Abstract
This article comments on:
Annunziata MG, Apelt F, Carillo P, Krause U, Feil R, Koehl K, Lunn JE, Stitt M. 2018. Response of Arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment. Journal of Experimental Botany 69, 4881–4895.
Keywords: Circadian clock, fluctuating light, growth, metabolism, photosynthesis, variable temperature
Light and temperature are highly dynamic environmental factors, but plant science research frequently ignores this variation to allow reproducible growth-chamber experiments. Using Arabidopsis and looking at changes over a 24 h period, Annunziata et al. (2018) scrutinized changes in metabolites and gene expression of circadian oscillator components under naturally varying light and temperature. They show that fluctuations modify systems organization and outputs, and propose that diel metabolism is adapted to covariation of light and temperature.
Climate chambers have become indispensable for plant science research. We benefit greatly from the possibility of controlling environmental variables such as photoperiod, light intensity, air temperature and relative air humidity, and this enables the study of plant responses to specific environments and repeated plant cultivation and experiments under defined conditions. Typically, conditions inside climate chambers are kept constant except when the illumination is switched on/off to simulate day/night. Whilst such quasi-binary environments are relatively easy to reproduce and use for growing plants, we know that they are very different from field conditions in which environmental fluctuations are the rule rather than the exception (Box 1). So what changes in plants when we grow them in fluctuating versus constant environments?
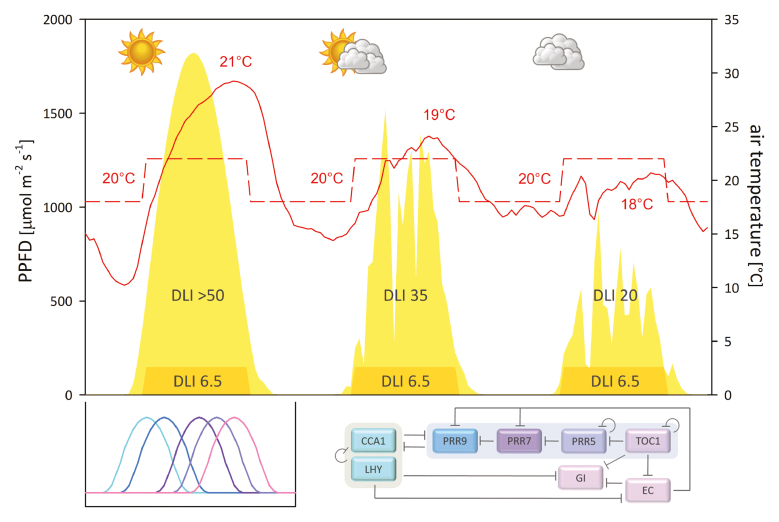
Box 1. Diel variations in light, temperature and gene expression of the plant circadian oscillator
The diurnal time course of light intensity exhibits a sine curve under a clear sky (upper panel, yellow area on the left). Photosynthetically active photon flux density (PPFD) peaks around noon, reaching >1800 μmol m–2 s–1. In this example (all data from June 2017, near Bonn, Germany; photoperiod ~16.5 h) air temperature (solid red line) increases by >10 °C in 4–5 h from dawn and continues to increase until late afternoon, followed by a rapid decline also by >10 °C till dusk. The daily mean temperature is 21 °C with a diurnal variation of 19 °C. The orange areas at the bottom and the broken red line represent a square-wave light regime in a climate chamber at PPFD 150 μmol m–2 s–1 and 22 °C/18 °C day/night (daily mean 20 °C). While the conditions in the climate chamber stay the same, light and temperature vary from day to day in the field. With increasing cloud cover (in the middle and on the right) the daily light integral (DLI) and daily maximum PPFD as well as the daily mean and maximum temperature decrease while the daily minimum temperature increases. Cloud movement causes fluctuations of irradiance. In reality light fluctuation is far more dynamic than shown in this figure which is based on 30-min average data.
The panel at bottom left depicts sequential gene expression of circadian oscillator components over 24 h. The x-axis matches the time axis of the first diel cycle in the upper panel. Different colours of the lines correspond to the colours of oscillator components shown on the right. For clarity, expression curves are drawn with equal peak height and width. Their peak times are according to the observations by Annunziata et al. (2018) under a sinusoidal light regime (photoperiod 12 h; max. PPFD 465 μmol m–2 s–1; DLI 12 mol m–2 d–1) at 21–22 °C/20 °C. At dawn CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LONG ELONGATED HYPOCOTYL (LHY) suppress expression of all the other components, namely PSEUDO RESPONSE REGULATOR (PRR) genes (PRR9, PRR7, PRR5 and PRR1 alias TIMING OF CAB EXPRESSION 1, TOC1), GIGANTEA (GI) and evening complex (EC) genes including LUX ARRHYTHMO, EARLY FLOWERING 3 (ELF3) and ELF4 (not shown). Sequential expression of PRRs then represses CCA1 and LHY from noon to evening and TOC1 represses all the others but ELF3 in the evening. Later EC maintains the repression of PRR9, PRR7 and GI. Additionally, CCA1 and LHY as well as the different PRRs repress each other’s expression. The simplified illustration and description of the negative transcriptional feedback loops of the plant circadian oscillator are both according to Nohales and Kay (2016).
Plants growing in fluctuating light
In a range of species leaf dry mass per unit leaf area (LMA), a key functional trait related to photosynthesis, growth and life history strategy of leaves (Wright et al., 2004), increases in the field compared to controlled environments, presumably because the daily light integral (DLI) is much higher and temperature much lower in the field (Poorter et al., 2016). For a given combination of DLI and temperature, however, LMA is influenced by temporal variation in irradiance. LMA declines in Arabidopsis under fluctuating light compared to 12 h/12 h light/dark (square-wave light) conditions (Vialet-Chabrand et al., 2017), suggesting a decrease in effective DLI under fluctuating light. Indeed, upon sudden increase or decrease in irradiance photosynthetic light use efficiency is limited by enzyme activation in Calvin–Benson cycle and CO2 diffusion (Pearcy, 1990; Kaiser et al., 2018) or slow relaxation of energy dissipation (Zhu et al., 2004; Kromdijk et al., 2016), respectively. Daily carbon gain and growth diminish in fluctuating light compared to square-wave light of the same DLI (Leakey et al., 2002; Alter et al., 2012; Vialet-Chabrand et al., 2017) although species may differ in their responses (Watling et al., 1997).
Concomitant with LMA and growth reduction, plants in fluctuating light also show other responses that are not found in square-wave light. At low DLI (3.5 or 5 mol m–2 d–1) square-wave light promotes light energy utilization and leaf expansion in Arabidopsis whereas fluctuating light, which comprises short lightflecks triggered under low-intensity background light (situations for inner-canopy leaves and understorey plants), enhances photoprotective energy dissipation and scavenging of reactive oxygen species (Alter et al., 2012). In contrast, conditions mimicking natural light fluctuation at higher DLI (10 or 20 mol m–2 d–1; situations for sun-exposed leaves) were shown to increase the maximum CO2 assimilation per unit leaf mass compared to the corresponding square-wave light. Hence Arabidopsis leaves maintain the maximum CO2 assimilation per unit area as they become thinner and the total leaf area declines under fluctuating light (Vialet-Chabrand et al., 2017).
Recently Annunziata et al. (2017) reported remarkable changes in leaf metabolism occurring under natural fluctuating light. Compared to square-wave or sinusoidal light regimes, natural illumination in otherwise similar environmental conditions attenuated connectivity between C and N metabolism in Arabidopsis. Natural light varies from day to day depending on the weather whereas the same light programme repeats every day in controlled environments (Box 1). Both within- and between-day fluctuations of irradiance thus affect metabolic profiles of plants under natural illumination. Notably, metabolic and photosynthetic properties were marginally altered in Arabidopsis when different light sources (fluorescent tubes versus light-emitting diodes) and regimes (square-wave versus sinusoidal) were compared under controlled environments (Annunziata et al., 2017; Köhl et al., 2017). These findings further underpin a major impact of light fluctuation.
Plants growing in fluctuating light and temperature
Deviation from reality is incidental not only to light but also to temperature conditions in controlled environments (Box 1). While the majority of experiments in climate chambers are conducted at ~20 °C and with day/night variations (if any) of <6 °C, the daily mean temperature in temperate zones is ~10 °C in spring and 15–20 °C in summer, but with diurnal variations of 12–13 °C (Poorter et al., 2016). Getting one more step closer to the field, Annunziata et al. (2018) extended the study to include temperature fluctuation. The authors compared sinusoidal light and natural illumination at 20–22 °C (Annunziata et al., 2017) versus natural illumination at variable temperature. The daily mean temperature under the latter conditions ranged over 14–22 °C during the experiment, with diurnal variations of 4–19 °C (on average 10 °C). The DLI on harvest day was 12–14 mol m–2 d–1 in all conditions.
The picture emerging from their metabolome analysis (Annunziata et al., 2018) illustrates how light and temperature fluctuations modify systems organization and outputs. Diel (over a 24 h period) C and N metabolism are strongly and positively correlated in Arabidopsis leaves under sinusoidal light at constant temperature. At the same DLI and temperature, fluctuating light dramatically weakens this correlation by partially uncoupling C and N metabolism and suppressing the accumulation of major amino acids and some organic acids. Combined variation of light and temperature, without further weakening or restoring the metabolic connectivity, brings about changes in specific compounds, such as retention of starch and sugars at dawn or marked increase in anthocyanins, amino acids (including proline) and proteins.
The variable responses of amino acids and organic acids were interpreted as an indication of weak buffering of the related pathways against environmental fluctuations (Annunziata et al., 2018). One may also wonder whether flexible adjustment of metabolic connectivity is part of a system-level buffer to cope with fluctuations; it was not selected against during evolution (in the field). Seeing the changes in these compounds under variable light and temperature, the authors point to a striking resemblance to low temperature acclimation (Strand et al., 1999; Stitt and Hurry, 2002). Accordingly, low night temperature slows down nocturnal starch mobilization and growth while up-regulating gene expression of ribosomal proteins (Usadel et al., 2008) to enhance translation. The resulting increase in leaf protein content may augment photosynthesis in fluctuating light and promote growth during the day (Annunziata et al., 2018). This hypothesis, which has yet to be tested, provides a possible explanation for apparent lack of growth penalty under natural light and variable temperature conditions.
The circadian clock in natural fluctuating environments
In parallel with the metabolic reprogramming, Annunziata et al. (2018) identified peculiar changes in gene expression of circadian oscillator components under fluctuating environments. Particularly, activation of dawn-phased MYB transcription factor genes CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LONG ELONGATED HYPOCOTYL (LHY) was delayed by ~2 h under natural illumination and variable temperature, which was accompanied by increased or earlier expression of PSEUDORESPONSE REGULATOR 9 (PRR9), PRR7 and GIGANTEA (GI) in the morning. The central oscillator of the plant circadian clock is composed of interlocked transcriptional and translational feedback loops (Box 1). Whilst the period length of circadian-regulated gene expression is robust against fluctuations in the field (Nagano et al., 2012), cool nights seem to fine-tune the timing and amplitude of specific clock gene expression (Annunziata et al., 2018). What could such fine-tuning be good for?
Circadian clocks allow organisms to synchronize physiological and developmental processes, including growth, metabolism, stress responses and flowering, with diel and seasonal cycles of their environment (Nohales and Kay, 2016). In the case of low temperature acclimation, CCA1 and LHY are in charge of circadian-gated induction of C-REPEAT BINDING FACTOR 1 (CBF1), CBF2 and CBF3 encoding AP2/ERF family transcription factors which regulate chilling/freezing tolerance (Dong et al., 2011). CCA1 and LHY also act as pacemakers of nocturnal starch mobilization, preventing premature starch exhaustion before dawn (Graf et al., 2010). Their importance is manifested in the Arabidopsis cca1lhy (or lhycca1) mutant and CCA1-overexpressor. These plants suffer from starch exhaustion and high starch retention, respectively, both resulting in nocturnal growth inhibition (Yazdanbakhsh et al., 2011; Ruts et al., 2012; Apelt et al., 2017), although this does not apply to hypocotyl elongation, which is controlled by the evening complex (EC) and light (Nozue et al., 2007; Nusinow et al., 2011).
Does slow activation of CCA1 and LHY following a cool night prolong starch mobilization after dawn and thereby redirect C to sucrose and amino acids (Annunziata et al., 2018)? Does the higher or earlier expression of PRR9, PRR7 and GI (Annunziata et al., 2018) also modulate circadian-regulated processes as the temperature rises quickly in the morning (Box 1)? Interestingly, these five components (CCA1, LHY, PRR9, PRR7 and GI) are implicated in temperature compensation of clock oscillation (Nohales and Kay, 2016), which would gain more importance under fluctuating temperature. The study by Annunziata et al. (2018) raises many questions and inspires further investigation of the roles of the plant circadian clock in natural fluctuating environments.
Future perspectives
More realistic though the results may be, experiments under natural irradiance and temperature have the significant drawback of low reproducibility. Moreover, preceding weather can influence the observations in present-day conditions, as noted by Annunziata et al. (2018), thus complicating data analysis and interpretation. Whilst such previous history effects are not always a problem (and can also be a fascinating subject of research), it is difficult to test hypotheses and validate models if we are not able to repeat the experiments. The good news: with the latest advances in climate chamber technology it is becoming possible to simulate light and temperature fluctuations in controlled environments. While factors like wind, rain and rhizosphere are not considered, light and temperature simulation will help us identify mechanisms and components which facilitate plant growth and confer resilience in fluctuating environments. The first examples demonstrating the power and utility of this approach for photosynthetic phenotyping have been reported in recent years (Cruz et al., 2016; Rungrat et al., 2016).
Plants can anticipate recurrent changes in their environment, above all in light and temperature. Plants are fairly safe in expecting a particular temporal pattern of light and temperature covariation in the field: lower temperature and dim or no light from dusk to dawn followed by a parallel increase in both in the morning. Even stochastic fluctuations of light, which are caused by clouds, wind and neighbouring plants, can be expected to occur during the daytime, in much the same way as the operation of photosynthesis. Given that seasonal changes in mean temperature and photoperiod or DLI are correlated in all major climate regions but in distinct ways (Hut et al., 2013; Poorter et al., 2016), and since photoperiod is known to be measured by the clock, anticipation and detection of the temperature–photoperiod relationship could be another important function of the clock in plant adaptation and acclimation to different environments. As we try to mimic natural light and temperature conditions in climate chambers, we may more often come across the ‘biological clockwork’ in the act of preparing for and responding to ever-fluctuating environments.
Acknowledgements
Climatic data in Box 1 were obtained in the experimental field site of the German Plant Phenotyping Network (Deutsches Pflanzenphänotypisierungsnetzwerk, DPPN) at Campus Klein-Altendorf (Rheinbach, Germany) in June, 2017. SM thanks the Campus Klein-Altendorf and Forschungszentrum Jülich teams for maintaining the weather stations and sensors, and Uwe Rascher and Onno Muller for valuable comments.
References
- Alter P, Dreissen A, Luo FL, Matsubara S. 2012. Acclimatory responses of Arabidopsis to fluctuating light environment: comparison of different sunfleck regimes and accessions. Photosynthesis Research 113, 221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata MG, Apelt F, Carillo P, et al. 2017. Getting back to nature: a reality check for experiments in controlled environments. Journal of Experimental Botany 68, 4463–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata MG, Apelt F, Carillo P, Krause U, Feil R, Koehl K, Lunn JE, Stitt M. 2018. Response of Arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment. Journal of Experimental Botany 69, 4881–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelt F, Breuer D, Olas JJ, Annunziata MG, Flis A, Nikoloski Z, Kragler F, Stitt M. 2017. Circadian, carbon, and light control of expansion growth and leaf movement. Plant Physiology 174, 1949–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JA, Savage LJ, Zegarac R, Hall CC, Satoh-Cruz M, Davis GA, Kovac WK, Chen J, Kramer DM. 2016. Dynamic environmental photosynthetic imaging reveals emergent phenotypes. Cell Systems 2, 365–377. [DOI] [PubMed] [Google Scholar]
- Dong MA, Farré EM, Thomashow MF. 2011. CIRCADIAN CLOCK-ASSOCIATED 1 and LATE ELONGATED HYPOCOTYL regulate expression of the C-REPEAT BINDING FACTOR (CBF) pathway in Arabidopsis. Proceedings of the National Academy of Sciences USA 108, 7241–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM. 2010. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proceedings of the National Academy of Sciences USA 107, 9458–9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Paolucci S, Dor R, Kyriacou CP, Daan S. 2013. Latitudinal clines: an evolutionary view on biological rhythms. Proceedings of the Royal Society B 280, doi: 10.1098/rspb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Morales A, Harbinson J. 2018. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiology 176, 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhl K, Tohge T, Schöttler MA. 2017. Performance of Arabidopsis thaliana under different light qualities: comparison of light-emitting diodes to fluorescent lamp. Functional Plant Biology 44, 727–738. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Press MC, Scholes JD, Watling JR. 2002. Relative enhancement of photosynthesis and growth at elevated CO2 is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant, Cell and Environment 25, 1701–1714. [Google Scholar]
- Nagano AJ, Sato Y, Mihara M, Antonio BA, Motoyama R, Itoh H, Nagamura Y, Izawa T. 2012. Deciphering and prediction of transcriptome dynamics under fluctuating field conditions. Cell 151, 1358–1369. [DOI] [PubMed] [Google Scholar]
- Nohales MA, Kay SA. 2016. Molecular mechanisms at the core of the plant circadian oscillator. Nature Structural & Molecular Biology 23, 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. 2007. Rhythmic growth explained by coincidence between internal and external cues. Nature 448, 358–361. [DOI] [PubMed] [Google Scholar]
- Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farré EM, Kay SA. 2011. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW. 1990. Sunflecks and photosynthesis in plant canopies. Annual Review of Plant Physiology and Plant Molecular Biology 41, 421–453. [Google Scholar]
- Poorter H, Fiorani F, Pieruschka R, Wojciechowski T, van der Putten WH, Kleyer M, Schurr U, Postma J. 2016. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytologist 212, 838–855. [DOI] [PubMed] [Google Scholar]
- Rungrat T, Awlia M, Brown T, et al. 2016. Using phenomic analysis of photosynthetic function for abiotic stress response gene discovery. The Arabidopsis Book 14, e0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruts T, Matsubara S, Wiese-Klinkenberg A, Walter A. 2012. Aberrant temporal growth pattern and morphology of root and shoot caused by a defective circadian clock in Arabidopsis thaliana. The Plant Journal 72, 154–161. [DOI] [PubMed] [Google Scholar]
- Stitt M, Hurry V. 2002. A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Current Opinion in Plant Biology 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Strand A, Hurry V, Henkes S, Huner N, Gustafsson P, Gardeström P, Stitt M. 1999. Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiology 119, 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Poree F, Höhne M, Günter M, Trethewey R, Kamlage B, Poorter H, Stitt M. 2008. Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non-freezing range. Plant, Cell & Environment 31, 518–547. [DOI] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Matthews JS, Simkin AJ, Raines CA, Lawson T. 2017. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiology 173, 2163–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling JR, Ball MC, Woodrow IE. 1997. The utilization of lightflecks for growth in four Australian rain-forest species. Functional Ecology 11, 231–239. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh N, Sulpice R, Graf A, Stitt M, Fisahn J. 2011. Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant, Cell & Environment 34, 877–894. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP. 2004. The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. Journal of Experimental Botany 55, 1167–1175. [DOI] [PubMed] [Google Scholar]