Abstract
The L-type pyruvate kinase (L-PK) gene is regulated by diet and hormones and expressed at high levels in the hepatocytes, enterocytes, and proximal tubular cells of the kidney and at low levels in the endocrine pancreatic cells. Two regulatory regions have been shown to be important in transgenic mice to confer on a reporter gene a similar tissue-specific and diet-responsive expression: a proximal promoter fragment, with binding sites for the tissue-specific hepatocyte nuclear factors 1 and 4, and presence of the glucoseresponse element (G1RE) and a distal activator corresponding to a liver-specific hypersensitive site at −3000 bp with respect to the cap site. Although the proximal promoter is able to confer by itself tissue-specific expression on a reporter gene, its activity in vivo is strongly stimulated by the distal activator. To determine the possible role of the distal region on diet responsiveness and tissue specificity of the L-PK gene expression, we have created lines of transgenic mice in which the gene for SV40 T antigen (Tag) was directed by composite regulatory sequences consisting of the L-PK promoter and different enhancers: either the SV40 early enhancer (SV) or the H enhancer of the aldolase A gene (H). The induction of the composite H-PK/Tag and SV-PK/Tag transgenes by a carbohydrate-rich diet in the liver was similar to that of the endogenous L-PK gene. This suggests that in fasted mice the L-PK promoter, and especially the G1RE, is able to silence the activating influence of a strong viral enhancer such as the SV40 enhancer. The H-PK/Tag mice expressed the transgene similarly to the endogenous gene, except in the pancreas, where expression was practically undetectable. Consistently, whereas L-PK/Tag mice develop insulinomas, H-PK/Tag mice develop only hepatomas. In contrast, the transgene expression was partly aberrant in SV-PK/Tag mice. In addition to a normal activation of the transgene in the liver, a strong expression was also detected in the kidney medulla, whereas the transgene was practically silent in enterocytes. Finally, the effect of the distal region (−2070 to −3200) on an ubiquitous promoter was tested by ligating the distal L-PK gene fragment in front of a thymidine kinase/CAT transgene. Such a transgene was constantly expressed in the pancreas and, strikingly, in the brain. It appears, therefore, tha t the L-PK distal activator exhibits, by itself, a certain neuropancreatic specificity required in combination with the proximal promoter for L-PK gene expression in pancreas endocrine cells.
Keywords: L-type pyruvate kinase gene, Nutrient gene regulation, Targeted carcinogenesis, Transgenic mice, Tissue-specific expression
BOTH promoters and enhancers, by themselves and in combination, can be involved in gene tissue-specific expression. The L-type pyruvate kinase (L-PK) gene offers the particular example of a gene controlled by two different pairs of promoter/distal region combination, one specific to the hepatocytes, enterocytes, and kidney tubular cells, and the other to erythroid cells (28,39).
Recently, it was shown that the enhancer of the erythroid pair was itself erythroid specific and was able to specifically enhance the activity of the L′ erythroid-specific promoter (33). Results from DNase 1 hypersensitivity analysis (3), transient transfection assays in hepatocytes or hepatoma cells (9), and creation of transgenic mouse lines (3,14,43) have shown that a 183-bp proximal promoter of the L-PK gene, called promoter L, was sufficient to direct a tissue-specific expression of a transgene into the liver and liver cells, kidney, and enterocytes, and to confer a responsiveness to diet and hormones (15); however, in transgenic mice this L promoter was stimulated 10- to 50-fold by the presence of a distal fragment spanning from −2070 to −3200 bp upstream of the L start site of transcription. This fragment contains a distal liver-specific hypersensitive site termed HSS2.
In addition, we have shown that a hybrid trans-gene, in which the SV40 large T and small t sequences are put under the direction of the L regulatory regions of the L-PK gene included in a 3.2-kb upstream fragment, leads to development of malignant insulinomas, due to the fact that the L-PK gene is expressed at a relatively low level in endocrine pancreas (7). As a matter of fact, the L-PK gene can also be expressed in fetal and cancerous exocrine pancreas (38).
Tissue specificity of the proximal L promoter is related to binding of two tissue-enriched factors, hepatocyte nuclear factor 1 (HNF1) and hepatocyte nuclear factor 4 (HNF4) (47). In the pancreas, the level of L-PK gene expression parallels the level of HNF4 in the different cells at different stages of development or malignant transformation (38). Up to now, our experiments have provided no information on the putative contribution of the distal (−2070/ −3200) activating element on tissue specificity of L-PK gene expression. To address this question we used two types of strategies in transgenic mice: i) replacing the L distal element by ubiquitous enhancers from viral or cellular origin, and ii) testing the influence of the distal region on the expression of a reporter chloramphenical acetyl transferase (CAT) gene directed by a truncated Herpes simplex virus thymidine kinase (HSV-TK) ubiquitous promoter.
From our results, we can conclude that the distal element, in addition to its stimulatory effect on the activity of the L promoter, is mainly involved in the pancreatic specificity of the transgenes. The proximal promoter can dictate its tissue specificity and dietary regulatory properties over ubiquitous enhancers. However, the strong SV40 enhancer can widen this specificity to new tissues and alter the expression in enterocytes.
MATERIALS AND METHODS
Construction of the Transgenes
A plasmid containing 2.7 kb of the coding sequence of the SV40 Large T and little t antigens placed behind the regulatory sequences of the rat L-type pyruvate kinase gene (−3200 to +700 pb) has been previously described (L-PK/Tag) (7,10). We realized hybrid constructs containing either the 72-bp repeats of the SV40 enhancer spanning from position 95 to 270, inserted into the ClaI site (−1004) of the L-PK/Tag construct (SV-PK/ Tag), or the enhancer H of the human aldolase A gene, extending from position +2610 to +3100 (11), and subcloned into the ClaI site (−1004) of the L-PK/Tag construct (H-PK/Tag). The insertion of both ubiquitous enhancers was preceded by the deletion of a EcoRI-ClaI L-PK fragment spanning from −3200 to −1004.
A second group of hybrid constructs was performed from the plasmid pBLCAT2 containing the HSV-TK promoter (nt −105 to +51) placed in front of the coding sequence of the chloramphenicol acetyl transferase gene (TK/CAT) (36). The L-PK fragment containing the distal hypersensitive site HSS2 (3) spanning from −3200 to −2070 nt was subcloned in both orientations into the XbaI site of the pBLCAT2 plasmid (HSS2-TK/CAT, HSS2b-TK/CAT).
Obtention and Detection of Transgenic Animals
Plasmids SV-PK/Tag and H-PK/Tag were digested by EcoRI and PvuI, whereas all constructs TK/CAT or HSS2TK/CAT were digested by HindIII and ClaI. The restriction fragments containing the chimeric constructs were purified on Elutip-d columns (according to the instructions of the supplier Schleicher & Schuell), and microinjected into fertilized mouse eggs by the method reported by Tremp et al. (43). Transgenic mice were detected by Southern blot analysis of 10 pg of DNA isolated from tails of 2-week-old mice, digested by appropriate restriction enzymes and hybridized with specific probe, SV40 T and t antigens fragment (2700 bp) for the PK/Tag contructs and a CAT fragment (1500 bp) for the other transgenes.
Total RNA Isolation, Northern Blot, and RT-PCR Amplification Analyses
Total RNA were isolated from homogenized tissues using the guanidium thiocyanate procedure (8). Total RNA (10 μg) was electrophoresed on 1.5% (w/v) agarose-formaldehyde gels, transferred to nylon membranes, probed, and washed as previously described (7).
First strand cDNA synthesis and PCR amplification were performed as previously described (38). The sequences of synthetic oligonucleotides used in PCR amplification experiments are: 5′-GAACACTCTACCCCAGCAGC-3′, reverse primer identical to a segment of the erythroid-specific PK first exon; 5′-GCAACGTAGCAGCATGG AAG-3′, reverse primer identical to a segment of the liver-specific PK first exon; and 5′-GCATCCCAGAAGCCTCCAAAG-3′, forward primer complementary to a segment of the SV40 T antigen. Oligonucleotides specific to the rat β-actin gene were used as internal standard (38). Amplification products were run on 8% (w/v) polyacrylamide gel (39:1), transferred onto a nylon membrane, and hybridized with radiolabeled specific oligonucleotides.
Immunocytochemical Studies
The SV40 large T antigen was immunolocalized on frozen tissue sections by using a specific rabbit polyclonal antibody (kindly provided by Dr. D. Hanahan, University of California, San Francisco). Kidneys, pancreas, livers, and midportions of the intestine from the different strains of trans-genic mice were dissected, cut into small pieces, and rapidly frozen on a plastic block precooled with liquid nitrogen. Tissues were then kept at −80°C before use. Frozen sections (7 μm) were cut with a cryostat (Bright), placed on gelatin-treated slides, and processed for immunofluorescence. Sections were fixed with 2% (v/v) para-formaldehyde for 10 min at room temperature, incubated with 0.25% (v/v) nonidet P-40 (Boeh-ringer), rinsed in phosphate-buffered saline (PBS), and incubated with the anti-large T antigen polyclonal antibody (dilution 1:2000) for 18 h at room temperature. After washing, specimens were incubated with biotinylated anti-rabbit IgG antibody (dilution 1:200, Vector) and streptavidin-fluorescein (dilution 1:200, Vector). Preparations were examined under a Zeiss photomicroscope equiped with epifluorescence optics.
CAT Assays
Tissues samples were treated as described by Cuif et al. (14), and CAT activity was assayed by standard methods (22).
RESULTS
Expression of the Different T Antigen Transgenes: RNA Analyses
Figure 1 summarizes the different features of the lines harboring three types of SV40 T antigen (Tag) transgenes directed either by the 3.2 kb of L-PK upstream regulatory sequence (L-PK/Tag) or by composite regulatory elements consisting of about 1 kb of L-PK upstream regulatory sequence in front of which were ligated two different types of ubiquitous enhancer: the enhancer H of the aldolase A gene (11) (H-PK/Tag) or the SV40 enhancer (SV-PK/Tag). Two lines of L-PK/Tag mice and three lines of the other two types of transgenic mice were obtained, with either a high number (≈ 20) or a low number (2–4) of integrated copies.
FIG. 1.
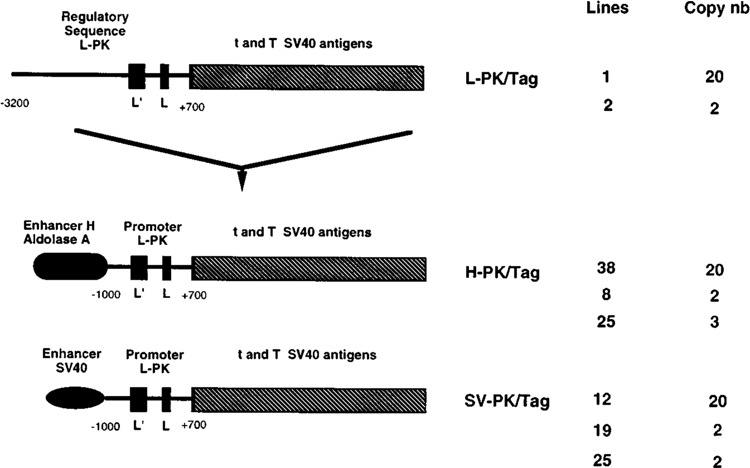
Structure of the hybrid pyruvate kinase transgenes. The L-PK/Tag transgene was composed of 2.7 kbp of the SV40 t and T antigens sequences (hatched rectangles) directed by 3.2 kbp of 5′ flanking regions of the rat L-PK gene (7,10). The black rectangles represent the erythroid (L′) and liver (L) specific exons of the L-PK gene. The H-PK/Tag and SV-PK/Tag were created by excision of 2.2 kbp of L-PK regulatory sequences between −3200 and −1000 nt of the L-PK/Tag clone. This region was replaced by enhancer H of aldolase A (H-PK/Tag) or SV40 enhancer (SV-PK/Tag). The designation of each mouse line and corresponding copy number are given on the right part of the figure.
Figure 2 compares Tag mRNA accumulation in the different types of transgenic mice with high or low transgene copy number. Transgene expression in the liver, kidney, and small intestine was similar in L-PK/Tag and H-PK/Tag lines, characterized by a strong mRNA accumulation in the liver, and a smaller accumulation in the kidney and small intestine (Fig. 2A); the slight variations in the ratio of mRNA abundance observed in these tissues between the different lines were probably due to some weak positional effect.
FIG. 2.

Northern blot analysis of transgene expression in various tissues. Northern blot analyses were performed with 10 μg of total RNAs from the liver, kidney, intestine, and spleen of transgenic mice fed a high-carbohydrate diet. The membranes were successively hybridized with Tag specific probe and with rRNA (18S) probe (R45) used as a standard (12). Mouse lines are indicated above the panels: (A) L-PK/Tag and H-PK/Tag lines and (B) L-PK/Tag and SV-PK/Tag lines.
The SV-PK/Tag transgene was also expressed in the liver, kidney, and small intestine (Fig. 2B), indicating that specificity was roughly conserved because it was not expressed in the brain (Fig. 3) and heart (not shown). However, mRNA abundance in the kidney was regularly stronger than that in the liver and was very low in the small intestine. In addition, some Tag mRNA was detected in the spleen in variable amount according to the lines, at a specially high level in SV-PK/ Tagl9 mice (Fig. 2B). Because all of the transgenes contained the erythroid-specific L′ promoter, which could be stimulated by upstream ubiquitous enhancers, we differentiated L and L′ transcripts (i.e., transcripts containing either exon L′ or exon L) by polymerase chain reaction (PCR) (Fig. 3). The only transcripts detected in spleen were of the L′ type; they were detected in all lines by reverse transcriptase PCR whereas Northern blot, a much less sensitive method, detected them in SV-PK/Tag mice and, at a lower level, in H-PK/Tag mice. Accumulation of the L′-Tag transcripts in spleen from SV-PK/Tag mice is most likely explained by the effect of the SV40 enhancer on the L′ promoter in erythroid cells (33). Surprisingly, both L′ and L transcripts were also detected by PCR in the thymus of SV-PK/ Tag mice, but not in L-PK/Tag (Fig. 3) or H-PK/ Tag (not shown) mice.
FIG. 3.

RT-PCR analysis of L and L′-PK/Tag transcripts in the different types of transgenic mice. Total RNAs from 500 ng of various tissues were used for reverse transcriptase PCR amplifications. The L-PK-Tag amplified fragment is 490 bp long and the L′-PK-Tag amplified fragment is 550 bp long. The mouse β-actin specific amplified fragment is 241 bp long and served as standard. Southern blotting of poly-acrylamide gel and hybridization were described in the Materials and Methods section.
PCR analyses of the L transcripts showed clearly that mRNA accumulation in the pancreas of L-PK/Tag mice was higher than in the pancreas of H-PK/Tag and SV-PK/Tag mice (Fig. 3), suggesting that neither the H enhancer nor the SV40 enhancer is as efficient as the L-PK distal activating element in sustaining expression in the pancreas. Another difference between specificity conferred by the different activators used concerned the intestine: expression of the endogenous gene (38) as well as of the L-PK/Tag transgene is very weak in the colon compared to the duodenum and jejunum. In contrast, the H enhancer seemed to disturb this gradient of expression with the same level of expression in the colon and in the jejunum.
Figure 4 compares by Northern blot analysis mRNA accumulation in the liver, kidney, and small intestine of the different types of transgenic mice either fasted for 24 h or fasted then refed a carbohydrate-rich (75% carbohydrates) diet for 16 h. Neither H nor SV40 enhancers disturbed diet responsiveness of the transgene, whose expression was strongly stimulated in refed mice. In the spleen from a SV-PK/Tag mice, the abundance of Tag mRNA was insensitive to the carbohydrate-rich diet, as expected because the transcripts are synthesized under the control of the erythroid-specific L′ promoter, which is glucose insensitive (3).
FIG. 4.

Northern blot analysis of transgene dietary regulation. Northern blot analyses were performed with 10 μg of total RNAs from liver, kidney, intestine, and spleen of transgenic mice fasted for 24 h (F) or refed a high-carbohydrate diet (75% of glucides) (R). We used 2.7 kb of SV 40 T-antigen sequences to reveal the transgene expression (t, T antigens) and the 18S R45 probe as a standard.
In Situ Immunofluorescence Analysis of H-PK/ Tag and SV-PK/Tag Transgene Expression
The results from Northern blot and RT-PCR experiments clearly showed that the levels of Tag transcripts widely differed from one line of transgenic mice to another. To define the expression of the transgenes at the cell level, indirect immunofluorescence studies were undertaken on frozen tissue sections taken from 2–4-month-old transgenic mice fed a high carbohydrate-rich diet. For this purpose, a polyclonal antibody raised against large T antigen (26) was used to detect T antigen positivity in the nuclei.
The livers of L-PK/Tag1, H-PK/Tag38, and SV-PK/Tag12 transgenic mice (Fig. 5A–C) exhibited a positive nuclear staining. All nuclei were labeled with antibody raised against the T antigen and no gradient in labeling intensity was observed between periportal and centrolobular areas (Fig. 5A-C). However, nuclei from hepatocytes of H-PK/Tag transgenic mice appeared abnormal (Fig. 5B), suggesting thus that rapid transformation process occurred in the liver of these animals.
FIG. 5.

Nuclear T antigen immunolocalization in livers and intestines from transgenic mice. Frozen sections from livers (A-C) and small intestines (D-I) of L-PK/Tag1 (A, D, G), H-PK/Tag38 (B, E, H), and SV-PK/Tag12 (C, F, I) transgenic mice were processed for the immunodetection of Tag using a specific polyclonal antibody as described in the Materials and Methods section. In the three lines of transgenic mice, 100% of liver cells exhibited a nuclear large T antigen positivity (A-C). Note the presence of numerous mitotic cells in the liver of H-PK/Tag transgenic mice (C). In the small intestine the pattern of labeling differed in the three lines of transgenic mice. In both L-PK/Tag1 and H-PK/Tag38 transgenic mice, an intense nuclear labeling was observed in enterocytes lining the crypt/villus axis (D, E, G, H). In contrast, the nuclear labeling was almost not detectable in small intestine sections of SV-PK/Tag12 transgenic mice (F, I)). Bars = 50 μm.
Important differences in nuclear labeling were observed in the small intestine. An intense nuclear labeling was detected in enterocytes lining the crypt/villus axis of the intestine of the L-PK/Tag transgenic mice (Fig. 5D, G). A similar pattern of the nuclear Tag labeling was found in the small intestine of H-PK/Tag transgenic mice (Fig. 5E, H). In contrast, the nuclear labeling was almost not detectable in enterocytes from SV-PK/Tag transgenic mice (Fig. 5F, I). In all lines, we could also detect a positive staining in cells present in the central areas of the villus walls (Fig. 5G-I).
Differences in the pattern of nuclear labeling were also observed in kidneys and pancreas of these transgenic mice. As shown in Fig. 6A-C, nuclei of all proximal tubule cells of the kidney cortex were labeled. Glomeruli, distal, and cortical collecting tubules were not stained. Tubule cells from kidney medulla sections of L-PK/Tag (Fig. 6D) and H-PK/Tag (Fig. 6E) were not stained, indicating thus that ascending and descending limb cells as well as medullary collecting tubule cells did not exhibited L-PK/Tag transcripts. In contrast, an intense nuclear labeling was detected on medulla sections from the ascending limb of SV-PK/Tag transgenic mice. All nuclei of small tubule sections, corresponding to ascending limb of Henle’s loop, were heavily stained (Fig. 6F). A discrete staining was also detected in some nuclei of large tubule sections, corresponding to medullary collecting tubules (Fig. 6F).Thus, these results suggested that the SV40 enhancer was responsible for the activation of the transgene in the more distal segments of the nephron, normally lacking of the L-PK gene expression (17).
FIG. 6.

Nuclear T antigen immunolocalization in kidneys and pancreas from transgenic mice. Frozen sections from kidney cortex (A-C), kidney medulla (D-F), and pancreas (G-I) of L-PK/Tag1 (A, D, G), H-PK/Tag38 (B, E, H), and SV-PK/Tag12 (C, F, I) transgenic mice were processed for the immunodetection of Tag using a specific polyclonal antibody as described in the Materials and Methods section. In kidney cortex the nuclear labeling was restricted to the proximal tubule cells in the three lines of trangenic mice (A, B, C). Glomeruli (arrowheads) and distal tubules (arrows) were not stained. No labeling was detected in the kidney medulla of L-PK/Tag1 (D) and H-PK/Tag38 (E) transgenic mice. In contrast, the nuclei from most tubules sections were labeled in kidney medulla of SV-PK/Tag12 mice (F). The pattern of labeling also varied in the pancreas from the various lines of transgenic mice. In L-PK/Tag1 mice (G), all nuclei from exocrine and endocrine (arrowhead) pancreas were labeled. Note that the nuclear labeling was more intense in the endocrine than in the exocrine pancreatic cells. Endocrine pancreatic cells from both H-PK/Tag38 (H) and SV-PK/Tag12 (I) transgenic mice also presented a positive nuclear labeling (arrowheads), whereas exocrine pancreatic cells were almost not labeled (H, I). Bars = 50 μm.
Analyses of the pattern of Tag labeling in the pancreas also showed differences between the three lines of transgenic mice. Endocrine and exocrine pancreatic cells were labeled in L-PK/Tag transgenic mice (Fig. 6G), with a more intense nuclear labeling in the former than in the latter. By contrast, exocrine pancreatic cells were almost not stained in H-PK/Tag (Fig. 6H), or in SV-PK/Tag transgenic mice (Fig. 6I). In these lines, pancreatic endocrine cells remained labeled in their nuclei (Fig. 6H, I).
Taken altogether, these results showed that expression of the transgene was maintained in hepatocytes, enterocytes, proximal tubule kidney cells, and endocrine pancreatic cells from L-PK/Tag transgenic mice fed a carbohydrate-rich diet, well correlated with the levels of Tag transcripts detected by Northern blot and RT-PCR analyses. The presence of distal enhancer H in H-PK/Tag transgenic mice did not affect the cellular and tissular distribution of Tag, except that exocrine pancreatic cells remained poorly labeled or not labeled at all. The most stricking differences were observed in SV-PK/Tag line of transgenic mice. In these mice, the expression of Tag was blunted in enterocytes and exocrine pancreatic cells and unexpectedely demasked in cells of the thick ascending limbs and medullary collecting tubules.
Tumor Development in Transgenic Mice Expressing the Tag Transgenes
In a previous report, we showed that L-PK/ Tag1 mice fed a carbohydrate-rich diet developed mainly malignant, aggresive insulinomas and, when the animals survived for a sufficient time, well-differentiated hepatomas; these results are recalled in Table 1. L-PK/Tag2 mice, with a smaller number of integrated copies, developed in most cases (90%) hepatomas after 1 year of age, and only occasional endocrine pancreas hyperplasia. Because the expression of the L-PK gene and transgene in the pancreas is intrinsically low compared to the liver, and the expression of L-PK transgenes is constantly related to the number of integrated copies (3,7,14,33,43), it could be that the abundance of Tag in the pancreas endocrine cells of L-PK/Tag2 mice is lower than a threshold beyond which tumorigenesis is triggered.
TABLE 1.
NEOPLASIA IN HYBRID PK/Tag LINES OF TRANSGENIC MICE
| Lines | Copy Number | No. of Animals | Age of Death | Pathology | |||
|---|---|---|---|---|---|---|---|
| Endocrine Pancreatic Tumor | Hepato-carcinoma | Thynoma | Lymphoma | ||||
| L-PK/Tag1 | ∼20 | 31 | 6–8 | 23 (80%) | 6(15%) | 0 | 0 |
| L-PK/Tag2 | 4 | 14 | 12–16 | 0* | 12 (90%) | 0 | 0 |
| H-PK/Tags8 | 2 | 24 | 14-? | 0 | 0 | 0 | 0 |
| H-PK/Tag25 | 3 | 35 | 14-? | 0 | 0 | 0 | 0 |
| H-PK/Tag38 | ∼20 | 10 | 6–8 | O† | 10(100%) | 0 | 0 |
| SV-PK/Tag12 | ∼20 | 76 | 4–5 | o† | 0 | 76(100%) | 22 (30%) |
| SV-PK/Tag19 | 2 | 14 | 7–8 | 0 | 0 | 13 (89%) | 10(80%) |
| SV-PK/Tag25 | 4 | 13 | 6.5–9 | 0 | 0 | 13(100%) | 9 (60%) |
In some cases, these mice developed endocrine pancreatic hyperplasia.
20% of these animals developed pancreatic adenoma.
This threshold effect probably explains that even high copy number H-PK/Tag38 and SV-PK/ Tag12 mice, whose transgene expression was low in the pancreas, did not develop insulinomas.
A similar explanation can be proposed to explain that hepatomas develop in H-PK/Tag38 mice, with a high copy number, whereas the mice harboring a low copy number of the same transgene remained free of hepatoma for more than 14 months.
In line with the ectopic expression of the Tag transgene in the thymus of SV-PK/Tag mice, these animals develop aggressive thymomas, lethal before the fifth month in high copy number mice and the eighth month in low copy number mice. In addition, B lymphomas are observed in these animals (de Ribains et al., in preparation).
As a whole, these results confirm our data that transgenes lacking the L-PK distal activating element encompassed into the −2070/−3200 fragment are less expressed in the pancreas than the transgene with the extended natural L-PK regulatory sequence, and, therefore, suggest that this L-PK distal element could be endowed with an intrinsic pancreas specificity. In addition, our data also confirm the well-established phenomenon of tissue specificity of the oncogene action (25,40): although Tag expression in SV-PK/Tag mice is maximal in the kidney, these animals presented no or only minimal kidney abnormalities. In contrast, despite a much lower Tag expression in the thymus than in the kidney, SV-PK/Tag mice developed thymomas. Although L-PK/Tag1 mice accumulated much more Tag in the liver than in the pancreas endocrine cells, they nevertheless develop insulinomas before hepatomas (7).
Effect of the L-PK Distal Activating Sequence on Activity of the Ubiquitous —105 TK Promoter in Transgenic Mice
Our results suggested that the activating −2070/−3200 L-PK distal element was important for the expression in the pancreas in which it could not be fully replaced by strong ubiquitous enhancers. In addition, we had previously established that a liver-specific DNase 1 hypersensitive site HSS2 was located in this fragment (3), which was able to stimulate strongly the activity of the L promoter in vivo (14,43) and ex vivo (9). To determine the intrinsic role of this element, we ligated it in both orientations in front of the – 105 HSVTK-CAT construct and several transgenic lines were obtained (Fig. 7A).
FIG. 7.

Tissue-specific expression of HSS2-TKCAT constructs in transgenic mice. (A) Structure of the HSS2-TK/ CAT hybrid gene. HSS2-TK/CAT and HSS2b-TK/CAT were obtained by the insertion of a 1111-bp-long fragment encompassing the HSS2 site in both orientation (hatched boxes), upstream of the thymidine kinase promoter from HSV spanning nt −105 to +51 and the CAT structural gene-SV40 poly(A) cassette (dot boxes). The designation of each mouse line and the corresponding copy number are given on the right part of the figure. (B) Tissue-specific expression of HSS2-TK/CAT constructs in transgenic mice. Samples from homogenates of the indicated organs assayed for CAT activity; 300 μg of proteins was used. The line numbers are indicated below each autoradiograph. Li, liver; P, pancreas; K, kidney; I, small intestine; S, spleen; B, brain; Lu, lung; C, cerebellum.
It is shown in Fig. 7B that the minimal TK/ CAT transgene was not at all expressed in three lines presented here. In fact, we obtained the same negative results for at least 10 similar lines created in our laboratory, without, in our series, any occurrence of enhancer trapping phenomenon [(33); Spitz, in preparation] leading to various types of expression (not shown).
In contrast, four out of the five HSS2-TK/ CAT lines, regardless of the orientation of the HSS2 element, synthesized CAT protein in the pancreas, and all of them synthesized CAT in the brain. Two lines exhibited a significant liver CAT expression, whereas CAT activity in the other tissues tested was either in the background level or was observed in only one line, therefore probably corresponding to a positional effect. In the pancreas, a CAT assay was performed in extracts from isolated islets of Langerhans and in the remaining pancreas tissue, showing similar activities (not shown). Similarly, CAT activity in different structures from microdissected brains was in the same range as shown in Fig. 7B in the cerebellum (lane C) of a line 10 mouse compared to nondissected mouse brain. Therefore, this analysis in transgenic mice confirmed the pancreas specificity conferred by the L-PK HSS2 distal element; in addition, unexpectedly, this fragment conferred a brain specificity on a ubiquitous promoter inactive by itself in transgenic mice.
DISCUSSION
Fine tuning of gene expression in different cell population at different stages of differentiation often requires cooperation between modular proximal promoters and distal enhancers. In the case of the L-type pyruvate kinase gene, we have previously shown that both tissue specificity and hormonal and dietary controls could be mainly ascribed to a 183-bp-long proximal promoter composed of four protein binding sites for, from 3′ to 5′: HNF1, nuclear factor 1 (NF1), HNF4, and upstream stimulatory factors (USFs) (16,46,47). Tissue specificity seems to be conferred by binding of HNF1 and HNF4, two factors enriched in the liver, proximal tubules of the kidney, and enterocytes (38), whereas the USF binding site is the glucose response element of the L-PK gene, required for both transcription activation by glucose and inhibition by cyclic AMP (2,16,35,45). NF1 appeared to be dispensable ex vivo (2) and in vivo (15).
Furthermore, a distal element located between −2070 and −3200 bp of the L start site of transcription and containing a liver-specific DNase 1 hypersensitive site, termed HSS2, has been shown to be necessary to stimulate in vivo, in transgenic mice, the activity of the L promoter by 10- to 50-fold (3,14,43).
The question was therefore raised of the contribution of this distal element to the complex specificity and regulation of the L-PK gene. To answer this question, we used two approaches: i) replacement of the L-PK distal element by strong but ubiquitous enhancers and looking at modifications of specificity and dietary response of trans-genes controlled by the L-PK promoter, and ii) cloning the L-PK distal element upstream of a minimal, ubiquitous promoter to evaluate its intrinsic influence on specificity and activity of this promoter in transgenic mice. In the first set of experiments, SV40 T antigen (Tag) was used as a reporter gene, such that it was not only possible to detect Tag mRNA and antigenicity in various organs, but also to correlate Tag expression with tumor development. Different conclusions can be drawn from our results.
The L-PK Promoter Is Able to Impose Most of its Tissue Specificity and Regulatory Properties to Strong Ubiquitous Enhancers
Although the enhancer H of the aldolase A gene is ubiquitous and particularly active in the heart, skeletal muscle, and actively dividing cells (12), the H-PK/Tag transgene was not at all expressed in these tissues and, in fact, exhibited mostly the same pattern of expression as the L-PK/Tag transgene. The SV40 enhancer/promoter was particularly active in choroid plexus, thymus, kidney, and skeletal muscle of transgenic mice (6,37), whereas the SV-PK/Tag transgene was not expressed in choroid plexus and skeletal muscle; however, expression of the transgene in kidney was quantitatively and qualitatively modified with respect to that of the L-PK/Tag transgene (see below). These results suggest that in tissues such as the heart, skeletal muscle, and brain, the L-PK promoter is not permissive for being activated by active enhancers, in spite of including a functional TATA box and binding sites for ubiquitous factors such as USF and NFL Two hypotheses, not mutually exclusive, can be proposed to explain this insensitivity of the L-PK promoter to the enhancer in most of the tissues in which the endogenous promoter is inactive: i) the HNF1 and HNF4 binding sites could play a positive m-acting role in tissues synthesizing these factors and a negative role in other tissues, and ii) in the absence of HNF1 and/or HNF4, the L-PK promoter could be intrinsically nonactivable. As a matter of fact, we have shown that HNF4 isobinders of the COUP-TF/ARP1/Ear family behave as inhibitors of the L-PK promoter when hyperproduced in hepatocytes (16); consequently, these ubiquitous factors could also contribute to turn off the L-PK promoter in tissues devoid of HNF4. In addition, Cognet et al. (9) have shown that the HNF1 binding site contributed to decrease the influence of the SV40 enhancer on the L-PK TATA box in transient transfection experiments in fibroblasts. These features rather argue for the first hypothesis. However, the influence of the SV40 enhancer in hepatocytes and hepatoma cells is also much more important on a −96-bp L-PK promoter fragment containing the TATA box and HNF1 binding site than on a −54 L-PK fragment containing only the TATA box (9); this indicated that a promoter binding HNF1 is much more activable by the SV40 enhancer than a promoter limited to the TATA box, in agreement with our second hypothesis.
It is also striking to observe that the presence of the strong ubiquitous enhancers upstream of the L-PK promoter did not alter dietary regulation, whereas the L-PK promoter appears by in vivo footprinting analysis to be charged with HNF1 and HNF4 regardless of the dietary and hormonal status (Lopez et al., in preparation). Therefore, it appears that the L-PK glucose response element (G1RE), which is also a USF binding site (2,16) is able to block in vivo, in fasted animals, the enhancer-dependent activation of the promoter binding HNF1 and HNF4 (i.e., behaves as a glucose-repressed silencer as well as a glucose-induced activator). This observation fits in with our previous data of transient transfection experiments in hepatocytes showing that the G1RE is a positive element in the presence of glucose and a negative element in the absence of glucose (2).
Ubiquitous Enhancers Cloned Upstream of the L-PK Promoter Are Able to Slightly Modify Tissue Specificity Imposed by the L-PK Promoter
Although the proximal L-PK promoter seems to be rather dominant with respect to the influence of heterologous enhancers, these are able to modulate the activity of this promoter and to extend its specificity. Such a specificity extension is weak for the enhancer H, which seems only to modify the expression gradient in the intestine, with abnormal expression of the transgene in the colon. In fact, the endogenous L-PK gene is itself slightly active in the colon as judged by PCR analysis (38). The presence of a certain amount of HNF1 and HNF4 transcription factors explains that the proximal promoter can be activable by an enhancer that itself is active in the colon. The SV40 enhancer stimulates the activity of the L′ erythroid promoter in the spleen, of both L and L′ promoters in the thymus, and of the L promoter in parts of the kidney in which it is usually inactive. We reasoned that such stimulations of expression should result from two phenomena: the intrinsic activity of the SV40 enhancer in some tissues (e.g., kidney, thymus) (4,6,21,24,30,37) and the presence in these tissues of low amounts of transcription factors binding to the L-PK promoter and making it activable by the SV40 enhancer. This hypothesis fits in with the presence of GATA 1 and 2 proteins in the spleen (5,44), binding to the erythroid L′ promoter (32,33). Thymocytes also contain GATA factors, especially GATA 3 (27,29), which could explain the activation of the L′ promoter by the SV40 enhancer in these cells.
HNF1α mRNA was detected by RNAse protection in rat thymus (1) and, recently, we have been able to detect by reverse-transcriptase PCR HNF1α mRNA in mouse thymic epithelial cells (de Ribains et al., in preparation), which could account for the SV40-dependent activation of the L-PK promoter in this tissue, with occurrence of thymoma of epithelial origin (de Ribains et al., in preparation). Finally, in the kidney, where the SV40 enhancer is especially strong (19,23), the ectopic localization of the Tag protein in the proximal convoluted tubules of SV-PK/Tag mice parallels the tissue distribution of HNF1α in these tissues (34). All of these results suggest that binding of HNF1 to the L-PK promoter is necessary and sufficient to make it transactivable by the SV40 enhancer, which confirms the previous results of Cognet et al. (9) in HepG2 hepatoma cells and hepatocytes.
The defect of the SV-PK/Tag transgene expression in enterocytes is difficult to explain in light of the discussed mechanisms because the L-PK promoter is active in these cells containing both HNF1 and HNF4, and the SV40 enhancer is not known to be extinguished in these cells. It could be that an additional mechanism explaining the enhancer/promoter cooperation involves, in addition to factors binding to promoters and enhancers, a third class of factors, eventually tissue specific, positively or negatively modulating the interaction between enhancer and promoter complexes. Such “bridging” or “auxiliary” factors important for functionally coupling enhancers and promoters have been described in various systems (20).
The L-PK Distal Element Plays an Essential Role in the Pancreas Specificity of the L-PK Gene Expression
The role of the −2070/−3200 L-PK distal fragment in the weak expression of the L-PK gene in the pancreas is attested by several results, i) This fragment confers by itself a dual brain and pancreas specificity on the ubiquitous HSV-TK promoter, ii) L-PK/Tag mice with a high copy number of transgenes develop diet-dependent insulinomas whereas mice in which the L-PK distal fragment was replaced by either enhancer H or enhancer SV40 and harboring a similar number of transgene copies do not develop this type of tumors. iii) Although the method is only semiquantitative, reverse transcription PCR analysis detects more L-PK transcripts in the pancreas of L-PK/ Tag mice than of SV-PK/Tag and H-PK/Tag mice. Tag detection by in situ immunofluorescence give results that are difficult to interpret, due to the very high sensitivity of this method: in spite of the low amount of Tag mRNA in the islets of Langerhans, they are intensively stained with the anti-Tag antibody and, in spite of the quasiabsence of this transcript in exocrine pancreas (7,38), this tissue is detectably stained in L-PK/Tag mice. It could be that Tag is very stable in nuclei of pancreatic cells; furthermore, immunofluorescence is clearly a very sensitive, nonquantitative detection method. In any case, nuclear Tag positive staining of pancreas exocrine cells in L-PK/Tag mice was almost lost in SV-PK/Tag and H-PK/ Tag mice. Such an observation is consistent with a lower general expression of the Tag transgene in the pancreas of mice harboring constructs devoid of the L-PK distal fragment. We can assume that the brain-pancreas specificity of the L-PK distal element cooperates with the L-PK promoter to restrict the expression to the endocrine pancreas: as previously discussed, the L-PK promoter is not activable in the brain devoid of HNF1 factors; in the pancreas, the L-PK promoter has an intrinsic higher activity, and is expected to be more sensitive to the distal element, in endocrine than in exocrine cells, because HNF4 is more abundant in the former cells than in the latter ones (38).
Finally, although exocrine as well as endocrine pancreas synthesize HNF1, the inefficiency of the SV40 and H enhancers in stimulating the L-PK promoter in this organ is not in contradiction with our former proposal that HNF1 binding to the L-PK promoter is necessary and sufficient for the activability by the SV40 enhancer: most viral and cellular enhancers, especially the SV40 enhancer, are known to be inefficient in pancreatic cells (41). It is striking that the L-PK distal fragment, containing the liver-specific HSS2 site and essential to stimulate activity of the L-PK promoter, in particular in the liver, does not seem to be itself endowed with a liver specificity. Our results suggest that, rather, the stimulating effect of this fragment in the liver is specific to the liver-specific L-PK promoter, through uncharacterized interactions. In contrast, the brain/exocrine/endocrine/ pancreas specificity of the L-PK distal element can be interpreted as a new example of regulatory sequences active in both endocrine pancreas and brain on the one hand, and in both exocrine and endocrine pancreas on the other. Endocrine pancreatic genes such as the insulin and glucagon genes are also expressed in the developing brain (18,42), whereas, conversely, a brain-specific gene such as the synaptophysin gene is also expressed in endocrine pancreas (48). The glucagon and elastase genes possess regulatory elements that seem to share common binding proteins in islets of Langerhans and exocrine pancreas (13,31).
In conclusion, our investigation of the combinatorial effect of the proximal promoter and distal activating region of the L-PK gene with either homologous or heterologous enhancers and promoter in transgenic mice shows that the main parameters for the specificity conferred by different couples of promoter/enhancer are: i) of course, the specificity of the promoters and enhancers, and ii) the activability of the promoter by enhancers, which seems to require the binding on the promoter of factors that, by themselves, could be not sufficient to stimulate the promoter. For instance, binding of HNF1 to the L-PK promoter is necessary and sufficient to make it activable by a strong viral enhancer such as the SV40 enhancer whereas the expression of the endogenous gene seems also to require the presence of HNF4, probably because the L-PK distal activating sequences do not behave as a strong “enhancer.” This mechanism allows to restrict gene activation to lesser tissues than those in which the enhancer is very active, because in some of these tissues the promoter is intrinsically not activable (e.g, the brain, heart, and skeletal muscle in the H-PK/Tag and SV-PK/Tag mice; the brain and exocrine pancreas in L-PK/Tag mice). In addition, the glucose response element (G1RE) of the L-PK promoter appears to be a master switch of gene expression, making it the promoter not activable in fasting animals and active in animals refed a carbohydrate-rich diet. Therefore, the insensitivity of the L-PK promoter to enhancers could be due to either the absence of necessary positive factors on the promoter or to constitution of a negative complex on the G1RE.
ACKNOWLEDGEMENTS
We are gratefull to Dr. D. Hanahan (University of California, San Franscisco) for the generous gift of his anti-large T antigen polyclonal antibody. We thank Dr. M. Raymondjean and Dr. D. Daegelen (INSERM U129, Paris, France) for helpful and stimulating discussion. We thank J. Gome (ARKA Laboratoire) for photographic works and V. Martinez for typing the text. L. Miquerol is a fellow of the Ligue Nationale contre le Cancer. This work was supported by the INSERM and grants from the Association pour la Rcherche sur le Cancer.
REFERENCES
- 1. Baumhueter S.; Mendel D. B.; Conley P. B.; Kuo C. J.; Turk C.; Graves M. K.; Edwards C. A.; Courtois G.; Crabtree G. R. HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LFB1 and APF. Genes Dev. 4:372–379; 1990. [DOI] [PubMed] [Google Scholar]
- 2. Bergot M.-O.; Diaz-Guerra M.-J. M.; Puzenat N.; Raymondjean M.; Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 20:1871–1878; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boquet D.; Vaulont S.; Tremp G. L.; Ripoche M.-A.; Daegelen D.; Jami J.; Kahn A.; Raymondjean M. DNaseI hypersensitivity analysis of the L-type pyruvate kinase gene in rats and transgenic mice. Eur. J. Biochem. 207:13–21; 1992. [DOI] [PubMed] [Google Scholar]
- 4. Botteri F. M.; van der Putten H.; Wong D. F.; Sauvage C. A.; Evans R. M. Unexpected thymic hyperplasia in transgenic mice harboring a neuronal promoter fused with simian virus 40 large T antigen. Mol. Cell. Biol. 7:3178–85; 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briegel K.; Lim K. C.; Plank C.; Beug H.; Engel J. D.; Zenke M. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 7:1097–1109; 1993. [DOI] [PubMed] [Google Scholar]
- 6. Brinster R. L.; Chen H. Y.; Messing A.; van Dyke T.; Levine A. J.; Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell 37:367–379; 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cartier N.; Miquerol L.; Tulliez M.; Lepetit N.; Levrat F.; Grimber G.; Briand P.; Kahn A. Diet-dependent carcinogenesis of pancreatic islets and liver in transgenic mice expressing oncogenes under the control of the L-type pyruvate kinase gene promoter. Oncogene 7:1413–1422; 1992. [PubMed] [Google Scholar]
- 8. Chirgwin J. M.; Przybyla A. E.; Mac Donald R. J.; Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294–5299; 1979. [DOI] [PubMed] [Google Scholar]
- 9. Cognet M.; Bergot M.-O.; Kahn A. Cis-acting DNA elements regulating expression of the liver pyruvate kinase gene in hepatocytes and hepatoma cells—evidence for tissue-specific activators and extinguisher. J. Biol. Chem. 266:7368–7375; 1991. [PubMed] [Google Scholar]
- 10. Cognet M.; Lone Y. C.; Vaulont S.; Kahn A.; Marie J. Structure of the rat L-type pyruvate kinase gene. J. Mol. Biol. 196:11–25; 1987. [DOI] [PubMed] [Google Scholar]
- 11. Concordet J.-P.; Maire P.; Kahn A.; Daegelen D. A ubiquitous enhancer shared by two promoters in the human aldolase A gene. Nucleic Acids Res. 19:4173–4180; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Concordet J.-P.; Salminen M.; Demignon J.; Moch C.; Maire P.; Kahn A.; Daegelen D. An opportunistic promoter sharing regulatory sequences with either a muscle-specific or ubiquitous promoter in the human aldolase A gene. Mol. Cell. Biol. 13:9–17; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cordier-Bussat M.; Morel C.; Philippe J. Homologous DNA sequences and cellular factors are implicated in the control of glucagon and insulin gene expression. Mol. Cell. Biol. 15:3904–3916; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuif M.-H.; Cognet M.; Boquet D.; Tremp G.; Kahn A.; Vaulont S. Elements responsible for hormonal control and tissue specificity of L-type pyruvate kinase gene expression in transgenic mice. Mol. Cell. Biol. 12:4852–4861; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuif M.-H.; Porteu A.; Kahn A.; Vaulont S. Exploration of a liver-specific glucose/insulin-responsive promoter in transgenic mice. J. Biol. Chem. 268:13769–13772; 1993. [PubMed] [Google Scholar]
- 16. Diaz-Guerra M.-J.; Bergot M.-O.; Martinez A.; Cuif M.-H.; Kahn A.; Raymondjean M. Functional characterization of the L-type pyruvate kinase gene glucose response complex. Mol. Cell. Biol. 13:7725–7733; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Domingo M.; Einig C.; Eigenbrodt E.; Reinacher M. Immunohistological demonstration of pyruvate kinase isoenzyme type L in rat with monoclonal antibodies. J. Histochem. Cytochem. 40:665–673; 1992. [DOI] [PubMed] [Google Scholar]
- 18. Efrat S.; Teitelman G.; Anwar M.; Ruggiero D.; Hanahan D. Glucagon gene regulatory region directs oncoprotein expression to neurons and pancreatic a cells. Neuron 1:605–613; 1988. [DOI] [PubMed] [Google Scholar]
- 19. Feigenbaum L.; Hinrichs S. H.; Jay G. JC virus and simian virus 40 enhancers and transforming proteins: Role in determining tissue specificity and pathogenicity in transgenic mice. J. Virol. 66:1176–1182; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forsberg M.; Westin G. Enhancer activation by a single type of transcription factor shows cell type dependence. EMBO J. 10:2543–2551; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garvin A. M.; Abraham K. M.; Forbush K. A.; Farr A. G.; Davison B. L.; Permultter R. M. Disruption of thymocyte development and lymphoma-genesis induced by SV40 T-antigen. Int. Immunol. 2:173–180; 1990. [DOI] [PubMed] [Google Scholar]
- 22. Gorman C.; Moffat L.; Howard B. Recombinant genomes which express chloramphenicol acetyl-transferase in mammalian cells. Mol. Cell. Biol. 2:1044–1051; 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Griffin B. E. Structure and genomic organization of SV40polyomavirus. In: Tooze J. ed. Molecular biology of tumor viruses: DNA tumor viruses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1981:61–123. [Google Scholar]
- 24. Grosveld F.; Kollias G. Ectopic expression of Thy-1 in the kidneys of transgenic mice induces functional and proliferative abnormalities. Cell 54:920; 1988. [DOI] [PubMed] [Google Scholar]
- 25. Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu. Rev. Genet. 22:479–519; 1988. [DOI] [PubMed] [Google Scholar]
- 26. Hanahan D. Heritable formation of pancreatic β-cell tumors in transgenic mice expressing recombinant insulin/simian virus SV40 oncogenes. Nature 315:115–122; 1985. [DOI] [PubMed] [Google Scholar]
- 27. Ho I. C.; Vorhees P.; Marin N.; Oakley B. K.; Tsai S. F.; Orkin S. H.; Leiden J. M. Human GATA-3: A lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 10:1187–1192; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kahn A.; Marie J. Pyruvate kinase from human erythrocytes and liver. Methods Enzymol. 90:131–141; 1982. [DOI] [PubMed] [Google Scholar]
- 29. Ko L. J.; Yamamoto M.; Leonard M. W.; George K. M.; Ting P.; Engel J. D. Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor delta gene enhancer. Mol. Cell. Biol. 11:2778–2784; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kollias G.; Evans D. J.; Ritter M.; Beech J.; Morris R.; Grosveld F. Ectopic expression of Thy-1 in the kidneys of transgenic mice induces functional and proliferative abnormalities. Cell 51:21–31; 1987. [DOI] [PubMed] [Google Scholar]
- 31. Kruse F.; Rose S. D.; Swift G. H.; Hammer R. E.; MacDonald J. An endocrine-specific element is an integral component of an exocrine-specific pancreatic enhancer. Genes Dev. 7:774–786; 1993. [DOI] [PubMed] [Google Scholar]
- 32. Lacronique V.; Boquet D.; Lopez S.; Kahn A.; Raymondjean M. In vitro and in vivo protein-DNA intensions on the rat erythroid-specific L′ pyruvate kinase gene promoter. Nucleic Acids Res. 20:5669–5676; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lacronique V.; Lopez S.; Miquerol L.; Porteu A.; Kahn A.; Raymondjean M. Identification and functional characterization of an erythroid-specific enhancer in the L-type pyruvate kinase gene. J. Biol. Chem. 270:14989–14997; 1995. [DOI] [PubMed] [Google Scholar]
- 34. Lazzaro D.; De Simone V.; De Magistris L.; Lehtonen E.; Cortese R. LFB1 and LFJ33 homeoproteins are sequentially expressed during kidney development. Development 114:469–479; 1992. [DOI] [PubMed] [Google Scholar]
- 35. Lefrancois-Martinez A. M.; Diaz-Guerra M. J. M.; Vallet V.; Kahn A.; Antoine B. Glucose-dependent regulation of the L-pyruvate kinase gene in a hepatoma cell line is independent of insulin and cyclic AMP. FASEB J. 8:89–96; 1994. [DOI] [PubMed] [Google Scholar]
- 36. Luckow B.; Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 15:5490; 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Messing A.; Chen H. Y.; Palmiter R. D.; Brinster R. L. Peripheral neuropathies, hepatocellular carcinomas and islet cell adenomas in transgenic mice. Nature 316:461–463; 1985. [DOI] [PubMed] [Google Scholar]
- 38. Miquerol L.; Lopez S.; Carder N.; Tulliez M.; Raymondjean, M ; Kahn A. Expression of the L-type pyruvate kinase gene and the hepatocyte nuclear factor 4 transcription factor in exocrine and endocrine pancreas. J. Biol. Chem. 269:8944–8951; 1994. [PubMed] [Google Scholar]
- 39. Noguchi T.; Yamada K.; Inoue H.; Matsuda T.; Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J. Biol. Chem. 262:14366–14371; 1987. [PubMed] [Google Scholar]
- 40. Sandgren E. P.; Quaife C. J.; Pinkert C. A.; Palmiter R. D.; Brinster R. L. Oncogene-induced liver neoplasia in transgenic mice. Oncogene 4:715–724; 1989. [PubMed] [Google Scholar]
- 41. Shibata M.; Puga A.; Salata K. F.; Bachurski C. J.; Lerman M. I.; Notkins A. L. Expression of a viral gene in insulin-producing cell lines renders them susceptible to immunological destruction. Diabetologia 32:709–715; 1989. [DOI] [PubMed] [Google Scholar]
- 42. Teitelman G. Insulin cells of pancreas extend neurites but do not arise from the neuroectoderm. Dev. Biol. 142:368–379; 1990. [DOI] [PubMed] [Google Scholar]
- 43. Tremp G. L.; Boquet D.; Ripoche M.-A.; Cognet M.; Yu-Chun L.; Jami J.; Kahn A.; Daegelen D. Expression of the rat L-type pyruvate kinase gene from its dual erythroid- and liver-specific promoter in transgenic mice. J. Biol. Chem. 264:19904–19910; 1989. [PubMed] [Google Scholar]
- 44. Tsai S. F.; Keller G.; Kuo F. C.; Weiss M.; Chen J.; Rosenblatt M.; Alt F. W.; Orkin S. H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371:221–226; 1994. [DOI] [PubMed] [Google Scholar]
- 45. Vaulont S.; Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 8:28–35; 1994. [DOI] [PubMed] [Google Scholar]
- 46. Vaulont S.; Puzenat M.; Kahn A.; Raymondjean M. Analysis by cell-free transcription of the liver-specific pyruvate kinase gene promoter. Mol. Cell. Biol. 9:4409–4415. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaulont S.; Puzenat N.; Levrat F.; Cognet M.; Kahn A.; Raymondjean M. Proteins binding to the liver-specific pyruvate kinase gene promoter. A unique combination of known factors. J. Mol. Biol. 209:205–219; 1989. [DOI] [PubMed] [Google Scholar]
- 48. Weidenmann B.; Franke W. W.; Kuhn C.; Moll R.; Gould V. E. Synaptophysin: A marker protein for neuroendocrine cells and neoplasms. Proc. Natl. Acad. Sci. USA 83:3500–3504; 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


