Abstract
Enzymatic hydrolysis of palm kernel cake to improve the quality of substrates with multi-response criteria based on the Taguchi orthogonal array. Nine experimental runs were performed based on an L9 orthogonal array. Percent substrate, incubation time, and enzyme units were optimized considering multiple performance characteristics. Analysis of variance was also applied to identify the most significant factors. Results determined percent substrate as the most important factor for enzymatic hydrolysis followed by incubation time and enzyme units. Enzymatic hydrolysis conditions were optimized as percent substrate, incubation time and enzyme units at 14%, 6 h and 750 units, respectively. Tests were conducted to compare experimental and model results. The experimental result (protein release) at optimal condition were three times higher than the predicted mode.
Keywords: Palm kernel cake, L9 orthogonal array, Enzymatic hydrolysis, Taguchi method
Introduction
Rapid growth of the palm oil industry in Thailand has resulted in an abundance of palm kernel cake (PKC) as a byproduct. A total of 10,944,000 tons of palm oil were produced by Thai palm oil millers in 2016 (OAE 2017). With the sustainable growth of palm oil in global markets, the amount of PKC has increased annually and its utilization as a source of valuable ingredients represents a significant economic and social benefit.
As a byproduct from palm kernel oil extraction, PKC provides moderate nutrition containing 14–18% crude protein (CP) and 13–15% crude fiber (CF) and is commonly used in ruminant diets as a protein source. However, PKC use is limited in monogastric animal diets as it contains mannan and non-starch polysaccharide (NSP) mainly mannan. Jaafar and Jarvis (1992) reported that the cell wall of PKC consists of 58% mannan, 12% cellulose and 4% xylan. Mannan, structure of PKC mannan, is hard and crystalline. Moreover, PKC have lack of several essential amino acids and high viscosity which have been proven to decrease nutrient uptake (Sundu and Dingle 2007). Mannanase, involved in the hydrolysis of linear mannans (pure mannan and glucomannan) (Chauhan et al. 2012), is mixed in animal feeds to increase digestion of NPS and improve nutrient utilization. Mixing mannanase into these diets results in decreased intestinal viscosity (Dhawan and Kaur 2007).
β-mannan and non-starch polysaccharide (NSP) have anti-nutritional properties that hinder the full utilization of nutrients in PKC by monogastric animals. Most mannan in PKC consists of a (1–4)-linked β-mannose backbone; however, small amounts of mannan with (1–6)-linked α-galactose (galactomannan) are also present and galactose substitution is low at 12–20% (Düsterhöft et al. 2006). Consequently, inclusion of 2–4% β-mannan slows growth and reduces feed efficiency to 20–30%. Therefore, removal of mannan in PKC is necessary. Addition of enzymes that digest β-mannan in feed ingredients can improve animal weight gain and feed efficiency. Enzymes widely used by the industry are hemicellulases that cleave the non-starch polysaccharide in viscous cereals (Ravindran and Son 2011).
PKC is classified as a medium grade protein feed because it contains 14.6–16.0% crude protein (Chin 2008). Due to its protein content and low cost, PKC is used for feed ingredient instead of soybean meal and other more expensive plant-proteins. However, PKC contains 30–35% β-mannan which is a strong anti-nutritive factor for monogastric animals. Degradation of β-mannan in PKC by an appropriate enzyme, therefore, releases sugars, manno-oligosaccharides, proteins and other nutritional values that can be absorbed and metabolized by monogastric animals. The application of such enzymatic hydrolysis can increase 30% mannose. Ng and Mohd Khan (2012) described an extract of PKC with a protein content of 68.50%. Therefore, preparation of PKC using physical, chemical, steam pretreatment or enzymatic hydrolysis can increase nutrient content and suitability as a feed additive for monogastric animals.
The feed enzymes market now amounts to US$150 million and 65% of poultry and 10% of swine feed already contain enzymes such as carbohydrases or phytase (van Beilen and Li 2002). Commercial mannanase has been widely used in animal feed to digest β-mannan and release nutritional value. A commercial mannanase was added in feedstuff to digest β-mannan in soybean meal and other plant protein such as sesame meal and cotton meal. Commercial mannanase is particularly effective with diets rich in soybean meal, sesame meal, palm kernel cake, copra meal, and guar meal which are high in β-mannan content. These enzymes contain β-mannan and xylanase as the primary active ingredients and act by degrading the anti-nutritive β-mannan (Dhawan and Kaur 2007). The aim of this research was to improve nutrient utilization of PKC as a feedstuff by enzymatic hydrolysis.
Materials and methods
Preparation of palm kernel cake
PKC from a palm oil factory in Southern Thailand was sieved through a 1-mm aperture mesh, then ground using a Braun coffee grinder for 10 min and re-sieved. This process was repeated twice. Materials that did not pass through the mesh after the third grinding were discarded.
Bacterial strain and culture condition
Bacillus amyloliquefaciens NT6.3, isolated from soil in Nakhon Pathom, Thailand (Phothichitto et al. 2006), at − 80 °C in medium containing 20% (w/v) glycerol was streaked for single colonies on medium agar. One colony from the overnight cultures was inoculated into 5 ml of medium and grown overnight at 37 °C, 250 rpm. The culture was transferred to a 250 ml Erlenmeyer flask containing 100 ml of LB medium as a starter.
Enzyme production using fermenter, enzyme harvest and determination of mannanase activity
A mineral salt medium was prepared according to the modified method of Ijah and Ukpe (1992), containing 0.018 (g/l) K2HPO4, 0.012 (g/l) KH2PO4, 0.04 (g/l) NH4NO3, 0.002 (g/l) MgSO4.7H2O, 0.001 (g/l) NaCl, 0.0001 (g/l) 0.00001 and 1% PKC and used as control for mannanase production. Supernatants of the culture medium were collected by centrifuging at 4 °C, 8000g for 20 min and kept at − 20 °C until used. Enzymes were assayed for mannanase activity in a reaction mixture containing 0.5 ml of 50 mM optimal pH and 1% locust bean gum with 0.5 ml of supernatant and incubation at a suitable temperature for 60 min. Amount of reducing sugar released was determined by the dinitrosalicyclic acid (DNS) method (Miller et al. 1959). The thermal stability of the enzyme was incubation at 50 °C at various times. Residual activities were determined at its optimal condition using the dinitrosalicyclic acid (DNS) method.
Optimization of enzymatic hydrolysis from palm kernel cake
Factors of PKC that affect enzymatic hydrolysis include percentage of substrate, incubation time and enzyme units. The level of each factor was varied.
To determine the optimal value, PKC was hydrolyzed by 500 units of mannanase with substrate percentage at 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20. Different levels of substrates were incubated in 0.1 M phosphate buffer pH 6 at 50 °C for 2 h. All supernatants were measured for nutrient release as reducing sugar, total sugar, and protein content. Reducing sugar assays (AOAC 2005) require hydrolysis of oligo- and polysaccharides to allow measurement of released monosaccharides from the supernatants. For total sugar, the supernatants were determined by the phenol–sulfuric acid method (DuBois et al. 1956) at maximum absorption wavelength (490 nm). Protein release was measured by the Bradford protein assay (Bradford 1976).
To optimize incubation times, 12% PKC was hydrolyzed by 500 units of mannanase at 2, 4, 6, 8, 10 and 12 h. Different conditions were incubated in 0.1 M phosphate buffer pH 6 at 50 °C. All supernatants were measured for nutrient release.
To optimize the enzyme units of both substrates, 12% PKC was hydrolyzed with 125, 250, 500, 750 and 1,000 units and incubated in 0.1 M phosphate buffer pH 6 at 50 °C for optimal incubation time (4 h). All supernatants were measured for nutrient release.
The performance derivable from a design should be ranked as the best case out of all which must be satisfied. This observation allows Taguchi methods to be extended to designs which have such necessity requirements by defining the signal-to-noise ratio (S/N) for all nutrients as:
where n is the representative number of measurements (9 in our case), and y is the experimental value.
After completing each factor test, an orthogonal experiment was carried out to determine interaction between the three factors with three levels of each. Combinations of experimental factors were chosen from an L9 (33) orthogonal test table to determine the optimal hydrolysis conditions of each substrate.
Chemical analysis
PKC and PKC hydrolysate, dried by a lyophilizer, were analyzed for pepsin digestibility following the method of Eggum et al. (1983), with proximate analysis using the Association of Official Analytical Chemist (AOAC 2005) recommended procedures and Van Soest detergent fiber analysis (Van Soest 1963).
Statistical analysis
All data were analyzed by the statistical software package SPSS version 17. Statistical significance was determined by one-way analysis of variance (ANOVA). All differences were considered by significant at P < 0.01 and P < 0.05.
Results and discussion
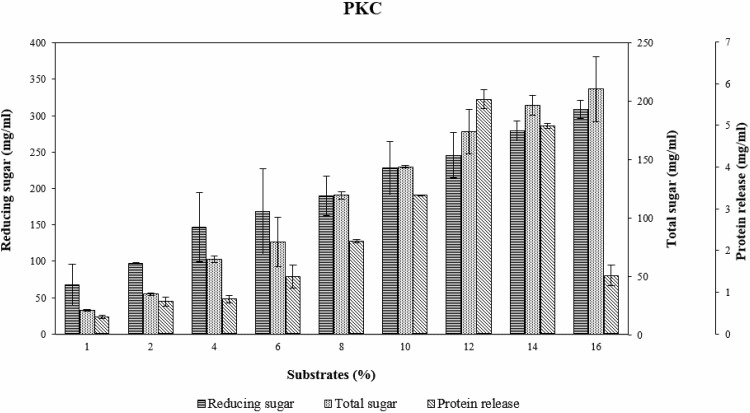
Effect of substrate percent on enzymatic hydrolysis
Substrate percent plays an important role in hydrolysis of PKC (Fig. 1). Nutrient release of PKC, reducing sugar and total sugar from enzymatic hydrolysis increased when substrate percent increased. Protein release increased when substrate percent increased, but decreased at 14% and 16%. At 14% and 16% of PKC were deposited on the bottle. The interaction between substrate and enzyme decreased due to the difficulty of enzyme to bind with substrate. Moreover, when substrates increase over 16% enzyme could not hydrolyze PKC because PKC absorbed all buffer. Mannanase enzyme, kind of hydrolase, is commonly used as biochemical catalysts that utilize water to break a chemical bond, without water mannanase then loss its efficient in hydrolysing substrate. This phenomenon was normally happened for insoluble substrates. Therefore, it was not necessary to use substrate at more than 16% to conduct the experiments.
Fig. 1.
The effect of percent substrates on nutrient release toward PKC
Effect of incubation time on enzymatic hydrolysis
Nutrient release was highest at 4 h for PKC hydrolyzed with mannanase (Fig. 2). Mannanase activity significantly decreased after 6 h incubation (data not shown) and it was not necessary to hydrolyze the substrate for more than 6 h. Thus, 6 h was selected as the highest level of incubation time using Taguchi orthogonal design.
Fig. 2.
The effect of incubated time on nutrient releases toward PKC
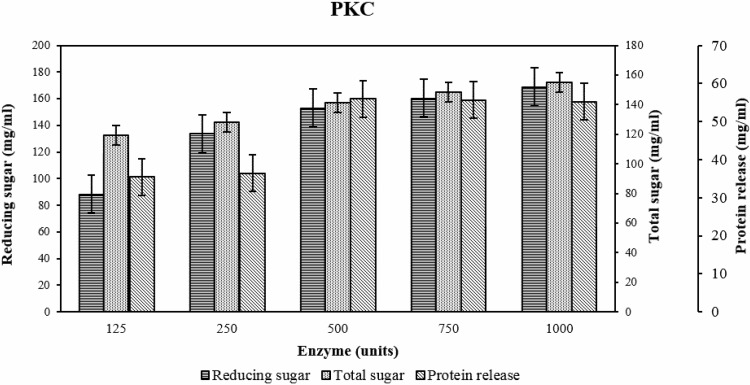
The effect of enzyme units
According to Fig. 3, at different levels of enzyme units, nutrient release of PKC gradually increased. However, nutrient release at 1000 units was nearly the same as at 750 units so it was not necessary to use enzyme at 1000 units.
Fig. 3.
The effect of enzyme unit on nutrient releases toward PKC
On the basis of this single factor experiment, the condition for hydrolyzing PKC was optimized by Taguchi orthogonal design. Nutrient release differed according to percent substrate, incubation time, and enzyme units (Fig. 4). Factors were selected to determine the optimal extraction conditions based on the single factor experiment. Factors and experimental results are listed in Table 1. Substrates were 10%, 12% and 14%, incubation times 2 h, 4 h and 6 h and enzyme units 250, 500 and 750.
Fig. 4.
Comparative results of response in terms of Nutrient releases of Taguchi L9 orthogonal array experiments; A,B,C and a,b,ccommon letters indicate highly significant differences (P < 0.01), 1,2,3common letters indicate significant differences (P < 0.05)
Table 1.
Results of orthogonal test
| Test number | Factors | Nutrient releases | ||||
|---|---|---|---|---|---|---|
| Substrates (A) (%) | Incubated times (B) (h) | Enzyme units (C) (U) | Reducing sugar (mg) | Total sugar (mg) | Protein release (mg/ml) | |
| Run 1 | 10 | 2 | 250 | 217.70 | 207.96 | 37.47 |
| Run 2 | 10 | 4 | 500 | 238.06 | 214.07 | 47.95 |
| Run 3 | 10 | 6 | 750 | 259.42 | 236.75 | 51.90 |
| Run 4 | 12 | 2 | 500 | 259.05 | 242.05 | 52.25 |
| Run 5 | 12 | 4 | 750 | 266.95 | 263.32 | 50.20 |
| Run 6 | 12 | 6 | 250 | 255.58 | 266.18 | 45.08 |
| Run 7 | 14 | 2 | 750 | 261.79 | 295.36 | 47.50 |
| Run 8 | 14 | 4 | 250 | 273.27 | 294.76 | 42.55 |
| Run 9 | 14 | 6 | 500 | 281.54 | 313.77 | 44.18 |
| K1 (reducing sugar) | 238.39 | 246.18 | 248.85 | |||
| K2 (reducing sugar) | 260.53 | 259.43 | 259.55 | |||
| K3 (reducing sugar) | 272.20 | 265.51 | 262.72 | |||
| R (reducing sugar) | 33.81 | 19.33 | 13.87 | |||
| K1 (total sugar) | 219.59 | 248.46 | 256.30 | |||
| K2 (total sugar) | 257.18 | 257.38 | 256.63 | |||
| K3 (total sugar) | 301.30 | 272.23 | 265.14 | |||
| R (total sugar) | 81.70 | 23.78 | 8.84 | |||
| K1 (protein release) | 45.77 | 45.74 | 41.70 | |||
| K2 (protein release) | 49.18 | 46.90 | 48.13 | |||
| K3 (protein release) | 44.74 | 47.05 | 49.87 | |||
| R (protein release) | − 1.03 | 0.15 | − 6.43 | |||
| Optimal levels (protein release) | A3 | B3 | C3 | |||
Orthogonal optimization for enzymatic hydrolysis parameters
An orthogonal table was referred to in orthogonal design. From the preliminary experimental data, three parameters (factors) were set at three levels. Table 1 shows that for reducing sugar and total sugar, effects of the three influencing factors on nutrient release followed percent substrate > incubation time > enzyme unit, whereas for reducing sugar and total sugar effects of the three influencing factors on nutrient release were incubation time > enzyme unit > percent substrate. Results of experiments on PKC hydrolysate concluded that all nutrients differed according to diverse percent substrate, incubation time and enzyme units (Table 1). Of the three nutrients measured, protein release was the main criterion because PKC hydrolysate is a protein source for monogastric animals. Therefore, the predicted condition was conducted based on protein release. All data were analyzed and optimal conditions predicted for hydrolyzing PKC. All nutrient release were the nutrients that were not necessary to digest in intestinal system and animal can absorb directly. To determine the relationship between independent and dependent variables, regression analysis is necessary (Kivak 2014). Here, the dependent variable was the protein release of PKC, one of all nutrient releases and main criteria, while independent variables were percent substrate, incubation time and enzyme unit. The predicted equation obtained by the linear regression model of PKC protein release in terms of coded factors was as follows:
where S is the percent substrate (%), T is the incubation time (h) and E is enzyme units (unit). R-squared = 68.9%, adj R-squared = 50.3%.
The predicted optimal condition of PKC hydrolysis from an orthogonal design was 14%, 6 h and 750 units at 90% confidence level with maximal nutrient release of reducing sugar, total sugar and protein at 65.91 ± 1.80 mg/ml, 294.59 ± 7.13 mg/ml and 46.70 ± 0.22 mg/ml, respectively, whereas the experimental optimal condition gave maximal nutrient release of reducing sugar, total sugar and protein at 100.94 mg/ml, 288.41 mg/ml and 58.60 ± 3.15 mg/ml, respectively. All nutrient releases of the experimental condition were higher than the predicted condition, indicating that the Taguchi method emphasized a mean performance characteristic value close to the target value, rather than a value within certain specification limits, thus improving product quality.
Chemical composition
Mannanase from B. amyloliquefaciens NT6.3 exhibited various enzyme activities other than mannanase including xylanase, glucanase, cellulase and amylase (data not shown). These enzymes can open and destroy plant cell walls which are composed of many different kinds of polysaccharides including cellulose, hemicellulose, and galactomannans called non-starch polysaccharide (NSP) (Carpita et al. 2000). NSP is an anti-nutrient for animal utilization and causes problems, especially in monogastric animals (Bedford 1995). The use of enzymes increases the rate of nutrient digestibility (Bedford 1995). For pepsin digestibility, 8 of 9 runs were significantly higher than raw PKC except for run no.4 (P < 0.05) (Table 2). Pepsin assay showed this difference and could be used to improve the digestibility of PKC. This indicated that mannanase destroyed or opened mannan structure as the main component in the plant cell wall and could increase nutrient digestibility and utilization.
Table 2.
Protein content and pepsin digestibility
| Sample | Protein content (%) | Pepsin digestibility (%) |
|---|---|---|
| PKC | 16.54 ± 0.02a | 75.24 ± 0.21b |
| Run 1 | 14.35 ± 0.50d | 79.20 ± 0.51a |
| Run 2 | 13.56 ± 0.50e | 79.69 ± 0.35a |
| Run 3 | 14.36 ± 0.17d | 79.93 ± 0.18a |
| Run 4 | 14.65 ± 0.03c | 73.36 ± 2.10c |
| Run 5 | 14.67 ± 0.05c | 79.54 ± 0.05a |
| Run 6 | 14.73 ± 0.09c | 78.60 ± 0.52a |
| Run 7 | 14.87 ± 0.05c | 79.23 ± 0.22a |
| Run 8 | 14.83 ± 0.21c | 78.66 ± 0.08a |
| Run 9 | 15.15 ± 1.12b | 78.96 ± 0.01a |
a,bMeans within columns with different superscripts are significantly different (p < 0.01)
Chemical compositions of PKC and PKC hydrolysate indicated that the latter was enriched by B. amyloliquefaciens NT6.3 in neutral detergent soluble (NDS), protein, starch, sugar, organic acids and pectin. On the other hand, non-starch polysaccharides as fiber, carbohydrate, hemicellulose and cellulose decreased (Table 3). Non-starch polysaccharides in PKC were linear mannans with very low galactose substitution (78% of total non-starch polysaccharides), followed by cellulose (12%) and small amounts of glucuronoxylans and arabinoxylans (3% each) (Düsterhöft et al. 2006). This indicated that mannanase enzymes from B. amyloliquefaciens NT6.3 could digest non-starch polysaccharide and open fiber structures to release nutrients.
Table 3.
Proximate and fiber composition
| Item | PKC | PKC hydrolysate |
|---|---|---|
| Moisture | 8.59 ± 0.14 | 6.75 ± 0.05 |
| Crude protein (%) | 16.95 ± 0.14 | 16.21 ± 0.17 |
| Crude fat (%) | 0.25 ± 0.00 | 0.21 ± 0.05 |
| Crude fiber (%) | 13.46 ± 0.19 | 12.71 ± 0.40 |
| Ash (%) | 4.69 ± 0.016 | 13.48 ± 0.09 |
| Carbohydrate (%) | 56.05 ± 0.21 | 50.65 ± 0.35 |
| Gross energy (kcal/kg) | 3911.7 ± 25.57 | 3505.67 ± 29.59 |
| Neutral detergent fiber; NDF (%) | 75.19 ± 0.22 | 66.31 ± 0.22 |
| Acid detergent fiber; ADF (%) | 45.67 ± 0.40 | 40.93 ± 0.52 |
| Acid detergent lignin; ADL (%) | 8.94 ± 0.28 | 8.10 ± 0.05 |
| Hemicellulose (%)a | 29.52 ± 0.44 | 25.39 ± 0.61 |
| Cellulose (%)b | 36.73 ± 0.14 | 32.83 ± 0.47 |
| Neutral detergent soluble; NDS (%)c | 24.81 ± 0.22 | 33.69 ± 0.22 |
aHemicellulose = NDF-ADF
bCellulose = ADF-ADL
cNDS are protein, starch, sugar, organic acids and pectin
Conclusions
Mannanase enzymes from B. amyloliquefaciens NT6.3 improved nutrient release of reducing sugar, total sugar and proteins. Nine Taguchi design experiments gave optimal condition of PKC hydrolysate as 14%, 6 h and 500 units, with predicted results 14%, 6 h and 750 units. Percent substrate was the most significant factor followed by incubation time and enzyme unit. Experiment results for optimal condition were higher than predicted for all nutrients with highest reducing sugar, total sugar and protein release at 100.94 mg/ml, 288.41 mg/ml and 197.40 mg/ml, respectively. Therefore, PKC hydrolysate may be suitable as a novel feed ingredient for monogastric animals.
Acknowledgements
This research was financially supported by a Research and Researchers for Industries (RRI) Ph.D. program (PHD56I0043), The Thailand Research Fund (TRF), Betagro Science Centre Co., Ltd., and Betagro Public Co. Ltd.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- AOAC; Horwitz W, Latimer GW Jr, editors. Official methods of analysis. 18. Gaithersburg: AOAC International; 2005. [Google Scholar]
- Bedford MR. Mechanism of action and potential environmental benefits from the use of feed enzymes. Anim Feed Sci Technol. 1995;53:145–155. doi: 10.1016/0377-8401(95)02018-U. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carpita N, McCann M. The cell wall. In: Buchanan B, Gruissem W, Jones R, editors. Biochemistry and molecular biology of plants. Rockville: Wiley; 2000. pp. 52–108. [Google Scholar]
- Chauhan PS, Puri N, Sharma P, Gupta N. Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol. 2012;93:1817–1830. doi: 10.1007/s00253-012-3887-5. [DOI] [PubMed] [Google Scholar]
- Chin FY (2008) Palm kernel cake (PKC) as a supplement for fattening and dairy cattle in Malaysia. In: 7th Meeting of the regional working group on grazing and feed resources. Forage Development in Southeast Asia: Strategies and Impacts. http://www.fao.org/ag/AGP/agpc/ doc/proceedings/manado/Chap.25.htm. Accessed 1 Mar 2018
- Dhawan S, Kaur J. Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol. 2007;27(4):197–216. doi: 10.1080/07388550701775919. [DOI] [PubMed] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Düsterhöft M, Posthumus A, Voragen AGJ. Non-starch polysaccharides from sunflower (Helianthus annuus) meal and palm-kernel (Elaeis guineensis) meal-investigation of the structure of major polysaccharides E-M. J Sci Food Agric. 2006;59(2):151–160. doi: 10.1002/jsfa.2740590204. [DOI] [Google Scholar]
- Eggum BO, Monowar L, Bach Knudsen KE, Munck L, Axtell J. Nutritional quality of sorghum and sorghum foods from Sudan. J Cereal Sci. 1983;1:127–137. doi: 10.1016/S0733-5210(83)80030-7. [DOI] [Google Scholar]
- Ijah UJJ, Ukpe LI. Biodegradation of crude oil by Bacillus Strains 28A and 61B isolated from oil spilled soil. Waste Manage (Oxford) 1992;12(1):55–60. doi: 10.1016/0956-053X(92)90009-8. [DOI] [Google Scholar]
- Jaafar MD, Jarvis MC. Mannans of oil palm kernels. Phytochemistry. 1992;31(2):463–464. doi: 10.1016/0031-9422(92)90017-K. [DOI] [Google Scholar]
- Kivak T. Optimization of surface roughness and flank wear using the Taguchi method in milling of Hadfield steel with PVD and CVD coated inserts. Measurement. 2014;50:19–28. doi: 10.1016/j.measurement.2013.12.017. [DOI] [Google Scholar]
- Miller GR, Blum R, Glennon W, Burton A. Measurement of carboxymethyl cellulase activity. Anal Biochem. 1959;2:127–132. [Google Scholar]
- Ng KL, Mohd Khan A. Enzymatic preparation of palm kernel expeller protein hydrolysate (PKEPH) Int Food Res J. 2012;19(2):721–725. [Google Scholar]
- Office of Agricultural Economics (OAE) (2017) Center for agricultural information ministry of agriculture and co-operatives. Background information of agricultural Economics. http://www.oae.go.th. Accessed 1 Mar 2018
- Phothichitto K, Nitisinprasert S, Keawsompong S. Isolation, screening and identification of mannanase producing microorganisms. Kasetsart J (Nat Sci) 2006;40:26–38. [Google Scholar]
- Ravindran V, Son JH. Feed enzyme technology: present status and feed developments. Recent Pat Food Nutr Agric. 2011;3:102–109. doi: 10.2174/2212798411103020102. [DOI] [PubMed] [Google Scholar]
- Sundu B, Dingle J. Use of enzymes to improve the nutritional value of palm kernel meal and copra meal. Proc Qld Poult Sci Symp Aust. 2011;11(14):1–15. [Google Scholar]
- Van Soest PJ. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J Ass Offic Anal Chem. 1963;46:829–835. [Google Scholar]
- Van Beilen JB, Li Z. Enzyme technology: an overview. Curr Opin Biotechnol. 2002;13(4):338–344. doi: 10.1016/S0958-1669(02)00334-8. [DOI] [PubMed] [Google Scholar]