Overexpression of Medicago SOC1a leads to precocious primary shoot axis elongation.
Keywords: Cell elongation, flowering, FTa1, gibberellins, internode, Medicago, MtSOC1a, MtSOC1b, MtSOC1c, primary shoot axis elongation
Abstract
Medicago flowering, like that of Arabidopsis, is promoted by vernalization and long days, but alternative mechanisms are predicted because Medicago lacks the key regulators CO and FLC. Three Medicago SOC1-like genes, including MtSOC1a, were previously implicated in flowering control, but no legume soc1 mutants with altered flowering were reported. Here, reverse transciption–quantitative PCR (RT–qPCR) indicated that the timing and magnitude of MtSOC1a expression was regulated by the flowering promoter FTa1, while in situ hybridization indicated that MtSOC1a expression increased in the shoot apical meristem during the floral transition. A Mtsoc1a mutant showed delayed flowering and short primary stems. Overexpression of MtSOC1a partially rescued the flowering of Mtsoc1a, but caused a dramatic increase in primary stem height, well before the transition to flowering. Internode cell length correlated with stem height, indicating that MtSOC1a promotes cell elongation in the primary stem. However, application of gibberellin (GA3) caused stem elongation in both the wild type and Mtsoc1a, indicating that the mutant was not defective in gibberellin responsiveness. These results indicate that MtSOC1a may function as a floral integrator gene and promotes primary stem elongation. Overall, this study suggests that apart from some conservation with the Arabidopsis flowering network, MtSOC1a has a novel role in regulating aspects of shoot architecture.
Introduction
The timing of flowering is a critical agronomic trait in plants because of its major effects on reproductive and vegetative productivity (Julier et al., 2007; Jung and Müller, 2009; Yeoh et al., 2011; Tadege et al., 2015). Plants have evolved sophisticated flowering gene networks that integrate different internal and external signals before making the decision to flower (Putterill et al., 2004; Srikanth and Schmid, 2011). In Arabidopsis thaliana (Arabidopsis), the MADS box transcription factor gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) and FLOWERING LOCUS T (FT) function as key floral integrator genes that promote the transition to flowering (Lee and Lee, 2010; Andrés and Coupland, 2012; Immink et al., 2012; Tao et al., 2012). In inductive long-day (LD) conditions, the FT florigen moves via the phloem to the shoot apex, where it rapidly activates SOC1 expression (Michaels et al., 2005; Turck et al., 2008; Berry and Dean, 2015; Putterill and Varkonyi-Gasic, 2016). SOC1 interacts with AGAMOUS-LIKE 24 (AGL24) and FRUITFULL (FUL) to activate LEAFY (LFY), which then triggers the expression of the floral meristem identity gene APETALA 1 (AP1) and thus floral commitment and the ensuing development of flowers on the flanks of the shoot apical meristem (Lee and Lee, 2010; Balanzà et al., 2014).
The Arabidopsis soc1 mutant is late flowering, and SOC1 overexpression causes Arabidopsis to flower early (Borner et al., 2000; Lee et al., 2000; Onouchi et al., 2000; Samach et al., 2000). In addition, analysis of soc1 ful double mutants uncovered an overlapping role of these genes in acceleration of flowering time in LDs, floral commitment, meristem determinacy, and in suppressing perennial characters in Arabidopsis (Melzer et al., 2008). AtSOC1 plays a redundant role with AGL24 and SHORT VEGETATIVE PHASE (SVP) in promoting the proliferation of the floral primordium during the early stages of flower development. It is then repressed, but transcript is detected at low levels later in flower development (Immink et al., 2012). Genome-wide studies indicate that AtSOC1 binds to and regulates a large number of flowering regulators including floral homeotic genes and AP2-like floral repressors. SOC1 also directly represses its own expression and that of its negative regulators such as SVP (Immink et al., 2012; Tao et al., 2012; Mateos et al., 2015).
SOC1 is in the TM3 clade of MIKCc MADS proteins which was present in all 27 flowering plant genomes analysed (Gramzow and Theißen, 2015), indicative of its general importance. In rice, the SOC1-like gene OsMADS50 promotes flowering while OsMADS56 represses flowering when overexpressed (Lee et al., 2004; Komiya et al., 2009; Ryu et al., 2009). The FvSOC1 gene also represses flowering in woodland strawberry (Mouhu et al., 2013). In maize, ZmMADS1 promotes the transition to flowering (Alter et al., 2016) and, in petunia, silencing of its three SOC1-like genes delays flowering (Preston et al., 2014). Several SOC1-like genes promote flowering when overexpressed, including orchid DoSOC1 that accelerates flowering in Arabidopsis and orchid (Ding et al., 2013), cotton GhSOC1 (Zhang et al., 2016) that promotes flowering in Arabidopsis, and tree peony PsSOC1 that accelerates tobacco flowering (Wang et al., 2015).
In legumes, a SOC1-like gene, GmGAL1/GmSOC1, from the tropical short-day (SD) plant soybean accelerates flowering when overexpressed in Arabidopsis (Zhong et al., 2012) and Lotus corniculatus (Na et al., 2013). However, loss-of-function soc1 mutations have not yet been associated with altered flowering time for any legume (Fudge et al., 2018). We are studying flowering time regulation in the temperate-climate reference legume Medicago truncatula (Medicago), using its genomic resources including a complete genome sequence and Tnt1 retroelement insertion mutant populations (Tadege et al., 2009; Young et al., 2011; Putterill et al., 2013). Although, like winter annual Arabidopsis, Medicago flowering is induced by vernalization followed by LD photoperiods (Clarkson and Russell, 1975), novel mechanisms of flowering time control are probably present because unlike Arabidopsis, Medicago lacks both the FLOWERING LOCUS C (FLC) clade of floral repressors and the CONSTANS (CO)-mediated photoperiodic switch function (Putterill et al., 2013; Weller and Ortega, 2015).
To dissect the Medicago flowering network, we isolated Medicago flowering time mutants including the early flowering spring mutants (Jaudal et al., 2013; Putterill et al., 2013; Yeoh et al., 2013) and identified flowering time regulators by reverse genetic approaches (Laurie et al., 2011; Jaudal et al., 2014, 2015, 2016). The Medicago FT-like gene, FTa1, strongly promotes the transition to flowering in Medicago and is a major integrator of the floral inductive signals of vernalization and LDs (Laurie et al., 2011). We also uncovered an important role for the predicted Polycomb complex member MtVERNALISATION2 (MtVRN2) as a flowering repressor, repressing FTa1 before vernalization (Jaudal et al., 2016). Thus, MtVRN2 has a different function from Arabidopsis VRN2 which stably represses FLC after vernalization (Berry and Dean, 2015).
Our previous results and other studies (Fudge et al., 2018) also implicated a SOC1-like gene, MtSOC1a, as a target of FTa1 in flowering in Medicago. Transcripts of MtSOC1a accumulated to higher levels in early flowering transgenic Medicago plants overexpressing FTa1 and in the Medicago spring and Mtvrn2 mutants which precociously expressed FTa1 (Jaudal et al., 2013, 2015, 2016; Yeoh et al., 2013). Interestingly, soybean GmSOC1 gene transcript levels were also elevated in early flowering soybean overexpressing an FT-like gene, GmFT2a (Nan et al., 2014).
Here, we focused on characterizing and functionally analysing the role of MtSOC1a by profiling its temporal and spatial expression, examining Arabidopsis and Medicago transgenic plants ectopically expressing MtSOC1a and determining the phenotype of a Mtsoc1a Tnt1 mutant. These analyses indicated that apart from its capacity to promote flowering, MtSOC1a also promotes elongation of the primary shoot axis of Medicago, a function that has not been shown with SOC1-like genes before. We also profiled the expression of the other two Medicago MtSOC1 genes, MtSOC1b and MtSOC1c, and found that they were strongly dependent on MtFTa1 for their expression as previously observed (Fudge et al., 2018), but had little effect on wild-type Arabidopsis flowering time when overexpressed.
Materials and methods
Bioinformatics
Reciprocal BLASTP searches were used to identify Medicago TM3 clade MADS box proteins in the JCVI Medicago genome (Mt4.0 http://blast.jcvi.org/Medicago-Blast/index.cgi). Additional SOC1 protein sequences were obtained from Hecht et al. (2005) and Gramzow and Theißen (2015) and from JCVI, TAIR (http://www.arabidopsis.org/), NCBI (http://www.ncbi.nlm.nih.gov/), and PHYTOZOME v10 (http://phytozome.jgi.doe.gov) (Goodstein et al., 2012). In silico analysis of normalized RNA sequencing (RNA Seq) data was from the MedicMine Medicago truncatula genome database (http://medicmine.jcvi.org/medicmine/begin.do). Medicago Tnt1 lines were identified by screening the FST database (https://medicago-mutant.noble.org/mutant/blast/blast.php).
Plant growth conditions and materials
Growth in controlled environment conditions under cool white fluorescent lights at ~22 oC in LDs (16 h of light/8 h of dark) or SDs (8 h light/16 h dark) with or without prior vernalization was done as described previously (Laurie et al., 2011; Yeoh et al., 2013; Jaudal et al., 2015).
Medicago truncatula (Medicago) wild-type R108_C3 (R108) (Trinh et al., 1998), the fta1 Tnt1 insertion mutant NF3307, and the FTa1 overexpression line 35S:FTa1 in R108 were previously reported (Laurie et al., 2011). Tnt1 insertion mutants (this work) for MtSOC1a (NF1705) and MtSOC1b (NF7041) in the R108 background were obtained from the Noble Research Institute (Ardmore, OK, USA). The Mtsoc1a (NF1705) homozygous mutant was backcrossed once to wild-type R108 and the segregating F2 population were used for linkage analysis and F3 homozygous mutants in some experiments. The 35S:MtSOC1a transgenic plants in the Mtsoc1a mutant background (NF1705) and Mtsoc1a mutant (NF1705) regeneration controls (RCs) were generated in this work. The 35S:MtSOC1a (5-1) transgenic line in the Mtsoc1a mutant (NF1705) background was also crossed with wild-type R108, and the F1 and segregating populations were analysed. Genotyping was done using gene-specific and Tnt1 primers (see Supplementary Table S1 at JXB online).
Agrobacterium EHA105 with the 35S:MtSOC1a construct (see below) was used to transform leaf tissues of Mtsoc1a. T0 transformants were selected using phosphinothricin and plants were regenerated via somatic embryogenesis as described previously (Cosson et al., 2006; Laurie et al., 2011). Transformed plants were rescued to soil and escapes were eliminated by spraying with Basta herbicide, and genotyping. Flowering time was measured in days after planting and the number of nodes on the primary axis at the time the plant flowered. The length of the primary shoot axis (main stem) was measured from the monofoliate leaf node to the uppermost shoot apical bud.
Epidermal leaf peels were obtained by lightly nicking the stem using a double-edged razor blade, then peeling using fine forceps. Peels were mounted in water on slides and photographed using bright field microscopy (Leica DMR). Plants were sprayed with 10 μM or 100 μM gibberellin (dissolved in 0.05% ethanol and 0.02% Tween) or control spray (0.05% ethanol and 0.02% Tween) twice per week unless otherwise specified.
Arabidopsis thaliana (Arabidopsis) wild-type Columbia (Col) was used. Transgenic plants overexpressing MtSOC1 genes (35S:MtSOC1a–c) or the Medicago AP1-like gene MtPIM (Medtr8g066260, 35S:MtPIM) were generated by inserting the R108 cDNAs into pB2GW7 (Karimi et al., 2002). Agrobacterium GV3101 containing the pB2GW7 vectors was applied using floral dipping, and transgenic T1 and T2 plants were selected by Basta spraying and genotyping as described previously (Martinez-Trujillo et al., 2004; Jaudal et al., 2015). Flowering time of Arabidopsis plants was measured as the total number of rosette and cauline leaves at flowering.
RNA extraction and reverse transcriptase–quantitative PCR (RT–qPCR) analysis
RT–qPCR was performed on Arabidopsis and Medicago samples using an oligo(dT) primer and SYBR Green PCR Master Mix on an Applied Biosystems 7900HT Sequence Detection System (Laurie et al., 2011; Yeoh et al., 2013; Jaudal et al., 2015). Each data point is derived from the mean of three biological replicates harvested in parallel, with each replicate consisting of a pool of tissues from at least three independent plants. Leaf and shoot apical samples (hand dissected by eye to remove leaves) were harvested separately. The identity of the PCR amplicons was checked by DNA sequencing. Gene expression was normalized to PROTEIN PHOSPHATASE 2A (PP2A) (Medtr6g084690) and At2g32170 for Arabidopsis. To calculate relative gene expression, the 2-ΔΔCT method was used (Livak and Schmittgen, 2001) with modifications (Bookout and Mangelsdorf, 2003). The sample with the highest gene expression relative to PP2A is given a value of 1 and the gene expression of the other samples is calculated relative to that. Primers are shown in Supplementary Table S1.
In situ hybridization
R108 seeds were vernalized at 4 °C for 7 d and then grown in LDs with day and night temperatures of 22 °C and 18 °C, respectively. The plants flowered between 4 and 6 weeks. Shoot apices at both vegetative and reproductive stages of wild-type R108 plants (4 weeks old) were fixed and in situ hybridization was performed as described previously (Zhou et al., 2010). A 285 bp cDNA fragment from the non-conserved region of MtSOC1a was used as a probe for hybridization.
Results
MtSOC1a encodes one of four TM3 clade MADS proteins in Medicago
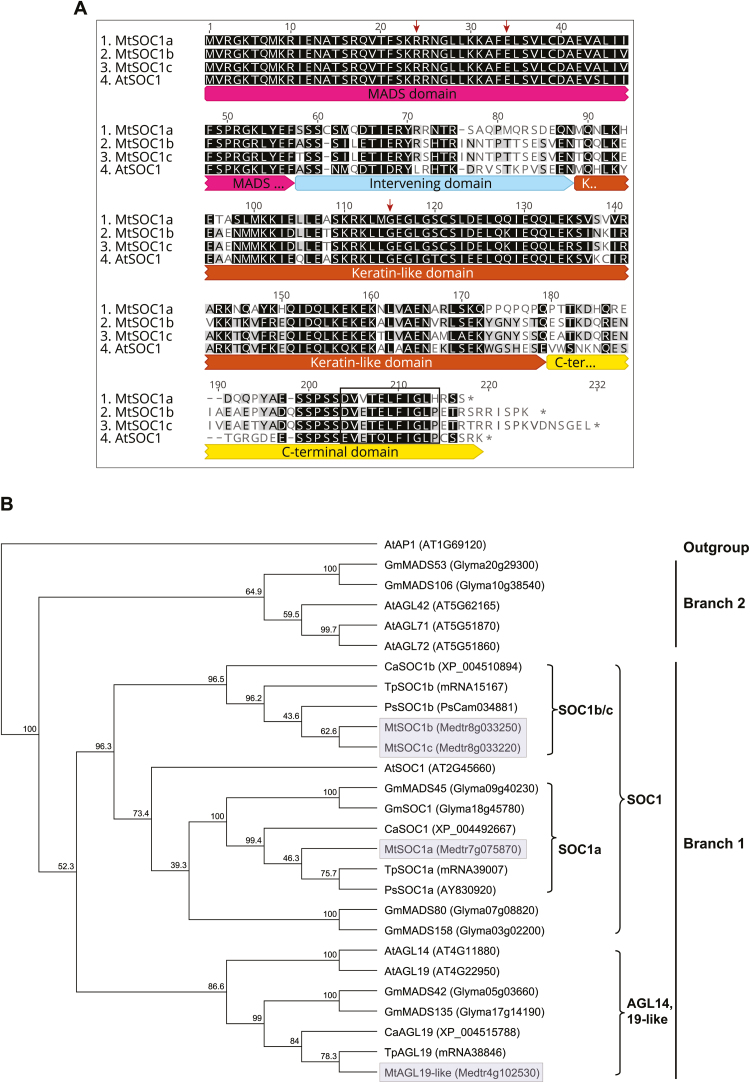
A previous phylogenetic analysis of TM3 MIKCc MADS proteins, that did not include Medicago proteins, indicated that an ancient gene duplication gave rise to two main branches: branch 1 included Arabidopsis SOC1, AGL14, and AGL19; and branch 2 had AGL42, 71, and 72, all of which are implicated in flowering regulation and other processes (Dorca-Fornell et al., 2011). Our analysis of the Mt4.0 genome (Tang et al., 2014) identified four members; the three SOC1-like proteins MtSOC1a, (Medtr7g075870), MtSOC1b (Medtr8g033250), and MtSOC1c (Medtr8g033220) (Hecht et al., 2005; Gramzow and Theißen, 2015; Fudge et al., 2018), and Medtr4g102530, more similar to AGL19, but not its top hit in reciprocal BLAST. MtSOC1a–c all shared ~66% amino acid sequence identity with AtSOC1. MtSOC1a was ~66% identical to MtSOC1b and c, while the latter two proteins, encoded by adjacent genes, were 93% identical (Fig. 1A).
Fig. 1.
Alignment and phylogenetic analysis of MtSOC1-like proteins. (A) Protein sequence alignment of AtSOC1, MtSOC1a, MtSOC1b, and MtSOC1c indicating the conserved MADS (M), intervening (I), and Keratin-like (K) domains of the MIKC MADS box proteins. The SOC1 motif (EVETQLFIGLP) (Ding et al., 2013) is boxed and the three functional residues identified by previous functional analysis of AtSOC1 (Wang et al., 2015) are marked with arrows. Identical and similar residues are highlighted in black. (B) A consensus phylogenetic tree based on the MIK domains of TM3 members from Arabidopsis, soybean, and the temperate legumes, Medicago, pea, chick pea, and red clover rooted on Arabidopsis APETALA1 (AtAP1). The tree was generated using the Neighbor–Joining (NJ) method via bootstrap resampling with a support threshold of 39%. The numbers indicate the bootstrap values based on 1000 replicates. The branch numbering is from Dorca-Fornell et al. (2011). The four Medicago proteins are highlighted in grey. At, Arabidopsis thaliana; Ca, Cicer arietinum (chick pea); Gm, Glycine max (soybean); Mt, Medicago truncatula; Ps, Pisum sativum (pea); Tp, Trifolium pratense (red clover).
Phylogenetic analysis of the MIK domains of TM3-like proteins from temperate legumes (Medicago, pea, chickpea, and red clover), Arabidopsis, and soybean (Fig. 1B) and one with additional TM3 proteins (Dorca-Fornell et al., 2011) (Supplementary Fig. S1A) indicated that all four Medicago TM3 proteins were in branch 1, suggesting that branch 2-type proteins had been lost or had diverged in Medicago. Branch 1 was subdivided into a SOC1-like clade with the three MtSOC1 proteins grouping with AtSOC1 and Medtr4g102530 grouping with other AGL14/19-like proteins (Fig. 1B). Within the SOC1 clade, the temperate legumes each had at least one SOC1a protein and one SOC1b/c protein, as recently observed using an IK domain phylogeny (Fudge et al., 2018). These legume SOC1a and SOC1b/c type paralogues probably originated during the whole-genome duplication event that pre-dated speciation of legumes ~58 million years ago (Bell et al., 2010; Young et al., 2011; Fudge et al., 2018). A more recent gene duplication event during speciation of the temperate legumes probably generated the two adjacent paralogues of the ‘b/c’ type gene that are only found in Medicago and alfalfa (Fudge et al., 2018).
Based on our in silico analysis of normalized RNA Seq data from different tissues (MedicMine Medicago truncatula genome database) (Supplementary Fig. S1B), the Medicago TM3 genes have gene-specific expression patterns, but all share the feature of being predominantly expressed in vegetative tissues, as previously observed for the MtSOC1 genes (Fudge et al., 2018). MtSOC1a and MtSOC1c are expressed at similar levels in the aerial tissues; leaf blades and vegetative buds, while MtSOC1b has a different pattern of expression because it is not detected in leaf blades, and only very weakly in vegetative buds.
RT–qPCR analysis of MtSOC1 expression in Medicago
Recently, the expression of the MtSOC1 genes was shown to be elevated by the environmental cues that induce flowering and regulated by FTa1 (Fudge et al., 2018). Our analysis by RT–qPCR of leaves and shoot apices in time courses, as opposed to a combination of total aerial tissues and apices in Fudge et al. (2018), gave similar results overall (Supplementary Figs S2–S4).
In different photoperiods, transcript of all three genes was more abundant in LDs than in SDs, with the greatest differential expression observed for MtSOC1a, which was highly elevated in the shoot apex of LD plants (Supplementary Fig. S2A, B). In the LD time course (Supplementary Fig. S2C, D), MtSOC1a transcript showed an elevation in the leaf and a more dramatic increase in the shoot apex, at 57 d old, prior to flowering. After flowering, expression dropped in the apex, but continued to rise in the leaf. There was a striking decrease in transcript levels in flower buds and it was further reduced in open flowers, as expected. MtSOC1b had a very different pattern of expression; its transcript was present at low levels during vegetative development, but it showed an abrupt increase after flowering (Supplementary Fig. S2E, F). There was a strong decline in MtSOC1b expression in flower buds, with even lower expression in open flowers. MtSOC1c transcript was present early in young plants and gradually increased through development in both leaves and apices (Supplementary Fig. S2G, H). At 57 d, a pronounced increase in MtSOC1c expression was observed in the apex, similar to MtSOC1a. It was weakly expressed in buds and was greatly reduced in open flowers, a feature it shares with the other MtSOC1 genes.
In a vernalized long day (VLD) time course carried out after vernalzing germinated seeds (Supplementary Fig. S3), the expression patterns of the three MtSOC1 genes and MtPIM, an AP1 orthologue (Benlloch et al., 2006; Cheng et al., 2018), were similar to those observed in LDs, except that more MtPIM expression was detected in the leaves of VLD- than in those of LD-grown plants (Jaudal et al., 2014). None of the four genes was expressed during cold treatment of germinated seeds. In a time course of vernalized fta1 mutant and wild-type seedlings in LDs (Supplementary Fig. S4), MtPIM expression in the fta1 mutant was barely detected, indicating dependence on FTa1 (Laurie et al., 2011). MtPIM transcript levels eventually increased at flowering in the shoot apices, indicating that other factors apart from FTa1 can regulate MtPIM expression (Supplementary Fig. S4A, B) (Laurie et al., 2011). MtSOC1a showed a much slower rate of increase in the fta1 mutant compared with the wild type, indicating that FTa1 is important for the rapid increase of MtSOC1a prior to flowering in the wild type (Supplementary Fig. S4C, D). There was almost no increase in the expression of MtSOC1b and MtSOC1c in the leaves and apices of fta1 mutants, indicating strong dependence of MtSOC1b and MtSOC1c on functional FTa1, in parallel with previous findings (Supplementary Fig. S4E–H) (Fudge et al., 2018). These results indicate that FTa1 is required for the correct temporal pattern of transcript accumulation of the MtSOC1 genes in the wild type. Consistent with this, in 35S:FTa1 transgenic plants, there was a very large increase in the expression levels of MtPIM and strong elevation of the MtSOC1 genes in the shoot apices (Supplementary Fig. S4J). In leaves, MtSOC1a was elevated, while only a modest increase was observed for MtPIM and MtSOC1b, and not much change for MtSOC1c (Supplementary Fig. S4I).
In summary, all the MtSOC1 genes were expressed in SDs, but at a higher level in LDs, and all were dependent to varying degrees on FTa1. MtSOC1a and MtSOC1c both showed steep elevation in transcript levels prior to flowering in the shoot apex, implicating them as candidate regulators of the transition to flowering in Medicago.
Overexpression of MtSOC1a accelerates wild-type Arabidopsis flowering time
To investigate if MtSOC1 or MtPIM genes can accelerate flowering in wild-type Arabidopsis, the Medicago genes were fused to the constitutive Cauliflower mosaic virus 35S promoter and transformed into Arabidopsis wild-type Col (Fig. 2).
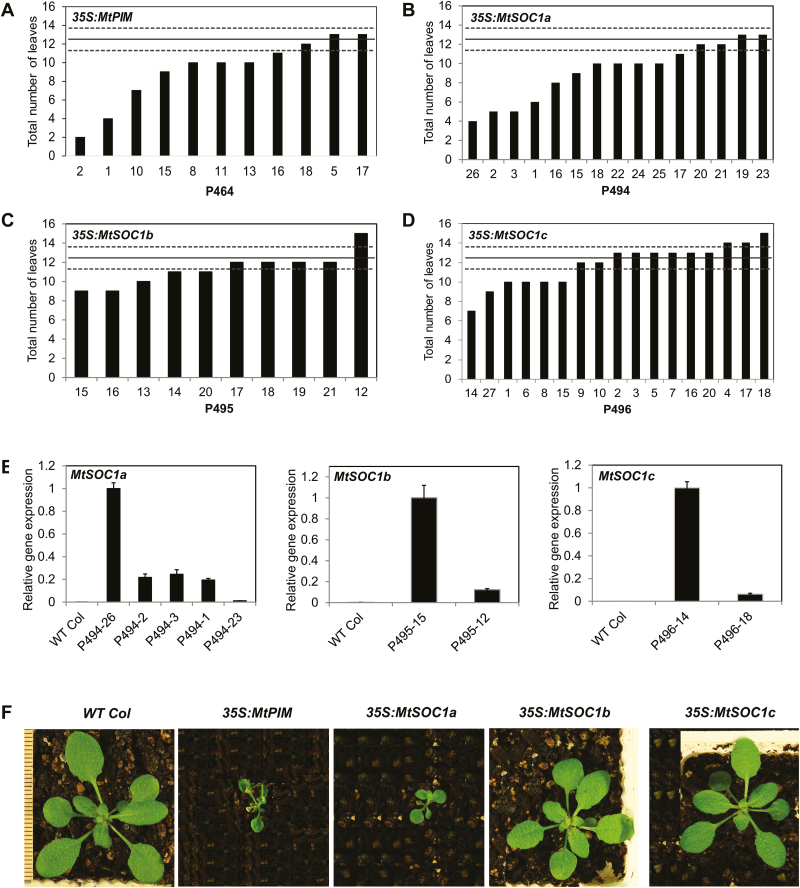
Fig. 2.
Flowering time and gene expression analyses of transgenic Arabidopsis plants with 35S:MtPIM and 35S:MtSOC1 transgenes in long-day (LD) conditions. (A–D) Graphs showing the flowering time in LDs of transgenic T1 Col plants transformed with 35S:MtPIM (A), 35S:MtSOC1a (B), 35S:MtSOC1b (C), and 35S:MtSOC1c (D). Flowering time was measured as the total number of rosette and cauline leaves at flowering. The solid line represents the average flowering time of Arabidopsis wild-type Columbia, 12.5 ± 1.10 (t.SE) leaves (0.05) (dashed lines). (E) Gene expression of MtSOC1a, MtSOC1b, and MtSOC1c in representative T1 transgenic 35S:MtSOC1 Arabidopsis plants shown in (B–D) as compared with wild-type Col plants. Gene expression was determined in selected T1 plants with a range of flowering times using RT–qPCR and normalized to At2g32170. The data are shown as the mean ±SE of three PCR technical replicates. Data are presented relative to the plant with the highest transgene expression for each overexpression construct. (F) Photographs at the time of flowering of wild-type Col plants, a T1 plant from 35S:MtPIM (P464-2), and representative T2 progeny from 35S:MtSOC1a (P494-3), 35S:MtSOC1b (P495-16), and 35S:MtSOC1c (P496-27). The scale bar has lines 1 mm apart.
The 35S:MtPIM T1 transgenic plants had a range of flowering times, with the earliest flowering transformants flowering much more rapidly than the wild type (Fig. 2A). The early flowering 35S:MtPIM plants were much smaller than the wild type, with tiny petioles, leaves, and flowers (Fig. 2F). Premature termination of the inflorescence was also observed due to formation of terminal flowers.
An extremely early flowering phenotype was also observed in four of 15 35S:MtSOC1a transgenic plants (Fig. 2B). The earliest flowering 35S:MtSOC1a transgenic plants were tiny at the time of flowering at 4–5 leaves (Fig. 2F). However, unlike MtPIM and MtFULb (Jaudal et al., 2015), overexpression of MtSOC1a did not result in the formation of terminal flowers. Analysis of MtSOC1a transgene expression by RT–qPCR indicated that the earliest flowering line (P494-26) had the highest levels of transgene expression while the latest flowering line (P494-23), which flowered similarly to the wild type, showed very low levels of transgene expression (Fig. 2B, E). Apart from early flowering, other phenotypes were also observed in some 35S:MtSOC1a plants including smaller plant size, looser flower structure, smaller flowers with shorter petals and sepals, and smaller and thinner siliques with low numbers of seeds that were dark in colour compared with the wild type (Supplementary Fig. S5).
The 35S:MtSOC1b lines flowered similarly to Col wild-type plants (Fig. 2C), although transgene expression was confirmed in selected lines (Fig. 2E). The 35S:MtSOC1b lines also had a similar plant architecture to the wild type (Fig. 2F).
The 35S:MtSOC1c transgenic plants also flowered at a similar time to the wild type (Fig. 2D), with one exception, P496-14 with earlier flowering and elevated MtSOC1c transcript levels relative to the other transgenic plant tested (Fig. 2D, E). As observed in some 35S:MtSOC1a lines (Supplementary Fig. S5), the earliest flowering 35S:MtSOC1c plant was much shorter in size and produced smaller flowers with curved petals and shorter sepals and smaller siliques with lower yield and dark seeds compared with the wild type.
Recently, Fudge et al. (2018) showed that all three MtSOC1 genes were able to promote flowering of Atsoc1 mutants, in some cases close to wild-type levels when overexpressed, but none flowered earlier than wild-type plants. Here, of the three MtSOC1 genes, overexpression of MtSOC1a appeared to have the strongest effect by dramatically accelerating flowering of four out of 15 transgenic plants compared with zero out of 10 of the 35S:MtSOC1b lines and one out of 17 of the 35S:MtSOC1c lines. The differences that we obtained with 35S:MtSOC1a compared with Fudge et al. (2018) may be due to the two different genetic backgrounds, the numbers of plants analysed, relative level of expression of the transgenes, or growth conditions. MtSOC1a shares 66% amino acid identity with MtSOC1b and MtSOC1c, while the latter two are 93% identical to each other, which raises the possibility that a coding region difference between MtSOC1a and MtSOC1b/c might contribute to the difference between the genes in this assay.
In situ hybridization with MtSOC1a
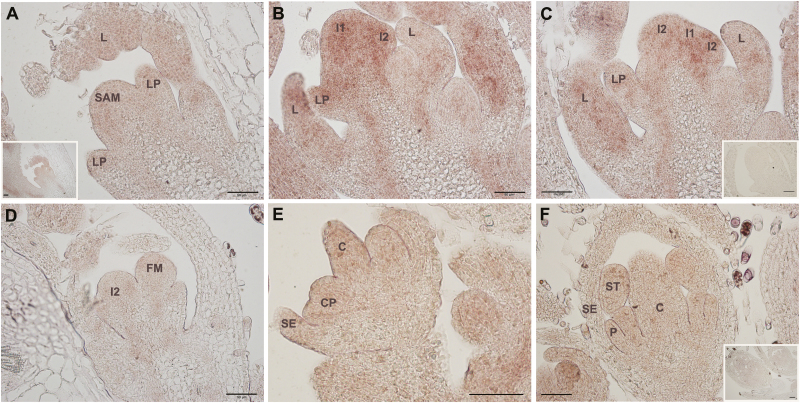
To analyse the function of MtSOC1a further, we examined the spatial expression pattern of MtSOC1a by in situ hybridization of longitudinal sections of shoot apices from 4-week-old VLD plants that had been vernalized for 1 week. These plants flowered when they were between 4 and 6 weeks old. In Arabidopsis, SOC1 expression is detected in the shoot apical meristem (SAM) and leaf primordia during the transition to flowering, but not in young floral meristems, yet reappears in the centre of the floral meristem at a later stage of floral development (Borner et al., 2000). MtSOC1a was detectable in the vegetative SAM and leaves (Fig. 3A). However, the MtSOC1a transcript level increased in the SAM, leaves, and primary (I1) and secondary (I2) inflorescence meristems at the initial reproductive stages (Fig. 3B, C), while weak expression was detected during the early stages of floral meristem development (Fig. 3D), during differentiation of floral organs (Fig. 3E), and in young flower buds (Fig. 3F).
Fig. 3.
In situ localization of MtSOC1a transcripts through the floral transition in longitudinal sections of wild-type R108 plants grown in LDs, after 1 week of vernalization. (A) Vegetative shoot apical meristem (SAM) with leaf primordium (LP) and leaf (L). The inset shows the same section at a lower magnification. (B and C) SAM at the initial reproductive stage. Increased transcript abundance was detected in SAM, L, LP, and primary (I1) and secondary (I2) inflorescence meristems. (D–F) Weak MtSOC1a expression was detected during early stages of floral meristem development (D), during differentiation of floral organs (E), and in young flower buds (F). No expression was detected using a sense control MtSOC1a probe (inset images in C and F). C, carpel; CP, common primordium for petal and stamen; P, petal; SE, sepal; ST, stamen. The black scale bar represents 50 µm.
In summary, our in situ hybridization result is analogous to our RT–qPCR data indicating that MtSOC1a transcript is expressed in Medicago during vegetative development and increases in level in the SAM during the transition to flowering.
Mtsoc1a mutant plants are late flowering and have shorter primary stems
To characterize MtSOC1a genetically, we used a mutant line (NF1705) segregating plants homozygous for a Tnt1 retrotransposon insertion in the first exon of MtSOC1a (Fig. 4A) and with greatly reduced expression of MtSOC1a compared with wild-type R108 plants (Fig. 4B).
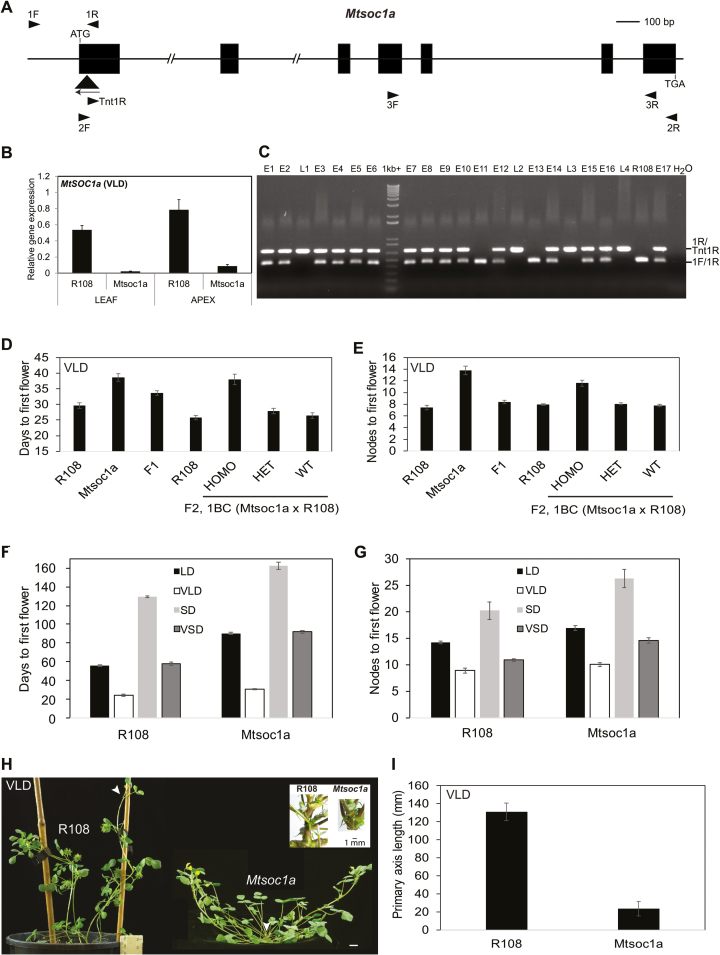
Fig. 4.
Mtsoc1a mutants have a recessive late flowering phenotype and reduced elongation of the primary stem. (A) Diagram of the MtSOC1a gene with the Tnt1 insertion (black triangle) in the Mtsoc1a mutant. Exons are the black boxes and introns are thin lines. Arrowheads indicate primers. (B) Relative expression of MtSOC1a in 14-day-old R108 and Mtsoc1a seedlings in VLDs. Gene expression was determined using RT–qPCR with 3F and 3R primers, and the data are the mean ±SE of three biological replicates, normalized to Medicago PP2A. Data are presented relative to the highest value. Tissues were harvested 2 h after dawn. (C) Photograph showing PCR genotyping fragments from segregating VLD F2 plants. Plants were scored as early (E) (like R108) or late (L) flowering relative to R108. Three genotyping primers were pooled in the PCR. (D and E) Graphs showing the flowering time in vernalized LDs (VLDs) scored as the number of days to flowering (D) or the number of nodes on the primary axis at flowering (E) of the F1 progeny (n=14) from a backcross of Mtsoc1a mutants to wild-type R108 plants compared with Mtsoc1a mutants (self-cross) (n=10) and R108 plants (n=13), and the segregating F2 progeny from this backcross (n=206: Mtsoc1a Tnt1 homozygotes, n=50; heterozygotes, n=111; wild-type segregants, n=45) with R108 wild-type control plants (n=28). The data are shown as the mean ± (t.SE) (0.05). (F and G) Graphs showing the flowering time in different conditions including short days (SDs) of Mtsoc1a mutants and R108 scored in either days (F) or nodes to first flower (G). The Mtsoc1a plants in LDs and VLDs were F3 plants after a backcross to R108. The mean ± (t.SE) (0.05) is presented (n=6–50). (H) Photographs of R108 wild-type plants with seed barrels and a prostrate, late flowering Mtsoc1a F2 homozygote with a very short primary axis taken at 45–47 d under VLDs. Arrowheads indicate the primary axis. Scale bar=1 cm. The inset image shows close-up photographs of primary shoot axes of 56-day-old LD wild-type R108 and Mtsoc1a F3 plants. (I) Graph showing the length of the primary axis of Mtsoc1a F2 homozygous mutants (n=39) after a backcross to R108 as compared with wild-type R108 (n=10). The measurements were taken at 45–50 d in VLDs and the data are shown as the mean ± (t.SE) (0.05).
We crossed the homozygous mutants with wild-type R108 plants and grew an F2 population in VLDs. Out of 206 F2 plants, approximately a quarter (50) were Mtsoc1a Tnt1 homozygotes and late flowering, approximately a half (111) were heterozygotes and flowered like wild-type R108, and approximately a quarter (45) were wild-type segregants and flowered like wild-type R108 (Fig 4C–E). Thus, there was 100% co-segregation between the homozygous Tnt1 insertion in MtSOC1a and the late flowering phenotype. These results indicated that the Tnt1 insertion in MtSOC1a was closely linked to the late flowering locus (within 1 cM) and that the mutation caused a recessive, late flowering phenotype.
In addition to their later flowering, Mtsoc1a mutant plants had a more prostrate appearance (Fig. 4H), because their primary shoot axis was shorter than that of the wild type in LDs and VLDs (Fig. 4H, I). This was associated with reduced internode elongation in the mutant (Fig 4H). Similar results were observed for a segregating population in LD conditions (Supplementary Fig. S6).
To investigate further the flowering response of the Mtsoc1a mutant, it was grown under different photoperiodic conditions with or without vernalization. The mutant flowered later than wild-type R108 plants under all conditions, in both days to flowering and in number of nodes on the primary shoot axis at the time of flowering (Fig. 4F, G). Thus, in summary, knock down of MtSOC1a in Medicago resulted in a delayed flowering time phenotype in the LD and SD conditions tested.
To analyse the molecular basis of the Mtsoc1a late flowering phenotype, candidate target genes were analysed using RT–qPCR in the leaves and shoot apices in VLDs (Supplementary Fig. S7). Only weak effects on gene expression were observed overall. MtSOC1b expression was slightly reduced in the leaves, while MtSOC1c had decreased expression in the leaves and shoot apices of Mtsoc1a. There was also a slight reduction in the transcript levels of MtFULa-b and MtPIM in the leaves of the mutants. All the other genes analysed including the five FT-like genes, FTa1, FTa2, FTc, FTb1, and FTb2; the candidate floral meristem identity gene, LFY; floral identity genes homologous to SEPALLATA (SEP3a and SEP3b); and candidate AP2-like genes, TEMPRANILLO (TEM1) and TOE1-like genes did not change much in either tissue of the Mtsoc1a mutants compared with the wild type.
Finally, we asked if mutations in the MtSOC1b/c genes can cause an altered flowering time or plant architecture phenotype. We could not identify a Mtsoc1c knock out mutant, but identified a Mtsoc1b line NF7041 with a homozygous Tnt1 insertion in the second exon of MtSOC1b and gene expression knock down (Supplementary Fig. S8A, B). However, in contrast to Mtsoc1a mutants, the Mtsoc1b Tnt1 insertion line did not display an altered flowering time phenotype or architecture in LD or VLD conditions (Supplementary Fig. S8C–F). A similar result was obtained with two other MtSOC1b Tnt1 mutant lines (Fudge et al., 2018).
Overexpression of MtSOC1a in Medicago causes precocious elongation of the primary stem
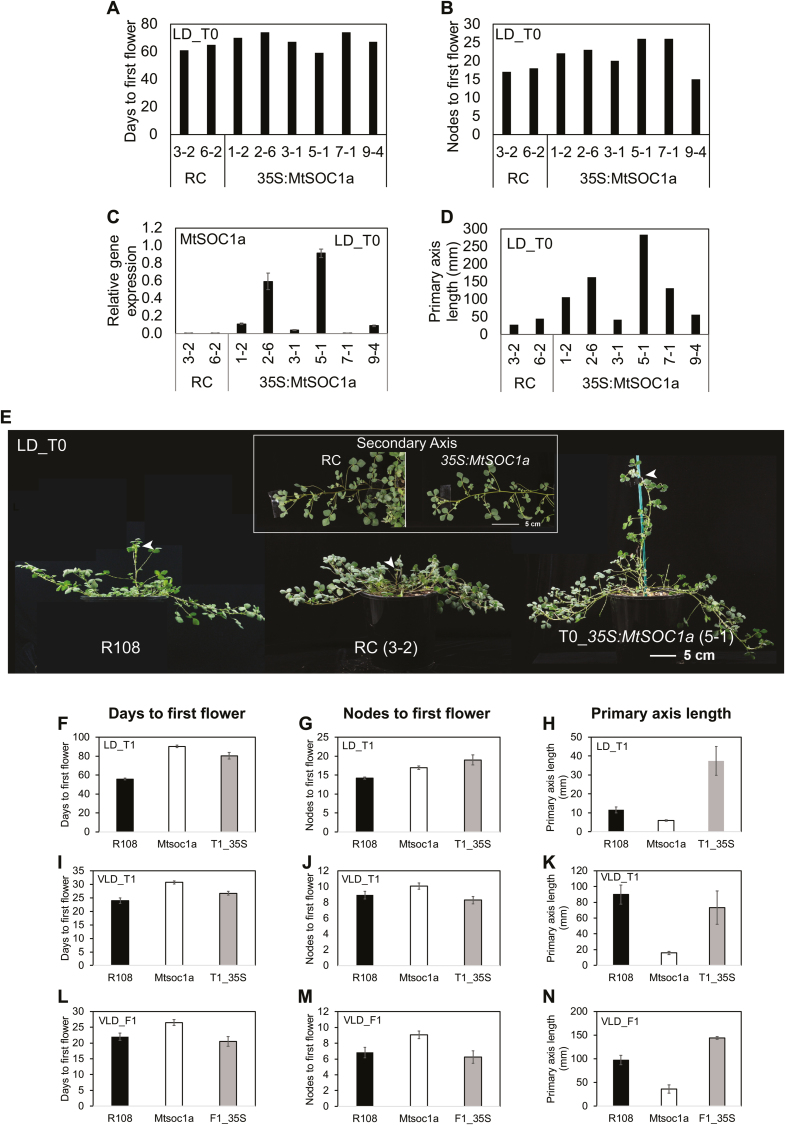
In order to investigate the effect of overexpressing MtSOC1a in Medicago, we transformed Mtsoc1a homozygous mutant leaf discs with the 35S:MtSOC1a construct. Transformant plants were regenerated via somatic embryogenesis and planted into soil in LDs. The six independent 35S:MtSOC1a/Mtsoc1a T0 lines had a range of gene expression levels, with some plants showing much higher expression of MtSOC1a than the regeneration controls (RCs) (Fig. 5C), but they all flowered at a similar time to Mtsoc1a RC at ~67 d with ~21 nodes on average (Fig 5A, B). Analysis of candidate flowering genes in the leaves and apices of transgenic plants also indicated that there was little change in their expression levels compared with RC plants (Supplementary Fig. S9).
Fig. 5.
Overexpression of MtSOC1a in Medicago results in elongation of the primary stem. (A and B) Flowering time in either days (A) or nodes (B) to first flower of individual regeneration controls (RCs) and T035S:MtSOC1a plants in LDs. (C) MtSOC1a transcript accumulation in leaves of RC and T035S:MtSOC1a plants (32–39 d old) at ZT4. Gene expression was determined using RT–qPCR with data shown as the mean ±SE of three biological replicates, normalized to PP2A and relative to the highest value. (D) Graph showing the length of the primary axis of RC and T035S:MtSOC1a plants (53–56 d in LDs). (E) Photographs of wild-type R108, RC, and T035S:MtSOC1a plants in LDs, 55–57 d after transfer of germinated seeds (for R108) or regenerated plantlets to soil. Arrowheads indicate the primary axis. The inset image shows close-up photographs of secondary branches from the primary axis of RC and T035S:MtSOC1a plants. (F–G, I–J) Graphs showing the flowering time of R108, Mtsoc1a mutants, and T135S:MtSOC1a (T1_35S) in LDs and VLDs scored in either days (F, I) or nodes (G, J) to first flower. The mean ± (t.SE) (0.05) is presented (n=7–18). (H, K) Graphs showing the length of the primary axis of T135S:MtSOC1a transformants in LDs (H) and VLDs (K) compared with Mtsoc1a and R108 plants. The measurements were taken at 33 d in LDs and 28 d in VLDs. The mean ± (t.SE) (0.05) is presented (n=7–18). The T135S:MtSOC1a plants in (F–K) were progeny of T035S:MtSOC1a (5-1). (L–N) Graphs showing the flowering time either in days (L) or nodes (M) to first flower and length of the primary axis measured at 28 d (N) in VLDs of the F1 progeny from a cross of T135S:MtSOC1a to wild-type R108 (F1_35S) compared with Mtsoc1a and R108 plants. The mean ± (t.SE) (0.05) is presented (n=4–16). The Mtsoc1a plants in (F–N) were F3 homozygous mutants after a backcross to R108.
Strikingly, however, four T0 transgenic plants had much longer primary shoot axes than RC and wild-type R108 plants (Fig. 5D, E). Thus, the short stem phenotype of the Mtsoc1a mutant was rescued by expression of 35S:MtSOC1a in these transgenic plants. Plants, such as 35S:MtSOC1a (5-1), with the highest level of MtSOC1a expression had the greatest primary shoot axis extension, indicating that primary stem elongation is sensitive to MtSOC1a levels. In contrast, the secondary branches of the 35S:MtSOC1a transgenic plants only displayed a slight increase in internode elongation compared with RCs (inset, Fig. 5E).
Next, we analysed the T1 progeny of 35S:MtSOC1a (5-1) in LD and VLD conditions. In LDs, the primary shoot was again much longer than in R108 wild-type controls, with little effect on flowering time (Fig. 5F–H). The stem elongation was striking because it was seen within the first month of growth and thus occurred well before the transition to flowering at ~80 d. These results indicate that it is possible to separate Medicago primary shoot elongation from the transition to flowering in LDs by ectopically overexpressing MtSOC1a.
In VLDs, in which R108 wild-type plants show much more rapid flowering and primary stem elongation than in LDs (Jaudal et al., 2013), the 35S:MtSOC1a (5-1) T1 plants had a similar primary shoot axis length to vernalized wild-type plants (Fig 5K). In terms of T1 flowering time, the delay in flowering seen in the Mtsoc1a mutant in VLDs was partially rescued (Fig. 5I, J). We observed that the 35S:MtSOC1a T1 plants had longer primary stems in VLDs than in LDs, indicating that they were responsive to vernalization, but this was a reduced response compared with R108 (Fig. 5H, K).
To investigate the effect of the 35S:MtSOC1a transgene in plants without the homozygous Mtsoc1a mutation, we crossed the 35S:MtSOC1a (5-1) line with R108. The VLD F1 plants with the 35S:MtSOC1a transgene flowered at a similar time to wild-type R108 plants, while their primary shoot lengths were slightly longer than those of vernalized wild-type plants (Fig. 5L–N). The same trend was observed in the LD F2 plants, in which the 35S:MtSOC1a plants without the Mtsoc1a mutation had very long primary shoot axes but with flowering time similar to R108 plants. R108 flowered at an average of 56.4 ± 0.68 (t.SE) d (0.05) and the 35S:MtSOC1a F2 segregants flowered at 55.7 ± 3.2 (t.SE) d (0.05).
In summary, the 35S:MtSOC1a transgene partially complements the late flowering of the Mtsoc1a mutant, but does not cause further acceleration of flowering in the MtSOC1a wild-type background. In contrast, the 35S:MtSOC1a transgene fully complements the short stem phenotype of the mutant and gives strikingly elongated primary shoot axes compared with the wild type, particularly in LD conditions.
Increased length of the primary shoot axis in 35S:MtSOC1a plants correlates with increased cell elongation
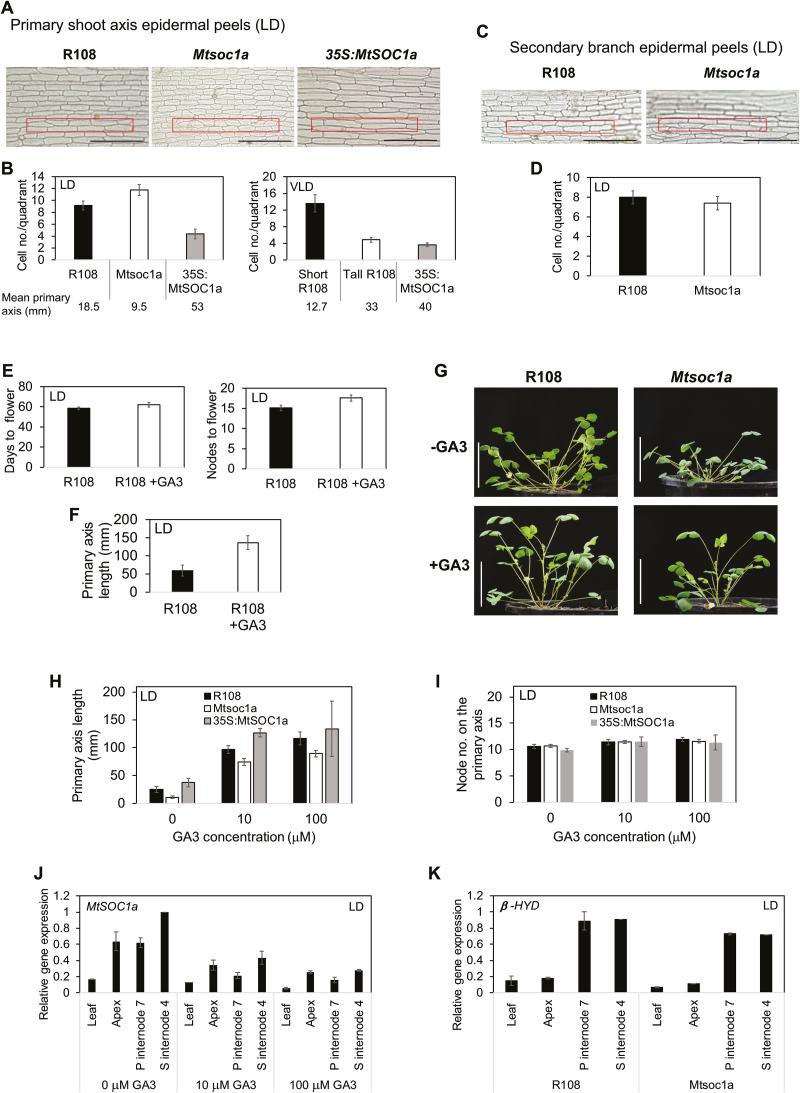
35S:MtSOC1a transgenic plants produced similar numbers of nodes to Mtsoc1a and wild-type plants in LDs (Fig. 5G), yet had much longer primary stems (Fig. 5H), suggesting that 35S:MtSOC1a promotes internode elongation. To test if cell size was affected, we analysed cell counts within a set area (quadrant) on epidermal peels of stem internodes (Fig. 6A–D).
Fig. 6.
MtSOC1a promotes cell and internode elongation in the Medicago primary shoot axis, but is not required for the response to GA3 spray. (A) Photographs of epidermal peels taken from the primary shoot axis of R108 (internode 6), Mtsoc1a mutant, and a 35S:MtSOC1a F2 plant (internode 6) (from a cross of T135S:MtSOC1a to wild-type R108) of 31- to 32-day-old plants in LDs. (B) Graphs showing average cell number ± (t.SE) (0.05) within the boxed area (400 μm×50 μm) as shown in (A) on 9–12 epidermal peels of LD plants (left graph) and 5–8 peels of VLD plants (right graph). (C) Photographs of epidermal peels taken from internode 4 of secondary branches of 50-day-old LD R108 and Mtsoc1a plants. (D) Graph showing counts of the average cell number ± (t.SE)(0.05) on 5–6 epidermal peels within the boxed area. The scale bars in (A) and (C) are 200 µm. (E and F) Graphs showing the flowering time (E) scored in either days or nodes to first flower and the length of primary axis (F) at 67 d of LD R108 plants sprayed with 100 μM GA3 or control spray. The mean ± (t.SE) (0.05) is presented (n=8). (G) Photographs of 29-day-old LD plants sprayed with 100 μM GA3 (+GA3) or control spray (–GA) with a scale bar of 5 cm. (H and I) Graphs showing the lengths of primary shoot axes (H) and their node number (I) of 33-day-old LD R108, Mtsoc1a, and T135S:MtSOC1a plants sprayed with 10 μM and 100 μM GA3 or control spray. Data are presented as the mean ± (t.SE) (0.05) (n=3–15). (J) Relative expression of MtSOC1a at ZT5 in the leaf, shoot apices of the primary and secondary branches (apex), internode 7 of the primary axis (P internode 7), and internode 4 of the longest secondary axis (S internode 4) of 42-day-old LD R108 plants sprayed with 10 μM and 100 μM GA3 or control spray. (K) Relative expression of GIBBERELLIN 3 BETA-HYDROXYLASE 1-LIKE (β-HYD, Medtr2g102570) in 42-day-old LD R108 and Mtsoc1a plants. Gene expression in (J–K) was determined using RT–qPCR, and data are the mean ±SE of two biological replicates, normalized to PP2A and presented relative to the highest value. The Mtsoc1a plants (C, D, G–K) were F3 homozygous mutants after a backcross to R108.
In LDs, the tall 35S:MtSOC1a (5-1) plants had longer epidermal cells, thus fewer cells/quadrant, than the short Mtsoc1a mutants which had shorter cells (Fig. 6A, B). In VLDs, which promote primary stem elongation in the wild type (Jaudal et al., 2013), the numbers of cells/quadrant were similar between a tall R108 plant and a 35S:MtSOC1a (5-1) plant of similar height, while a younger, shorter R108 plant had smaller cells, thus more cells/quadrant (Fig. 6B). In contrast, the secondary branches of R108 and Mtsoc1a, which were of similar lengths, had similar epidermal cell sizes and thus similar cell counts/quadrant (Fig. 6C, D).
These results indicate that an increase in primary shoot axis length positively correlates with the increased epidermal cell size and that this is promoted by MtSOC1a.
Wild-type and Mtsoc1a plants show increased shoot elongation in response to gibberellin
One of the classical roles of the hormone gibberellin (GA) is to promote stem elongation when applied to dwarf peas (Hedden and Sponsel, 2015). Thus, we hypothesized that the Mtsoc1a mutant with its shorter primary stem might have a defect in the GA response pathway. First, we tested the effect of applying 100 μM GA3 spray weekly to wild-type R108 plants in LDs. This led to a significant increase in primary shoot length (Fig. 6F, G). All plants had a similar node number at this stage (Fig. 6E), indicating that the GA3 spray had caused internode elongation, but did not promote flowering, with control plants flowering slightly earlier (Fig. 6E).
To compare the effect of GA3 application, we sprayed plants with GA3 (10 μM or 100 μM). The primary shoots were measured at 33 d old (Fig. 6H) when the plants had ~10 nodes on the primary shoot axis (Fig. 6I). Strikingly, all genotypes responded to GA3 with an overall increase in the primary shoot axis length that ranged from ~3-fold (35S:MtSOC1a), ~4-fold (R108), to ~10-fold (Mtsoc1a) with the 10 μM GA3 spray compared with the control spray, and with only a minor additional increase with the 100 μM spray. These results indicate that the Mtsoc1a short primary shoot phenotype does not appear to result from a defect in GA response in LDs.
The results also suggest that GA-mediated primary stem elongation in Medicago does not require MtSOC1a. Consistent with this, MtSOC1a expression in aerial tissues of wild-type plants, including stem internodes, was not elevated by GA3 application, rather it appeared to be slightly reduced, which may contribute to the slightly delayed flowering observed (Fig. 6J).
One alternative explanation for the short primary shoot stem of the Mtsoc1a mutant is that the mutant is defective in some aspect of GA production. Therefore, in an initial investigation, we examined the expression of a Medicago homologue of Mendel’s pea Le gene that is involved in pea internode elongation. The pea gene encodes a gibberellin 3 beta-hydroxylase involved in the production of bioactive GAs (Lester et al., 1997). However, we did not see any differences in the transcript level of this gene between the Mtsoc1a mutant and wild type (Fig. 6K).
Discussion
The transition to flowering in Medicago is promoted by vernalization and LD photoperiods, and is accompanied by changes in plant architecture including elongation of the primary stem (Chabaud et al., 2006; Jaudal et al., 2013). Our previous studies of Medicago Tnt1 insertion mutants (Jaudal et al., 2013, 2016; Yeoh et al., 2013) identified MtSOC1a as a potential target of FTa1, a potent floral integrator gene (Laurie et al., 2011). However, ours and others recent work indicate that MtSOC1a is one of three SOC1-like genes in Medicago that all depend on MtFTa1 for the magnitude and timing of their expression and can partially complement the Arabidopsis soc1 mutant, raising the possibility that they have redundant roles in flowering (Fudge et al., 2018). We tried to identify Tnt1 mutations in the three MtSOC1 genes. We and others (Fudge et al., 2018), identified several Mtsoc1b mutants, but none had a flowering time phenotype, while a Mtsoc1c mutant was not found. However, here we have identified a Mtsoc1a mutant with flowering time and growth phenotypes indicating that the MtSOC1 genes do not have fully overlapping roles.
In Medicago, our spatial and temporal analysis of MtSOC1a gene expression in aerial tissues indicated that MtSOC1a transcript was highest in the SAM during the transition to flowering, suggesting a potential role in floral induction. Consistent with this, the timing and magnitude of MtSOC1a expression was also partially regulated by FTa1 as MtSOC1a rapid accumulation was perturbed in the fta1 null mutants. However, MtSOC1a transcript eventually reached relatively high levels in the leaves and apices of fta1 mutants at flowering, indicating that other pathways or factors may turn on MtSOC1a expression in the absence of FTa1, as might be expected of a floral integrator gene (Fudge et al., 2018). MtSOC1a, in turn, does not appear to be the only target of FTa1 during floral promotion, because fta1 mutants flower later than Mtsoc1a mutants and have lost their responsiveness to vernalization (Laurie et al., 2011), unlike Mtsoc1a mutants.
The phenotype associated with a loss-of-function mutation in a SOC1a-like gene was not previously reported in legumes. Here we showed that MtSOC1a has the capacity to promote flowering in Medicago as shown in a Tnt1 mutant line, which had knock down expression of MtSOC1a and was late flowering in all conditions tested. However, unlike in Arabidopsis, we did not observe precocious flowering in 35S:MtSOC1a Medicago transgenic plants compared with wild-type plants.
MtSOC1a also has an important role in regulating primary shoot axis architecture. A striking feature of the Mtsoc1a mutants was their very short primary axis, which is a novel phenotype not observed in Arabidopsis soc1 mutants. Overexpression of MtSOC1a reversed this phenotype. Indeed, it caused a dramatic increase in internode elongation and precocious elongation of the primary shoot axis in the transgenic plants, which was not associated with the transition to flowering and not observed in the wild type in LDs. These results indicate that MtSOC1a promotes internode elongation of the primary shoot axis of Medicago, consistent with its expression in stem internode tissue (Fig. 6J) (Fudge et al., 2018).
Previously, we showed that VLD wild-type R108 plants had a much longer primary shoot axis compared with prostrate LD plants (Jaudal et al., 2013). Our analysis of the architecture of the spring early flowering mutants (Jaudal et al., 2013) also indicated that they had a much longer primary shoot axis compared with the wild type, while the late flowering fta1 null mutants had a very short primary shoot axis (Laurie et al., 2011) comparable with the Mtsoc1a mutants. These results suggest that the promotion of flowering by FTa1 is normally tightly linked with primary shoot stem elongation. However, our analysis of the phenotype of 35S:MtSOC1a transgenic plants provides the first evidence that primary shoot axis elongation and acceleration of flowering can be uncoupled in Medicago.
Further investigation of the 35S:MtSOC1a plant phenotype indicates that the increase in internode elongation and thus primary axis height is correlated with an increase in epidermal cell length. This increase in cell length was also observed in wild-type R108 that have elongated primary axes in response to vernalization. Thus, elongation of the primary shoot axis in Medicago is probably due at least in part to increased cell expansion.
Because of the important role of GA in stem elongation in pea and because AtSOC1 is downstream of GA-mediated floral promotion in Arabidopsis (Lee and Lee, 2010), we investigated if the short stem phenotype of Mtsoc1a mutants is due to a defect in responsiveness to GA. GA3 spray promoted stem internode elongation, but not early flowering in wild-type and Mtsoc1a plants in LDs. Thus, the short primary shoot phenotype of the mutant does not appear to result from a defect in GA response. This result also suggests that despite apparent similarities to the effect of 35S:MtSOC1a, GA spray-mediated primary stem elongation in Medicago does not require functional MtSOC1a. Consistent with this, MtSOC1a expression was not elevated by GA3 application. Thus, MtSOC1a and GA may be involved in different pathways that regulate primary stem elongation in Medicago.
Ectopic expression of the other two MtSOC1 genes, MtSOC1b and MtSOC1c, had little effect on flowering time of wild-type Arabidopsis plants in our study but, like MtSOC1a, they did partially complement the late flowering of the Atsoc1 mutant, in some cases near to wild-type levels (Fudge et al., 2018). However, Mtsoc1b mutants had no flowering or obvious architectural phenotypes, suggestive of functional redundancy (Fudge et al., 2018). In addition, the Medicago FUL-like genes displayed quite similar patterns of expression and FTa1 dependence (Jaudal et al., 2015). Thus, gene editing approaches in Medicago (Meng et al., 2017) targeting more than one of these genes at a time may serve to optimize future functional studies.
While many SOC1-like genes regulate flowering, others are known to affect other plant processes such as the duration of dormancy in kiwifruit (Voogd et al., 2015). Overall, our study indicates that MtSOC1a regulates Medicago flowering time and primary shoot axis elongation, thus showing perhaps both conservation and divergence with Arabidopsis SOC1 function. Since only weak changes in gene expression of candidate flowering time genes were observed in the Mtsoc1a mutant, genomic and biochemical analyses including hormone content may help to illuminate how MtSOC1a executes these roles.
Supplementary Data
Supplementary data are available at JXB online.
Fig. S1. Phylogenetic tree of TM3 proteins and analysis of normalized Medicago RNA Seq data.
Fig. S2. Expression of MtSOC1 genes in LDs and SDs, and overdevelopment in LDs.
Fig. S3. Expression of MtPIM and MtSOC1 genes in VLDs.
Fig. S4. Expression of MtPIM and MtSOC1 genes in fta1and 35S:FTa1 Medicago plants in LD vernalized seedlings.
Fig. S5. Plant architectures of transgenic Arabidopsis plants with 35S:MtSOC1 transgenes.
Fig. S6. Flowering time and stem lengths of a LD segregating population from R108×Mtsoc1a.
Fig. S7. Gene expression of candidate flowering genes in the Mtsoc1a mutant.
Fig. S8. Mtsoc1b Tnt1 mutant flowered at the same time as the wild type.
Fig. S9. Expression of candidate flowering time genes in T035S:MtSOC1a plants.
Table S1. List of primers.
Supplementary Material
Acknowledgements
We thank Thorben Krueger, Andrew Jiang, Davide Zazzaro, and Wendy Payne for technical assistance, and funders the Marsden Fund (http://www.royalsociety.org.nz/programmes/funds/marsden/) 10-UOA-200 and 14-UOA-125 (http://www.msi.govt.nz/) C10X0816.
References
- Alter P, Bircheneder S, Zhou LZ, Schlüter U, Gahrtz M, Sonnewald U, Dresselhaus T. 2016. Flowering time-regulated genes in maize include the transcription factor ZmMADS1. Plant Physiology 172, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés F, Coupland G. 2012. The genetic basis of flowering responses to seasonal cues. Nature Reviews. Genetics 13, 627–639. [DOI] [PubMed] [Google Scholar]
- Balanzà V, Martínez-Fernández I, Ferrándiz C. 2014. Sequential action of FRUITFULL as a modulator of the activity of the floral regulators SVP and SOC1. Journal of Experimental Botany 65, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CD, Soltis DE, Soltis PS. 2010. The age and diversification of the angiosperms re-revisited. American Journal of Botany 97, 1296–1303. [DOI] [PubMed] [Google Scholar]
- Benlloch R, d’Erfurth I, Ferrandiz C, Cosson V, Beltrán JP, Cañas LA, Kondorosi A, Madueño F, Ratet P. 2006. Isolation of mtpim proves Tnt1 a useful reverse genetics tool in Medicago truncatula and uncovers new aspects of AP1-like functions in legumes. Plant Physiology 142, 972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S, Dean C. 2015. Environmental perception and epigenetic memory: mechanistic insight through FLC. The Plant Journal 83, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, Mangelsdorf DJ. 2003. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nuclear Receptor Signaling 1, e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleissner R, Wisman E, Apel K, Melzer S. 2000. A MADS domain gene involved in the transition to flowering in Arabidopsis. The Plant Journal 24, 591–599. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Lichtenzveig J, Ellwood S, Pfaff T, Journet EP. 2006. Vernalization, crossings and testing for pollen viability. In: Mathesius U, Journet EP, Sumner LW, eds. Medicago truncatula handbook. Ardmore, OK: Noble Research Institute, 1–13. [Google Scholar]
- Cheng X, Li G, Tang Y, Wen J. 2018. Dissection of genetic regulation of compound inflorescence development in Medicago truncatula. Development 145, dev158766. [DOI] [PubMed] [Google Scholar]
- Clarkson NM, Russell JS. 1975. Flowering responses to vernalisation and photoperiod in annual medics (Medicago spp). Australian Journal of Agricultural Research 26, 831–838. [Google Scholar]
- Cosson V, Durand P, d’Erfurth I, Kondorosi A, Ratet P. 2006. Medicago truncatula transformation using leaf explants. Methods in Molecular Biology 343, 115–127. [DOI] [PubMed] [Google Scholar]
- Ding L, Wang Y, Yu H. 2013. Overexpression of DOSOC1, an ortholog of Arabidopsis SOC1, promotes flowering in the orchid Dendrobium Chao Parya Smile. Plant & Cell Physiology 54, 595–608. [DOI] [PubMed] [Google Scholar]
- Dorca-Fornell C, Gregis V, Grandi V, Coupland G, Colombo L, Kater MM. 2011. The Arabidopsis SOC1-like genes AGL42, AGL71 and AGL72 promote flowering in the shoot apical and axillary meristems. The Plant Journal 67, 1006–1017. [DOI] [PubMed] [Google Scholar]
- Fudge JB, Lee RH, Laurie RE, Mysore KS, Wen J, Weller JL, Macknight RC. 2018. Medicago truncatula SOC1 genes are up-regulated by environmental cues that promote flowering. Frontiers in Plant Science 9, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, et al. 2012. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Research 40, D1178–D1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramzow L, Theißen G. 2015. Phylogenomics reveals surprising sets of essential and dispensable clades of MIKCc-group MADS-box genes in flowering plants. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution 324, 353–362. [DOI] [PubMed] [Google Scholar]
- Hecht V, Foucher F, Ferrándiz C, et al. 2005. Conservation of Arabidopsis flowering genes in model legumes. Plant Physiology 137, 1420–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Sponsel V. 2015. A century of gibberellin research. Journal of Plant Growth Regulation 34, 740–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink RG, Posé D, Ferrario S, et al. 2012. Characterization of SOC1’s central role in flowering by the identification of its upstream and downstream regulators. Plant Physiology 160, 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaudal M, Monash J, Zhang L, Wen J, Mysore KS, Macknight R, Putterill J. 2014. Overexpression of Medicago SVP genes causes floral defects and delayed flowering in Arabidopsis but only affects floral development in Medicago. Journal of Experimental Botany 65, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaudal M, Yeoh CC, Zhang L, Stockum C, Mysore KS, Ratet P, Putterill J. 2013. Retroelement insertions at the Medicago FTa1 locus in spring mutants eliminate vernalisation but not long-day requirements for early flowering. The Plant Journal 76, 580–591. [DOI] [PubMed] [Google Scholar]
- Jaudal M, Zhang L, Che C, Hurley DG, Thomson G, Wen J, Mysore KS, Putterill J. 2016. MtVRN2 is a Polycomb VRN2-like gene which represses the transition to flowering in the model legume Medicago truncatula. The Plant Journal 86, 145–160. [DOI] [PubMed] [Google Scholar]
- Jaudal M, Zhang L, Che C, Putterill J. 2015. Three Medicago MtFUL genes have distinct and overlapping expression patterns during vegetative and reproductive development and 35S:MtFULb accelerates flowering and causes a terminal flower phenotype in Arabidopsis. Frontiers in Genetics 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julier B, Huguet T, Chardon F, Ayadi R, Pierre JB, Prosperi JM, Barre P, Huyghe C. 2007. Identification of quantitative trait loci influencing aerial morphogenesis in the model legume Medicago truncatula. Theoretical and Applied Genetics 114, 1391–1406. [DOI] [PubMed] [Google Scholar]
- Jung C, Müller AE. 2009. Flowering time control and applications in plant breeding. Trends in Plant Science 14, 563–573. [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. 2002. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science 7, 193–195. [DOI] [PubMed] [Google Scholar]
- Komiya R, Yokoi S, Shimamoto K. 2009. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136, 3443–3450. [DOI] [PubMed] [Google Scholar]
- Laurie RE, Diwadkar P, Jaudal M, et al. 2011. The Medicago FLOWERING LOCUS T homolog, MtFTa1, is a key regulator of flowering time. Plant Physiology 156, 2207–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I. 2000. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes & Development 14, 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee I. 2010. Regulation and function of SOC1, a flowering pathway integrator. Journal of Experimental Botany 61, 2247–2254. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim J, Han JJ, Han MJ, An G. 2004. Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. The Plant Journal 38, 754–764. [DOI] [PubMed] [Google Scholar]
- Lester DR, Ross JJ, Davies PJ, Reid JB. 1997. Mendel’s stem length gene (Le) encodes a gibberellin 3 beta-hydroxylase. The Plant Cell 9, 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo M, Limones-Briones V, Cabrera-Ponce JL, Herrera-Estrella L. 2004. Improving transformation efficiency of Arabidopsis thaliana by modifying the floral dip method. Plant Molecular Biology Reporter 22, 63–70. [Google Scholar]
- Mateos JL, Madrigal P, Tsuda K, Rawat V, Richter R, Romera-Branchat M, Fornara F, Schneeberger K, Krajewski P, Coupland G. 2015. Combinatorial activities of SHORT VEGETATIVE PHASE and FLOWERING LOCUS C define distinct modes of flowering regulation in Arabidopsis. Genome Biology 16, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T. 2008. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nature Genetics 40, 1489–1492. [DOI] [PubMed] [Google Scholar]
- Meng Y, Hou Y, Wang H, Ji R, Liu B, Wen J, Niu L, Lin H. 2017. Targeted mutagenesis by CRISPR/Cas9 system in the model legume Medicago truncatula. Plant Cell Reports 36, 371–374. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM. 2005. Integration of flowering signals in winter-annual Arabidopsis. Plant Physiology 137, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouhu K, Kurokura T, Koskela EA, Albert VA, Elomaa P, Hytönen T. 2013. The Fragaria vesca homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 represses flowering and promotes vegetative growth. The Plant Cell 25, 3296–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na X, Jian B, Yao W, Wu C, Hou W, Jiang B, Bi Y, Han T. 2013. Cloning and functional analysis of the flowering gene GmSOC1-like, a putative SUPPRESSOR OF OVEREXPRESSION CO1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in soybean. Plant Cell Reports 32, 1219–1229. [DOI] [PubMed] [Google Scholar]
- Nan H, Cao D, Zhang D, Li Y, Lu S, Tang L, Yuan X, Liu B, Kong F. 2014. GmFT2a and GmFT5a redundantly and differentially regulate flowering through interaction with and upregulation of the bZIP transcription factor GmFDL19 in soybean. PLoS One 9, e97669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onouchi H, Igeño MI, Périlleux C, Graves K, Coupland G. 2000. Mutagenesis of plants overexpressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. The Plant Cell 12, 885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Jorgensen SA, Jha SG. 2014. Functional characterization of duplicated SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1-Like genes in petunia. PLoS One 9, e96108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Laurie R, Macknight R. 2004. It’s time to flower: the genetic control of flowering time. BioEssays 26, 363–373. [DOI] [PubMed] [Google Scholar]
- Putterill J, Varkonyi-Gasic E. 2016. FT and florigen long-distance flowering control in plants. Current Opinion in Plant Biology 33, 77–82. [DOI] [PubMed] [Google Scholar]
- Putterill J, Zhang L, Yeoh C, Balcerowicz M, Jaudal M, Varkonyi Gasic E. 2013. FT genes and regulation of flowering in the legume Medicago truncatula. Functional Plant Biology 40, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Ryu CH, Lee S, Cho LH, et al. 2009. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant, Cell & Environment 32, 1412–1427. [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. 2000. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288, 1613–1616. [DOI] [PubMed] [Google Scholar]
- Srikanth A, Schmid M. 2011. Regulation of flowering time: all roads lead to Rome. Cellular and Molecular Life Sciences 68, 2013–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadege M, Chen F, Murray J, Wen J, Ratet P, Udvardi MK, Dixon RA, Mysore KS. 2015. Control of vegetative to reproductive phase transition improves biomass yield and simultaneously reduces lignin content in Medicago truncatula. BioEnergy Research 8, 857–867. [Google Scholar]
- Tadege M, Wang TL, Wen J, Ratet P, Mysore KS. 2009. Mutagenesis and beyond! Tools for understanding legume biology. Plant Physiology 151, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Krishnakumar V, Bidwell S, et al. 2014. An improved genome release (version Mt4.0) for the model legume Medicago truncatula. BMC Genomics 15, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Shen L, Liu C, Liu L, Yan Y, Yu H. 2012. Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. The Plant Journal 70, 549–561. [DOI] [PubMed] [Google Scholar]
- Trinh HT, Ratet P, Kondorosi E, Durand P, Kamaté K, Bauer P, Kondorosi A. 1998. Rapid and efficient transformation of diploid Medicago truncatula and Medicago sativa ssp. falcata lines improved in somatic embryogenesis. Plant Cell Reports 17, 345–355. [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G. 2008. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annual Review of Plant Biology 59, 573–594. [DOI] [PubMed] [Google Scholar]
- Voogd C, Wang T, Varkonyi-Gasic E. 2015. Functional and expression analyses of kiwifruit SOC1-like genes suggest that they may not have a role in the transition to flowering but may affect the duration of dormancy. Journal of Experimental Botany 66, 4699–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Beruto M, Xue J, Zhu F, Liu C, Yan Y, Zhang X. 2015. Molecular cloning and potential function prediction of homologous SOC1 genes in tree peony. Plant Cell Reports 34, 1459–1471. [DOI] [PubMed] [Google Scholar]
- Weller JL, Ortega R. 2015. Genetic control of flowering time in legumes. Frontiers in Plant Science 6, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh CC, Balcerowicz M, Laurie R, Macknight R, Putterill J. 2011. Developing a method for customized induction of flowering. BMC Biotechnology 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh CC, Balcerowicz M, Zhang L, Jaudal M, Brocard L, Ratet P, Putterill J. 2013. Fine mapping links the FTa1 flowering time regulator to the dominant spring1 locus in Medicago. PLoS One 8, e53467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ND, Debellé F, Oldroyd GE, et al. 2011. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480, 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wei J, Fan S, Song M, Pang C, Wei H, Wang C, Yu S. 2016. Functional characterization of GhSOC1 and GhMADS42 homologs from upland cotton (Gossypium hirsutum L.). Plant Science 242, 178–186. [DOI] [PubMed] [Google Scholar]
- Zhong X, Dai X, Xv J, Wu H, Liu B, Li H. 2012. Cloning and expression analysis of GmGAL1, SOC1 homolog gene in soybean. Molecular Biology Reports 39, 6967–6974. [DOI] [PubMed] [Google Scholar]
- Zhou R, Jackson L, Shadle G, Nakashima J, Temple S, Chen F, Dixon RA. 2010. Distinct cinnamoyl CoA reductases involved in parallel routes to lignin in Medicago truncatula. Proceedings of the National Academy of Sciences, USA 107, 17803–17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.