to its inhibition of NF-κB activity, we first coinjected either a control plasmid or the IκBα SR with the NF-κB reporter plasmid prior to 3 days of immobilization. As expected, the IκBα SR abolished the immobilization-induced increase in NF-κB activity (Fig. 7⤻ A). We subsequently coinjected the control or IκBα SR with the atrogin-1 or MuRF1 promoter reporter plasmid into the skeletal muscle of rats prior to 3 days of immobilization. Surprisingly, the increase in atrogin-1 and MuRF1 promoter activities with immobilization were unchanged by the IκBα SR (Fig. 7C, D⤻ ).
Figure 7.
Foxo3a, but not NF-κB, is required for the immobilization-induced increases in Atrogin-1 and MuRF1 promoter activities. A) NF-κB reporter activity from the soleus muscle of weight bearing and 3-day immobilized rats injected with either a control plasmid or the IκBα super repressor (IκBα SR). B) Foxo3a reporter activity from the soleus muscle of weight bearing and 3-day immobilized rats injected with a control plasmid or d.n.Foxo3a. C, D) Atrogin-1 (C) and MuRF1 (D) promoter reporter activity from the soleus muscles of weight bearing or 3-day immobilized rats injected with a control plasmid, the IκBα super repressor, or d.n.Foxo3a. Bars represent means ± se from 6 muscles. *P < 0.05 vs. weight-bearing control; †P < 0.05 vs. immobilized control.
Foxo3a is required for both atrogin-1 and MuRF1 transcription during disuse
Because atrogin-1 and MuRF-1 may both be regulated by Foxo, we next determined whether the inhibition of their promoter activities by Hsp70 could be due to the inhibition by Hsp70 of Foxo3a. To inhibit Foxo3a transcriptional activity we coinjected a control or d.n. Foxo3a expression plasmid with the Foxo3a reporter plasmid prior to 3 days of immobilization. As shown in Fig. 7B⤻ , the immobilization-induced increase in Foxo3a activity was abolished by expression of the d.n. Foxo3a. We then coinjected the control or d.n. Foxo3a plasmids with the atrogin-1 or MuRF1 reporter plasmids prior to 3 days of hind-limb immobilization. The increases in both atrogin-1 and MuRF1 promoter activities with immobilization were completely abolished in muscles injected with the d.n. Foxo3a (Fig. 7C, D⤻ ).
DISCUSSION
Skeletal muscle disuse has consistently been shown to result in the down-regulation of Hsp70, at both the mRNA and protein levels (5⤻ 6⤻ 7⤻ 8⤻ , 10)⤻ . The findings of this study are unique in that we use an in vivo model to show that overexpression of Hsp70 is sufficient to prevent skeletal muscle fiber atrophy caused by disuse, inhibit the widely reported increase in atrogin-1 and MuRF1 during disuse; and abolish the increase in Foxo3a and NF-κB activation during disuse.
Hsp70 overexpression prevents skeletal muscle fiber disuse atrophy
The decrease in Hsp70 expression during skeletal muscle disuse has long been speculated to contribute to the observed muscle atrophy. However, the functional role of Hsp70 in chaperoning nascent polypeptides during translation (6⤻ , 40)⤻ is the suggested mechanism. In this respect, a decrease in Hsp70 could slow the elongation rate and decrease protein synthesis. However, although decreased protein synthesis may contribute to muscle atrophy, increased protein degradation appears to play a much greater role (12⤻ , 41)⤻ . Furthermore, the rates of protein synthesis and translational efficiency were recently shown to be unaltered following 5 days of hind-limb immobilization despite significant muscle atrophy (42)⤻ .
Despite the speculated role of Hsp70, no study has actually tested for a direct role of Hsp70 in the maintenance of skeletal muscle mass during disuse. Therefore, we transduced rat soleus muscle with an Hsp70 expression plasmid to specifically overexpress Hsp70 in skeletal muscle during disuse. This transduction caused an approximately 5-fold increase in Hsp70 protein expression over endogenous levels, which we consider to be a physiological level of induction since physical exercise causes comparable increases (43⤻ , 44)⤻ . In contrast, Hsp70 transgenic mice are reported to show a 10- to 20-fold increase in skeletal muscle Hsp70 protein levels (45)⤻ , which may not be physiologically attainable. The level of Hsp70 overexpression in the current study completely prevented skeletal muscle fiber atrophy in those fibers overexpressing Hsp70 compared to fibers not overexpressing Hsp70. This finding shows, for the first time, a direct role for Hsp70 in regulating muscle mass during disuse.
Hsp70 regulates the expression of ubiquitin ligases
The increased expression of the E3 ligases, atrogin-1/MAFbx, MuRF1, and Nedd4, after 3 days of hind-limb immobilization, was significantly attenuated in immobilized muscles injected with Hsp70. Since these ubiquitin ligases catalyze the transfer of active ubiquitin to specific protein substrates (36)⤻ , the sum of their inhibition could explain the complete attenuation of total ubiquitinated proteins in muscles overexpressing Hsp70.
Although the protein substrates that are ubiquitinated by atrogin-1/MAFbx, MuRF1, and Nedd4 remain largely unknown, some evidence indicates that atrogin-1/MAFbx ubiquitinates and degrades MyoD (46)⤻ , MuRF1 ubiquitinates and degrades Troponin I (47)⤻ and myosin heavy chain (48)⤻ , and Nedd4 targets Notch1 (37)⤻ . Of these known substrates myosin heavy chain is perhaps the most functionally significant since myosin is a major contractile protein and therefore an important determinant of contractile function.
Hsp70 inhibits NF-κB and Foxo3a reporter activities
Our finding of an increase in NF-κB reporter activity during disuse muscle atrophy is congruent with the findings of others (17⤻ , 25⤻ 26⤻ 27)⤻ . It has also been shown that overexpression of the IκBα SR during disuse abolishes the increase in NF-κB activity and attenuates skeletal muscle fiber atrophy by 40–45% (17⤻ , 26)⤻ . In this study we show that overexpression of Hsp70, like the super repressor, completely abolishes the increase in NF-κB reporter activity following 3 and 7 days of immobilization. Although Hsp70 inhibits NF-κB activity in Cos-1, HeLa, 293 cells (38)⤻ , human T-lymphoma Molt4 cells (3)⤻ , and brain tissue (39)⤻ , to our knowledge this is the first work to show that Hsp70 can inhibit NF-κB activation in skeletal muscle, either in vitro or in vivo.
Because the magnitude of NF-κB inhibition seen in this study is similar to that reported in the IκBα super repressor studies, it seems likely that ∼40–45% of the atrophy attenuation by Hsp70 is due to its inhibition of NF-κB. The mechanism of atrophy attenuation through the inhibition of NF-κB signaling is presumed to be through down-regulation of NF-κB target genes that are involved in muscle atrophy. In this respect, MuRF1 (17⤻ , 49)⤻ , UbcH2 (an E2 protein, or ubiquitin carrier protein) (50)⤻ , the C3 proteasome subunit (51)⤻ , and atrogin-1/MAFbx (26)⤻ are all potential NF-κB target genes. Yet none of these genes have been identified as bona fide NF-κB target genes. In the current study, we show that complete inhibition of NF-κB reporter activity during immobilization, by overexpression of the IκBα super repressor, does not inhibit the increased promoter activities of MuRF1 or atrogin-1/MAFbx. This finding shows that NF-κB is not required for the increased transcription of either MuRF1 or atrogin-1/MAFbx during disuse and therefore suggests that NF-κB regulates the transcription of other atrophy genes that are required for disuse muscle atrophy.
Although we show here that NF-κB is not required for MuRF1 transcription during disuse, NF-κB is sufficient to increase MuRF1 transcription. This was shown in transgenic mice overexpressing a muscle-specific c.a. IKKβ (MIKK mice). MIKK mice showed a 15-fold increase in NF-κB activity that was associated with an increase in MuRF1 mRNA expression but not atrogin-1 (17)⤻ . In the same study TNF-α treatment of C2C12 myotubes caused a 4.6-fold increase in MuRF1 promoter activity, which was abolished by transfection of the IκBα SR. Therefore, unlike disuse, NF-κB is required for TNF-α-induced MuRF1 transcription.
Our finding that Hsp70 can inhibit Foxo3a reporter activity has not previously been shown in any cell type. Although the effects of Foxo3a inhibition in a physiological model of muscle atrophy are currently unknown, Foxo3a overexpression is sufficient to cause muscle atrophy in C2C12 myotubes and in whole muscle (19)⤻ . Therefore, the inhibition of Foxo3a reporter activity by Hsp70 likely explains, to some degree, the prevention of muscle fiber atrophy by Hsp70 during disuse. We show here that expression of a d.n. Foxo3a plasmid during disuse abolished the increase in Foxo3a reporter activity and inhibited the increase in atrogin-1/MAFbx and MuRF1 promoter activities. These are the same findings we observed with overexpression of Hsp70. It therefore seems likely that the inhibition of atrogin-1/MAFbx and MuRF1 promoter activities by Hsp70 are through its inhibition of Foxo3a.
Thus, these findings show, for the first time, that Foxo3a activity is required for atrogin-1/MAFbx and MuRF1 transcriptional activation in vivo during muscle atrophy. Our finding of a transcriptional regulation of atrogin-1/MAFbx by Foxo3a is in agreement with others (19)⤻ . Expression of a c.a. Foxo3a construct is sufficient to increase atrogin-1 promoter activity and cause atrophy in myotubes and in whole muscle (19⤻ , 52)⤻ . In addition, expression of a d.n. Foxo3a construct significantly attenuates the increase in atrogin-1 mRNA expression and myotube atrophy caused by dexamethasone treatment. However, since overexpression of atrogin-1 alone is not sufficient to cause either myotube or muscle atrophy (19)⤻ Foxo3a must regulate other atrophy genes that explain the atrophy caused by overexpression of c.a. Foxo3a. We show here that Foxo3a also regulates MuRF1.
Our finding of a direct transcriptional regulation of MuRF1 by Foxo3a has not previously been reported. However, some indirect evidence suggests that Foxo1 regulates MuRF1 since IGF-1 treatment blocks the dexamethasone-induced increase in MuRF1 mRNA expression in myotubes overexpressing WT Foxo1 (53)⤻ . In addition, intramuscular injection of IGF-1 inhibits the increase in MuRF1 mRNA expression with denervation (53)⤻ . Because IGF-1 leads to the phosphorylation and inactivation of both Foxo1 and Foxo3a (19⤻ , 54)⤻ , this inhibition of MuRF1 with IGF-1 injection could be regulated by either, or both, Foxos.
In summary, we demonstrate that increased levels of Hsp70 are sufficient to prevent skeletal muscle disuse atrophy, which appears to be mediated, at least in part, through Hsp70’s inhibition of Foxo3a and NF-κB signaling. While these data further support the notion that a decrease in Hsp70, as occurs during skeletal muscle atrophy, may enhance Foxo3a and NF-κB signaling, further investigation is clearly needed. Furthermore, although the current work focuses on the role of Hsp70 in regulating signaling pathways that lead to protein degradation pathways, we acknowledge that muscle atrophy is a result of reduced protein synthesis as well as increased protein degradation. It is therefore possible that the prevention of disuse muscle atrophy, by Hsp70, is also a function of maintained protein synthesis. However, further investigation is clearly needed to determine whether Hsp70 directly regulates both protein degradation and protein synthesis during muscle atrophy.
References
- 1.Liu, Y., Gampert, L., Nething, K., Steinacker, J. M. (2006) Response and function of skeletal muscle heat shock protein 70. Front. Biosci. ,2802-2827 [DOI] [PubMed] [Google Scholar]
- 2.Jaattela, M., Wissing, D., Kokholm, K., Kallunki, T., Egeblad, M. (1998) Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. ,6124-6134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzhova, I. V., Darieva, Z. A., Melo, A. R., Margulis, B. A. (1997) Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones ,132-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer, M. P., Bukau, B. (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. ,670-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selsby, J. T., Rother, S., Tsuda, S., Pracash, O., Quindry, J., Dodd, S. L. (2007) Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J. Appl. Physiol. ,1702-1707 [DOI] [PubMed] [Google Scholar]
- 6.Naito, H., Powers, S. K., Demirel, H. A., Sugiura, T., Dodd, S. L., Aoki, J. (2000) Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J. Appl. Physiol. ,359-363 [DOI] [PubMed] [Google Scholar]
- 7.Lawler, J. M., Song, W., Kwak, H. B. (2006) Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve ,200-207 [DOI] [PubMed] [Google Scholar]
- 8.Stevenson, E. J., Giresi, P. G., Koncarevic, A., Kandarian, S. C. (2003) Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J. Physiol. ,33-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecker, S. H., Jagoe, R. T., Gilbert, A., Gomes, M., Baracos, V., Bailey, J., Price, S. R., Mitch, W. E., Goldberg, A. L. (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. ,39-51 [DOI] [PubMed] [Google Scholar]
- 10.Oishi, Y., Taniguchi, K., Matsumoto, H., Kawano, F., Ishihara, A., Ohira, Y. (2003) Upregulation of HSP72 in reloading rat soleus muscle after prolonged hindlimb unloading. Jpn. J. Physiol. ,281-286 [DOI] [PubMed] [Google Scholar]
- 11.Selsby, J. T., Dodd, S. L. (2005) Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am. J. Physiol. ,R134-R139 [DOI] [PubMed] [Google Scholar]
- 12.Thomason, D. B., Biggs, R. B., Booth, F. W. (1989) Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am. J. Physiol. ,R300-R305 [DOI] [PubMed] [Google Scholar]
- 13.Costelli, P., Baccino, F. M. (2003) Mechanisms of skeletal muscle depletion in wasting syndromes: role of ATP-ubiquitin-dependent proteolysis. Curr. Opin. Clin. Nutr. Metab. Care ,407-412 [DOI] [PubMed] [Google Scholar]
- 14.Jagoe, R. T., Goldberg, A. L. (2001) What do we really know about the ubiquitin-proteasome pathway in muscle atrophy?. Curr. Opin. Clin. Nutr. Metab. Care ,183-190 [DOI] [PubMed] [Google Scholar]
- 15.Cao, P. R., Kim, H. J., Lecker, S. H. (2005) Ubiquitin-protein ligases in muscle wasting. Int. J. Biochem. Cell Biol. ,2088-2097 [DOI] [PubMed] [Google Scholar]
- 16.Bodine, S. C., Latres, E., Baumhueter, S., Lai, V. K., Nunez, L., Clarke, B. A., Poueymirou, W. T., Panaro, F. J., Na, E., Dharmarajan, K., Pan, Z. Q., Valenzuela, D. M., DeChiara, T. M., Stitt, T. N., Yancopoulos, G. D., Glass, D. J. (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science ,1704-1708 [DOI] [PubMed] [Google Scholar]
- 17.Cai, D., Frantz, J. D., Tawa, N. E., Jr, Melendez, P. A., Oh, B. C., Lidov, H. G., Hasselgren, P. O., Frontera, W. R., Lee, J., Glass, D. J., Shoelson, S. E. (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell ,285-298 [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. W., Dai, G., Hu, Z., Wang, X., Du, J., Mitch, W. E. (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J. Am. Soc. Nephrol. ,1537-1545 [DOI] [PubMed] [Google Scholar]
- 19.Sandri, M., Sandri, C., Gilbert, A., Skurk, C., Calabria, E., Picard, A., Walsh, K., Schiaffino, S., Lecker, S. H., Goldberg, A. L. (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell ,399-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer, E. L., Brunet, A. (2005) FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene ,7410-7425 [DOI] [PubMed] [Google Scholar]
- 21.Giresi, P. G., Stevenson, E. J., Theilhaber, J., Koncarevic, A., Parkington, J., Fielding, R. A., Kandarian, S. C. (2005) Identification of a molecular signature of sarcopenia. Physiol. Genomics ,253-263 [DOI] [PubMed] [Google Scholar]
- 22.Kamei, Y., Mizukami, J., Miura, S., Suzuki, M., Takahashi, N., Kawada, T., Taniguchi, T., Ezaki, O. (2003) A forkhead transcription factor FKHR up-regulates lipoprotein lipase expression in skeletal muscle. FEBS Lett. ,232-236 [DOI] [PubMed] [Google Scholar]
- 23.Kamei, Y., Miura, S., Suzuki, M., Kai, Y., Mizukami, J., Taniguchi, T., Mochida, K., Hata, T., Matsuda, J., Aburatani, H., Nishino, I., Ezaki, O. (2004) Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. ,41114-41123 [DOI] [PubMed] [Google Scholar]
- 24.Kawamura, I., Morishita, R., Tomita, N., Lacey, E., Aketa, M., Tsujimoto, S., Manda, T., Tomoi, M., Kida, I., Higaki, J., Kaneda, Y., Shimomura, K., Ogihara, T. (1999) Intratumoral injection of oligonucleotides to the NF kappa B binding site inhibits cachexia in a mouse tumor model. Gene Ther. ,91-97 [DOI] [PubMed] [Google Scholar]
- 25.Hunter, R. B., Kandarian, S. C. (2004) Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J. Clin. Invest. ,1504-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judge, A. R., Koncarevic, A., Hunter, R. B., Liou, H. C., Jackman, R. W., Kandarian, S. C. (2007) Role for IkappaBalpha, but not c-Rel, in skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. ,C372-C382 [DOI] [PubMed] [Google Scholar]
- 27.Hunter, R. B., Stevenson, E., Koncarevic, A., Mitchell-Felton, H., Essig, D. A., Kandarian, S. C. (2002) Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J. ,529-538 [DOI] [PubMed] [Google Scholar]
- 28.Storz, P., Doppler, H., Toker, A. (2005) Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol. Cell. Biol. ,8520-8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dijkers, P. F., Birkenkamp, K. U., Lam, E. W., Thomas, N. S., Lammers, J. W., Koenderman, L., Coffer, P. J. (2002) FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. ,531-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traenckner, E. B., Pahl, H. L., Henkel, T., Schmidt, K. N., Wilk, S., Baeuerle, P. A. (1995) Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. ,2876-2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell-Felton, H., Kandarian, S. C. (1999) Normalization of muscle plasmid uptake by Southern blot: application to SERCA1 promoter analysis. Am. J. Physiol. ,C1269-C1276 [DOI] [PubMed] [Google Scholar]
- 32.Alzghoul, M. B., Gerrard, D., Watkins, B. A., Hannon, K. (2004) Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. ,221-223 [DOI] [PubMed] [Google Scholar]
- 33.Rana, Z. A., Ekmark, M., Gundersen, K. (2004) Coexpression after electroporation of plasmid mixtures into muscle in vivo. Acta Physiol. Scand. ,233-238 [DOI] [PubMed] [Google Scholar]
- 34.Booth, F. W., Kelso, J. R. (1973) Production of rat muscle atrophy by cast fixation. J. Appl. Physiol. ,404-406 [DOI] [PubMed] [Google Scholar]
- 35.Ahtikoski, A. M., Koskinen, S. O., Virtanen, P., Kovanen, V., Risteli, J., Takala, T. E. (2003) Synthesis and degradation of type IV collagen in rat skeletal muscle during immobilization in shortened and lengthened positions. Acta Physiol. Scand. ,473-481 [DOI] [PubMed] [Google Scholar]
- 36.Gomes, M. D., Lecker, S. H., Jagoe, R. T., Navon, A., Goldberg, A. L. (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. U. S. A. ,14440-14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koncarevic, A., Jackman, R. W., Kandarian, S. C. (2007) The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J. ,427-437 [DOI] [PubMed] [Google Scholar]
- 38.Ran, R., Lu, A., Zhang, L., Tang, Y., Zhu, H., Xu, H., Feng, Y., Han, C., Zhou, G., Rigby, A. C., Sharp, F. R. (2004) Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. ,1466-1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, Z., Kim, J. Y., Ma, H., Lee, J. E., Yenari, M. A. (2007) Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J. Cereb. Blood Flow Metab. [DOI] [PubMed] [Google Scholar]
- 40.Ku, Z., Yang, J., Menon, V., Thomason, D. B. (1995) Decreased polysomal HSP-70 may slow polypeptide elongation during skeletal muscle atrophy. Am. J. Physiol. ,C1369-C1374 [DOI] [PubMed] [Google Scholar]
- 41.Lecker, S. H., Solomon, V., Mitch, W. E., Goldberg, A. L. (1999) Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J. Nutr. ,227S-237S [DOI] [PubMed] [Google Scholar]
- 42.Krawiec, B. J., Frost, R. A., Vary, T. C., Jefferson, L. S., Lang, C. H. (2005) Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am. J. Physiol. Endocrinol. Metab. ,E969-E980 [DOI] [PubMed] [Google Scholar]
- 43.Milne, K. J., Noble, E. G. (2002) Exercise-induced elevation of HSP70 is intensity dependent. J. Appl. Physiol. ,561-568 [DOI] [PubMed] [Google Scholar]
- 44.Thompson, H. S., Scordilis, S. P., Clarkson, P. M., Lohrer, W. A. (2001) A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiol. Scand. ,187-193 [DOI] [PubMed] [Google Scholar]
- 45.Broome, C. S., Kayani, A. C., Palomero, J., Dillmann, W. H., Mestril, R., Jackson, M. J., McArdle, A. (2006) Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. ,1549-1551 [DOI] [PubMed] [Google Scholar]
- 46.Tintignac, L. A., Lagirand, J., Batonnet, S., Sirri, V., Leibovitch, M. P., Leibovitch, S. A. (2005) Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J. Biol. Chem. ,2847-2856 [DOI] [PubMed] [Google Scholar]
- 47.Kedar, V., McDonough, H., Arya, R., Li, H. H., Rockman, H. A., Patterson, C. (2004) Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc. Natl. Acad. Sci. U. S. A. ,18135-18140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke, B. A., Drujan, D., Willis, M. S., Murphy, L. O., Corpina, R. A., Burova, E., Rakhilin, S. V., Stitt, T. N., Patterson, C., Latres, E., Glass, D. J. (2007) The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell. Metab. ,376-385 [DOI] [PubMed] [Google Scholar]
- 49.Mourkioti, F., Kratsios, P., Luedde, T., Song, Y. H., Delafontaine, P., Adami, R., Parente, V., Bottinelli, R., Pasparakis, M., Rosenthal, N. (2006) Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Invest. ,2945-2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, Y. P., Lecker, S. H., Chen, Y., Waddell, I. D., Goldberg, A. L., Reid, M. B. (2003) TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J. ,1048-1057 [DOI] [PubMed] [Google Scholar]
- 51.Du, J., Mitch, W. E., Wang, X., Price, S. R. (2000) Glucocorticoids induce proteasome C3 subunit expression in L6 muscle cells by opposing the suppression of its transcription by NF-kappa B. J. Biol. Chem. ,19661-19666 [DOI] [PubMed] [Google Scholar]
- 52.Sandri, M., Lin, J., Handschin, C., Yang, W., Arany, Z. P., Lecker, S. H., Goldberg, A. L., Spiegelman, B. M. (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. U. S. A. ,16260-16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stitt, T. N., Drujan, D., Clarke, B. A., Panaro, F., Timofeyva, Y., Kline, W. O., Gonzalez, M., Yancopoulos, G. D., Glass, D. J. (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell ,395-403 [DOI] [PubMed] [Google Scholar]
- 54.Dehoux, M., Gobier, C., Lause, P., Bertrand, L., Ketelslegers, J. M., Thissen, J. P. (2007) IGF-I does not prevent myotube atrophy caused by proinflammatory cytokines despite activation of Akt/Foxo and GSK-3beta pathways and inhibition of atrogin-1 mRNA. Am. J. Physiol. Endocrinol. Metab. ,E145-E150 [DOI] [PubMed] [Google Scholar]