Abstract
Helminths have strong immunoregulatory properties that may be exploited in treatment of chronic immune disorders, such as multiple sclerosis and inflammatory bowel disease. Essential players in the pathogenesis of these diseases are proinflammatory macrophages. We present evidence that helminths modulate the function and phenotype of these innate immune cells. We found that soluble products derived from the Trichuris suis (TsSP) significantly affect the differentiation of monocytes into macrophages and their subsequent polarization. TsSPs reduce the expression and production of inflammatory cytokines, including IL-6 and TNF, in human proinflammatory M1 macrophages. TsSPs induce a concomitant anti-inflammatory M2 signature, with increased IL-10 production. Furthermore, they suppress CHIT activity and enhance secretion of matrix metalloproteinase 9. Short-term triggering of monocytes with TsSPs early during monocyte-to-macrophage differentiation imprinted these phenotypic alterations, suggesting long-lasting epigenetic changes. The TsSP-induced effects in M1 macrophages were completely reversed by inhibiting histone deacetylases, which corresponded with decreased histone acetylation at the TNF and IL6 promoters. These results demonstrate that TsSPs have a potent and sustained immunomodulatory effect on human macrophage differentiation and polarization through epigenetic remodeling and provide new insights into the mechanisms by which helminths modulate human immune responses.—Hoeksema, M. A., Laan, L. C., Postma, J. J., Cummings, R. D., de Winther, M. P. J., Dijkstra, C. D., van Die, I., Kooij, G. Treatment with Trichuris suis soluble products during monocyte-to-macrophage differentiation reduces inflammatory responses through epigenetic remodeling.
Keywords: helminth, multiple sclerosis, autoimmunity, TNF, IL-10
The increasing incidence and prevalence of autoimmune and allergic diseases in Western countries is associated with a markedly decreased prevalence of infections caused by lifestyle changes such as improvements in sanitation. These diseases include inflammatory bowel disease (IBD), allergies, type 1 diabetes, and multiple sclerosis (MS). According to the hygiene hypothesis, infections in the first years of life confer protection against inappropriate immune responses (1–3). Recent research in line with this concept has revealed that exposure to helminths has long-lasting immunomodulatory effects (4). These findings have raised great interest in the mechanisms by which helminths exert their immunoregulatory properties, and their possible therapeutic potential in the treatment of inflammatory diseases. The porcine nematode Trichuris suis is currently under investigation in clinical trials in several immune disorders (5). Early observational studies showed promising effects of helminth therapy in IBD and MS (6–8), but larger trials have shown disappointing results in IBD (9), and debate is ongoing about the setup and effectiveness of worm therapy. To enable optimization of helminth-based treatment regimens for inflammatory disorders, there is an urgent need to obtain fundamental mechanistic knowledge of how T. suis interacts with the immune system and to define the helminth-specific derivatives that are responsible for the immunomodulatory effects.
Studies of the complex interplay between T. suis and the immune system have shown that T. suis soluble products (TsSPs) can significantly reduce clinical pathology in murine experimental autoimmune encephalomyelitis [EAE, a well-established model for MS (10)] and hyperreactivity in a model of allergy (11). Such results indicate that parasite-derived products, in the absence of living parasites, can induce anti-inflammatory effects. Immunoregulatory effects of helminths have been attributed to the suppression of inflammatory T helper (Th)1 and -17 responses and an induction of Th2 and regulatory T-cell responses via multiple pathways, including modulation of dendritic cell (DC) functions and the induction of alternatively activated macrophages (10, 12–14). We have shown that TsSPs suppress proinflammatory Th1 responses in human DCs (10), which may partly explain their protective potential in inflammatory diseases. Besides adaptive immune responses, innate immune cells, including macrophages, are key players in inflammatory diseases (15). On the basis of activation protocol and phenotype, macrophage subsets have been classified as classically (M1) or alternatively (M2) activated. Although the classification is oversimplified, these phenotypes can be viewed as a spectrum in which the M1 or M(IFNγ+LPS) and M2 or M(IL-4) represent the most extreme phenotypes (16–18). M1 macrophages are proinflammatory macrophages that are induced by the Th1-cytokine IFNγ and bacterial products, such as LPS, whereas M2 macrophages dampen inflammatory responses and promote tissue repair and can be induced by the Th2-cytokine IL-4, the anti-inflammatory cytokine IL-10, or helminth products (17, 19, 20).
Helminths provide protection in murine IBD and MS animal models by inducing M2 macrophages (21, 22). Moreover, we found that TsSPs induce anti-inflammatory human monocyte subsets (13) and that these helminth products could dampen the proinflammatory profile of activated macrophages (14). During chronic inflammation and T. suis administration, TsSPs may interact with monocytes in the circulation before they are recruited to tissues and differentiate into a monocyte-derived macrophage subset. Thus, in the present study, we explored the effects of TsSPs during both human monocyte-to-macrophage differentiation and macrophage polarization. We hypothesized that treatment with TsSPs would suppress the induction of the inflammatory M1 phenotype and boost an M2 profile. Moreover, because macrophage subtypes are highly regulated by epigenetic mechanisms (23), we assessed whether TsSPs would induce epigenetic remodeling in monocyte-derived macrophages.
In our study, treatment with TsSPs significantly reduced the expression and production of proinflammatory cytokines, such as IL-6 and TNF, as well as chitinase (CHIT) activity in M1 macrophages, and increased the M2 signature in both naïve macrophages and polarized M2 macrophages. We observed that even brief priming with TsSPs early during monocyte-to-macrophage differentiation imprinted phenotypic changes on proinflammatory cytokine genes in subsequent M1 polarized macrophages. The TsSP-induced effects on repressed genes in M1 macrophages were completely reversed by inhibiting histone deacetylases (HDACs), which corresponded with histone deacetylation of the IL6 and TNF promoters, thereby providing the first evidence that TsSPs can exert potent anti-inflammatory effects on human macrophages through epigenetic remodeling.
MATERIALS AND METHODS
Preparation of TsSPs
TsSPs were prepared as described elsewhere (12). In brief, T. suis worms were harvested from the colon and cecum of infected pigs and extensively washed with 0.98% NaCl. For the preparation of TsSPs, the worms were frozen in liquid nitrogen and crushed into powder with a mortar and pestle. The powder was dissolved in double-distilled H2O, and the solution was centrifuged for 30 min at 10,000 g, 4°C. The supernatants were filtered (0.45 μm) and stored at −80°C until use. Endotoxin levels were determined with a limulus amebocyte lysate assay (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA), to assess the presence of lipopolysaccharides (LPSs) in the TsSPs; endotoxin levels were below 0.2 EU/ml.
Monocyte isolation, cell culture, and experimental setup
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy human donor buffy coats (Sanquin Blood Bank, Amsterdam, The Netherlands) by using a Ficoll density gradient (Lymphoprep, Axis-Shield, Oslo, Norway) (24). Monocytes were obtained from the PBMCs by using CD14 MicroBeads (Miltenyi Biotec, Leiden, The Netherlands) and a MACS MultiStand, by percolating 3 ml MACS buffer (2 mM EDTA and 1% fetal calf serum in PBS) through an LS column according to the manufacturer’s protocol.
For macrophage differentiation, monocytes were cultured in 6-well plates (Greiner Bio-One, Alphen aan de Rijn, The Netherlands) at a concentration of 2 × 106 cells/ml in macrophage medium (DMEM; Thermo Fisher Scientific Life Sciences, Breda, The Netherlands) supplemented with 5% (v/v) normal human serum (NHS; Bio Whittaker, East Rutherford, NJ, USA), and 1% (v/v) penicillin-streptomycin-glutamine (Thermo Fisher Scientific Life Sciences), at 37°C, 5% CO2. Monocytes were induced to differentiate into macrophages in the course of 6 d in the presence or absence of TsSPs (40 μg/ml) for 1 or 6 d, after which the products were washed away and fresh medium added. After 6 d, the macrophages were washed and incubated with 4 mg/ml lidocaine (Sigma-Aldrich, Zwijndrecht, The Netherlands), scraped from the wells, and reseeded in a concentration of 1 × 106 cells/ml, and polarization was achieved by culturing the macrophages for 48 h in macrophage medium in the absence or presence of M1/M2 stimuli. M1 macrophages were obtained by culturing the macrophages in the presence of 100 U/ml recombinant human IFNγ (U-Cytech, Utrecht, The Netherlands) for 24 h and for an additional 24 h, also in the presence of 10 ng/ml Escherichia coli LPS (026:B6; Sigma-Aldrich). M2 macrophages were generated in the presence of 10 ng/ml recombinant human IL-4 (Immunotools, Friesoythe, Germany) for 48 h, and control macrophages (M0) were cultured without polarizing stimuli.
Inhibition of chromatin-modifying enzymes
Monocytes were pretreated with various chromatin-modifying enzyme (CME) inhibitors: the histone demethylase inhibitor (HDMi) pargyline (Sigma-Aldrich), the histone methyltransferase inhibitor (HMTi) 5′-methylthioadenosine (MTA; Sigma-Aldrich), the histone deacetylase inhibitor (HDACi) givinostat (ITF2357; a generous gift from Italfarmaco, Cinisello Balsamo, Italy), and the histone acetyltransferase inhibitor (HATi) epigallocatechin-3-gallate (EGCG; Sigma-Aldrich). The inhibitors were added 45 min before the TsSPs for 24 h. The cells were washed to remove the TsSPs and the inhibitors, and fresh medium was added. After 6 d of differentiation, the macrophages were incubated with M1 stimuli (see above) to skew them toward the M1 phenotype.
Flow cytometry
To analyze the expression of intracellular and surface markers for differentiated macrophages, we performed flow cytometry. First, macrophages were harvested after 8 d of differentiation. For analysis of intracellular marker expression, the cells were washed with PBS and fixed with 2% paraformaldehyde for 15 min on ice. They were then washed with PBS and incubated for 10 min at room temperature with either PBS supplemented with 0.1% (v/v) saponin (Riedel-de Haen, Seelze, Germany) and 0.1% (w/v) bovine serum albumin (BSA; Roche, Basel, Switzerland), for detection of surface and intracellular mannose receptor (MR), or with PBS with 0.1% (w/v) BSA for detection of surface CD68, CD40, major histocompatibility class (MHC)-II, and CD163. The cells were incubated with primary antibodies for 1 h, washed twice, incubated with the fluorescently labeled secondary antibody for 20 min at room temperature (Table 1), and analyzed on a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) in combination with CellQuest Pro software, version 3.3 (BD Biosciences) and FlowJo software, version 9.4.0 for Windows (Tree Star, Inc., Ashland, OR, USA). Cell viability was assessed with a viability dye, 7-aminoactinomycin D (Thermo Fisher Scientific Life Sciences, Leiden, The Netherlands). Antibodies and dilutions are listed in Table 1.
TABLE 1.
Antibodies and working concentrations used in FACS analysis
| Antigen | Species | Working concentration (μg/ml) | Manufacturer |
|---|---|---|---|
| CD68 | Mouse IgG1ĸ | 2.37 | DakoCytomation |
| CD40 | Mouse IgG2α | 4.00 | AbD Serotec |
| MHC II | Mouse IgG2β | 11.33 | Thermo Fisher |
| CD163 | Mouse IgG1ĸ | 18.06 | AbD Serotec |
| MR | Mouse IgG1ĸ | 10.00 | BioLegend |
| Isotype | Mouse IgG1ĸ | 10.00 | BioLegend |
| Alexa Fluor 488 F(ab′)2 fragment | Goat anti-mouse IgG (H+L) | 5.00 | Thermo Fisher |
Quantitative real-time PCR
Polarized M0, M1, and M2 macrophages were harvested after 4 h and stored at −80°C. mRNA was isolated by capturing poly(A+)RNA in streptavidin-coated tubes by using the mRNA Capture Kit (Roche) and was transcribed into cDNA with a reverse transcription kit (Promega, Madison, WI, USA) (25). Quantitative real-time RT-PCR (qPCR) was performed with a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific Life Sciences) and SYBR Green analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. The primer sequences are listed in Table 2.
TABLE 2.
Primers used for qPCR
| Gene | Forward | Reverse |
|---|---|---|
| TNF | GCCCATGTTGTAGCAAACCCT | ATGAGGTACAGGCCCTCTGATG |
| IL12 | ACCACTCCCAAAACCTGCTGA | TTGTCTGGCCTTCTGGAGCAT |
| CXCL9 | TGCAAGGAACCCCAGTAGTGA | TAGTCCCTTGGTTGGTGCTGA |
| IL10 | CGCTGTCATCGATTTCTTCCCT | AGGCATTCTTCACCTGCTCCAC |
| CCL17 | ACTTCAAGGGAGCCATTCCC | CCCTGCACAGTTACAAAAACGA |
| MR | GTCTTGGGCCACAGGTGAA | AAGGCGTTTGGATAGCCACA |
| CHIT1 | TGGACTTAGATGACTTTGCCG | CCTGGTTTTGGAACTTCAAGC |
| CD40 | CAAATACTGCGACCCCAACCTA | TTTCTGAGGTGCCCTTCTGCT |
| GAPDH | CCATGTTCGTCATGGGTGTG | GGTGCTAAGCAGTTGGTGGTG |
| CHI3L1 | CTTCTGAGACTGGTGTTGGAG | CGAGGATTCTATGGACTGTGG |
| TNFprom | CCTCTCTCCCCTGGAAAGGA | AGAGGCTGAGGAACAAGCAC |
| IL6prom | GGAGTCACACACTCCACCTG | CTGTGTTCTGGCTCTCCCTG |
| IL10enh(ex3) | CGCCTTGATGTCTGGGTCTT | CCTCCGCCAATCTCTCACTC |
Enh(ex3), enhanced expression ×3; prom, promoter.
Cytokine assays
The production of pro- and anti-inflammatory mediators was assessed by ELISA in cell-free supernatant samples, which were harvested after 24 h (monocytes), 6 d (macrophages) and 8 d (M0, M1, and M2 macrophages, respectively). TNF was measured with a human TNF ELISA kit (Thermo Fisher Scientific Life Sciences), and IL-10 and -6 were measured with a PeliPair reagent set (Sanquin Blood Bank), according to the manufacturer’s protocol. Absorbance at 450 nm was determined on a spectrophotometer and analyzed with Microplate Manager software (both from Bio-Rad, Hemel Hempstead, United Kingdom).
CHIT assay
CHIT activity in the supernatant of d 8 macrophages was determined with 4-methylumbelliferyl-N,N′,N′′-triacetyl chitotrioside [4-MU-(GlcNAc)3; Sigma-Aldrich] as a substrate. Reaction mixtures contained 50 µl of cell supernatant and 50 µl of 20 µM 4-MU-(GlcNAc)3 in 50 mM citrate/phosphate buffer (pH 5). The assay was performed for 1 h at 37°C, after which the reaction was stopped with 100 µl sodium hydroxide–buffered 1 M glycine (pH 10). Liberated methylumbelliferone was measured on a Fluostar fluorescence spectrometer (BMG Labtech, Ortenberg, Germany) with excitation and emission wavelengths of 360 and 450 nm, respectively. Values of samples containing 4-MU-galactopyranoside were subtracted from original data to control for nonspecific substrate hydrolysis.
Gelatin zymography
To assess MMP activity, gelatin zymography was performed. First, d 8 macrophages were treated with 4 mg/ml lidocaine in PBS, collected, and reseeded in a 96-well plate (2 × 105 cells/well) in 80 μl of DMEM supplemented with 1% (v/v) penicillin-streptomycin-glutamine (Thermo Fisher Scientific Life Sciences) without NHS, because NHS interferes with the signal of MMPs. The cells were incubated for 6 h at 37°C, after which supernatants were collected. SDS-PAGE (8% polyacrylamide) was performed with 10 mg/ml gelatin, 30 μl cell-free supernatant, and 15 μl 4× nonreducing Laemmli buffer [0.25 M Tris-HCl (pH 6.8), 8% SDS, 40% glycerol, and 0.04% bromophenol blue]. After electrophoresis, the proteins were renatured in 2.5% Triton X-100 for 30 min. Gels were incubated for 20 h in developing solution [1 M Tris-HCl (pH 7.4), 4 M NaCl, 0.5 M CaCl2, 1 mM ZnCl2, and 0.2% Brij35) and stained (7.5% acetic acid, 5% EtOH; 87.5% distilled water, and 2.5 g Coomassie Brilliant Blue (R-250)] for 1 h. The gels were destained (7.5% acetic acid and 5% methanol) for at least 1.5 h, after which MMP-9 (72 kDa) was detectable, as clear bands against a blue background. Band areas were quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Chromatin immunoprecipitation
Cell lysis and chromatin immunoprecipitation (ChIP) were performed with the True MicroChIP kit (Diagenode, Liège, Belgium) with minor modifications. monocytes/macrophages (105) were lysed in 125 µl of buffer tL1 and incubated for 5 min on ice; then, 375 µl HBSS was added, and the cells were sonicated on the Bioruptor Pico (Diagenode) for 35 cycles (30 s on, 30 s off). For immunoprecipitation, 2 μl AcH3K9/14 (Cell Signaling Technology, Leiden, The Netherlands) was added to the diluted chromatin. Precipitated DNA was isolated with MicroChIP DiaPure columns (Diagenode). ChIP-qPCR was performed on a ViiA7 Real-Time PCR System, with SYBR Green Fast (both from Thermo Fisher Scientific Life Sciences). Relative enrichments are presented as percentage input. Primer sequences are listed in Table 2.
Statistical analysis
Results are expressed as means ± sem. Data were analyzed with the unpaired Student’s t test, 1-way ANOVA with Bonferroni post hoc, or 2-way ANOVA with Bonferroni multiple testing in Prism 5.0 (GraphPad, San Diego, CA, USA), depending on the experimental setup. Values of P < 0.05 indicated significant results.
RESULTS
Treatment with TsSPs during macrophage differentiation induces an intermediate phenotype in naïve macrophages
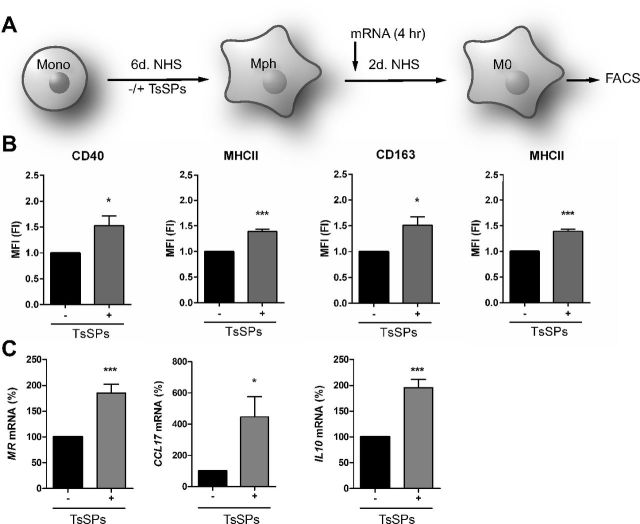
To characterize the effect of TsSPs on monocyte-to-macrophage differentiation, human monocytes were induced to differentiate into macrophages in the presence or absence of TsSPs. After 6 d, the cells were washed to remove TsSPs, cultured for another 2 d (Fig. 1A), and analyzed for the expression of M1 and -2 markers. The presence of TsSPs during differentiation had no effect on the surface expression of the macrophage marker CD68 (data not shown). In the CD68+ macrophages, we used flow cytometry to analyze the expression of the typical proinflammatory M1 markers, CD40 and MHCII, as well as the anti-inflammatory M2 markers CD163 and MR (26). As shown in Fig. 1B, macrophages treated with TsSPs during their differentiation showed significantly higher expression of both M1 and -2 surface markers, indicating an intermediate phenotype rather than polarization toward a specific macrophage subset. At the gene expression level, we observed a significant upregulation of the M2 markers MR, CCL17, and IL10 (Fig. 1C), whereas gene transcripts of the M1 markers TNF, IL12, and CD40 were unaffected (data not shown). Altogether, we observed a transcriptional shift toward M2 marker genes by treatment with TsSPs, but no clear shift to either one of the macrophage subtypes based on the surface marker expression. To further investigate the effects of TsSPs on macrophage phenotype and function, we polarized the differentiated macrophages toward an M1 or -2 subtype.
Figure 1.
TsSPs affect expression of pro- and anti-inflammatory markers in naive macrophages. A) Overview of the cell culture setup. Monocytes, isolated from healthy donors, were differentiated into macrophages with NHS in the course of 6 d, in the presence or absence of TsSPs (40 μg/ml), after which the products were washed away. Macrophages were further differentiated for 2 d and further analyzed, and RNA was extracted after 4 h. B) The expression of CD40, MHCII, CD163, and the MR was assessed by FACS on CD68+ macrophages (n = 4). Data are represented as fold induction compared to M0 macrophages not exposed to TsSPs. C) Gene expression levels of several anti-inflammatory mediators were determined by qPCR. Expression was determined relative to GAPDH (n = 7). Results are presented as the mean ± sem and analyzed with an unpaired Student’s t test. *P < 0.05, ***P < 0.001.
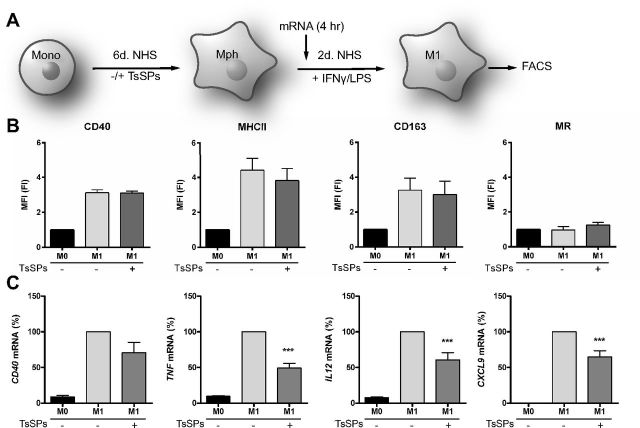
Treatment with TsSPs during macrophage differentiation dampens the expression of proinflammatory marker genes in M1 macrophages
To assess how treatment with TsSPs during macrophage differentiation affects the inflammatory phenotype of IFNγ/LPS-induced human M1 macrophages, we analyzed the expression of pro- and anti-inflammatory markers in these cells. For M1 polarization, macrophages were skewed toward the M1 phenotype by treatment with IFNγ and LPS (Fig. 2A). We then used flow cytometry to analyze the expression of proinflammatory (CD40 and MHCII) and anti-inflammatory (CD163 and MR) surface markers. TsSPs did not affect the expression of the proinflammatory surface markers CD40 and MHCII or the anti-inflammatory markers CD163 and MR (Fig. 2B). However, the expression of 3 inflammation-related genes, coding for the cytokines TNF and IL12 and the chemokine CXCL9, was significantly reduced by TsSPs and a similar, but not significant, trend was observed for CD40 gene expression (Fig. 2C). Overall, we concluded that TsSPs predominantly have repressive effects on the gene expression of proinflammatory mediators, but that these helminth products do not affect the expression of typical M1 surface markers.
Figure 2.
TsSPs repress the expression of marker genes in M1 macrophages. A) Overview of the cell culture setup. After 6 d of differentiation in the presence or absence of TsSPs, macrophages were skewed toward the M1 phenotype by incubation with 100 U/ml IFNγ for 48 h, with 10 ng/ml LPS added for the last 24 h. RNA was extracted after 4 h. B) The expression of CD40, MHC-II, CD163, and the MR was assessed by FACS (n = 4) on CD68+ macrophages. Data are represented as fold induction of M0 macrophages not exposed to TsSPs. C) Gene expression levels of inflammation-related genes CD40, TNF, IL12, and CXCL9 were determined by qPCR after 4 h polarization, relative to the expression in control macrophages. Expression was determined relative to GAPDH (n = 4–8). Results are presented as means ± sem, 1-way ANOVA with Bonferroni post hoc test. M1+TsSPs vs. M1 alone. ***P < 0.001.
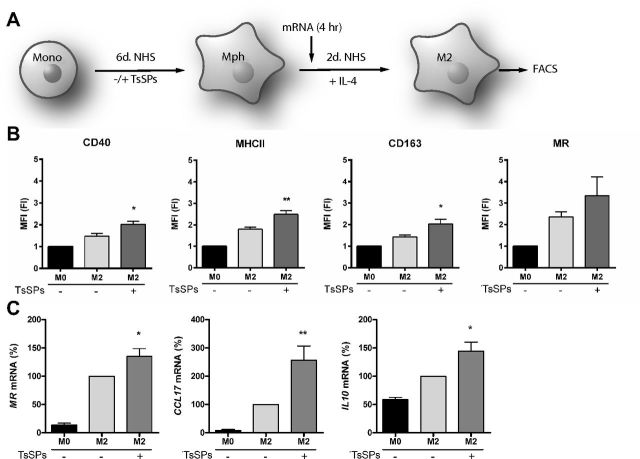
Treatment with TsSPs during differentiation enhances the expression of anti-inflammatory mediators in M2 macrophages
We next determined whether monocyte-to-macrophage differentiation in the presence of TsSPs can boost the phenotype of M2 macrophages. Macrophages were stimulated with IL-4 (Fig. 3A) and further analyzed for the expression of surface markers and of M2-associated genes. In M2 polarized macrophages, TsSPs significantly upregulated the surface expression of the proinflammatory CD40 and MHCII and the anti-inflammatory marker CD163 (Fig. 3B), similar to findings in naïve macrophages (Fig. 1B). At the gene expression level, we observed that the anti-inflammatory marker genes MR, CCL17, and IL10 were significantly increased by TsSPs (Fig. 3C). Together, these results demonstrate that TsSPs significantly affect monocyte-to-macrophage differentiation in IL-4-induced M2 macrophages by skewing gene expression toward an anti-inflammatory profile.
Figure 3.
TsSPs further enhance the expression of M2 marker genes in M2 macrophages. A) Overview of the cell culture setup. After 6 d of differentiation in the presence or absence of TsSPs, macrophages were skewed toward the M2 phenotype with 10 ng/ml IL-4 for 48 h, RNA was extracted after 4 h. B) The expression of CD40, MHC-II, CD163, and the MR was assessed by FACS of CD68+ macrophages (n = 4). Data are represented as fold induction of M0 macrophages not exposed to TsSPs. C) Gene expression levels of M2 genes MR, CCL17, and IL10 were determined after 4 h by qPCR, relative to the expression in control macrophages. Expression was determined relative to GAPDH (n = 4–8). Results are presented as means ± sem; 1-way ANOVA with Bonferroni post hoc test. M2+TsSPs vs. M2. *P < 0.05, **P < 0.01.
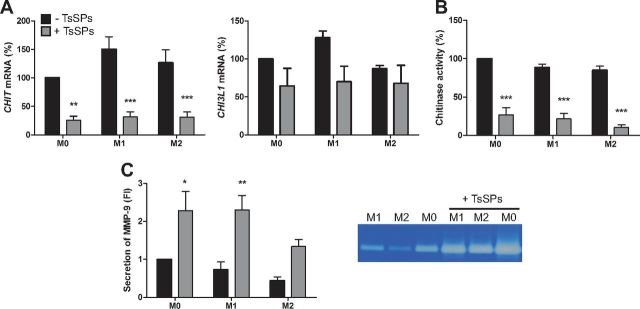
TsSPs suppress CHIT transcription and activity in M0, -1, and -2 macrophages
To assess whether TsSPs affect functional characteristics of macrophages, we measured the activity and expression of CHITs, key mediators in various human inflammatory disorders (27–30). We determined the expression of the most relevant CHITs in macrophages: chitotriosidase or CHIT 1 (encoded by the CHIT1 gene), CHIT 3-like 1 (CHI3L1), and acidic mammalian CHIT (AMCase) (31). We observed that CHIT1 expression was significantly decreased in all macrophage subtypes, whereas there was no significant decrease in CHI3L1 expression (Fig. 4A), and AMCase expression was almost nil in these human monocyte-derived macrophages (data not shown). Moreover, we assessed total CHIT activity in naïve (M0), M1, and -2 macrophages. We found that TsSPs decreased CHIT activity in all macrophage subtypes (Fig. 4B), and no significant differences were observed between the different subsets. Furthermore, we determined the secretion of matrix metalloproteinases (MMPs), which can be associated with inflammation, but are also important contributors to the wound-healing properties of macrophages (32, 33). We found that secretion of MMP9 in M0 and -1 macrophages increased significantly after treatment with TsSPs and a similar but nonsignificant trend was observed in M2 macrophages (Fig. 4C). Altogether, these results demonstrate that treatment with TsSPs decreases CHIT activity and CHIT1 expression in all subtypes and increases MMP9 secretion in M0 and -1 macrophages.
Figure 4.
TsSPs suppress CHIT transcription and activity in naïve, M1, and M2 macrophages. A) After 4 h of skewing toward the M0, -1, or -2 phenotype, qRT-PCR revealed expression levels of the CHIT genes CHIT and CHI3L1. Data show expression relative to GAPDH. Experiments were performed in cells of 6 (CHIT1) or 3 (CHI3L1) human donors. B) After 48 h of skewing toward the M0, -1, or -2 phenotype, the supernatant was incubated with 4-methylumbelliferyl-N,N ′,N′′-triacetyl chitotrioside (20 μM), and liberated methylumbelliferone was measured on a fluorescence spectrometer to determine CHIT activity. Experiments were performed in cells of 4 human donors. C) Gelatin zymography revealed secretion of MMP9, expressed relative to controls in fold induction (FI), 1 of 4 representative experiments is shown on the gel. Results are presented relative to M0 macrophages not exposed to TsSPs as means ± sem. Two-way ANOVA with Bonferroni post hoc test, −TsSPs vs. +TsSPs in all macrophage subsets. *P < 0.05, **P < 0.01, ***P < 0.001.
Downregulation of TNF and IL-6 by TsSPs can be reversed by HDAC inhibition
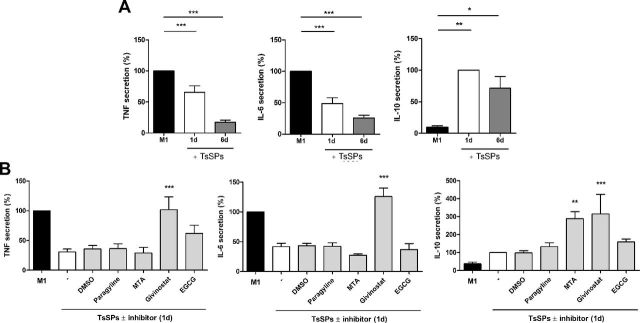
Our observation that the presence of TsSPs during differentiation resulted in a reduction of proinflammatory cytokine gene expression in M1 macrophages, although the TsSPs were absent during polarization, suggests that priming with TsSPs induces long-lasting changes leading to the reduction of cytokine transcripts. To test this hypothesis, we assessed whether brief incubations with TsSPs would accomplish similar anti-inflammatory effects on cytokine expression levels. We measured cytokine production in M1 macrophages treated with TsSPs during only the first day of monocyte-to-macrophage differentiation and found that both TNF and IL-6 secretion was still significantly reduced despite the short-term triggering by TsSPs (Fig. 5A). Furthermore, IL-10 secretion in M1 macrophages was enhanced by the single day of incubation with TsSPs. These data demonstrate that short-term priming with TsSPs can cause imprinting of long-lasting anti-inflammatory effects.
Figure 5.
TsSP priming effects on TNF and IL-6 can be reversed through HDAC inhibition. A) Primary human monocytes were incubated with TsSPs for 24 h or 6 d during differentiation, and the macrophages were skewed toward the M1 phenotype with IFNγ for 48 h, with LPS added for the last 24 h. Afterward, TNF, IL-6, and IL-10 secretion was determined by ELISA. B) Primary human monocytes were pretreated with various CMEs or left untreated for 45 min, followed by 24 h of incubation with TsSPs. After 6 d of differentiation, macrophages were incubated for 48 h with IFNγ with LPS added for the last 24 h, to skew them to the M1 phenotype. Afterward, TNF, IL-6, and IL-10 secretion was determined by ELISA. Experiments were performed on cells from at least 3 human donors. Results are presented as means ± sem, relative to M1 macrophages not exposed to TsSPs. One-way ANOVA with Bonferroni post hoc vs. control (A), or DMSO/TsSPs (B). *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether these TsSP-induced effects are mediated through epigenetic mechanisms that in turn affect gene transcription responses, we evaluated whether the effects of TsSPs can be reversed by targeting epigenetic enzymes with broad-spectrum epigenetic inhibitors: the histone demethylase inhibitor (HDMi) pargyline, the histone methyltransferase inhibitor (HMTi) MTA, the HDAC inhibitor givinostat, and the histone acetyltransferase inhibitor (HATi) EGCG. As shown above, we found that 1-d priming with TsSPs repressed TNF and IL-6 secretion (Fig. 5B). Only HDAC inhibition with givinostat significantly reversed this repression for TNF and IL-6 secretion. For IL-10, givinostat did not reverse the TsSP-induced effects, but further enhanced the production. HMT inhibition with MTA had a similar effect on IL-10 secretion. We therefore conclude that 1-d priming with TsSPs during monocyte-to-macrophage differentiation can confer imprinted long-lasting anti-inflammatory effects that are partly mediated through HDAC activity.
Priming with TsSPs decreases histone acetylation on TNF and IL6 promoters
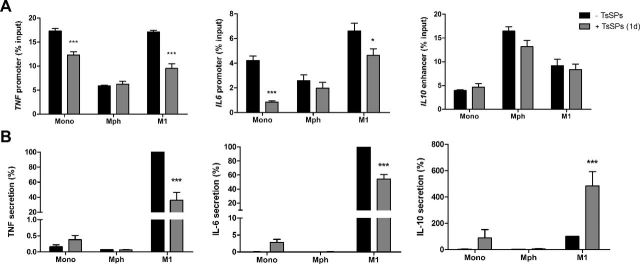
As the repressive effects of priming with TsSPs on TNF and IL-6 secretion was reversed through HDAC inhibition, we then tested the hypothesis that TsSPs induce histone deacetylation, given that decreased histone acetylation is associated with gene repression (34). Accordingly, we evaluated the effects of the treatment of TsSPs on histone acetylation during the first day of differentiation. We found that incubating monocytes with TsSPs decreased histone acetylation at the promoters of TNF and IL6 (Fig. 6A) after 24 h. During this period, TNF and IL-6 secretion by the monocytes was increased, most likely because of the release of premade cytokines, which had an acute effect on pathogen interaction (Fig. 6B). Afterward, the cells were washed and further differentiated into naïve macrophages for another 5 d. The TsSP-treated cells showed no differences in histone acetylation (Fig. 6A). However, when the TsSP-treated naïve macrophages were polarized toward the M1 phenotype, histone acetylation was suppressed at both the TNF and IL6 promoters. These data correspond with decreased TNF and IL-6 secretion by the M1 macrophages when primed with TsSPs (Fig. 6B). In contrast, the increased production of IL-10 by TsSP-primed M1 macrophages did not correlate with changes in histone acetylation at the IL10 enhancer (Fig. 6A). Overall, these results demonstrate that treatment with TsSPs induces long-lasting epigenetic changes, which underlie the epigenetic memory resulting in the repressed expression and secretion of TNF and IL-6.
Figure 6.
TsSP priming decreases histone acetylation of the TNF and IL6 promoters. Primary human monocytes were incubated with TsSPs for 24 h, washed, and further differentiated for 5 d. The macrophages were then skewed toward the M1 phenotype with IFNγ for 48 h, with LPS added for the last 24 h. A) Histone acetylation on the TNF and IL-6 promoters and IL-10 enhancer was determined with ChIP for AcH3K9/14, immediately after TsSPs treatment, after 6 d differentiation, and after 48 h M1 skewing. Enrichment of histone acetylation is presented as a percentage of input DNA; 1 experiment representative of 3 is shown. B) Also, TNF, IL-6, and IL-10 secretion was determined by ELISA at the same time points. Experiments were performed on cells from 3 donors, and results are presented as means ± sem; 2-way ANOVA with Bonferroni post hoc test vs. controls. *P < 0.05, ***P < 0.001.
DISCUSSION
Probiotic helminth administration of the porcine nematode T. suis has potential immunomodulatory capacities in the treatment of chronic inflammatory and autoimmune diseases (6–9, 35). Macrophages, in particular through their proinflammatory M1 phenotype, are key players in these diseases that can increase disease severity by releasing proinflammatory mediators (15). Consequently, dampening of the inflammatory immune response may be a potent therapeutic strategy to combat these diseases (36). In contrast, helminth-induced M2 macrophages have been shown to be protective in murine IBD and MS models by repressing inflammatory responses (21, 22). Unraveling the underlying mechanisms to show how helminth-derived molecules exert anti-inflammatory effects can contribute to new therapeutic strategies for a broad range of inflammatory and autoimmune diseases. In the current study, TsSPs functionally and phenotypically reprogrammed human macrophages by dampening the proinflammatory M1 phenotype and boosting an anti-inflammatory M2 profile, thereby revealing a new immunomodulatory mechanism of action of helminths.
We showed that treatment with TsSPs during monocyte-to-macrophage differentiation affects naïve macrophages and skews them toward a cell population with enhanced M2 properties by increasing the expression of anti-inflammatory genes, such as IL10 and the Th2-recruiting chemokine CCL17 (37). Moreover, secretion of TNF and IL-6 was significantly suppressed in M1 macrophages after treatment with TsSPs, whereas secretion of IL-10 was highly increased, which together further indicates that TsSPs repress M1 properties and induce an M2 phenotype. When polarized to M2 macrophages, TsSP treatment during monocyte-to-macrophage differentiation further enhanced the expression of M2 markers. These findings demonstrate that treatment with TsSPs may represent an effective strategy for shifting macrophages to an anti-inflammatory phenotype, by repressing the proinflammatory responses in M1 macrophages and increasing the expression of anti-inflammatory properties in both naïve and M2 macrophages. In line with our findings, it was has been shown that products of other helminths can polarize macrophages toward an M2 phenotype. For example, products of both Fasciola hepatica (38) and Schistosoma mansoni (20, 39) can induce an M2 macrophage phenotype, probably through the secreted antioxidant peroxiredoxin (40). Further research is warranted to identify the soluble products of helminths with immunomodulatory capacity.
A further characterization of the TsSP-induced macrophages revealed that TsSPs reduce the production of CHITs by all macrophage subsets studied. Several studies in mice have shown that CHIT expression is a characteristic of anti-inflammatory M2 macrophages (41, 42), but in contrast, studies in humans suggest that CHITs, such as chitotriosidase (CHIT1) and CHIT 3-like 1 (CHI3L1), exacerbate inflammation in IBD and MS (28–30). Furthermore, recent evidence has shown that secretion of these molecules enhances the migratory capacity of macrophages across an in vitro blood–brain barrier model (30). We found that monocyte-to-macrophage differentiation in the presence of TsSPs considerably decreased CHIT activity in all macrophage subsets tested. The TsSP-reduced CHIT activity is probably caused by the decreased expression of CHIT1, as CHI3L1 expression was not significantly decreased by TsSPs, and the expression of AMCase was low in these human monocyte-derived macrophages. Macrophages may produce active CHITs as a defense against chitin-containing pathogens (31). We hypothesize that T. suis may suppress the activity of CHITs for its own survival, which may explain part of the beneficial side effects in inflammatory disorders.
Besides its anti-inflammatory effects, TsSPs induce proinflammatory features in macrophages, as illustrated by the enhanced CD40 and MHCII levels in M0 macrophages and the enhanced production of MMP9 by M0 and M1 macrophages. MMP9 may play an important role in the inflammatory responses. In IBD, it is thought that upregulation of MMP9 leads to tissue damage and increased disease activity in patients (43–45). In MS, levels of MMP9 in cerebrospinal fluid are significantly increased and correlate with blood–brain barrier disruption (46). However, it has also been suggested that in the CNS, MMP2 and -9 are involved in regenerative mechanisms, such as neurogenesis, regeneration of axons, and remyelination (47), suggesting a neuroprotective role for enhanced MMP9 secretion. It should be mentioned that it is surprising to see that TsSPs suppress TNF secretion but not that of MMP9, given that TNF reduction has been thought to be the mechanism behind MMP9 reduction in patients with IBD treated with TNF-blocking antibodies (48).
Our data reveal that the TsSP-induced properties in monocyte-derived macrophages reprogrammed these cells and potently diminished their proinflammatory capacity. We found that epigenetic imprinting explains the long-lasting repression of IL-6 and TNF secretion by the M1 macrophages. It has been shown that the polarization toward M2 macrophages is highly regulated by epigenetic mechanisms (17, 23, 49). In helminth-induced macrophage polarization, this regulation is shown for example by Nippostrongylus brasiliensis, which regulates polarization to the M2 phenotype through activity of the epigenetic enzyme Jmjd3 (50). Epigenetic mechanisms are known to regulate memory responses, which can lead to tolerance, with reduced responses to prevent further tissue damage, or training, resulting in a stronger and more effective immune response. After training with Candida albicans or β-glucans (components of the skeletal cell wall of C. albicans), monocytes respond with increased cytokine production upon a second inflammatory trigger (51–53). By contrast, prestimulation of monocytes or macrophages with high doses of the bacterial cell wall component LPS can induce tolerance. Tolerized macrophages show reduced inflammatory responses, thereby preventing further tissue damage (54). This effect is accomplished by a tight, epigenetic, and transcriptional regulation of which genes are repressed at a second hit and which ones are not (55).
In our current study, we found that a brief (1 d) encounter with TsSPs is sufficient to dampen macrophage activation caused by a second stimulation with IFNγ/LPS, which suggests the induction of tolerance in TsSP-treated M1 macrophages. In a previous study of LPS tolerance, treatment of macrophages with pargyline, an inhibitor of HDMs, prevented IL6 silencing in tolerant macrophages, indicating that demethylase activity is essential for shutting down tolerizable genes (55). We applied a similar approach in which we used epigenetic inhibitors for the 4 most important classes of epigenetic enzymes (inhibitors for HDMs, HMTs, HDACs, and HATs). We demonstrated that only inhibition of HDACs could significantly reverse the repressive effects of TsSPs on TNF and IL6 expression. HDACs repress gene transcription, as histone acetylation is associated with open chromatin and gene transcription (34). We therefore hypothesized that TsSPs induce histone deacetylation. Using ChIP for acetylated [3H] on Lys9 and Lys14 in the TNF and IL6 promoters in monocytes, we indeed observed decreased histone acetylation after 1 d treatment with TsSPs. We no longer observed this difference after differentiation of these monocytes to macrophages (6 d), suggesting the involvement of other epigenetic mechanisms, such as the placement of repressive histone marks, to maintain repression of these genes. When triggered with IFNγ/LPS, however, we again observed a marked decrease in histone acetylation of both promoters, indicating epigenetic imprinting of the TsSP-induced effects. The TsSP-induced enhanced expression of IL10 could not be reversed with epigenetic inhibitors, but rather was increased by HDAC inhibition, which may be attributable to additional properties of HDAC inhibitors (56). No differences were observed on histone acetylation of the IL10 enhancer, suggesting that alternative transcriptional mechanisms, such as increased PKCδ signaling (13), may play a role.
In conclusion, we have demonstrated that priming with TsSPs during monocyte-to-macrophage differentiation has long-lasting effects in human macrophages by dampening the M1 phenotype and boosting an M2 profile. At a transcriptional level, our results unravel the long-lasting memory effects of a brief encounter with TsSPs, which are caused by histone deacetylation of the TNF and IL6 promoters. We concluded that TsSPs are strong anti-inflammatory mediators that can tolerize proinflammatory cytokine genes for the long term through epigenetic changes. Together, these findings provide new fundamental insights into the underlying mechanism on T. suis-induced modulation of monocyte-derived macrophage maturation and polarization. This knowledge contributes to the understanding of the complex interplay between T. suis and the innate immune system, and we postulate that TsSP treatment not only dampens pathogenic proinflammatory responses in patients, but simultaneously induces protective M2 responses and thereby restores the disturbed M1/M2 balance that characterizes various chronic inflammatory and autoimmune disorders.
Acknowledgments
The authors thank Dr. Helene Kringel (University of Copenhagen, Copenhagen, Denmark) for providing the Trichuris suis worms. This work was supported by Dutch Multiple Sclerosis Research Foundation Grant MS11-771 (to I.vD.), and by U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases Grant AI101982 (to R.D.C.). M.P.J.D.W. is an established investigator of the Netherlands Heart Foundation (2007T067), supported by Netherlands Heart Foundation Grant 2010B022, NWO Grant TOP91208001, Netherlands Cardiovascular Research Initiative Grant CVON2011-19 (GENIUS), and European Union Grant Horizon2020 ITN (EPIMAC), and holds an Academisch Medisch Centrum (AMC) fellowship. The authors declare no conflicts of interest.
Glossary
- AMCase
acidic mammalian chitinase
- BSA
bovine serum albumin
- CHI3L
CHIT 3-like
- ChIP
chromatin immunoprecipitation, CHIT, chitinase
- CME
chromatin modifying enzyme
- DC
dendritic cell
- EGCG
epigallocatechin-3-gallate
- FACS
fluorescence-activated cell sorting
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HDM
histone demethylase
- HMT
histone methyltransferase
- IBD
inflammatory bowel disease
- MACS
magnetic-activated cell sorting
- MHC
major histocompatibility class
- MMP
matrix metalloproteinase
- MR
mannose receptor
- MS
multiple sclerosis
- MTA
5′-methylthioadenosine
- NHS
normal human serum
- PBMC
peripheral blood mononuclear cell
- Th
T helper
- TsSP
Trichuris suis soluble product
REFERENCES
- 1.Fleming J. O. (2013) Helminth therapy and multiple sclerosis. Int. J. Parasitol. , 259–274 [DOI] [PubMed] [Google Scholar]
- 2.Yazdanbakhsh M., Kremsner P. G., van Ree R. (2002) Allergy, parasites, and the hygiene hypothesis. Science , 490–494 [DOI] [PubMed] [Google Scholar]
- 3.Maizels R. M., McSorley H. J., Smyth D. J. (2014) Helminths in the hygiene hypothesis: sooner or later? Clin. Exp. Immunol. , 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott D. E., Weinstock J. V. (2012) Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann. N. Y. Acad. Sci. , 83–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming J. O., Weinstock J. V. (2015) Clinical trials of helminth therapy in autoimmune diseases: rationale and findings. Parasite Immunol. , 277–292 [DOI] [PubMed] [Google Scholar]
- 6.Fleming J. O., Isaak A., Lee J. E., Luzzio C. C., Carrithers M. D., Cook T. D., Field A. S., Boland J., Fabry Z. (2011) Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult. Scler. , 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summers R. W., Elliott D. E., Urban J. F. Jr., Thompson R., Weinstock J. V. (2005) Trichuris suis therapy in Crohn’s disease. Gut , 87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers R. W., Elliott D. E., Urban J. F. Jr., Thompson R. A., Weinstock J. V. (2005) Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology , 825–832 [DOI] [PubMed] [Google Scholar]
- 9.Garg S. K., Croft A. M., Bager P. (2014) Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. , CD009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuijk L. M., Klaver E. J., Kooij G., van der Pol S. M., Heijnen P., Bruijns S. C., Kringel H., Pinelli E., Kraal G., de Vries H. E., Dijkstra C. D., Bouma G., van Die I. (2012) Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol. Immunol. , 210–218 [DOI] [PubMed] [Google Scholar]
- 11.Ebner F., Hepworth M. R., Rausch S., Janek K., Niewienda A., Kühl A., Henklein P., Lucius R., Hamelmann E., Hartmann S. (2014) Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy , 1489–1497 [DOI] [PubMed] [Google Scholar]
- 12.Klaver E. J., Kuijk L. M., Laan L. C., Kringel H., van Vliet S. J., Bouma G., Cummings R. D., Kraal G., van Die I. (2013) Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int. J. Parasitol. , 191–200 [DOI] [PubMed] [Google Scholar]
- 13.Kooij G., Braster R., Koning J. J., Laan L. C., van Vliet S. J., Los T., Eveleens A. M., van der Pol S. M., Förster-Waldl E., Boztug K., Belot A., Szilagyi K., van den Berg T. K., van Buul J. D., van Egmond M., de Vries H. E., Cummings R. D., Dijkstra C. D., van Die I. (2015) Trichuris suis induces human non-classical patrolling monocytes via the mannose receptor and PKC: implications for multiple sclerosis. Acta Neuropathol. Commun. , 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottow M. K., Klaver E. J., van der Pouw Kraan T. C., Heijnen P. D., Laan L. C., Kringel H., Vogel D. Y., Dijkstra C. D., Kooij G., van Die I. (2014) The helminth Trichuris suis suppresses TLR4-induced inflammatory responses in human macrophages. Genes Immun. , 477–486 [DOI] [PubMed] [Google Scholar]
- 15.Wynn T. A., Chawla A., Pollard J. W. (2013) Macrophage biology in development, homeostasis and disease. Nature , 445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. , 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeksema M. A., Stöger J. L., de Winther M. P. (2012) Molecular pathways regulating macrophage polarization: implications for atherosclerosis. Curr. Atheroscler. Rep. , 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., Natoli G., Saeij J. P., Schultze J. L., Shirey K. A., Sica A., Suttles J., Udalova I., van Ginderachter J. A., Vogel S. N., Wynn T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity , 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuch O., Akira S. (2011) Epigenetic control of macrophage polarization. Eur. J. Immunol. , 2490–2493 [DOI] [PubMed] [Google Scholar]
- 20.Wolfs I. M., Stöger J. L., Goossens P., Pöttgens C., Gijbels M. J., Wijnands E., van der Vorst E. P., van Gorp P., Beckers L., Engel D., Biessen E. A., Kraal G., van Die I., Donners M. M., de Winther M. P. (2014) Reprogramming macrophages to an anti-inflammatory phenotype by helminth antigens reduces murine atherosclerosis. FASEB J. , 288–299 [DOI] [PubMed] [Google Scholar]
- 21.Reyes J. L., Espinoza-Jiménez A. F., González M. I., Verdin L., Terrazas L. I. (2011) Taenia crassiceps infection abrogates experimental autoimmune encephalomyelitis. Cell. Immunol. , 77–87 [DOI] [PubMed] [Google Scholar]
- 22.Smith P., Mangan N. E., Walsh C. M., Fallon R. E., McKenzie A. N., van Rooijen N., Fallon P. G. (2007) Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. , 4557–4566 [DOI] [PubMed] [Google Scholar]
- 23.Van den Bossche J., Neele A. E., Hoeksema M. A., de Winther M. P. (2014) Macrophage polarization: the epigenetic point of view. Curr. Opin. Lipidol. , 367–373 [DOI] [PubMed] [Google Scholar]
- 24.van Stijn C. M., van den Broek M., van de Weerd R., Visser M., Taşdelen I., Tefsen B., van Die I. (2009) Regulation of expression and secretion of galectin-3 in human monocyte-derived dendritic cells. Mol. Immunol. , 3292–3299 [DOI] [PubMed] [Google Scholar]
- 25.García-Vallejo J. J., van Liempt E., da Costa Martins P., Beckers C., van het Hof B., Gringhuis S. I., Zwaginga J. J., van Dijk W., Geijtenbeek T. B., van Kooyk Y., van Die I. (2008) DC-SIGN mediates adhesion and rolling of dendritic cells on primary human umbilical vein endothelial cells through LewisY antigen expressed on ICAM-2. Mol. Immunol. , 2359–2369 [DOI] [PubMed] [Google Scholar]
- 26.Vogel D. Y., Glim J. E., Stavenuiter A. W., Breur M., Heijnen P., Amor S., Dijkstra C. D., Beelen R. H. (2014) Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology , 695–703 [DOI] [PubMed] [Google Scholar]
- 27.Kzhyshkowska J., Gratchev A., Goerdt S. (2007) Human CHITs and CHIT-like proteins as indicators for inflammation and cancer. Biomark. Insights , 128–146 [PMC free article] [PubMed] [Google Scholar]
- 28.Mizoguchi E. (2006) CHIT 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology , 398–411 [DOI] [PubMed] [Google Scholar]
- 29.Comabella M., Fernández M., Martin R., Rivera-Vallvé S., Borrás E., Chiva C., Julià E., Rovira A., Cantó E., Alvarez-Cermeño J. C., Villar L. M., Tintoré M., Montalban X. (2010) Cerebrospinal fluid CHIT 3-like 1 levels are associated with conversion to multiple sclerosis. Brain , 1082–1093 [DOI] [PubMed] [Google Scholar]
- 30.Correale J., Fiol M. (2011) CHIT effects on immune cell response in neuromyelitis optica and multiple sclerosis. Mult. Scler. , 521–531 [DOI] [PubMed] [Google Scholar]
- 31.Nance J. P., Vannella K. M., Worth D., David C., Carter D., Noor S., Hubeau C., Fitz L., Lane T. E., Wynn T. A., Wilson E. H. (2012) CHIT dependent control of protozoan cyst burden in the brain. PLoS Pathog. , e1002990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. , 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gause W. C., Wynn T. A., Allen J. E. (2013) Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. , 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neele A. E., Van den Bossche J., Hoeksema M. A., de Winther M. P. (2015) Epigenetic pathways in macrophages emerge as novel targets in atherosclerosis. Eur. J. Pharmacol. (Pt A), 79–89 [DOI] [PubMed] [Google Scholar]
- 35.Weinstock J. V., Elliott D. E. (2009) Helminths and the IBD hygiene hypothesis. Inflamm. Bowel Dis. , 128–133 [DOI] [PubMed] [Google Scholar]
- 36.Bogie J. F., Stinissen P., Hendriks J. J. (2014) Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. , 191–213 [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. , 677–686 [DOI] [PubMed] [Google Scholar]
- 38.Donnelly S., O’Neill S. M., Sekiya M., Mulcahy G., Dalton J. P. (2005) Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. , 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaccone P., Burton O. T., Gibbs S., Miller N., Jones F. M., Dunne D. W., Cooke A. (2010) Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J. Biomed. Biotechnol. , 795210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donnelly S., Stack C. M., O’Neill S. M., Sayed A. A., Williams D. L., Dalton J. P. (2008) Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. , 4022–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. , 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreider T., Anthony R. M., Urban J. F. Jr., Gause W. C. (2007) Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. , 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravi A., Garg P., Sitaraman S. V. (2007) Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm. Bowel Dis. , 97–107 [DOI] [PubMed] [Google Scholar]
- 44.Faubion W. A. Jr., Fletcher J. G., O’Byrne S., Feagan B. G., de Villiers W. J., Salzberg B., Plevy S., Proctor D. D., Valentine J. F., Higgins P. D., Harris J. M., Diehl L., Wright L., Tew G. W., Luca D., Basu K., Keir M. E. (2013) EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am. J. Gastroenterol. , 1891–1900 [DOI] [PubMed] [Google Scholar]
- 45.Liu H., Patel N. R., Walter L., Ingersoll S., Sitaraman S. V., Garg P. (2013) Constitutive expression of MMP9 in intestinal epithelium worsens murine acute colitis and is associated with increased levels of proinflammatory cytokine Kc. Am. J. Physiol. Gastrointest. Liver Physiol. , G793–G803 [DOI] [PubMed] [Google Scholar]
- 46.Avolio C., Ruggieri M., Giuliani F., Liuzzi G. M., Leante R., Riccio P., Livrea P., Trojano M. (2003) Serum MMP-2 and MMP-9 are elevated in different multiple sclerosis subtypes. J. Neuroimmunol. , 46–53 [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg G. A. (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. , 205–216 [DOI] [PubMed] [Google Scholar]
- 48.Gao Q., Meijer M. J., Schlüter U. G., van Hogezand R. A., van der Zon J. M., van den Berg M., van Duijn W., Lamers C. B., Verspaget H. W. (2007) Infliximab treatment influences the serological expression of matrix metalloproteinase (MMP)-2 and -9 in Crohn’s disease. Inflamm. Bowel Dis. , 693–702 [DOI] [PubMed] [Google Scholar]
- 49.Ivashkiv L. B. (2013) Epigenetic regulation of macrophage polarization and function. Trends Immunol. , 216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satoh T., Takeuchi O., Vandenbon A., Yasuda K., Tanaka Y., Kumagai Y., Miyake T., Matsushita K., Okazaki T., Saitoh T., Honma K., Matsuyama T., Yui K., Tsujimura T., Standley D. M., Nakanishi K., Nakai K., Akira S. (2010) The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. , 936–944 [DOI] [PubMed] [Google Scholar]
- 51.Netea M. G., Quintin J., van der Meer J. W. (2011) Trained immunity: a memory for innate host defense. Cell Host Microbe , 355–361 [DOI] [PubMed] [Google Scholar]
- 52.Quintin J., Saeed S., Martens J. H., Giamarellos-Bourboulis E. J., Ifrim D. C., Logie C., Jacobs L., Jansen T., Kullberg B. J., Wijmenga C., Joosten L. A., Xavier R. J., van der Meer J. W., Stunnenberg H. G., Netea M. G. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe , 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saeed S., Quintin J., Kerstens H. H., Rao N. A., Aghajanirefah A., Matarese F., Cheng S. C., Ratter J., Berentsen K., van der Ent M. A., Sharifi N., Janssen-Megens E. M., Ter Huurne M., Mandoli A., van Schaik T., Ng A., Burden F., Downes K., Frontini M., Kumar V., Giamarellos-Bourboulis E. J., Ouwehand W. H., van der Meer J. W., Joosten L. A., Wijmenga C., Martens J. H., Xavier R. J., Logie C., Netea M. G., Stunnenberg H. G. (2014) Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science , 1251086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Álvarez-Errico D., Vento-Tormo R., Sieweke M., Ballestar E. (2015) Epigenetic control of myeloid cell differentiation, identity and function. Nat. Rev. Immunol. , 7–17 [DOI] [PubMed] [Google Scholar]
- 55.Foster S. L., Hargreaves D. C., Medzhitov R. (2007) Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature , 972–978 [DOI] [PubMed] [Google Scholar]
- 56.Han S. B., Lee J. K. (2009) Anti-inflammatory effect of Trichostatin-A on murine bone marrow-derived macrophages. Arch. Pharm. Res. , 613–624 [DOI] [PubMed] [Google Scholar]