Abstract
The current work is focused on establishing therapeutic protocol using unconventional drugs of herbal origin and studying their mechanism of action at molecular level in the treatment of bovine sub-clinical mastitis. It explores the potential of different cytokines which can be used for diagnosis, prognosis and monitoring of bovine sub-clinical mastitis. Prosopis juliflora alkaloids was administered intramammarily in 24 sub-clinically affected quarters once a day for 5 consecutive days at the rate of 10 ml of 1% formulation. In 18 disease control quarters, sterile normal saline was infused. The bacterial cultural examination, somatic cell count (SCC) and cytokines (IL-1β, IL-6, IL-8, IL-12, GM-CSF, IFN-γ, TNF-α) expression by real-time PCR were evaluated on day 7, 14, 21 and 28 post-last treatment from milk samples. Around 75.0% of treatment group quarters showed significant (p < 0.05) reduction in SCC on day 28 post-last treatment, whereas 94.4% control group quarters did not show any significant decline in SCC. 58.3% of treated quarters showed both bacteriological cure as well as significant (p < 0.05) reduction in SCC on day 28 post-last treatment. While, among control group quarters, 83.3% quarters not only remained bacteriological positive, they also did not show any significant decline in SCC. The in vitro antimicrobial activity of alkaloids of P. juliflora was evaluated. Lower concentrations of alkaloids (0.25% and 0.50%) dissolved in normal saline showed zone of inhibition against 12 out of 15 isolates, however higher concentration (1, 1.5, 2, 2.5 and 5%) showed zone of inhibition against all 15 bacterial isolates. The gene expression level of IL-1β, IL-8 and IFN-γ cytokines exhibited significant difference between healthy and sub-clinically affected quarters highlighting the potential of these cytokines in the diagnosis of bovine sub-clinical mastitis. Down-regulation of IL-1, IL-6, IL-8 and IFN-γ cytokines in treated quarters can be explored for making the prognosis and monitoring post-treatment disease progression of bovine sub-clinical mastitis. The P. juliflora alkaloid demonstrated strong in vitro and in vivo antibacterial activity, along with causing immunomodulation by enhancing post-treatment gene expression of IL-1, IL-6 and IL-8 cytokines. Therefore, P. juliflora alkaloids hold a strong claim as an effective alternative herbal therapy in bovine sub-clinical mastitis.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1438-1) contains supplementary material, which is available to authorized users.
Keywords: Alkaloids, Bovine sub-clinical mastitis, Cytokines, Prosopis juliflora, Real-time PCR, Somatic cell count, Treatment
Introduction
Mastitis, an inflammation of the mammary gland, has a profound impact on milk production and milk quality leading to great economic losses to dairy industry in general and dairy farmers in particular. In India, the economic losses incurred due to mastitis have shown tremendous increase during the last five decades and lately the annual losses due to mastitis have been estimated to the tune of Rs. 7165.51 crore (Bansal et al. 2011). Since mastitis affects the milk quality, its consequences are not just restricted to the dairy farm but expand beyond that. Increasing concerns among the consumers about the antimicrobial residues, antimicrobial resistance, milk quality and animal welfare further demand proper policies in place to effectively prevent and control mastitis.
During mastitis there is huge influx of polymorphonuclear (PMN) leukocytes into the infected udder with secretion of inflammatory mediators into the milk compartment (Paape et al. 2002). The process of inflammation enables the immune system of the host to protect it and clear the pathogen from system. Milk PMNs have been indicted to play crucial role in udder defense mechanism against bacterial infection (Stevens et al. 2012).
Cytokines are immuno-regulatory mediators that play pivotal role in regulating the immune response against different infections. The use of cytokines in the immunotherapy, diagnosis and prognosis of mastitis is rising with the knowledge of cytokine network in bovine mammary gland and the development of efficient diagnostic techniques (Alluwaimi 2004). The expression of some cytokines has been historically difficult (Gonzalez et al. 2013). Since, cytokines’ messenger RNA (mRNA) is expressed in low quantities, accurate methods are required for their measurement. Different methods for quantitation of cytokine expression at the protein level (ELISA, Elispot, biological assays, intracellular cytokine staining) (Kabilan et al. 1990) and at the mRNA level (Northern blots), in situ hybridisation, ribonuclease protection assay, reverse transcriptase polymerase chain reaction (RT-PCR) are reported (Dallman et al. 1991).
Among various techniques being reported, quantitative real-time PCR (qPCR) is a highly sensitive technique which enables to quantify minimal physiologic changes in gene expression. qPCR has the ability of detection of 10- to 100-fold higher than other methods (Bustin 2000) which has rendered it being one of the best methods of choice in various studies since it provides good reproducibility and wide range of quantification (Pfaffl et al. 2003).
Up till now, mastitis control strategies have largely involved implementation of strict hygiene practices on the farm and the direct administration of antibiotics into udder quarters exhibiting clinical signs of infection. The side-effects and limitations associated with the use of antibiotic have necessitated the need to look for alternative measures to formulate effective control strategies against bovine mastitis. In order to address the issue of increasing multi-drug resistance microbes, the current study explores the treatment efficacy with a herbal concoction prepared from the leaves of P. juliflora.
Prosopis juliflora (family Leguminosae, sub-family Mimosoideae) has been introduced in arid and semi-arid regions since it exhibits fast growth, drought resistance, salt tolerance, nitrogen fixation, acts as a sand stabiliser and provides fuel wood. Prosopis commonly called mesquite is known to contain a total of 21 flavonoids, one of the most bioactive compounds. The leaves are known to contain monocyclic diketone, prosopidione. The therapeutic importance attributed to P. juliflora have been carried out by Ahmad et al. (1986). They have reported the isolation and structural elucidation of alkaloids juliflorine, julifloricine and julifloridine. Further Ahmad et al. (1989a, b) isolated two new alkaloids juliprosinene and juliflorinine. The alkaloid juliprosinene has showed antibacterial activity against Escherichia coli strains, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus and Shigella sonnei (Ahmad et al. 1989a, b).
In the present study, alkaloids separated from the chloroform extract of P. juliflora leaves were intramammarily infused in the infected udder quarters included in the study and the treatment efficacy was evaluated in terms of bacterial cultural examination, somatic cell count and their cytokine profile was compared with that of disease control group by real-time PCR.
Materials and methods
Screening of quarters for sub-clinical mastitis
Animals and milk collection
The present study was conducted on a total of 69 lactating cows comprising 35 triple cross (Kankrej × Jersey × Holstein Friesian), 26 Kankrej and 8 Gir maintained at the Livestock Research Station (LRS) affiliated with College of Veterinary Science and Animal Husbandry, Anand Agricultural University, Anand. All the cows were in lactation during the sampling period. The animals were housed, fed and managed under identical management practices as per the schedule followed at the LRS. Clinical examination of individual quarter of each animal included in this study was carried out for abnormalities in the shape and size of udder, udder oedema or atrophy, and consistency, i.e. lumps, fibrosis or hardening of udder. Similarly, each quarter milk sample was checked at the time of milking for any gross abnormality in the milk. Based on these examinations, the animals having apparently healthy quarters were selected for the present investigation. The approval from the Institutional Animal Ethics Committee (IAEC) was taken and is mentioned in detail at section termed “Ethical Approval”.
Microbiological culturing
Loopful of milk from foremilk sample from each quarter was streaked on blood agar plate (containing 5% sheep blood) and simultaneously on MacConkey agar plate for primary bacterial isolation and subsequently the plates were incubated at 37 °C for 24 h. Following incubation, the plates were examined for bacterial growth and the morphological characteristics of bacterial colonies were recorded. Characterisation of bacterial isolates was performed as per the method described by Cowan and Steel (1970).
Somatic cell count (SCC)
Somatic cell count of foremilk samples was carried out using Fossomatic™ minor cell counter (Foss Electric, Hillerod, Denmark) as per the method described by Gonzalo et al. (2003). The SCC value more than 500 cells/µl of milk indicated the presence of sub-clinical mastitis.
Identification of infected quarters
By correlating the results of SCC and bacteriological culture examination, infected quarters were identified following the guidelines of Indian Dairy Federation (IDF).
Preparation of P. juliflora leaf alkaloids
Preparation of chloroform extract of P. juliflora leaves
Leaves of P. juliflora were collected from their natural habitat and were identified and authenticated by the Department of Medicinal and Aromatic Plants, B. A. College of Agriculture, A.A.U., Anand. The leaves were dried in shade and subjected to repeated extraction in Soxhlet apparatus using chloroform (Chhabra 1992).
Separation of alkaloids from chloroform extract by solvent method
For the separation of alkaloids from the chloroform extract of P. juliflora leaves, the chloroform extract was heated with water at 50 °C approximately for 30 min. The resultant water-soluble fraction was filtered by using Whatman filter paper Grade 1 and the residue was discarded. The filtrate was evaporated to obtain residue which was brownish in colour having semi-solid consistency.
Therapeutic studies: in vivo evaluation of P. juliflora leaf alkaloids in sub-clinical mastitis
Prior to in vivo studies, the in vitro antimicrobial activity of alkaloids of P. juliflora were evaluated against four typed cultures (Pseudomonas aeruginosa ATCC27853, Bacillus subtilis ATCC6633, Staphylococcus aureus ATCC25923, Streptococcus agalactiae NCIB8778) of bacteria obtained from National Chemical Laboratory (NCL), Pune (India) and 11 organisms (Escherichia coli, Klebsiella sp., Enterobacter sp., Serratia marcescens, Micrococcus varians, Staphylococcus chromogenes, Staphylococcus epidermidis, Staphylococcus hyicus, Staphylococcus intermedius, Staphylococcus aureus, Streptococcus agalactiae) recovered from cases of sub-clinical mastitis. Lower concentrations of alkaloids (0.25% and 0.50%) dissolved in normal saline showed zone of inhibition against 12 out of 15 isolates, however higher concentrations (1, 1.5, 2, 2.5 and 5%) showed zone of inhibition against all 15 bacterial isolates (unpublished data). Based on the affirmative effect of alkaloids in inhibiting bacterial growth in vitro they were used for further in vivo studies.
Forty-two quarters belonging to three breeds of cows (triple cross, Kankrej and Gir) affected with pathogen-specific mastitis were selected and included in the present study. The selection of healthy and infected quarters was done on the basis of results obtained on microbiological culture examination and SCC following the IDF guidelines (1987). These quarters were divided in two groups in each breed of cows. One group comprised quarters given alkaloids treatment and another group consisting of quarters without any treatment, which served as diseased control.
Evaluation of acute dermal and oral toxicity of alkaloids
Based on the results of the acute dermal and oral toxicity test carried out on male Wistar rats (as per OECD New Draft Guideline 434) the use of P. juliflora alkaloids was approved by the Institution Animal Ethics Committee (IAEC 2007/VPT/26). This study was conducted separately.
Treatment of specific mastitis quarters with intramammary infusion of Prosopis juliflora alkaloids
Twenty-four quarters harbouring specific mastitis (distributed in three breeds of cows) were diagnosed and infused with 10 ml of 1% formulation of alkaloids dissolved in normal saline, whereas 18 quarters (distributed in three breeds of cows, viz. 9 in Triple cross, 3 in Kankrej and 6 in Gir) were infused with sterile normal saline solution which served as diseased control.
Udder and teats were first washed with 0.01% (w/v) potassium permanganate solution properly after milking. Following washing, the udder and teats were wiped with tissue paper and then the teat and teat orifices were scrubbed with 70% ethanol. Subsequently, 1% P. juliflora alkaloids formulation was administered by intra-mammary infusion in infected quarters using 10 ml sterile syringe once daily for 5 consecutive days.
Evaluation of Prosopis juliflora leaf alkaloids treatment
Quarter milk samples (qms) from treated and diseased control quarters were collected on day 7, 14, 21 and 28 after last infusion with alkaloids. The qms were evaluated for improvement in diseased condition of quarters using mastitis markers (SCC and bacteriological culture examination).
Cytokine expression in milk somatic cells of healthy quarters, disease control quarters and pathogen-specific mastitic quarters
To study the expression profile of IL-1β, IL-6, IL-8, IL-12, GM-CSF, IFN-γ and TNF-α, six healthy quarters from each breed. While 12, 9, and 21 sub-clinically infected (specific mastitis) quarters of Gir, Kankrej and triple-cross breed of cow, respectively, were selected. They were further categorised into treatment and disease control quarters, viz. Gir (12: 6 DC, 6 T); Kankrej (9: 3 DC, 6 T) and triple cross (21: 9 DC, 12 T). The quarters in treatment group were given P. juliflora alkaloids (1% formulation in normal saline) treatment.
Collection of milk samples
Milk samples were collected from the selected healthy and diseased (specific mastitis) udder quarters for separation of somatic cells for the study of cytokine expression. The selection of healthy and infected quarters was done on the basis of results obtained on doing bacteriological culture examination and SCC following the IDF guidelines (1987).
RNA extraction
Separation of somatic cells from milk samples
Isolation and formation of pellets of somatic cells was carried out as per the method described by Verdi and Barbano (1991). Milk was centrifuged at 1000×g for 15 min in 50 ml tubes and pellet was collected after discarding fat layer and milk. The pellet was washed twice in sterile normal saline solution (pH 7.2) prepared in DEPC-treated water. The pellet was suspended in 150 µl of NSS.
Extraction of RNA from somatic cell pellets
Extraction of RNA from the pellets of somatic cells was carried out using TRIZOL method as described by Chomczynski (1993). Total RNA from the milk cell pellet was extracted by TRI Reagent®.
Reverse transcription (cDNA synthesis from RNA)
cDNA was prepared from extracted RNA by using Thermo Scientific Verso™ cDNA synthesis kit following manufacturer’s instruction.
Quantitative real-time polymerase chain reaction of cDNA samples
Quantitative real-time PCR was carried out for study of cytokine expression by the method as described by Higuchi et al. (1993). The forward and reverse sequences of primers used for real-time PCR are mentioned in Table 1. Expression of cytokines mRNA was quantified by real-time PCR and analysed using Applied Biosystems 7500 SDS software.
Table 1.
Primer sequences (forward and reverse) used for real-time PCR
| Genes | Primer sequence (5´–3´) | T m (°C) | GC content (%) |
|---|---|---|---|
| IL-1β | F: GCTCTTGGGGTAGACTTTGGGGT R: CCCGAGCCCTGCTGATCCAT |
64.2 63.5 |
56.5 65.0 |
| IL-6 | F: TCATTAAGCGCATGGTCGACAAA R: TCAGCTTATTTTCTGCCAGTGTCT |
58.9 59.3 |
43.5 41.7 |
| IL-8 | F: CACTGTGAAAATTCAGAAATCATTGTTA R: CTTCACAAATACCTGCACAACCTTC |
57.8 61.3 |
28.6 44.0 |
| IL-12 | F: TTATTGAGGTCGTGGTAGAAGCTG R: GGTCTCAGTTGCAGGTTCTTGG |
61.0 62.1 |
45.8 54.5 |
| GM-CSF | F: AGTAATGACACAGAAGTCGTCTCTG R: GCCGTTCTTGTACAGCTTCAGG |
61.3 62.1 |
44.0 54.5 |
| IFN-γ | F: TCATTAAGCGCATGGTCGACAAA R: TCAGCTTATTTTCTGCCAGTGTCT |
58.9 59.3 |
43.5 41.7 |
| TNF-α | F: TCTTCTCAAGCCTCAAGTAACAAGC R: CCATGAGGGCATTGGCATAC |
61.3 59.4 |
44.0 55.0 |
| GAPDH | F: GGCGTGAACCACGAGAAGTATAA R: CCCTCCACGATGCCAAAGT |
60.6 58.8 |
47.8 57.9 |
Relative quantification
Cytokine quantification was achieved using the comparative Ct (cycle threshold) method and was expressed as ‘‘n-fold up regulation of cytokine transcription’’ in relation to a calibrator which is represented by the smallest signal detectable for that specific cytokine. The expression of each gene was analysed using the relative quantification method described by Pfaffl (2001). For relative quantitation by the comparative Ct method, values were expressed relative to a reference sample, called the calibrator (healthy milk sample). The Ct for the target gene and the Ct for the internal control (reference housekeeping gene) were determined for each sample and the calibrator. The expression of selected cytokine genes were normalised by that of the reference gene, bovine GAPDH, at each time point and converted to the relative expression ratio (fold of expression), where
where ∆∆Ct is the ∆Ct for target gene − ∆Ct for reference gene, ∆Ct for target gene is the average Ct of sample − average Ct of calibrator, ∆Ct for reference gene is the average Ct of sample − average Ct of calibrator.
The term ∆Ct for target gene refers to the difference in Ct values between the sample and calibrator (Ct-sample − Ct-calibrator) for the target gene sequence and the term ∆Ct for reference gene refers to the difference in Ct values between the sample and calibrator (Ct-sample − Ct-calibrator) for the reference gene sequence (housekeeping gene).
Statistical analysis
Statistical analysis of data pertaining to various parameters (somatic cell count and cytokine expression) was done as per the standard methods and one-way ANOVA was conducted by Statistical software package (SPSS Version 17.0) to analyse expression on day 7, 14, 21 and 28 post-treatment with alkaloids in treatment group and in disease group post-normal saline infusion. Paired t test was conducted between disease control and treatment group for each cytokine on each day. Bars in the figures indicate standard error (SE) and has been used in the entire study for cytokine profile study and SCC.
Results
For the screening of sub-clinical mastitis somatic cell count (SCC) and bacteriological culture examination were employed on a total of 69 lactating cows comprising 35 triple cross (Kankrej × Jersey × Holstein Friesian), 26 Kankrej and 8 Gir to study the effect of alkaloids on treatment of bovine sub-clinical mastitis. Out of 274 quarters, 42 quarters from Gir (12), Kankrej (9) and triple-cross breed (21) were selected; 24 as treatment group and 18 as untreated control (disease control) group. Additionally, six quarters each from healthy cattle of each breed were selected. Ten ml of 1% formulation of P. juliflora alkaloids was administered once a day for 5 consecutive days in 24 sub-clinically affected mastitic quarters belonging to three breeds. Relative fold expressions of seven cytokine genes, viz. IL-1β, IL-6, IL-8, IL-12, GM-CSF, IFN-γ and TNF-α in milk somatic cells of sub-clinically affected mastitic quarters were determined vis-à-vis normal quarters.
In bacterial cultural examination, complete absence of colony forming unit (CFU) on blood agar was monitored for 3 consecutive days. Thus, according to the IDF guidelines, complete absence of microorganisms on 2 days out of 3 consecutive days and SCC below 500,000 cells/ml for all 30 days would classify the quarter as healthy.
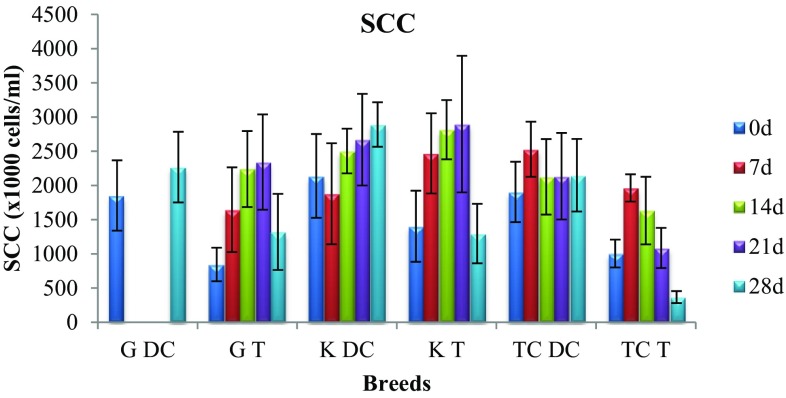
In figures, G indicates cattle from Gir breed, K indicates cattle from Kankrej breed, TC indicates triple-cross breed, DC means disease control and T means treatment.
In Gir breed, mean SCC (× 1000 cells/ml) rose from 1854 to 2269 in disease control, while in treatment it rose from 845 to 2342 on day-21 post-last treatment, while decreasing to 1320 on day-28 post-last treatment. In Kankrej breed, mean SCC (× 1000 cells/ml) rose from 2140 to 2892 in disease control, while in treatment it rose from 1403 to 2898 on day-21 post-last treatment, while decreasing to 1297 on day-28 post-last treatment. In triple-cross breed, mean SCC (× 1000 cells/ml) rose from 1906 to 2150 in disease control, while in treatment it rose from 1005 to 1087 on day-21 post-last treatment, while decreasing to 367 on day-28 post-last treatment (Fig. 1).
Fig. 1.
Mean somatic cell count in treatment and disease control group of three breeds
In the present study, the distribution of treatment and disease control quarters among three breeds can be summarised, viz. Gir (12: 6 DC, 6 T); Kankrej (9: 3 DC, 6 T) and triple cross (21: 9 DC, 12 T). The trend of SCC based on per cent quarters exhibited that on subjecting the qms to somatic cell count, 18 (75.0%) treatment group quarters showed significant (p < 0.05) reduction in SCC on day-28 post-last treatment, whereas 17 (94.44%) control group quarters did not show any significant decline in SCC. Out of 24 treated quarters, 14 (58.33%) quarters showed both bacteriological cure as well as significant (p < 0.05) reduction in SCC on day-28 post-last treatment. Among control group quarters, 15 (83.33%) quarters not only remained bacteriological positive, they also did not show any significant decline in SCC.
The real-time PCR conditions established resulted in expected amplicon size and single-band amplification for all genes. Amplification specificity was further validated by melting curve analysis, generated at the end of each PCR reaction. Genes exhibiting single peak in the melting curve were selected, which indicates absence of primer-dimer formation during the reaction and specificity of the amplification.
The target gene was calibrated with reference gene (GAPDH) and expressed as fold of induction over the healthy quarters at every instance. This was done to normalise the process for the variability in the number of cells in the original samples, for differences in extraction efficiency, for mRNA degradation in the starting material, and for reverse transcriptase reaction efficiency (Leutenegger et al. 2000).
The expression of housekeeping gene GAPDH was observed to be steady among different samples and breeds. The relative quantification was done by using GAPDH for normalisation.
Across all three breeds, the cytokines IL-1β, IL-8 and TNF-α showed significant difference (p < 0.1) between healthy and mastitic quarters. Breed-wise examination revealed highly significant difference (p < 0.01) for IL8 and TNF-α, and significant difference (p < 0.1) for IFN-γ between healthy and mastitic quarters of Kankrej breed. However, in case of IL1, significant difference (p < 0.1) was observed between healthy and mastitic quarters of Gir breed.
As one would have expected, non-significant difference was observed in the expression of GM-CSF, IFN-γ and TNF-α of disease control and treatment group belonging to Gir, Kankrej and triple-cross cattle. For IL-12, non-significant difference was observed between disease control and treatment group belonging to Kankrej and triple-cross cattle. Similarly for IL-1β and IL-8, non-significant difference was observed in Gir and triple-cross cattle between disease control and treatment group. In case of IL-6, only triple-cross cattle exhibited non-significant difference between disease control and treatment group.
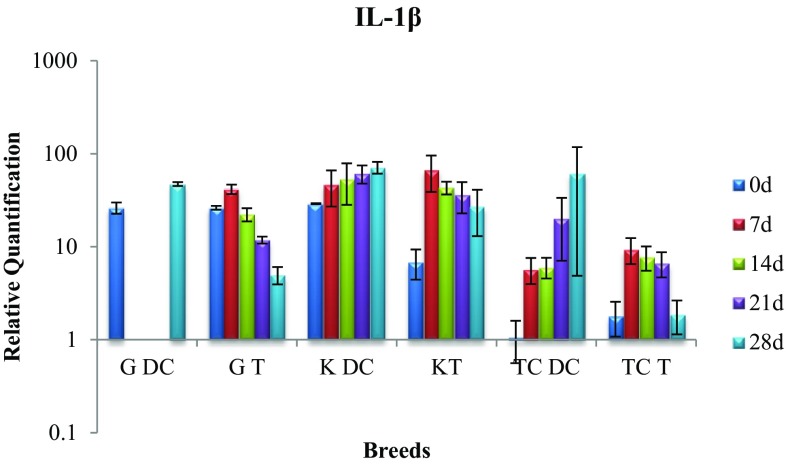
Expression of IL-1β gene
In disease control and treatment group of Gir cattle, the gene expression on day 0 and 28 exhibited significant difference (p < 0.05). While the expression rose in disease control cows, it dropped in treatment group indicating positive therapeutic effect of P. juliflora alkaloid (Fig. 2).
Fig. 2.
Expression of IL-1β mRNA in sub-clinically mastitic (treatment and control group)
The disease control cows of Kankrej breed showed significant difference (p < 0.05) in the gene expression levels on day 0 and 28. However, in treatment group the gene expression rose steeply on day 7 which showed significant drop on day 28 and came close to the expression level of healthy cattle indicating therapeutic efficacy of intramammary infusion.
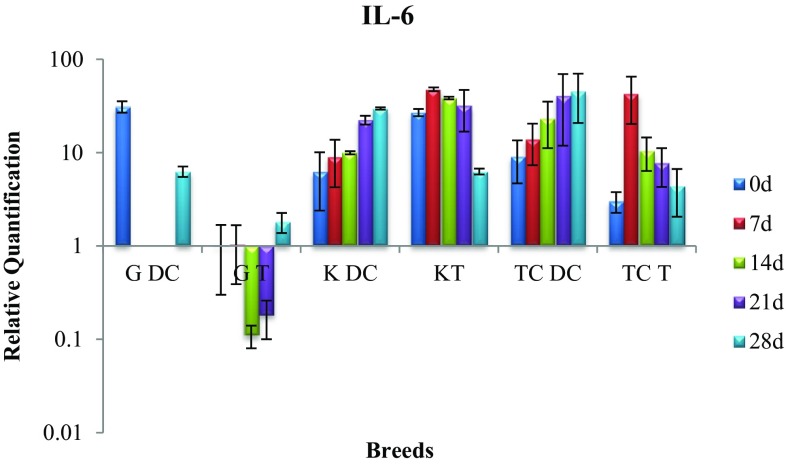
Expression of IL-6 gene
In disease control group of Kankrej breed there was significant (p < 0.05) rise in IL-6 expression on day 28 compared to day zero. However, the treatment group showed rise in gene expression on day-7 post-treatment, but it dropped significantly (p < 0.05) by day 28 and reached the expression level of healthy group indicating positive effect of infusion of alkaloids during treatment (Fig. 3).
Fig. 3.
Expression of IL-6 mRNA in sub-clinically mastitic (treatment and control group)
In case of Gir cattle, there was significant (p < 0.05) drop in IL-6 activity in disease control cows on day 28, whereas the treatment group continued to exhibit gene expression level equivalent to healthy group throughout. However, on day 28, significant (p < 0.05) difference was noted between disease control and treatment group with latter demonstrating low level of gene expression.
In the treatment group cows of triple cross, IL-6 gene expression exhibited a significant (p < 0.05) rise on day 7 which dropped to the level shown by healthy group by day 28. The expression on day 28 between two groups was significantly (p < 0.05) different with low expression in treatment group indicating affirmative effect of therapeutic efficacy of alkaloids.
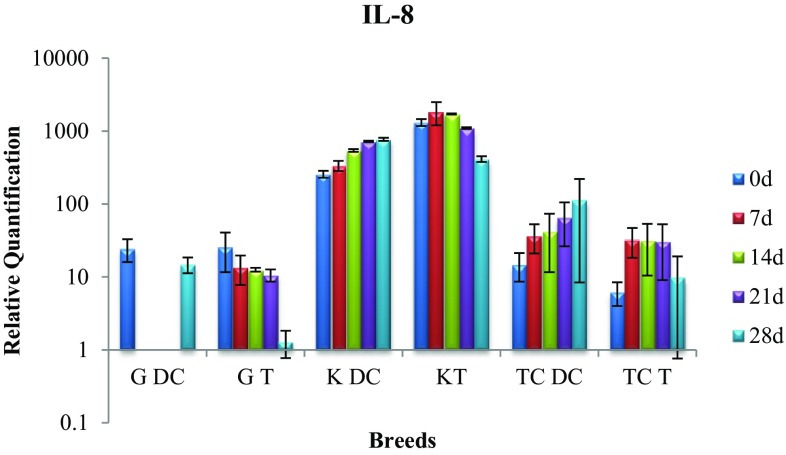
Expression of IL-8 gene
The disease control cows of Kankrej breed exhibited significant (p < 0.05) rise in gene expression between day 0 and day 28. In contrast, in treatment group the IL-8 level rose on day 7, but with a significant (p < 0.05) decrease in the gene expression by day 28 thus suggesting therapeutic efficacy of P. juliflora alkaloids in the treatment of sub-clinical mastitis (Fig. 4).
Fig. 4.
Expression of IL-8 mRNA in sub-clinically mastitic (treatment and control group)
In Gir breed, the difference between the two groups was significant (p < 0.05) on day 28 with treatment group exhibiting lower expression suggesting therapeutic efficacy of alkaloids.
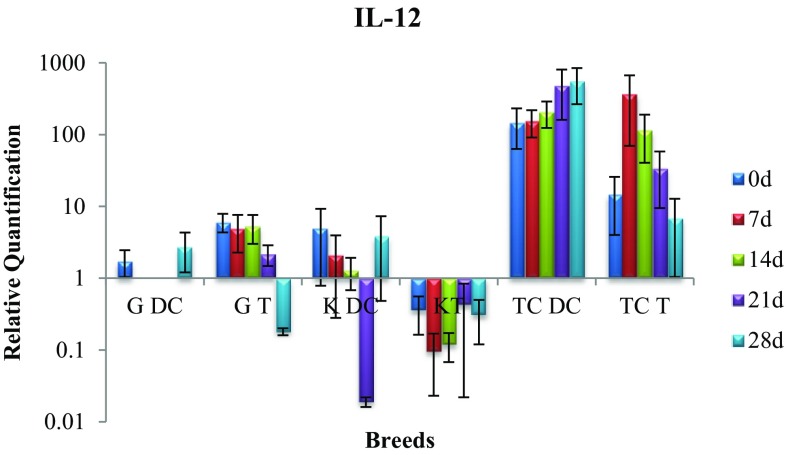
Expression of IL-12 gene
The non-significant rise and drop in IL-12 gene of disease control and treatment group belonging to Gir, Kankrej and triple-cross cattle did not indicate any therapeutic effect of intramammary infusion of alkaloids (Fig. 5). However, on day 28 in triple-cross treatment group, the gene expression level was less than disease control on any given day and day 0 of treatment group itself.
Fig. 5.
Expression of IL-12 mRNA in sub-clinically mastitic (treatment and control group)
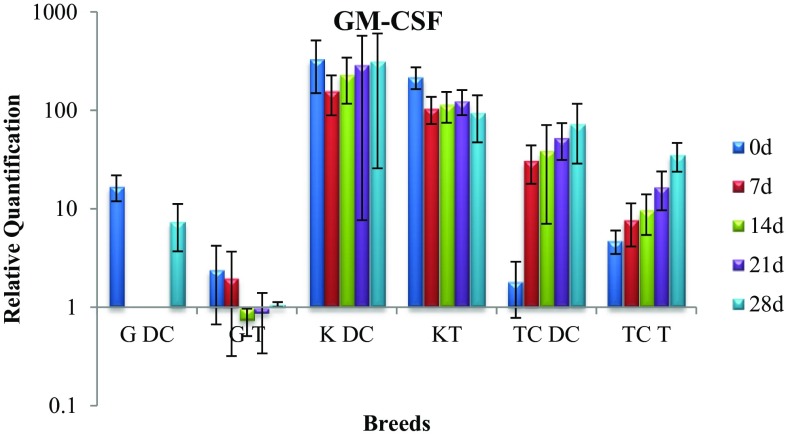
Expression of GM-CSF gene
In Kankrej cattle, the GM-CSF gene expression in disease control group throughout remained the same, however, in treatment group there was significant (p < 0.05) drop in gene expression that was equivalent to the level exhibited by healthy cattle suggesting positive effect of intramammary therapy (Fig. 6).
Fig. 6.
Expression of GM-CSF mRNA in sub-clinically mastitic (treatment and control group)
The gene expression in disease control group of triple-cross cattle revealed non-significant increase, whereas significant (p < 0.05) drop was observed in treatment group suggesting therapeutic efficacy of alkaloids. Additionally, on day 28 significant (p < 0.05) difference in the gene expression was observed between the two groups with disease control cattle demonstrating substantially high level.
In Gir cattle, significant (p < 0.05) rise was observed in disease control group which was contrary to non-significant drop noticed in the treatment group.
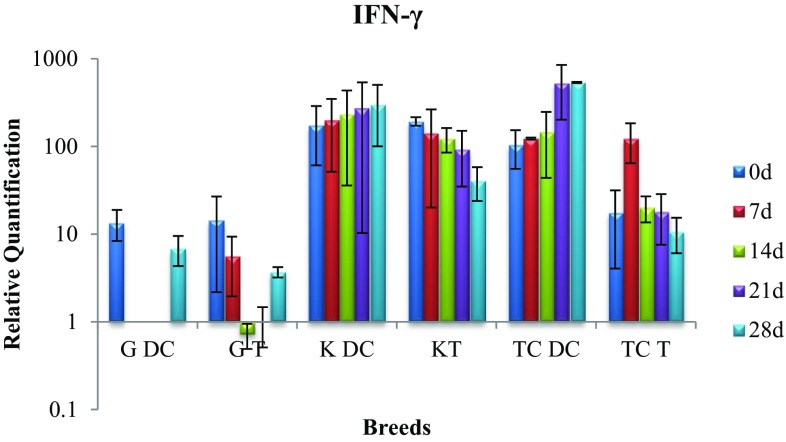
Expression of IFN-γ gene
On comparison of gene expression between disease control and treatment group on day 28, the difference was significant (p < 0.05) with the gene expression in disease control cows being very high (Fig. 7).
Fig. 7.
Expression of IFN-γ mRNA in sub-clinically mastitic (treatment and control group)
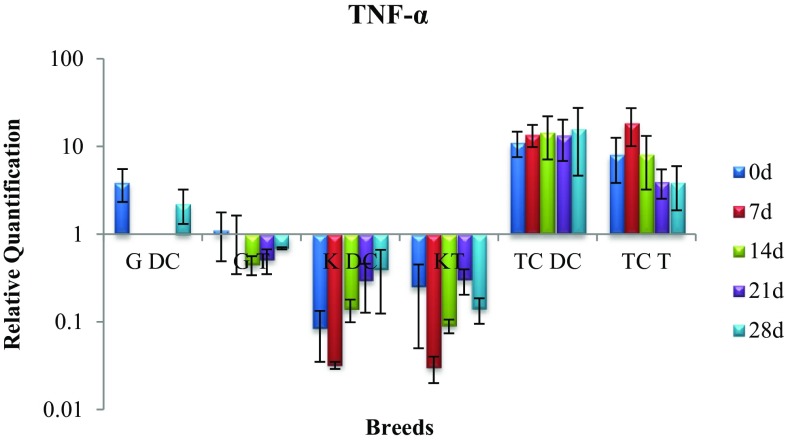
Expression of TNF gene
In Kankrej cattle there was significant (p < 0.05) rise in disease control group and significant (p < 0.05) drop in treatment group indicating positive effect of treatment with alkaloid (Fig. 8).
Fig. 8.
Expression of TNF-α mRNA in sub-clinically mastitic (treatment and control group)
Discussion
The contemporary treatment of bovine mastitis employs administration of antibiotics, whose impacts are perceived as presence of antimicrobial residues in milk and increased antimicrobial resistance in host which may confound the treatment procedure. Additionally, the effect of mastitis is felt beyond mammary system to the reproduction ability of the affected animal. This factor compounds the economic losses incurred due to mastitis to a staggering number when glanced at national level (Bansal and Gupta 2009; Sinha et al. 2014).
Under prevailing conditions, the treatment of subclinical mastitis by some alternative strategy needs to be re-explored. The use of herbal extracts or the traditional ethno-veterinary medicinal practices in the treatment of various veterinary diseases in developing countries is well known; however, the reports of ethno-veterinary medicinal practices in treatment of bovine mastitis is lesser known and reported. Feyera et al. (2017) reported that botanical ethnoveterinary therapies are the mainstay of livestock health care system in Fafan Zone, Eastern Ethiopia. Aziz et al. (2018) investigated 73 medicinal plants used by indigenous communities for veterinary practices in Pakistan. Mukherjee et al. (2010), in an effort to search the potential of ethnoveterinary application, evaluated therapeutic efficacy of Tinospora cordifolia against bovine mastitis. In another study an alkaloid possessing antimicrobial activity called juliflorine was separated from P. juliflora, which was implicated in bringing out some immunomodulatory or immunostimulating effect in rabbits infected with Listeria hemolysin along with Freund’s complete adjuvant (Ahmad et al. 1992).
Prosopis juliflora are rich source of piperidine alkaloids. Many alkaloids such as juliflorine, julifloricine and julifloridine (Ahmad et al. 1978), juliprosine (Daetwyler et al. 1981), juliprosinene and juliflorinine (Ahmad et al. 1989a, b), 3′-oxojuliprosopine, sceojuliprosopinol, 3-oxojuliprosine and 3′-oxo-juliprosine (Nakano et al. 2004) have been isolated from leaves and have proven to be pharmacologically active (Ahmad et al. 1989a, b; Aqeel et al. 1989); however, less work has been done to evaluate biological activity and chemical characterisation of other parts of the plants.
The results of the present study indicated that the 1% formulation of P. juliflora alkaloids not only eliminated mastitis pathogens from 14 out of 24, i.e. 58% of treated quarters, it also caused significant reduction in the somatic cell count thereby decreasing the inflammatory response in the affected quarters.
The gene expression levels of IL-8, and IFN-γ cytokines were observed to be significantly higher, while the levels of IL-1 and IL-6 were higher in milk somatic cells of SCM quarters as compared to healthy quarters suggesting potential of these cytokines for diagnosis of bovine sub-clinical mastitis. For IFN-γ, it is reported that its transcription is significantly correlated with IL-12 expression (Alluwaimi and Cullor 2002). In the present study, the expression level of IFN is observed to correlate with that of IL-12 in treatment group of triple-cross breed.
In our study, we observed that in several instances the cytokine expression (IL-1β, IL-6, IL8, IL12, IFN-γ, TNF-α) rose significantly on day-7 post-treatment. Considering three breeds collectively, the gene expression of IL-1, IL-6 and IL-8 showed considerable increase on day 7 in treatment group indicating the immunomodulation potential of P. juliflora alkaloids compared to control group. By day 28, the gene expression level for these three genes reached below the level of control group with subsequent decrease in somatic cell counts and reducing bacterial load in udder quarters. The bacterial cultural examination revealed reduction in bacterial load and consequently clearing of the bacterial PAMPs (pathogen associated molecular patterns for instance lipopolysaccharide and endotoxins activate innate immune response) in nearly 58.3% treatment udders along with reduction in SCC, while in 83.3% control udders, neither the bacterial infection was removed nor SCC lowered. The reduction in bacterial infection can be attributed to direct effect of alkaloids on bacteria. While in untreated udder quarters, cytokines remained elevated as a result of bacterial–somatic cell interaction. Apart from exerting antibacterial activity following infusion of alkaloids which decimated the intensity of sub-clinical mastitis, it mediated enhancement of somatic cell infiltration in the milk suggesting chemoattractant property of the alkaloids. These findings are consistent with previous studies in cows treated with some biological response modulator (De and Mukherjee 2013), or with cell wall product of Mycobacterium phlei in bovine SCM (Mukherjee et al. 2004) and in guinea pigs treated with peptidoglycan subunit by intraperitoneal route (Nagao et al. 1990).
Similar observation of reduction in bacterial load due to increased number of SCC and cytokines secreted from inflammatory cells in response to biological response modifier were reported (Trebichevsky et al. 1993; Salinas-Carmona et al. 1999; Eisenblatter et al. 2002). It has been explained earlier that in response to bacterial stimuli the leukocytes travel into the infected udder from central pool. The effect is accentuated by some proinflammatory cytokines which are considered to induce migration of leukocytes into the udder (Person Waller et al. 2003; McClenahan et al. 2006). In such instances during migration the primed PMNs gain activation for phagocytosis and clear up the intramammary infection.
Significant down-regulation in the gene expression of IL-1, IL-6, IL-8 and IFN-γ on day-28 post-treatment with P. juliflora alkaloids indicated the importance of these cytokines in the prognosis and monitoring disease progression after treatment of bovine sub-clinical mastitis.
Advancement of such therapeutic protocol can help in averting acquired drug resistance of microbes and presence of antibiotic residue in the milk that has been associated with the use of conventional antibiotics in the treatment of bovine mastitis.
Conclusion
The study involved alkaloids separated from the chloroform extract of leaves of P. juliflora to be intramammarily infused in the infected udder quarters included in the study. The treatment efficacy was evaluated in terms of bacterial cultural examination, somatic cell count and their cytokine profile was compared with that of disease control group by real-time PCR. The ultimate objective was to address the issue of increasing multi-drug resistance microbes. Simultaneously, the study can be demonstrated as a pilot in a village in India, where normally P. juliflora is observed to grow in abundance in wasteland. The farmers can be trained for preparation of this extract and they can treat their cattle from bovine sub-clinical mastitis. This would also help in reduction of economic loss due to restoration of milk quality and reducing expense incurred in antibiotic treatment, thereby elevating farmer income which is critical for balanced socio-economic and political stability of any country. From medical biotechnological perspective, down-regulation of IL-1, IL-6 and IL-8 cytokines in treated quarters can be exploited by pharmaceutical companies for making the prognosis and monitoring kits which will be very well accepted among farmers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to Government of India’s Rashtriya Krishi Vikas Yojana (RKVY) Stream-I Project (B.H. 18442) for providing financial support. KS is appreciative of Dr. Vaibhav Bhatt, Dr. Pradeep Kumar Sharma, Dr. Maulik Upadhyay and Dr. Kamlesh Sadaria for the invaluable interactions. KS expresses his gratitude towards the milkers of Livestock Research Station of Anand Agricultural University for extending their support during the entire study.
Funding
This study was funded by Rashtriya Krishi Vikas Yojana (RKVY) (Stream-I), Government of India. Money earmarked for RKVY projects is given to Government of Gujarat, which is allocated to different universities by former. The funds were allocated by Anand Agricultural University under the Budget Head (B.H.) 18442.
Ethical approval
Based on the results of the acute dermal and oral toxicity test carried out on male Wistar rats (as per OECD New Draft Guideline 434) the use of P. juliflora alkaloids was approved by the Institution Animal Ethics Committee (IAEC 2007/VPT/26). All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
Prof. Dr. Dev S. Nauriyal has received research grant from Rashtriya Krishi Vikas Yojana (RKVY) (Stream-I), Government of India, as Principal Investigator for project entitled “Use of Recent Molecular Techniques to Reduce Economic Losses Incurred due to Bovine Mastitis by Evolving Diagnostic, Therapeutic and Preventive Measures” under the B.H. 18442. Prof. Dr. C. G. Joshi is Co-PI in this project. The authors declare that they have no conflict of interest.
References
- Ahmad VU, Basha A, Haque W. New alkaloids from Prosopis juliflora. Z Naturforsch. 1978;33:347–348. doi: 10.1515/znb-1978-0322. [DOI] [Google Scholar]
- Ahmad A, Khan KA, Ahmad VU, Qazi S. Antibacterial Activity of juliflorine isolated from Prosopis juliflora. Planta Med. 1986;4:285–288. doi: 10.1055/s-2007-969153. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Khursheed AK, Sabiha Q, Viqaruddin A. Antifungal activity of some hydrosoluble Prosopis juliflora alkaloids. Fitoterapia. 1989;60:86–89. [Google Scholar]
- Ahmad VU, Sultana A, Qazi S. Alkaloids from the leaves of Prosopis juliflora. J Nat Prod. 1989;5:497–501. doi: 10.1021/np50063a005. [DOI] [Google Scholar]
- Ahmad A, Khan KA, Ahmad V. Immunomodulating effect of juliflorine on the antibody response to Listeria hemolysin. J Islam Acad Sci. 1992;5:189–193. [Google Scholar]
- Alluwaimi AM. The cytokines of bovine mammary gland: prospects for diagnosis and therapy. Res Vet Sci. 2004;77:211–222. doi: 10.1016/j.rvsc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Alluwaimi AM, Cullor JS. Cytokines gene expression patterns of bovine milk during mid and late stages of lactation. J Vet Med B. 2002;49:105–110. doi: 10.1046/j.1439-0450.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- Aqeel A, Khursheed AK, Viqaruddin A, Sabiha Q. Antimicrobial activity of julifloricine isolated from Prosopis juliflora, Arzneim. Forsch Drug Res. 1989;39:652–655. [PubMed] [Google Scholar]
- Aziz MA, Khan AH, Adnan M, Ullah H. Traditional uses of medicinal plants used by indigenous communities for veterinary practices at Bajaur Agency, Pakistan. J Ethnobio Ethnomed. 2018;14:11. doi: 10.1186/s13002-018-0212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal BK, Gupta DK. Economic analysis of bovine mastitis in India and Punjab—a review. Indian J Dairy Sci. 2009;62:337–345. [Google Scholar]
- Bansal BK, Bajwa NS, Randhawa SS, Ranjan R, Dhaliwal PS. Elimination of erythromycin in milk after intra-mammary administration in cows with specific mastitis: relation to dose, milking frequency and udder health. Trop Anim Health Prod. 2011;43:323–329. doi: 10.1007/s11250-010-9692-1. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Chhabra SC, Shao JF, Mshiu EN. Antifungal activity among traditionally used herbs in Tanzania. Dar-es-Salam Med J. 1992;9:68–73. [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- Cowan ST, Steel JK. Manual for the identification of the medical bacteria. 3. Cambridge: Cambridge University Press; 1970. [Google Scholar]
- Daetwyler P, Ott-Longoni R, Schöpp E, Hesse M. Juliprosine, a further alkaloid from Prosopis juliflora A. DC. Helv Chim Acta. 1981;64:1959–1963. doi: 10.1002/hlca.19810640629. [DOI] [Google Scholar]
- Dallman MJ, Montgomery RA, Larsen CP, Wanders A, Wells AF. Cytokine gene expression: analysis using northern blotting, polymerase chain reaction and in situ hybridization. Immunol Rev. 1991;119:163–179. doi: 10.1111/j.1600-065X.1991.tb00583.x. [DOI] [PubMed] [Google Scholar]
- De UK, Mukherjee R. Dynamics of milk leukocytes in response to a biological response modifier during bovine subclinical mastitis. Res Vet Sci. 2013;95:352–357. doi: 10.1016/j.rvsc.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Eisenblätter M, Buchal A, Gayum H, Jasny E, Renner Viveros P, Ulrichs T, Schneider T, Schumann RR, Zweigner J, Ignatius R. Nocardia farcinica activates human dendritic cells and induces secretion of interleukin-23 (IL-23) rather than IL-12p70. Infect Immun. 2012;80:4195–4202. doi: 10.1128/IAI.00741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyera T, Mekonnen E, Wakayo BU, Assefa S. Botanical ethnoveterinary therapies used by agro-pastoralists of Fafan Zone, Eastern Ethiopia. BMC Vet Res. 2017;13:232. doi: 10.1186/s12917-017-1149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DD, Rimondi A, Perez Aguirreburualde MS, Mozgovoj M, Bellido D, Wigdorovitz A, Dus Santos MJ. Quantitation of cytokine gene expression by real-time PCR in bovine milk and colostrum cells from cows immunized with a bovine rotavirus VP6 experimental vaccine. Res Vet Sci. 2013;95:703–708. doi: 10.1016/j.rvsc.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Gonzalo C, Martinez JR, Carriedo JA, Primitivo FS. Fossomatic cell-counting on ewe milk: comparison with direct microscopy and study of variation factors. J Dairy Sci. 2003;86:138–145. doi: 10.3168/jds.S0022-0302(03)73593-0. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Satoshi K, Yamanaka H, Kirisawa R, Iwai H. Detection of cytokines in bovine colostrum. Vet Immunol Immunopathol. 2000;76:183–190. doi: 10.1016/S0165-2427(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology. 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- International Dairy Federation Bovine mastitis. Definition and guidelines for diagnosis. Brussels, Belgium. IDF Bull. 1987;211:24. [Google Scholar]
- Kabilan L, Andersson G, Lolli F, Ekre HP, Olsson T, Troye-Blomberg M. Detection of intracellular expression and secretion of interferon-gamma at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur J Immunol. 1990;20:1085–1089. doi: 10.1002/eji.1830200521. [DOI] [PubMed] [Google Scholar]
- Leutenegger CM, Alluwaimi AM, Smith WL, Perani L, Cullor JS. Quantitation of bovine cytokine mRNA in milk cells of healthy cattle by real-time Taq Man polymerase chain reaction. Vet Immunol Immunopathol. 2000;77:275–287. doi: 10.1016/S0165-2427(00)00243-9. [DOI] [PubMed] [Google Scholar]
- McClenahan D, Krueger R, Lee HY, Thomas C, Kehrli ME, Jr, Czuprynski C. Interleukin-8 expression by mammary gland endothelial and epithelial cells following experimental mastitis infection with E. coli. Comp Immunol Microbiol Infect Dis. 2006;29:127–137. doi: 10.1016/j.cimid.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Ram GC, Dash PK, Goswami T. The activity of milk leukocytes in response to a water-soluble fraction of Mycobacterium phlei in bovine sub-clinical mastitis. Vet Res Commun. 2004;28:47–54. doi: 10.1023/B:VERC.0000009531.25317.5d. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, De UK, Ram GC. Evaluation of mammary gland immunity and therapeutic potential of Tinospora cordifolia against bovine subclinical mastitis. Trop Anim Health Prod. 2010;42:645–651. doi: 10.1007/s11250-009-9471-z. [DOI] [PubMed] [Google Scholar]
- Nagao S, Masayuki N, Hiroshi K, Katsuro Y, Shoichi K, Tetsuo S, Atsushi T, Shozo K, Nakano H, Nakajima E, Hiradate H, Fujii Y, Yamada K, Shigemori H, Hasegawa A. Macrophages are stimulated by muramyl dipeptide to induce polymorphonuclear leukocyte accumulation in the peritoneal cavities of guinea pigs. Infect Immun. 1990;58:536–542. doi: 10.1128/iai.58.2.536-542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Nakajima E, Hiradate H, Fujii Y, Yamada K, Shigemori H, Hasegawa A. Growth inhibitory alkaloids from mesquite (Prosopis julispora (Sw.) DC.) leaves. Phytochemistry. 2004;65:587–591. doi: 10.1016/j.phytochem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Paape M, Mehrzad J, Zhao X, Detilleux J, Burvenich C. Defense of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J Mammary Gland Biol Neoplasia. 2002;7:109–121. doi: 10.1023/A:1020343717817. [DOI] [PubMed] [Google Scholar]
- Person Waller K, Colditz IG, Lun S, Ostensson K. Cytokines in mammary lymph and milk during endotoxin-induced bovine mastitis. Res Vet Sci. 2003;74:31–36. doi: 10.1016/S0034-5288(02)00147-9. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:E45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Wittmann SL, Meyer HH, Bruckmaier RM. Gene expression of immunologically important factors in blood cells, milk cells, and mammary tissue of cows. J Dairy Sci. 2003;86:538–545. doi: 10.3168/jds.S0022-0302(03)73632-7. [DOI] [PubMed] [Google Scholar]
- Salinas-Carmona MC, Torres-Lopez E, Ramos AI, Licon-Trillo A, Gonzalez-Spencer D. Immune response to Nocardia brasiliensis antigens in an experimental model of actinomycetoma in BALB/c mice. Infect Immun. 1999;67:2428–2432. doi: 10.1128/iai.67.5.2428-2432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha Mukesh Kr., Thombare N. N., Mondal Biswajit. Subclinical Mastitis in Dairy Animals: Incidence, Economics, and Predisposing Factors. The Scientific World Journal. 2014;2014:1–4. doi: 10.1155/2014/523984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MG, De Spiegeleer B, Peelman L, Boulougouris XJ, Capuco AV, Burvenich C. Compromised neutrophil function and bovine E. coli mastitis: is C5a the missing link? Vet Immunol Immunopathol. 2012;149:151–156. doi: 10.1016/j.vetimm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Trebichavsky I, Barot-Ciorbaru R, Charley B, Splíchal I. Induction of inflammatory cytokines by Nocardia fractions. Folia Biol. 1993;39:243–249. [PubMed] [Google Scholar]
- Verdi RJ, Barbano DM. Properties of proteases from milk somatic cells and blood leucocytes. J Dairy Sci. 1991;74:2077–2081. doi: 10.3168/jds.S0022-0302(91)78379-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.