Abstract
Programed cell death-1/programed death ligand-1 (PD-1/PD-L1) blockade represents an affirmed reality in the treatment of advanced non-small-cell lung cancer (NSCLC) patients. Atezolizumab, an anti-PD-L1 agent, figures among the drugs that provide previously unenvisaged outcomes in the pretreated setting of metastatic NSCLC. Increasing evidence vouches for the early administration of PD-1/PD-L1 blockers in untreated patients, encompassing atezolizumab combinations with chemotherapy and the anti-angiogenic agent bevacizumab. Moreover, the development of atezolizumab allowed to derive several hints regarding clinical and immunological factors predictive of its activity and efficacy, some of them exclusive among this class of drugs. This review provides an overview of atezolizumab development throughout clinical trials toward its applicability in the routine practice, with a particular focus on patient selection based on clinical and immune-related factors.
Keywords: non-small cell lung cancer, NSCLC, immune checkpoint blockers, ICB, PD-1, PD-L1, atezolizumab development, biomarkers
Background
Immunotherapy in thoracic tumors
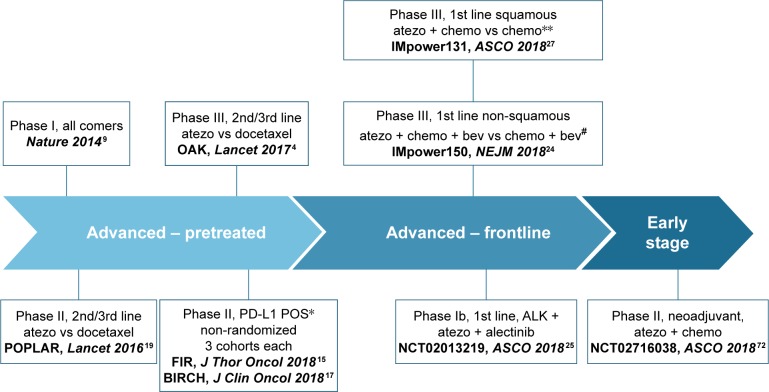
Immunotherapy agents have impetuously entered the stage with regard to the treatment of a wide spectrum of malignancies. The strategy of unleashing the immune response through the modulation of immune checkpoint has provided previously unhoped-for survival for patients suffering from lung cancer. In non-small-cell lung cancer (NSCLC), the development of therapeutic antibodies directed against programed cell death-1 (PD-1; nivolumab and pembrolizumab) and programed death ligand-1 (PD-L1; atezolizumab, durvalumab, and avelumab) has followed the classical trajectory from the advanced, pretreated settings toward the locally advanced and early stages (Figure 1). Table 1 recapitulates the approved anti-PD-1/PD-L1 antibodies in NSCLC, with regard to the differential disease settings and the PD-L1 expression levels mandatory for their prescription.1–8 As in other malignant diseases, a variety of combinatorial strategies are being currently envisaged in NSCLC, looking for the increase of both cure rates and survival outcomes across all the disease stages.
Figure 1.
Clinical development of atezolizumab in non-small-cell lung cancer.
Notes: The listed trials are the ones with available preliminary or definitive data. *PD-L1 positivity stated as TC 2/3 or IC 2/3: ≥5% PD-L1 expression on TCs or ICs. **This trial contemplates three treatment arms, the results of two of which have been recently presented (see “Activity and efficacy data of atezolizumab” section and Table 2). #This trial contemplates three treatment arms, the results of two of which have been recently published (see “Activity and efficacy data of atezolizumab” section and Table 2).
Abbreviations: Atezo, atezolizumab; bev, bevacizumab; chemo, chemotherapy; TC, tumor cell; IC, immune cell.
Table 1.
Anti-PD-1/PD-L1 agents currently approved for the treatment of advanced and locally advanced NSCLC
| Drug | PD-L1 expression on tumor cells |
|---|---|
| Advanced, pretreated setting | |
| Nivolumab1,2 | Any (or unknown) |
| Pembrolizumab3 | ≥1% |
| Atezolizumab4 | Any (or unknown) |
| Advanced, first-line setting | |
| Pembrolizumab5 | ≥50% |
| Platin-pemetrexed and pembrolizumab7,8,a | Any (or unknown) |
| Locally advanced disease, after chemo-radiotherapy | |
| Durvalumab6 | Any (or unknown) |
Note:
At the time of this study, this combination had US FDA approval for the treatment of non-squamous histologies only. EMA approval was still underway.
Abbreviation: FDA, US Food and Drug Administration.
Atezolizumab and NSCLC
Atezolizumab (MPDL3280A; Hoffman-La Roche Ltd., Basel, Switzerland) is a high-affinity human monoclonal immunoglobulin-G1 (IgG1), specifically binding to PD-L1 and preventing its interaction with PD-1 and B7.1 (also known as CD80).9 The antibody leaves the interaction of PD-1 with its alternative ligand PD-L2 (also called B7-DC or CD273) intact: PD-1/PD-L2 binding is indeed supposed to have a key role in maintaining peripheral tolerance and immune homeostasis, particularly in the lung.10,11 Atezolizumab is moreover engineered with a crystallizable fragment (Fc) domain modification, eliminating antibody-dependent cellular cytotoxicity. This mechanism avoids potential loss of PD-L1-expressing T-effector (Teff) cells and reduced anticancer immunity.12,13
After the anti-PD-1 nivolumab and pembrolizumab, atezolizumab is the first anti-PD-L1 agent to show robust activity and efficacy in advanced NSCLC patients. The present paper reviews the development of atezolizumab in advanced NSCLC (Figure 1) and its place in the clinical practice, with peculiar insights dedicated to clinical and molecular patient selection and upcoming scenarios.
Activity and efficacy data of atezolizumab
Early clinical data
The first data regarding the activity of atezolizumab in NSCLC patients refer to the “all comers” dose-escalation and dose-expansion Phase I study.9,14 This trial enrolled 88 patients with advanced pretreated NSCLC. The majority of patients had already received three lines of systemic therapy before study entry. Atezolizumab was administered three-weekly and the dose did not exceed 20 mg/kg intravenously. Objective response rate (ORR) resulted 21%; 24 weeks progression-free survival (PFS) and 1-year overall survival (OS) were 42% and 89%, respectively (Table 2). A detailed focus on molecular biomarkers associated with atezolizumab activity and efficacy already emerging from the Phase I trial (with special regard to PD-L1 expression)9 is provided in the section “Immune-related determinants of atezolizumab activity and efficacy in advanced NSCLC”.
Table 2.
Atezolizumab in advanced non-small-cell lung cancer: trials with published or presented data
| Study | Study population | Treatment arm(s) | Primary outcome(s) |
|---|---|---|---|
| Phase I | |||
| Horn et al14 | Advanced pretreated NSCLC, regardless PD-L1 expression 88 pts evaluable for activity and safety | Atezolizumab ≥20 mg/kg q3w | • Overall population: ORR 21%, mDOR 67 weeks |
| Liu et al23 | Advanced NSCLC patients regardless PD-L1 expression, candidate to first-line treatment combination of atezolizumab and platinum-based chemotherapy 76 pts evaluable for safety and activity | Atezolizumab 15 mg/kg plus Arm C: carboplatin/paclitaxel Arm D: carboplatin/pemetrexed Arm E: carboplatin/nab-paclitaxel |
Acceptable safety profile • Arm C ORR: 36%, mPFS 7.1 mo, mOS 12.9 mo • Arm D ORR: 64%, mPFS 8.4, mOS 19.3 mo • Arm E ORR: 46%, mPFS 5.7, mOS 14.8 mo |
| Kim et al25 | Advanced NSCLC ALK-positive regardless PD-L1 status candidate to first-line therapy with ALK-TKI 21 pts evaluated for safety and tolerability | Alectinib 600 mg BID for 7 days followed by alectinib 600 mg BID and atezolizumab 1,200 mg q3w | No grade 4–5 adverse events ORR 81% Median PFS 21.7 mo, median DOR 20.3 mo |
| Phase II | |||
| Spigel et al, FIR trial15 | Stage IIIB/IV NSCLC PD-L1-positive patients. 3 cohorts: 1) chemo-naive patients; 2) ≥2 line patients without brain metastases; 3) ≥2 line patients with asymptomatic treated brain metastases 138 pts (137 evaluable for efficacy) | Atezolizumab 1,200 mg q3w | • Overall population: 1) cohort ORR 29%; 2) cohort ORR 19%; 3) cohort ORR 23% |
| Peters et al, BIRCH trial17 | Stage IIIB/IV NSCLC patients with PD-L1 positivity. 3 cohorts: 1) first-line (139 pts); 2) second-line (268 pts); 3) third-line (252 pts) | Atezolizumab 1,200 mg q3w | • Overall population: 1) cohort ORR 22%; 2) cohort ORR 19%; 3) cohort ORR 18% |
| Fehrenbacher et al, POPLAR trial19 | Stage IIIB/IV NSCLC patients progressed to one line of platinum-based chemotherapy, regardless PD-L1 expression (287 pts) | Atezolizumab 1,200 mg q3w or docetaxel 75 mg/m2 q3w | • Overall population: OS 12.6 vs 9.7 mo (P=0.040) |
| Phase III | |||
| Rittmeyer et al4 and Fehrenbacher et al19 | Stage IIIB/IV NSCLC patients progressed to one line of platinum-based chemotherapy, regardless PD-L1 expression (850 pts for primary efficacy analysis; 1,225 pts for final analysis) | Atezolizumab 1,200 mg q3w or docetaxel 75 mg/m2 q3w | • Overall population: OS 13.3 vs 9.8 mo (P=0.0012); ORR 13.7% vs 11.8%; median DOR 23.9 vs 6.3 mo; PFS 2.7 vs 3.9 mo (P=0.498) |
| Socinski et al, IMpower150 trial24 | Stage IV or recurrent NSCLC patients with non- squamous histology, chemotherapy-naïve, regardless PD-L1 expression (1,202 pts) | Carboplatin AUC 6 and paclitaxel 200 mg/m2 q3w plus Arm A: atezolizumab 1,200 mg Arm B: bevacizumab 15 mg/kg Arm C: atezolizumab and bevacizumab |
Results regarding Arm C vs Arm B in WT patients • WT-overall population: PFS 8.3 vs 6.8 mo (P<0.001), ORR 63.5% vs 48%, DOR 9 vs 5.7 mo, OS 19.2 vs 14.7 mo • WT high Teff patients: PFS 11.3 vs 6.8 mo (P<0.001), ORR 69.3% vs 53.5%, DOR 11.2 vs 5.7 mo |
| Jotte et al, IMpower131 trial27 | Advanced NSCLC patients with squamous histology, chemotherapy naïve regardless PD-L expression (1,021 pts) | Arm A: atezolizumab, carboplatin, paclitaxel Arm B: atezolizumab, carboplatin, nab-paclitaxel Arm C: carboplatin, nab-paclitaxel |
Results regarding Arm B vs Arm C in WT patients • Overall population: PFS 6.3 vs 5.6 mo (P=0.0001), ORR 49% vs 40%, OS 14 vs 13.9 (P=0.693) |
Abbreviations: BID, bis in die (twice daily); DOR, duration of response; m, median; mo, months; NSCLC, non-small-cell lung cancer; ORR, overall response rate; OS, overall survival; PD-L1, programed death ligand-1; pts, patients; Teff, T-effector gene signature; PFS, progression-free survival; WT, wild-type; q3w, every three weeks.
Multiarm, non-comparative Phase II trials
Considering these results and the notable durable responses observed (median of 67 weeks in the overall population), Phase II studies were designed to confirm atezolizumab activity. The FIR trial15 is a Phase II study in which atezolizumab was administered as monotherapy every 3 weeks at a flat dose of 1,200 mg. This posology was thereafter adopted in further clinical studies and in clinical practice. The study included only patients with medium–high (≥5%) PD-L1 expression in tumor cells (TCs) or immune cells (ICs) (see “Immune-related determinants of atezolizumab activity and efficacy in advanced NSCLC” section) that were enrolled in three different cohorts: 1) untreated patients; 2) patients who had received at least one treatment and without brain metastases; 3) patients who had received at least one treatment and with asymptomatic treated brain metastases. Activity of atezolizumab was confirmed in all the cohorts of patients, both pretreated or not (Table 2). Outcomes of activity and efficacy, such as ORR (the primary objective of the trial), resulted better when considering modified (for immunotherapy) RECIST criteria compared to conventional RECIST version 1.1, in line with the noncanonical patterns of response sometimes observed with immune checkpoint blockade (ICB).16
Another Phase II trial (BIRCH) tested the efficacy of atezolizumab in both untreated and pretreated patients diagnosed with advanced NSCLC and at least 5% of PD-L1 positivity on TCs or tumor-infiltrating ICs.17 Again, three cohorts of patients were evaluated: 1) patients treated in first-line setting; 2) patients treated in second-line setting; 3) patients treated in third or subsequent lines (Table 2). Patients with brain metastases were excluded from the trial. The primary endpoint was objective response rate (ORR) by independent review, compared with pre-specified historical controls. ORR resulted ~20% in the three treatment arms (Table 2). Patients in cohort 1 presented higher median PFS (5.4 months) and OS (23.5 months), while median PFS resulted 2.8 months in both cohort 2 and 3, and median OS reached 15.5 months in cohort 2 and 13.2 months in cohort 3. In responder patients, the median duration of response (DOR) was 9.8 months in cohort 1, not reached for cohort 2, and 11.8 months for cohort 3. Recently, updated results from first-line cohort of BIRCH trial, collected after ~3 years of follow-up, have been presented.18
POPLAR trial
The POPLAR study is a Phase II, open-label, multicentric trial that tested the activity of atezolizumab vs standard chemotherapy in patients who had already received one or two lines of systemic chemotherapy.19 Patients were 1:1 randomly assigned to received atezolizumab or three-weekly docetaxel. The availability of tumor specimen was mandatory for enrolment, and patients were stratified according to PD-L1 tumor-infiltrating IC positivity. Of note, PD-L1-negative patients were not excluded. Other stratification factors were histology and previous line of therapy. For patients in the atezolizumab arm, treatment beyond progression was allowed and continued as long as patients obtained clinical benefit. Since the primary endpoint was OS, crossover was not permitted. Overall, the study enrolled 287 patients and met its primary endpoint, demonstrating that patients treated with atezolizumab reached longer median OS (12.6 months, 95% confidence interval [95% CI] 9.7–16.4) if compared with patients in docetaxel arm (9.7 months, 95% CI 8.6–12) (Table 2). This difference was statistically significant (hazard ratio [HR] 0.73; 95% CI 0.53–0.99, P=0.04), and as previously noted, the activity of atezolizumab seemed to be related to PD-L1 expression (see “Immune-related determinants of atezolizumab activity and efficacy in advanced NSCLC” section). With a median follow-up of 38 months, the updated results of the study confirmed atezolizumab to be superior to docetaxel in terms of both OS and DOR across all the histologies and PD-L1 expression subpopulations, with 2 and 3 years OS rates of 32% and 19%, respectively.20
OAK trial
Moving to the Phase III study, OAK trial randomized 1,225 patients, who had already received one or two lines of systemic therapy, to receive either atezolizumab or docetaxel.4,21 Overall, 613 and 612 patients were assigned to atezolizumab and docetaxel arm, respectively. The primary efficacy analysis included 425 patients in each arm, and OS was chosen as primary endpoint.4 Both the treatments were administered until unacceptable toxicity or disease progression, but atezolizumab could be continued beyond progression, and crossover was not allowed. However, it should be noted that 17% of patients treated with docetaxel received another immunotherapeutic agent after a documented progression as per clinical practice vs only 4% of patients treated with atezolizumab. Overall, the study reached its primary endpoint demonstrating an improvement in OS in patients treated with atezolizumab, who presented a median OS of 13.8 vs 9.6 months in patients treated with docetaxel (HR 0.73; 95% CI 0.62–0.87, P=0.0003)4 (Table 2). Although there was no significant difference in terms of PFS between the two treatment arms, patients receiving atezolizumab experienced a longer DOR (median 16.3 months) compared to patients treated with docetaxel (median 6.2 months, P<0.0001). The efficacy of atezolizumab was demonstrated to be consistent across all the subgroups of patients stratified according to PD-L1 expression, although higher benefit was noted in patients with higher expression (see “Immune-related determinants of atezolizumab activity and efficacy in advanced NSCLC” section). Based on these results, atezolizumab was approved for the treatment of advanced NSCLC patients who progressed to first-line chemotherapy, regardless of PD-L1 expression. The efficacy of atezolizumab was confirmed by the updated analyses conducted in primary (850 patients) and secondary (overall 1,225 patients) populations. In fact, median OS resulted ~4 months longer in patients treated with atezolizumab and HR resulted 0.75 (95% CI 0.64–0.89, P=0.0006) and 0.80 (95% CI 0.70–0.92, P=0.0012) in primary and secondary efficacy populations, respectively.21 Moreover, the benefit in long-term survival was irrespective of radiological response and significant also for patients who received atezolizumab beyond progression. In fact, among 332 patients who experienced progression of disease as their best response, 168 (51%) continued atezolizumab beyond progression, and the disease was found to be under control (7% partial response, 49% stable disease) at subsequent radiological assessments.22
Moving atezolizumab combinations to the first-line setting
Non-squamous NSCLC
Favorable safety and activity data regarding the combination of atezolizumab with chemotherapy (Table 2)23 prompted its further development as first-line treatment in non-squamous histology. Indeed, the preliminary results from a Phase III trial in non-squamous NSCLC (IMpower150), testing the efficacy of adding atezolizumab to carboplatin and paclitaxel chemotherapy with or without the anti-angiogenic agent bevacizumab, have been recently published.24 The study enrolled 1,202 patients with stage IV non-squamous NSCLC who were randomized to receive carboplatin, paclitaxel and atezolizumab (arm ACP), carboplatin, paclitaxel, atezolizumab and bevacizumab (arm ABCP) or carboplatin, paclitaxel, and bevacizumab (standard-control arm, BCP) for four to six cycles. In every arm, maintenance with atezolizumab, bevacizumab, or both was allowed, whereas crossover was not permitted. The study was designed to assess the effect of adding immunotherapy to chemotherapy (arm ABCP vs BCP) and changing bevacizumab with atezolizumab (arm ACP vs BCP). However, to date, only results regarding the comparison between chemotherapy with and without atezolizumab (arm ABCP vs arm BCP) have been released. During the study, the primary-analysis populations were amended with the exclusion of patients carrying EGFR or ALK alterations and by replacing the analysis of PD-L1 expression with the identification of an effector T-cell (Teff) gene signature in the tumor. This trial was designed with subsequent co-primary endpoints: PFS in the wild-type (WT) population and in the Teff-high subgroup of patients and OS in the WT population. Results of analysis after a median follow-up of ~15 months confirm that all co-primary endpoints have been met. In fact, PFS was significantly longer in ABCP arm in both WT and WT/Teff-high populations. In particular, in WT population, median PFS resulted 8.3 months in ABCP arm and 6.8 months in BCP arm (HR 0.62, 95% CI 0.52–0.74, P<0.001), while in WT/Teff-high population, the difference was even higher (11.3 vs 6.8 months, HR 0.51, 95% CI 0.38–0.68, P<0.001). With regard to OS in the WT population, the interim analysis confirmed the superiority of combination arm that reached median OS of 19.2 vs 14.7 months in chemotherapy arm (HR 0.78, 95% CI 0.64–0.96, P=0.02). ORR was also higher in the combination arm, both in WT and WT/Teff-high populations, and again median DOR was longer in patients treated with immunotherapy. In summary, results of this trial support the utility of combining immunotherapy and chemotherapy as first-line treatment in all patients with advanced non-squamous NSCLC. The safety profile of combination treatment is defined by authors as consistent with the profile of singular drugs, and no significant new safety issues emerged. While the majority of immune-related adverse events were G1 or G2 and none led to death, five patients in the ABCP arm died from bleeding events, ostensibly related to bevacizumab. Results of comparison between ACP and BCP arms are largely awaited to understand what could be the better combination of treatment choice. Interestingly, the benefit of ABCP therapy was also evident in patients harboring EGFR or ALK alterations, although they represented a minority, opening a new scenario for immunotherapy in this specific group of patients in whom results of immunotherapy alone have always been disappointing. Also, this paves the way for new strategies of combination between atezolizumab and tyrosine kinase inhibitors (TKIs). Data from Phase Ib study of alectinib plus atezolizumab in advanced ALK-positive NSCLC have been recently presented, with encouraging results in terms of both activity and safety.25
Still preliminary if compared to the robust documentation of the relevancy of combinatorial treatments involving pembrolizumab,7,8 platin/pemetrexed-based chemotherapy benefits from the addition of atezolizumab at least in terms of PFS, as recently stated by a press release on IMpower132 trial.26
Squamous NSCLC
Other promising results have been recently presented at 2018 ASCO annual meeting regarding the squamous counterpart of NSCLC. The trial IMpower131 randomized 1,021 patients with advanced squamous NSCLC to receive atezolizumab, carboplatin or paclitaxel (arm A), atezolizumab, carboplatin and nab-paclitaxel (arm B), or carboplatin and nab-paclitaxel (arm C).27 Planned cycles ranged from 4 to 6, then atezolizumab was administered as maintenance and cross-over was not allowed. Also this trial had two co-primary endpoints: PFS as per investigators’ assessment and OS, and results regarding the comparison between arm B (343 patients) and arm C (340 patients) have been announced. After a median follow-up of 17.1 months, patients receiving combination treatment presented globally a longer PFS (6.3 vs 5.6 months), and the difference was statistically significant (HR 0.71, 95% CI 0.60–0.85, P=0.0001). Although brief follow-up does not allow to draw definitive conclusions about long-term survival, the first interim analysis of OS showed no difference between the two treatment arms in the overall population, even if the percentage of patients reaching 24 months OS in arm B was higher than in arm C. However, post-progression treatments could have influenced this outcome, considering that 42% of patients in arm C (vs 5% in arm B) received single-agent immunotherapy as second-line therapy. In addition, OS in high PD-L1 patients resulted significantly longer in the combination arm (23.6 vs 14.1 months, HR 0.56, 95% CI 0.32–0.99).
Clinico-pathological parameters associated with atezolizumab benefit
Several clinical parameters have been explored by subgroup analyses in the four randomized trials including atezolizumab in the experimental arms (OAK, POPLAR, IMpower150, and IMpower131 trials), to assess their potential role as clinical predictive factors (Figure 1; Table 3).
Table 3.
Efficacy of atezolizumab according to clinical/pathological parameters in randomized trials
| Clinical/pathological parameters | OAK triala,4,21 II–III line (1,225 pts), Phase III | POPLAR triala,19 II line (287 pts), Phase II | IMpower150 trialb,24 I line (800 pts), Phase III | IMpower131 trialc,27 I line (1,021 pts), Phase III |
|---|---|---|---|---|
| ECOG performance status | ||||
| 0 | 37% (HR 0.80)d | 32% (NA) | 41% (HR 0.55) | 33% (HR 0.68) |
| 1 | 63% (HR 0.77) | 68% (NA) | 58% (HR 0.64) | 67% (HR 0.70) |
| Age | ||||
| <65 years | 54% (HR 0.84)d | NA | 54% (HR 0.65) | 48% (HR 0.77) |
| ≥65 years | 46% (HR 0.69) | NA | NA | NA |
| 65–74 years | NA | NA | 36% (HR 0.52) | 41% (HR 0.66) |
| 75–84 years | NA | NA | 9% (HR 0.78)c | 11% (HR 0.51) |
| Sex | ||||
| Male | 62% (HR 0.79) | 59% (NA) | 61% (HR 0.55) | 82% (HR 0.71) |
| Female | 38% (HR 0.81)d | 41% (NA) | 39% (HR 0.73) | 18% (HR 0.66) |
| Smoking | ||||
| Current/previous smoker | 83% (HR 0.78) | 81% (HR 0.75)d | 84% (HR 0.58) | 92% (HR 0.70) |
| Never smoker | 17% (HR 0.91)d | 19% (HR 0.55)d | 16% (HR 0.80)d | 8% (HR 0.77)d |
| Liver metastases | ||||
| Yes | NA | NA | 14% (HR 0.54)* | 20% (HR 0.77)d |
| No | NA | NA | 86% (HR 0.63) | 80% (HR 0.68) |
| Brain metastases | ||||
| Yes | 10% (HR 0.59) | NA | NA | NA |
| No | 90% (HR 0.82) | NA | NA | NA |
| Histology | ||||
| Squamous | 74% (HR 0.79) | 34% (HR 0.66)d | NAe | NAf |
| Non-squamous | 26% (HR 0.79)d | 66% (HR 0.69) | ||
| Molecular status | ||||
| EGFR mutation | 9% (HR 1.19)d | 7% (NA) | NA | NA |
| EGFR wild type | 75% (HR 0.76) | 51% (NA) | NA | NA |
| KRAS mutation | 7% (HR 0.82)d | 9% (NA) | 12% (HR 0.50) | NA |
| KRAS wild type | 24% (HR 0.93)d | 16% (NA) | 18% (HR 0.47) | NA |
| KRAS unknown | 69% (HR 0.76) | 75% (NA) | 71% (HR 0.67) | NA |
| ALK translocation | 0.4% (NA) | 1% (NA) | NA | NA |
| ALK wild type | 49% (NA) | 40% (NA) | NA | NA |
| EGFR/ALK alteration | NA | NA | 13% (HR 0.54)d,* | NA |
| EGFR/ALK wild type | NA | NA | 87% (HR 0.62) | NA |
Notes:
The hazard ratios of OAK and POPLAR studies refer to overall survival.
Hazard ratios of comparison between arms B (atezolizumab + bevacizumab + chemotherapy) and C (bevacizumab + chemotherapy) only. The hazard ratios of IMpower150 study refer to progression-free survival for all parameters, with the exception of “Liver metastases – yes” and “EGFR/ALK alteration” the hazard ratios of which refer to overall survival (*).
Hazard ratios of comparison between arms B (atezolizumab + chemotherapy) and C (chemotherapy) only. The hazard ratios of IMpower131 study refer to progression-free survival.
Confidence intervals of hazard ratio through the unit.
Only patients with non-squamous histology were enrolled in IMpower150 study.
Only patients with squamous histology were enrolled in IMpower131 study.
Abbreviations: NA, not available; pts, patients.
As shown in Table 3, the benefit of atezolizumab, in terms of PFS or OS, seems to be confirmed irrespective of ECOG PS, age, presence of brain or liver metastases, gender, and smoking history. Moreover, the ethnicity and the number of previous therapy lines (one or two) seem to not impact on drug efficacy, either.4,21 The prevalence of molecular alterations (EGFR, ALK, and KRAS) in the mentioned randomized studies was very low (Table 3), so it is difficult to establish the real benefit of atezolizumab in these subgroups. Of note, both in published non-randomized Phase II studies15,17 and in prospective randomized trials (Table 3), enrollment of patients with ECOG PS ≥2 or with autoimmune diseases was not permitted; therefore, in patients with these conditions, the benefit (and safety) of atezolizumab is not yet known.
At least eight meta-analyses (from published data), including atezolizumab among immunotherapy agents have been performed to better address the role of clinicopathological parameters as possible predictive factors of benefit from immunotherapy in advanced NSCLC.28–35 Regarding EGFR status, three meta-analyses showed that EGFR mutation could be a potential negative predictive biomarker for survival in the pretreated setting (HRs 1.05, 1.11, and 1.40 in EGFR-mutated subgroup and HRs 0.66, 0.66, and 0.67 in EGFR-WT subgroup, respectively).32,34,35 KRAS status also seems to have a predictive value, in terms of OS, as documented in three meta-analyses (HRs 0.63, 0.65, and 0.64 in KRAS-mutated subgroup and HRs 0.86, 0.86, and 0.88 in KRAS-WT subgroup, respectively).29,30,34 Age of patients (cutoff 65 years),28,29,34,35 as well as sex,29,33,35,36 had no predictive value. Lastly, smoking history seems to predict benefit from immunotherapy in four meta-analyses (HRs 0.71, 0.69, 0.70, and 0.71 in current/former smokers and HRs 0.79, 0.79, 0.79, and 0.88 in never smokers),29,31,34,35 likely encompassing the impact of smoking exposure on tumor mutational burden (TMB) (see “Immune-related determinants of atezolizumab activity and efficacy in advanced NSCLC” section).
Immune-related determinants of atezolizumab activity and efficacy in advanced NSCLC
Hints from early clinical trial
As approached in “Activity and efficacy data of atezolizumab” section, in its first-in-human trial, a total of 88 patients suffering from advanced NSCLC received atezolizumab.9,14 Besides being the most represented among tumor types, several predictive hints concerning potential predictive immunological markers of drug activity and efficacy were driven from lung cancer patients.
First, PD-L1 was assessed through immunohistochemistry (IHC) on both tumor and tumor-infiltrating ICs with SP142 clone (Ventana, Tucson, AZ, USA) on an automated staining platform (Benchmark; Ventana). The proportion of TCs expressing PD-L1 was estimated as the percentage of total TCs. With regard to the counterpart of ICs, specimens were scored as IHC 0 in the case of PD-L1-positive cells <1%, IHC 1 if PD-L1 ≥1% but <5%, 2 if PD-L1 ≥5% but <10%, or 3 if PD-L1 ≥10% (Table 4). The association between response to atezolizumab treatment and tumor-infiltrating IC PD-L1 expression reached statistical significance (P=0.015). Of note, no association was observed when PD-L1 expression was assessed on TCs (P=0.92). Exploring additional markers, no correlation between immune inhibitory factors (such as PD-2, IDO1, FOXP3, LAG3, TIM3, CTLA4, B7-H3, and B7-H4) assessed by mRNA expression levels and lack of response to atezolizumab was seen, with a trend toward increased response in PD-L1-positive patients who expressed a second negative regulator. Serial on-treatment biopsies were furthermore performed in 28 patients (four of whom suffering from NSCLC) within the entire cohort of enrolled patients. Albeit no conclusion can be driven with regard to lung cancer, tumor shrinkage was globally accompanied by an increase in PD-L1 expression on both tumor-infiltrating ICs and tumor cells, itself correlated with an increase in tumor interferon-γ (IFN-γ) expression (the role of which in inducing PD-L1 is known).37 In addition, the analysis of mRNA transcripts from regressing lesions documented a generalized activation of CD8 and TH1 T-cell responses (absent in progressing cases). Of major relevance, for the first time, three typical tissue patterns were associated with the lack of response to anti-PD-L1 blockade, by analyzing posttreatment samples: 1) scarce or no tumor-infiltrating IC infiltration (“immunological ignorance”); 2) minimal to no expression of PD-L1 by the intra-tumor immune infiltrate (“non-functional immune response”); 3) presence of an immune infiltrate exclusively around the outer edge of the TC mass (“excluded infiltrate”).9
Table 4.
PD-L1 immunohistochemistry (IHC) scoring definition on tumor cell (TC) and immune cell (IC) compartments, as reported in clinical studies from the randomized Phase II trial POPLAR in 201617
| PD-L1 TC scoring
|
PD-L1 IC scoring
|
||
|---|---|---|---|
| Score | Percentage of PD-L1 expressing cells | Score | Percentage of PD-L1 expressing cells |
| TC3 | ≥50 | IC3 | ≥10 |
| TC2 | ≥5 and <50 | IC2 | ≥5 and <10 |
| TC1 | ≥1 and <5 | IC1 | ≥1 and <5 |
| TC0 | <1 | IC0 | <1 |
PD-L1 in Phase II and III trials
Phase II and III clinical studies evaluating atezolizumab monotherapy further confirmed the association between high PD-L1 scores and better clinical outcomes, as already observed in atezolizumab early development studies and for other ICB in similar settings (Tables 5 and 6). Moving from the continuous quantification of the Phase I trial, PD-L1 expression in TCs was thereafter categorized as the percentage of PD-L1-positive cells: TC0 <1%, TC1 ≥1% and <5%, TC2 ≥5% and <50%, TC3 ≥50%.4,19 PD-L1 IHC in ICs remained as percentage of tumor area: IC0 <1%, IC1 ≥1% and <5%, IC2 ≥5% and <10%, IC3 ≥10% (Table 4). In these trials, every case was provided with its higher score, either in TC or in IC. Still, the impact of PD-L1 expression by IC appeared stronger than TC in predicting better outcomes.4 Moreover, FIR trial documented the concordance of PD-L1 expression between recent and archival tumor tissues, with higher rates of positivity observed in surgical samples compared to small biopsies.15 Of note, PD-L1 was stained using the mentioned SP142 clone. Across clinical trials enrolling patients regardless of PD-L1 expression levels,19,21,24,27 ~15% of the cases were scored as TC3 or IC3, with slighter incidence of TC2 or IC2 staining (Tables 5 and 6). TC1 or IC1 group represented around a third of the patients, and 30%–45% of the tumor specimens were labeled as PD-L1 negative (TC0 and IC0), these latter accounting for half of the cases in the mentioned Phase III trials evaluating atezolizumab combination in the first-line setting (Tables 5 and 6). Interestingly, when compared to second- or third-line docetaxel, early measures of benefit such as better response rate or PFS were achieved with atezolizumab only in TC3 or IC3 subgroups.4,19 Nevertheless, OS improvements were observed in the entire cohort and, moreover, across every level of PD-L1 expression, even in the TC0 and IC0 subgroups. The remarkable DOR of ~2 years achieved in atezolizumab-treated patients, according to the latest follow-up, is not affected by PD-L1 status.21
Table 5.
Activity and efficacy of atezolizuamb as emerged from the multiarm Phase II trials FIR and BIRCH
| Trial
|
FIR15
|
BIRCH17
|
||||
|---|---|---|---|---|---|---|
| Cohorts | 1 | 2 | 3 | 1 | 2 | 3 |
| 1st line | ≥2nd line, no BM | ≥2nd line, treated BM | 1st line | 2nd line | ≥3rd line | |
| Objective response rate (ORR), % | ||||||
| All (TC2/3 or IC2/3) | 29% | 19% | 23% | 26% | 20% | 20% |
| pts | 9/31 | 17/92 | 3/13 | 36/139 | 53/268 | 50/252 |
| TC3 or IC3 | 43% | 26% | 25% | 35% | 26% | 31% |
| pts | 3/7 | 9/38 | 2/8 | 23/65 | 32/112 | 36/115 |
| TC2 or IC2 | NA | NA | NA | 18% | 14% | 10% |
| pts | 13/74 | 20/146 | 14/136 | |||
| Overall survival (OS), months | ||||||
| All (TC2/3 or IC2/3) | 14.4 | 9.3 | 6.8 | 24 | 15.5 | 13.2 |
| pts | 31 | 93 | 13 | 138 | 269 | 252 |
| TC3 or IC3 | 15.8 | 22.2 | 7.0 | 26.9 | 16.6 | 17.5 |
| pts | 7%–23% | 38%–41% | 8%–62% | 65%–47% | 122%–46% | 115%–46% |
| TC2 or IC2 | NA | NA | NA | 23.5 | 15.5 | 11.0 |
| pts | 73%–53% | 146%–54% | 136%–54% | |||
Notes: ORRs are reported according to conventional RECIST criteria version 1.1, nevertheless, ORRs measured in line with modified Recist (the primary objective of FIR trial) were higher.
Abbreviations: BM, brain metastases; NA, not available; pts, patients; TC, tumor cell; IC, immune cell.
Table 6.
Efficacy outcomes in terms of median overall survival (OS) in atezolizumab randomized trials, according to PD-L1 expression
| Differential populations as for PD-L1 scoring | OAK21 | Poplar19 | IMpower150 (WT population)a,24 | IMpower131a,27 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atezo (months) | Doc (months) | HR P-value | Atezo (months) | Doc (months) | HR P-value | ABCP (months) | BCP (months) | HR P-value | ACnP (months) | CnP (months) | HR P-value | |
| ITT (n) pts |
13.3 613 |
9.8 612 |
0.80 0.0012 |
12.6 144 |
9.7 143 |
0.69 0.011 |
19.2 359 |
14.7 337 |
0.78 0.02 |
14.0 343 |
13.9 340 |
0.96b 0.6931 |
| TC3 or IC3 pts |
20.5 89 (14%) |
9.7 85 (14%) |
0.45 ,0.0001 |
NR 24 (17%) |
11.1 23 (16%) |
0.45 0.033 |
25.2 | 15 | 0.70b | 23.6 53 (15%) |
14.1 48 (14%) |
0.56 |
| 136 (20%) | ||||||||||||
| TC2/3 or IC2/3 pts |
16.6 168 (27%) |
11.4 182 (30%) |
0.64 0.0012 |
15.1 50 (35%) |
7.4 55 (38%) |
0.50 0.003 |
NA | NA | NA | NA | NA | NA |
| TC1/2/3 or IC1/2/3 pts |
14.3 347 (57%) |
10.8 337 (55%) |
0.77 0.0045 |
15.1 93 (65%) |
9.2 102 (71%) |
0.59 0.003 |
NA | NA | NA | NA | NA | NA |
| TC2 or IC2 pts |
12.6 | 13 | 0.98b | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 177 (14%) | ||||||||||||
| TC1/2 or IC1/2 pts |
NA | NA | NA | NA | NA | NA | 20.3 | 16.4 | 0.80b | 12.4 129 (38%) |
16.6 121 (36%) |
0.70 |
| 226 (32%) | ||||||||||||
| TC0/1/2 or IC0/1/2 pts |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| TC1 or IC1 pts |
12.7 | 9.7 | 0.87b | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 334 (27%) | ||||||||||||
| TC0 and IC0 pts |
11.8 260 (42%) |
8.9 271 (44%) |
0.84b 0.0887 |
9.7 51 (35%) |
9.7 41 (29%) |
0.88b 0.601 |
17.1 | 14.1 | 0.82b | 13.8 160 (47%) |
12.5 171 (50%) |
0.81b |
| 339 (49%) | ||||||||||||
Notes:
Preliminary results with regard to OS, given the limited follow-up.
95% confidence interval (95% CI) crossing the unit.
Abbreviations: ABCP, atezolizumab, bevacizumab, carboplatin, paclitaxel; AcnP, atezolizumab, carboplatin, nab-paclitaxel; Atezo, atezolizumab; BCP, bevacizumab, carboplatin, paclitaxel; CnP, carboplatin, nab-paclitaxel; Doc, docetaxel; HR, hazard ratio; ITT, intention to treat; NA, not available; pts, number of patients; TC, tumor cell; IC, immune cell; WT population, including only EGFR and ALK wild-type patients.
The putative lack of sensitivity of SP142 clone38 has been mitigated by the evaluation of 400 cases enrolled in the OAK Phase III trial with the 22C3 clone by Ventana, allowing PD-L1 quantification on TCs only.39 In this study, 77% of “negative” SP142 cases were also 22C3 “negative.”39 Looking at OS outcomes, comparable results were observed in PD-L1-negative subgroups when PD-L1 was defined by both the assays. The latter considerations sustain that, besides being an extremely complex biologic milieu, the issues that arose in PD-L1 determination are more likely technical and, hopefully, have been addressed.38
Looking at the most recent clinical data presented in extenso24 and as interim results,27 PD-L1 IHC maintains its role in predicting atezolizumab efficacy when combined with chemotherapy or chemotherapy and bevacizumab in squamous and non-squamous histologies, respectively (Table 6).
TMB
The attention of the lung cancer community for the quantification of DNA mutations and its relationship with benefit from ICB originated from the seminal work by Rizvi et al.40 The assessment of the TMB through whole exome sequencing (WES) allowed to determine that the most mutated NSCLC patients were indeed the ones to benefit the most from pembrolizumab administration in the pretreated setting. Similar results were observed in the first-line setting, with the demonstration of a strong predictive role of TMB, evaluated retrospectively, in addressing the outcomes of patients undergoing nivolumab or chemotherapy.41 More recently, high TMB prospectively emerged as a robust biomarker predicting positive outcomes in NSCLC patients who received first-line ICB combination with nivolumab and the CTLA-4 inhibitor ipilimumab.42,43
With regard to atezolizumab, TMB implications were retrospectively evaluated by gathering the data from the three Phase II studies FIR, BIRCH, and POPLAR.44 The median number of mutations documented by WES, assessed as 9.9/megabase pair (Mbp), correlated with the one obtained with FoundationOne targeted sequencing panel of more than 300 genes, thus envisaging the potential estimation of TMB in clinical practice. Higher TMB was correlated with better disease response, PFS and OS (with the limitation of a limited number of evaluable patients and events) under atezolizumab, whereas no predictive impact was documented regarding patients receiving docetaxel.44 Given the evolving technology in this field, circulating tumor DNA (ctDNA)45 can represent a useful and relevant tool for TMB quantification in blood samples. Evidence regarding the potential applicability of ctDNA for TMB measurement has recently been provided,46 as observed in the two randomized trials of atezolizumab vs docetaxel in pretreated patients.4,20 Retrospectively, a total of 794 baseline plasma samples underwent next-generation sequencing with a 394-gene panel and the cutoff defining high TMB was set at 16 mutations/Mbp. Of note, 16.2 mutations/Mbp corresponded to the 75% quantile observed in the mentioned study of TMB quantification in tumor samples,44 and indeed, 27% of the patients had a TMB ≥16 mutations/Mbp as for ctDNA analysis.46 Patients with high TMB obtained a benefit from atezolizumab compared to docetaxel even in terms of PFS (observed only in the TC3 or IC3 subgroup in the OAK Phase III trial),4 whereas TMB did not influence the outcomes of patients receiving docetaxel.46 Atezolizumab provided better OS compared to docetaxel regardless of TMB, with survival medians apparently similar in the ≥16 and <16 mutations/Mbp groups. As revealed for PD-L1 expression, higher values of TMB correlated with longer PFS and OS in patients receiving atezolizumab. Of note, higher ctDNA TMB was documented in plasma samples of patients with current or previous tobacco exposure. Importantly, in terms of intrinsic validity and potential widespread clinical application, concordance between TMB measured in tumor samples and matched ctDNA was observed. As noticed in the experiences with nivolumab and ipilimumab,41,43 no correlation between TMB quantification in plasma and PD-L1 expression was registered. Of note, ctDNA is the material of choice in a current clinical trial randomizing NSCLC patients with high TMB detected in plasma to receive either first-line atezolizumab or chemotherapy.47
Immune gene expression profiles
When looking specifically at the baseline status of the anti-tumor immune compartment, atezolizumab studies were the first to dig into the relevance of the expression of immune-related genes in cancer-associated T-cells. Moving from the preliminary results emerging from the mentioned Phase I trial,9 once again the randomized trials POPLAR and OAK provided evidence in this sense. Pretreatment tumor samples were indeed analyzed with the Fluidigm-based gene-expression platform.48 CD8A, GZMA, GZMB, IFN-γ, EOMES, CXCL9, CXCL10, and TBX21, defining the Teff and IFN-γ signatures as associated with activated T-cells, cytolytic activity and IFN-γ expression, were shown to have high co-expression in tumor specimens of POPLAR patients.19 PD-L1, PD-1, PD-L2, and B7.1 gene expression was moreover assessed within POPLAR study. Using the median values of expression as cutoffs, high mRNA levels significantly improved the outcomes of patients receiving atezolizumab (median OS 12.6 vs 9.7 months, HR 0.73, P=0.04), whereas no apparent difference in OS was appreciated between atezolizumab and docetaxel in patients with low levels.19 No impact of the gene expression profiles was detected in patients receiving docetaxel. The Teff-associated and IFN-γ-associated gene signature was related to PD-L1 expression on tumor-infiltrating ICs only (ie, not with PD-L1 expression on TCs). PD-L1 expression on tumour-infiltrating ICs could therefore serve as an indicator of pre-existing immunity within the tumor tissue. Similar observations emerged when considering only PD-L1, CXCL9, and IFN-γ in samples from the OAK trial.49 The population of metastatic non-squamous NSCLC with a high expression of Teff gene signature, defined according to the mRNA levels of the three genes just mentioned, was the specific setting with a dedicated primary objective, of the IMpower150 trial. The available results of the study in terms of PFS documented that the high Teff group benefitted the most from adding atezolizumab to the regimen containing carboplatin, pacli-taxel, and bevacizumab (see “Activity and efficacy data of atezolizumab” section).24
Ongoing studies
Table 7 summarizes the ongoing (recruiting patients or going to recruit) clinical trials of atezolizumab for the treatment of metastatic NSCLC in different clinical settings, gathering the data obtained starting from searching “lung cancer AND atezolizumab” in ClinicalTrials.gov.
Table 7.
Ongoing trials of atezolizumab in NSCLC
| ClinicalTrials.gov ID | Phase | Treatment arm(s) | Study population | Primary outcome(s) |
|---|---|---|---|---|
| First-line treatment | ||||
| NCT02599454 | I | Atezolizumab + SBRT | Unresectable stage I NSCLC | • MTD |
| NCT02013219 | Ib | Atezolizumab + erlotinib/alectinib | Locally advanced or metastatic EGFR-TKI naïveEGFR-positive or untreated ALK-positive NSCLC | • DLT |
| • RP2D | ||||
| NCT02848651(B-F1RST) | II | Atezolizumab | Locally advanced or metastatic NSCLC | • ORR |
| • PFS in positive vs negative bTMB groups | ||||
| NCT03526900(ATEZO-BRAIN) | II | Atezolizumab + CBDCA + pemetrexed | Non-squamous stage IV NSCLC that have untreated asymptomatic brain metastasis | • PFS |
| NCT02409342(IMpower110) | III | • Atezolizumab | Non-squamous or squamous PD-L1-selected stage IV | • OS |
| • CDDP/CBDCA + pemetrexed/gemcitabine | NSCLC | |||
| NCT02409355(IMpower111) | III | • Atezolizumab | Squamous PD-L1-selected stage IV NSCLC | • PFS |
| • CDDP/CBDCA + gemcitabine | ||||
| NCT02367781(IMpower130) | III | • Atezolizumab + CBDCA + nab-paclitaxel | Non-squamous stage IV NSCLC | • PFS |
| • CBDCA + nab-paclitaxel | • OS | |||
| NCT02657434(IMpower132) | III | • Atezolizumab + CDDP/CBDCA + pemetrexed | Non-squamous stage IV NSCLC | • PFS |
| • CDDP/CBDCA + pemetrexed | • OS | |||
| NCT03191786(IPSOS) | III | • Atezolizumab | Locally advanced or metastatic untreated NSCLC unsuitable for platinum-containing therapy (ECOG PS 2–3) | • OS |
| • Vinorelbine/gemcitabine | ||||
| Second and subsequent lines treatment | ||||
| NCT02400814 | I | • Atezolizumab + SBRT (concurrent cohort) | Previously treated stage IV NSCLC | • Safety |
| • Atezolizumab → Atezolizumab + SBRT (induction cohort) | • ORR | |||
| • PFS | ||||
| • SBRT → Atezolizumab (sequential cohort) | ||||
| NCT03023423(DARZALEX) | Ib/II | • Atezolizumab + daratumumab | Previously treated stage IIIB-IV NSCLC | • ORR |
| • Atezolizumab | ||||
| NCT03455556 | I/II | Atezolizumab + anetumab ravtansine | Previously treated stage IIIA-IV NSCLC | • MTD |
| • ORR | ||||
| NCT03014648 | II | Atezolizumab | Stage IIIB-IV non-squamous and squamous NSCLC previously treated with anti PD-1 therapy | • ORR |
| NCT02813785a(IMpower210) | III | • Atezolizumab | Locally advanced or metastatic NSCLC who have | • OS |
| • Docetaxel | progressed during or following a platinum-containing regimen | |||
| NCT03285763(TAIL) | IV | Atezolizumab | Previously treated stage IIIB-IV NSCLC | • Safety |
| NCT03232593b | IV | Atezolizumab | NSCLC, according to the locally approved indications | • Safety |
| Basket trials | ||||
| NCT03138889(PROPEL) | I | Atezolizumab + CD122-Biased Cytokine (NNTR-214) | Solid tumors, among which previously treated stage IV | • Safety |
| NSCLC | • MTD | |||
| • RP2D | ||||
| NCT02655822 | I | Atezolizumab + CPI-444 | Solid tumors, among which previously treated stage IV | • Pharmacokinetics parameters |
| NSCLC | • Safety | |||
| • ORR | ||||
| NCT03289962 | I | Atezolizumab + RO7198457 (personalized cancer vaccine) | Solid tumors, among which stage IV NSCLC | • Safety |
| • MTD | ||||
| • RP2D | ||||
| NCT03170960 | Ib/II | Atezolizumab + cabozantinib | Solid tumors, among which non-squamous stage IV | • MTD |
| NSCLC | • RP2D | |||
| • ORR |
Notes: Bolded NCT numbers refer to ongoing trials currently recruiting participants.
Study conducted in East Asian countries;
Study conducted in Republic of Korea.
Abbreviations: bTMB, blood tumor mutational burden; CBDCA, carboplatin; CDDP, cisplatin; DLT, dose-limiting toxicities; ECOG PS, Eastern Cooperative Oncology Group performance status; MTD, maximum tolerated dose; NSCLC, non-small-cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RP2D, recommended Phase II dose; SBRT, stereotactic body radiation therapy; TKI, tyrosine kinase inhibitor.
First-line setting
Beyond pretreated NSCLC patients, atezolizumab is being extensively studied frontline. IMpower110 is a large randomized Phase III study that compares atezolizumab monotherapy to standard platinum-doublet in PD-L1-positive untreated stage IV NSCLC, regardless of histology, with OS as primary endpoint (NCT02409342). A trial specifically addressed to untreated PD-L1-positive squamous NSCLC is currently evaluating atezolizumab monotherapy vs standard gemcitabine plus carboplatin/cisplatin (IMpower111, NCT02409355). Not least, IPSOS Phase III trial is assessing the efficacy of single atezolizumab in a special population of NSCLC patients unsuitable for platinum-containing therapy due to poor ECOG Performance Status (NCT03191786).
Combination treatment with atezolizumab and platinum-based chemotherapy in chemotherapy-naïve advanced non-squamous NSCLC is being explored in two large Phase III, randomized, multicenter trials: IMpower130 (NCT02367781) and IMpower132 (NCT02657434). In IMpower130, patients are randomized in a 2:1 ratio to receive carboplatin and nab-paclitaxel doublet for four/six cycles either in combination with atezolizumab or alone. Atezolizumab will be provided in the experimental arm during maintenance treatment phase until loss of clinical benefit. Concerning the control arm, maintenance phase will consist of best supportive care or switch to pemetrexed. An amended version of the protocol admitted patients in the control arm to cross over to atezolizumab monotherapy until disease progression (NCT02367781). Interestingly, a Roche’s press release announced that the trial met its co-primary endpoints of PFS and OS, adding evidence to the proven efficacy of atezolizumab in NSCLC;50 final results are largely awaited.
In IMpower132, atezolizumab is being studied in the same IMpower130 category of advanced untreated non-squamous NSCLC patients. In the induction phase, platinum and pemetrexed doublet ± atezolizumab are administered for four cycles; subsequently, a maintenance treatment with pem-etrexed ± atezolizumab is continued until disease progression. Even in this trial, similarly to IMpower130, OS and PFS are co-primary endpoints (NCT02657434). Atezolizumab + chemotherapy efficacy in a selected population of non-squamous NSCLC patients with asymptomatic brain metastases will be further assessed in ATEZO-BRAIN Phase II trial (NCT03526900). In oncogene-addicted patients, a Phase Ib trial estimates safety and pharmacokinetics of atezolizumab combined with erlotinib or alectinib in untreated EGFR-or ALK-positive NSCLC, respectively (NCT02013219).
Subsequent lines of treatment
A single-arm Phase II trial is aimed at evaluating the efficacy of PD-L1 inhibition with atezolizumab in stage IV NSCLC patients who had already received a previous anti-PD-1 therapy with either nivolumab or pembrolizumab; patients are being enrolled in three different cohorts, defined by the best response to previous anti PD-1, in order to account for the variability of response kinetics to anti PD-1 (NCT03014648). An additional Phase III trial conducted in East Asian countries is comparing atezolizumab to docetaxel as second-line treatment for NSCLC (NCT02813785). In the same clinical setting, a Phase IV multicentric study is ongoing (NCT03285763). Beyond monotherapy in pretreated patients, a wide variety of new target drugs are being studied in combination with atezoli-zumab in early studies, including daratumumab (anti-CD38 monoclonal antibody, NCT03023423), anetumab ravtansine in mesothelin-positive only patients (anti-mesothelin antibody–drug conjugate, NCT03455556,), NKTR-214 (CD122-biased cytokine, NCT03138889), CPI-444 (inhibitor of adenosine-A2A receptor, NCT02655822), RO7198457 (personalized cancer vaccine, NCT03289962), and cabozantinib (multitargeted TKI, NCT03170960).
Patient selection and place in therapy
Along with nivolumab and pembrolizumab, the role of atezolizumab for the treatment of NSCLC in the pretreated setting is established, as documented by the largest studies performed in this setting, now supplemented by data of prolonged follow-up.4,21 The translation of nivolumab into clinical practice has been successful, even when patient populations were less selected compared to clinical trials (with the relevant exception of patients with poor performance status, whose dismal prognosis cannot be reverted by ICB).51,52 Real-life data of atezolizumab are awaited too (NCT03285763); nevertheless, no particular element is expected to scale down its effectiveness. Among the cited ICB approved for the second-line treatment, atezolizumab retains the advantage of being administered every 3 weeks, with no limitations according to PD-L1 IHC status. Similarly to nivolumab indeed, “unknown” (ie, due to the lack of tissue to be tested) or negative (<1%) PD-L1 statuses allow atezoli-zumab to be prescribed, differently from pembrolizumab (PD-L1 ≥1% required). The benefit observed with atezoli-zumab in the TC0/IC0 PD-L1 cases across clinical studies (Table 4) supports this possibility. From a pragmatic point of view, the three-weekly schedule (similar to pembrolizumab one) appears more convenient than nivolumab bi-weekly one; nevertheless, the latter agent can now be infused at the flat dose of 480 mg once monthly.53 No remarkable differences in the disimmune toxicity54 arising as a consequence of atezolizumab treatment have been observed when compared to other ICB.55 As mentioned in the “Background” section, the peculiar targeting of PD-L1 instead of PD-1, together with its engineered Fc domain, could account for the reduced incidence of pulmonary adverse events.56 In addition, whether the toxicities induced by anti-PD-1 agents can be modulated by the alternative blocking of PD-L1, as in a report of reverted inflammatory polyarthritis,57 is still to be proven. Besides benefits in terms of activity and efficacy, clinical trials showed a positive role of atezolizumab with regard to patient-reported outcomes, as exemplified by the significant improvement in health-related quality of life indexes (such as time to deterioration in physical and role function) in OAK study.58
However, not all NSCLC patients progressing to first-line chemotherapy could benefit from atezolizumab, as well as other ICB. Patient-associated factors historically labeled as exclusion criteria for entering into clinical studies evaluating ICB, like HIV or viral hepatitis infection and autoimmune diseases, are approached more tolerantly in the current clinical practice, as growing evidence sustains their cautious feasibility.59–61 If “indolent” cancers may be the ideal candidates to ICB,62 “aggressive” diseases (ie, the ones determining disease progression under chemotherapy and/or with a significant tumor burden) could potentially benefit more from second-line chemotherapy combined with anti-angiogenic agents.63 As approached in the “Clinico-pathological parameters associated with atezolizumab benefit” section, age itself does not seem to preclude ICB activity and efficacy,64 differently from a poor performance status and the need of medium to high dose of steroids; these latter two factors were clearly as sociated to dismal outcomes under immunotherapy.52,65,66 Alongside clinical and molecular predictors of atezolizumab benefit, clinicians’ interest is addressed toward potential biomarkers useful to identify patients at risk of undergoing hyper-progression under ICB,67,68 in order to avoid the detrimental effect elicited by ICB administration in this peculiar subgroup of patients.
As atezolizumab has entered the scenario of NSCLC treatment after nivolumab and pembrolizumab, the large majority of evidence regarding ICB behavior in the clinical setting is attributed by the latter two drugs. According to available data, we do not think that atezolizumab harbors specific characteristics significantly differentiating it from other ICB, with regard to patient selection. In this sense, ICB in general and atezolizumab in specific have shown reduced activity and efficacy in EGFR-mutated and, with a less extent due to the limited population studies, in ALK-rearranged patients (Figure 2).32,34,69,70 On the contrary, KRAS-mutated tumors are more responsive to atezolizumab and other ICB.29,30 The association between smoking exposure and positive KRAS mutational status could additionally account for this observation, as tumors arising in smokers are accompanied by higher TMB, encompassing better outcomes under ICB (Figure 2).
Figure 2.
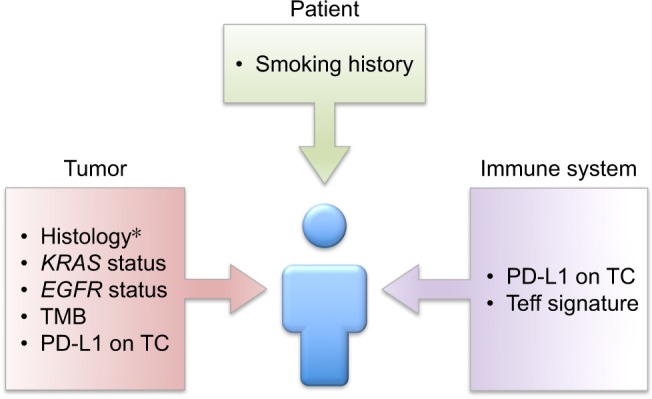
Elements addressing patient selection emerging from atezolizumab development.
Note: *Histology addresses differential combinations with atezolizumab in the frontline setting.
Abbreviations: IC, immune cells; PD-L1, programed death ligand-1; TC, tumor cells; Teff, T-effector gene signature; TMB, tumor mutation burden.
Data regarding the prognostic and predictive relevance of TMB in NSCLC (see “Immune-related determinants of atezolizumab activity and efficacy in advanced NSCLC” section) are abundant and significant. Nevertheless, their applicability in the daily clinical scenario strongly depends on the widespread availability of such a complex technique. Similarly, the documentation of a specific genetic signature intrinsic to Teff, predictive of atezolizumab benefit, represents an impactful element to better understand the underpinnings of ICB actions (Figure 2). Again, the clinical applicability of this gene panel remains difficult for practical reasons.
Approaching the first-line setting, the recent available data go in the same direction, sustaining the upfront administration of ICB, either in reciprocal combination (nivolumab/ipilimumab in high-TMB cases) or associated with chemotherapy and potentially with anti-angiogenic agents, or as monotherapy (pembrolizumab in the strong PD-L1 expressing population).5 The first-line scenario is thus a rapidly evolving field, and the depiction of putative treatment algorithms, when combinations will be hopefully available, goes beyond the aims of this review. While this manuscript is being written (after 2018 ASCO meeting), data corroborating the administration of atezolizumab combinations in the upfront setting is not as strong as pembrolizumab ones, in both non-squamous and squamous histologies. However, with regard to atezolizumab, we do believe that its association with carboplatin/taxanes-based regimens (with or without the potential benefit generated by nab-paclitaxel), moreover combined with bevacizumab,24,27 will find its place in this busy scenario, strongly supported by the rationale of synergic role of immunotherapy and anti-angiogenesis.71
Again, clinical and molecular factors will be pivotal in addressing patients to the adequate first-line therapy (Figure 2). Albeit ICB monotherapy is not the treatment of choice in oncogene-addicted NSCLC, we hope that combinations with TKIs, as alectinib associated with atezolizumab in ALK-positive cases,25 could significantly prolong disease control.
Conclusion
Atezolizumab definitely has its place in the setting of pretreated NSCLC patients, and the current abundant data of atezolizumab efficacy in first-line will help to define the best therapeutic strategies. The overviewed clinical, pathological, molecular, and immune-related factors predicting the outcomes to atezolizumab will be of pivotal relevance in addressing every single patient to her/his best suitable treatment option, in line with a personalized approach.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 4.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 6.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemo-radiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 7.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto K, Fukuyama S, Eguchi-Tsuda M, et al. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun. 2008;365(1):170–175. doi: 10.1016/j.bbrc.2007.10.156. [DOI] [PubMed] [Google Scholar]
- 11.Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3(1):81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacs JD, Wing MG, Greenwood JD, Hazleman BL, Hale G, Waldmann H. A therapeutic human IgG4 monoclonal antibody that depletes target cells in humans. Clin Exp Immunol. 1996;106(3):427–433. doi: 10.1046/j.1365-2249.1996.d01-876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warncke M, Calzascia T, Coulot M, et al. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol. 2012;188(9):4405–4411. doi: 10.4049/jimmunol.1200090. [DOI] [PubMed] [Google Scholar]
- 14.Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study. 2015. J Clin Oncol. 2015;33:8029. [Google Scholar]
- 15.Spigel DR, Chaft JE, Gettinger S, et al. FIR: Efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with non-small-cell lung cancer. J Thorac Oncol. 2018 May 15; doi: 10.1016/j.jtho.2018.05.004. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolchok JD, Hoos A, O’Day S, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 17.Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH) J Clin Oncol. 2017;35(24):2781–2789. doi: 10.1200/JCO.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carcereny E, Felip E, Reck M, et al. Updated efficacy results from the BIRCH Study: First-line atezolizumab therapy in PD-L1–selected patients with advanced NSCLC. J Thorac Oncol. 2017;(12):S1791–S1792. [Google Scholar]
- 19.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 20.Park K, Lewanski C, Gadgeel S, et al. MA 10.03 3-year survival and duration of response in randomized phase II study of atezolizumab vs docetaxel in 2L/3L NSCLC (POPLAR) J Thorac Oncol. 2017;12(11):S1840. [Google Scholar]
- 21.Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(8):1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Gandara DR, von Pawel J, Sullivan RN, et al. Atezolizumab treatment beyond disease progression in advanced NSCLC: results from the randomized Ph III OAK study. J Clin Oncol. 2017;35:9001. doi: 10.1016/j.jtho.2018.08.2027. [DOI] [PubMed] [Google Scholar]
- 23.Liu SV, Camidge DR, Gettinger SN, et al. Atezolizumab (atezo) plus platinum-based chemotherapy (chemo) in non-small cell lung cancer (NSCLC): Update from a phase Ib study. J Clin Oncol. 2017;35:9092. [Google Scholar]
- 24.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 25.Kim D-W, Gadgeel SM, Gettinger SN, et al. Safety and clinical activity results froma a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC) J Clin Oncol. 2018;36 abstract 9009. [Google Scholar]
- 26.Roche’s Tecentriq in combination with pemetrexed and platinum-based chemotherapy reduced the risk of disease worsening or death in the initial treatment of people with advanced lung cancer. Media Release. 2018. [Accessed August 28, 2018]. Available from: https://www.roche.com/media/releases/med-cor-2018-07-19.htm.
- 27.Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36(18 Suppl):LBA9000. [Google Scholar]
- 28.Elias R, Giobbie-Hurder A, Mccleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer. 2018;6(1):26. doi: 10.1186/s40425-018-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Kim HS, Kim BJ. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: A meta-analysis and review. Oncotarget. 2017;8(29):48248–48252. doi: 10.18632/oncotarget.17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH, Kim HS, Kim BJ. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Oncotarget. 2017;8(54):93149–93155. doi: 10.18632/oncotarget.18703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer – a meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Botticelli A, Onesti CE, Zizzari I, et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget. 2017;8(59):99336–99346. doi: 10.18632/oncotarget.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Rahman O. Smoking and EGFR status may predict outcomes of advanced NSCLC treated with PD-(L)1 inhibitors beyond first line: a meta-analysis. Clin Respir J. 2018;12(5):1809–1819. doi: 10.1111/crj.12742. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Pang Z, Ding N, et al. The efficacy and potential predictive factors of PD-1/PD-L1 blockades in epithelial carcinoma patients: a systematic review and meta analysis. Oncotarget. 2016;7(45):74350–74361. doi: 10.18632/oncotarget.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Rahman O. Does a patient’s sex predict the efficacy of cancer immunotherapy? Lancet Oncol. 2018;19(6):716–717. doi: 10.1016/S1470-2045(18)30270-5. [DOI] [PubMed] [Google Scholar]
- 37.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Büttner R, Gosney JR, Skov BG, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35(34):3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- 39.Gadgeel S, Kowanetz M, Zou W, et al. Clinical efficacy of atezolizumab (Atezo) in PD-L1 subgroups defined by SP142 and 22C3 IHC assays in 2L+ NSCLC: Results from the randomized OAK Study. Ann Oncol. 2017;28(Suppl 5):460–461. [Google Scholar]
- 40.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters S, Creelan B, Hellmann MD, et al. Abstract CT082: Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage IV or recurrent non-small cell lung cancer: An exploratory analysis of CheckMate 026. Cancer Res. 2017;77(13 Suppl):CT082. [Google Scholar]
- 42.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipili-mumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33(5):843–852. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kowanetz M, Zou W, Shames D, et al. Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J Thorac Oncol. 2017;12(1):S321–S322. [Google Scholar]
- 45.Sacher AG, Komatsubara KM, Oxnard GR. Application of plasma genotyping technologies in non-small cell lung cancer: a practical review. J Thorac Oncol. 2017;12(9):1344–1356. doi: 10.1016/j.jtho.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 46.Gandara DR, Kowanetz M, Mok TSK, Tsk M, et al. Blood-based biomarkers for cancer immunotherapy: Tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK) Ann Oncol. 2017;28(Suppl 5):460. [Google Scholar]
- 47.Tsk M, Gadgeel S, Kim ES, et al. Blood first-line ready screening trial (B-F1RST) and blood first assay screening trial (BFAST) enable clinical development of novel blood-based biomarker assays for tumor mutational burden (TMB) and somatic mutations in 1L advanced or metastatic NSCLC. Ann Oncol. 2017;28:494–495. [Google Scholar]
- 48.Schleifman EB, Desai R, Spoerke JM, et al. Targeted biomarker profiling of matched primary and metastatic estrogen receptor positive breast cancers. PLoS One. 2014;9(2):e88401. doi: 10.1371/journal.pone.0088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowanetz M, Zou W, Mccleland M, et al. Pre-existing immunity measured by Teff gene expression in tumor tissue is associated with atezolizumad efficacy in NSCLC. J Thorac Oncol. 2017;12(11):S1817–S1818. [Google Scholar]
- 50.Phase III IMpower130 study showed Roche’s Tecentriq (atezolizumab) plus chemotherapy (carboplatin and Abraxane) helped people with metastatic non-squamous NSCLC live significantly longer compared to chemotherapy alone. Investor Update. 2018. [Accessed August 28, 2018]. Available from: https://www.roche.com/investors/updates/inv-update-2018-05-29.htm.
- 51.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Dudnik E, Moskovitz M, Daher S, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: the real-life data. Lung Cancer. 2017 Nov 23; doi: 10.1016/j.lungcan.2017.11.015. Epub. [DOI] [PubMed] [Google Scholar]
- 53.Bristol-Myers Squibb’s Opdivo® (nivolumab) Now the First and Only FDA-Approved PD-1 Inhibitor to Offer Every Four-Week Dosing. [Accessed August 28, 2018]. Media Release. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibbs-opdivo-nivolumab-now-first-and-only-fda-
- 54.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 55.Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer. 2018;124(2):271–277. doi: 10.1002/cncr.31043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 57.Swami U, Lenert P, Furqan M, Abu Hejleh T, Clamon G, Zhang J. Atezolizumab after nivolumab-induced inflammatory polyarthritis: can anti-PD-L1 immunotherapy be administered after anti-PD-1-related immune toxicities? J Thorac Oncol. 2018;13(6):e102–e103. doi: 10.1016/j.jtho.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Bordoni R, Ciardiello F, von Pawel J, et al. Patient-reported outcomes in OAK: a phase III study of atezolizumab versus docetaxel in advanced non-small-cell lung cancer. Clin Lung Cancer. 2018 May 31; doi: 10.1016/j.cllc.2018.05.011. Epub. [DOI] [PubMed] [Google Scholar]
- 59.Ostios-Garcia L, Faig J, Leonardi GC, et al. Safety and efficacy of PD-1 inhibitors among HIV-positive patients with non-small cell lung cancer. J Thorac Oncol. 2018;13(7):1037–1042. doi: 10.1016/j.jtho.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 60.Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: a case series. Melanoma Res. 2018;28(2):1–158. doi: 10.1097/CMR.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 61.Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36(19):1905–1912. doi: 10.1200/JCO.2017.77.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Facchinetti F, Veneziani M, Buti S, et al. Clinical and hematologic parameters address the outcomes of non-small-cell lung cancer patients treated with nivolumab. Immunotherapy. 2018;10(8):681–694. doi: 10.2217/imt-2017-0175. [DOI] [PubMed] [Google Scholar]
- 63.Reck M, Garassino MC, Imbimbo M, et al. Antiangiogenic therapy for patients with aggressive or refractory advanced non-small cell lung cancer in the second-line setting. Lung Cancer. 2018;120:62–69. doi: 10.1016/j.lungcan.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Ferrara R, Mezquita L, Auclin E, Chaput N, Besse B. Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: does age really matter? Cancer Treat Rev. 2017;60:60–68. doi: 10.1016/j.ctrv.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Popat S, Ardizzoni A, Ciuleanu T, et al. Nivolumab in previously treated patients with metastatic squamous NSCLC: results of a European single-arm, phase 2 trial (CheckMate 171) including patients aged ≥70 years and with poor performance status. Ann Oncol. 2017;28(Suppl 5):463. [Google Scholar]
- 66.Arbour KC, Mezquita L, Long N, et al. Deleterious effect of baseline steroids on efficacy of PD-(L)1 blockade in patients with NSCLC. J Clin Oncol. 2018;36(abstr 9003) doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 67.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara R, Caramella C, Texier M, et al. Hyperprogressive disease (HPD) is frequent in non-small cell lung cancer (NSCLC) patients (pts) treated with anti PD1/PD-L1 monoclonal antibodies (IO) Ann Oncol. 2017;28(Suppl 5):464–465. [Google Scholar]
- 69.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manegold C, Dingemans AC, Gray JE, et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol. 2017;12(2):194–207. doi: 10.1016/j.jtho.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Shu CA, Grigg C, Chiuzan C, et al. Neoadjuvant atezolizumab + chemotherapy in resectable non-small cell lung cancer (NSCLC) J Clin Oncol. 2018;36 abstr 8532. [Google Scholar]