Abstract
Receptors for leukocyte chemoattractants, including chemokines, are traditionally considered to be responsible for the activation of special leukocyte functions such as chemotaxis, degranulation, and the release of superoxide anions. Recently, these G-protein-coupled serpentine receptors have been found to transduce signals leading to gene transcription and translation in leukocytes. Transcription factors, such as NFκB and AP-1, are activated upon stimulation of the cells with several chemoattractants at physiologically relevant concentrations. Activation of transcription factors through these receptors involves Gprotein coupling and the activation of protein kinases. The underlying signaling pathways appear to be different from those utilized by TNF-α, a better characterized cytokine that induces the transcription of immediate-early genes. Chemoattractants stimulate the expression of several inflammatory cytokines and chemokines, which in turn may activate their respective receptors and initiate an autocrine regulatory mechanism for persistent cytokine and chemokine gene expression.
Keywords: Transcription activation, NFκB, AP-1, Chemoattractant, Receptors, G-protein
LEUKOCYTE infiltration is characteristic of inflammatory reaction to tissue injuries caused by trauma, invasion of foreign particles, ischemia-reperfusion, cancer, autoimmune diseases, and other conditions. This process begins with activation of leukocytes by a collection of cell-attracting chemicals termed chemoattractants. Two classes of chemoattractants have been identified. The classical chemoattractants consist of the bacterially derived N-formyl peptide fMet-Leu-Phe (FMLP), the activated complement component C5a, the lipid mediator platelet-activating factor (PAF), and the arachidonic acid product leukotriene B4 (LTB4) (30). These chemoattractants are well characterized and are generally short-lived at sites of inflammation. The more recently discovered chemotactic cytokines (chemokines) consist of a group of 8–10-kDa polypeptides secreted by leukocytes, endothelial cells, and certain tumor cells. Chemokines have longer lives and higher specificity for leukocyte subtypes. They can be further divided into the CXC (α) and CC (β) classes depending on the location of the first two of the four cysteines in the molecules (3,22).
Chemoattractants are pleiotropic activators of leukocyte functions. At subnanomolar concentrations, chemoattractants induce directed migration of leukocytes along a chemical concentration gradient (chemotaxis). Chemoattractants such as FMLP also cause shedding of l-selectin and activation of integrins. At higher concentrations (10–100 nM), chemoattractants stimulate phagocyte degranulation and the generation of superoxide anions (30). Attention has been drawn recently to an additional function of chemoattractants: induction of the synthesis and secretion of proinflammatory cytokines, including chemokines. Because chemoattractants are one of the first factors that leukocytes encounter at sites of inflammation (31), their ability to induce the expression of cytokine genes may be significant with respect to the recruitment of additional leukocytes and the modulation of a local cytokine network.
Monocytes and neutrophils are major sources of proinflammatory cytokines, including chemokines. Here we present results from our current study of the mechanisms for chemoattractant receptor-mediated gene transcription. Our data suggest that chemoattractant binding to these receptors leads to activation of NFκB and AP-1, both of which are transcription activators for the expression of immediate-early genes. The activation process requires functional coupling to G-proteins and appears to utilize signaling pathways different from those employed by several other inducers of NFκB activation, such as TNF-α and PMA. The activation of transcription leading to the expression of cytokines and chemokines might reflect a fundamental role of chemoattractants. A more detailed understanding of the signaling mechanisms involved will likely reveal valuable sites for therapeutic intervention.
MATERIALS AND METHODS
Reagents
Platelet-activating factor (C-16) was obtained from Calbiochem (San Diego, CA). Cholera and pertussis toxins were from List Laboratory (Campbell, CA). Lipopolysaccharide (LPS) was isolated from lyophilized Salmonella minnesota Re595 bacteria. The PI3-kinase inhibitor LY294002 was generously provided by Dr. Chris Vlahos (Eli Lilly & Company, Indianapolis, IN). Rabbit polyclonal antibodies against the subunits of NFκB/Rel were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody against a COOH-terminal peptide (residues 289–317) of IκBα was a gift from Dr. Warner C. Greene (University of California, San Francisco, CA) (33). The mouse IκB cDNA and the IκB-CAT constructs (6) were kindly provided by Dr. Inder M. Verma (The Salk Institute, La Jolla, CA).
Oligonucleotides and their complementary strands were from Promega (Madison, WI) and Santa Cruz Biotechnology. The sequences are: murine intronic κ chain κ B site (underlined), 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (NFκB) (29), and a mutant κ B site with the G → C substitution (underlined) in the NFκB DNA binding motif, 5′ -AGTTGAGGCGACTTTCCCAGGC-3′.
Double-stranded oligonucleotide (5 pmol) was 32P-labeled with T4 polynucleotide kinase.
Preparation of Monocytes From Peripheral Blood
Heparinized human peripheral blood from healthy donors was fractionated on Percoll density gradients. The separated cells were then washed and monocytes were isolated using gelatin/plasma-coated flasks. The purity of monocytes was greater than 95% as determined by staining with the anti-CD 14 Ab MY4. Cell viability was greater than 98% as measured by trypan blue exclusion. Monocytes were resuspended in RPMI-1640 medium (Irvine Scientific) with 10% (v/v) heat-inactivated fetal bovine serum.
RNA Extraction and Northern Blotting
Total RNA was isolated from monocytes by a modified guanidinium-acetic phenol method (7). About 20 μg of total RNA was subjected to electrophoresis in agarose gels containing 6% formaldehyde and transferred to the Hybond-N plus membranes (Amersham). A 616-bp HindIII-XbaI fragment of the human HB-EGF cDNA was synthesized from U937 total RNA by reverse transcriptase polymerase chain reaction (RT-PCR). For Northern hybridization, the fragment was labeled with [32P]dATP to a specific activity of > 5 × 108 cpm/ μg DNA, by random priming. Blots were prehybridized in 6 × SSC, 5 × Denhardt’s solution, and 0.1% SDS at 65°C for 2 h and further hybridized with the 32P-labeled probe (1 × 106 cpm/ml) for 16 h at the same temperature. Filters were washed with 2 × SSC and 0.1% SDS at 65°C, followed by another wash with 0.1 x SSC and 0.1% SDS. Relative hybridization was analyzed for quantitation of actual radioactivity by a phosphorimaging System (Molecular Dynamics, CA). The quantity of samples in each lane was standardized against relative staining of the 18S and 28S RNA after gel electrophoresis.
Preparation of Nuclear Extracts
Nuclear extracts were prepared by a modified method of Dignam et al. (9). Cells were washed three times with ice-cold PBS, harvested, and re-suspended in 0.4 ml of buffer A (10 mM HEPES, pH 7.9, 10 mM KCI, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride). After 10 min, 23 μl of 10% Nonidet P-40 was added and mixed for 2 s. Nuclei were separated from cytosol by centrifugation at 13,000 × g for 10 s and were resuspended in 50 μl of buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 0.1 mM PMSF). After 30 min at 4°C, lysate was separated by centrifugation (13,000 × g, 30 s) and supernatants containing nuclear proteins were transferred to new vials. The protein concentration of extracts was measured using the BCA reagents (Bio-Rad) with bovine serum albumin as standard and samples were diluted to equal concentration in buffer B for use directly or storage at − 80°C.
Electrophoretic Mobility Shift Assay (EMSA)
Electrophoretic mobility shift assays were performed by incubating 2.5 μg of the nuclear extract in 12 μ1 of binding buffer [5 mM HEPES, pH 7.8, 5 mM MgCl2, 50 mM KC1, 0.5 mM dithiothreitol, 0.4 mg/ml poly(dl-dC) (Pharmacia), 0.1 mg/ml sonicated double-stranded salmon sperm DNA, and 10% glycerol] for 10 min at room temperature. Then approximately 20–50 fmol of 32P-labeled oligonucleotide probe (30,000–50,000 cpm) was added and the reaction mixture was incubated for 10 min at room temperature. The samples were analyzed on 5% or 6% acrylamide gels, which were made in 50 mM Tris-borate buffer containing 1 mM EDTA (TBE) or 50 mM Tris/380 mM glycine/2 mM EDTA (TGE buffer) and were preelectrophoresed for 2 h at 12 V/cm. Electrophoresis was carried out at the same voltage for 2–2.5 h. Gel contents were dried on Whatman DE-81 paper and exposed for 3–5 h at −80°C with an intensifying screen.
Chloramphenicol Acetyltransferase (CAT) Assay
Five micrograms each of the IκBα promoter-CAT plasmids p0.2kb(WT)CAT and p0.2kb(M)-CAT (6) and the pSVLCAT plasmid were separately transfected into CHO-PAFR cells, together with 1 μg pCMVβ plasmid (Clonetech), by using the cationic lipid DOTAP (Boehringer Mannheim). Seven hours after transfection, cells were washed, stimulated with agonists for 2 h, and collected for CAT assay. CAT activities were measured in crude cellular extracts using [14C]chloramphenicol (Amersham) as substrate, followed by thin-layer chromatography. The relative transfection efficiency was determined by measurement of coexpressed β-galactosidase activities.
Ultraviolet (UV) Cross-Linking Analysis
Double-stranded 32P-radiolabeled photoreac-tive oligonucleotide probe containing the kB site was prepared as described (4,21). UV cross-linking was performed in solution by irradiation (300 nm, 7000-mW/cm illuminator, Fotodyne) of the respective binding reaction with 10 μg nuclear extract and [32P]BrdU (5′-bromo-2′-deoxyuridine 5′-triphosphate) κB-probe for 30 min. After UV cross-linking, the oligonucleotide-protein adduct was boiled for 2 min in 0.5% SDS, diluted fivefold with TSN buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% Nonidet P-40), and immunoprecipitated by incubation at room temperature for 30 min with 5 μg of normal rabbit IgG or anti-p50, anti-p65, and anti-c-Rel antibodies. Finally, the 32P-labeled products were directly analyzed by SDS-PAGE (8% discontinuous gel) under reducing conditions. In parallel, a [32P]BrdU probe binding protein complexes were UV cross-linked in native EMSA gel, excised, and analyzed by SDS-PAGE.
Immunoblotting
Approximately 10 μg of cytoplasmic extracts, collected after the Nonidet P-40 lysis and centrifugation steps, was mixed with loading dye, boiled, electrophoresed on a 10% SDS gel, and transferred to Hybond-ECL nitrocellulose (Amersham). Filter strips were incubated with primary antibody against the IκBα carboxyl-terminus (1:2500 dilution) for 30 min at room temperature, followed by addition of peroxidase-conjugated goat anti-rabbit IgG at 1:10,000 for 30 min and analysis with enhanced chemiluminescence reagents (Du Pont-NEN).
RESULTS
Chemoattractant receptors belong to the super-family of G-protein-coupled receptors with seven putative transmembrane domains (19). Agonist binding of the receptors triggers a series of signaling events leading to specific leukocyte functions (30). To examine whether chemoattractants directly stimulate transcription activation, peripheral blood monocytes were treated with PAF, and the subsequent changes in the levels of the message for heparin binding EGF-like growth factor (HB-EGF) were measured. PAF, a lipid chemoattractant, induced an elevation of the HB-EGF message in a dose- and time-dependent fashion (Fig. 1A). Similarly, PAF stimulated the upregulation of the message for IL-1β (Fig. IB). The expression of the IL-1β gene was known to be regulated in part by the transcription activator NFκB (8), although it was not clear how HB-EGF gene expression was regulated (11). The effect of PAF on NFκB was then examined. As shown in Fig. 2, at nanomolar concentrations PAF activated NFκB to an extent similar to the previously characterized activators TNF-α and PMA. The time course of these responses suggested that PAF-stimulated NFκB may be responsible for the elevated message levels. This notion was supported by the observation that the inhibitor for NFκB, pyrrolidine dithi-ocarbamate (PDTC), dose-dependently blocked PAF-induced NFκB activation as well as elevation of the HB-EGF message levels (Fig. 3).
FIG. 1.
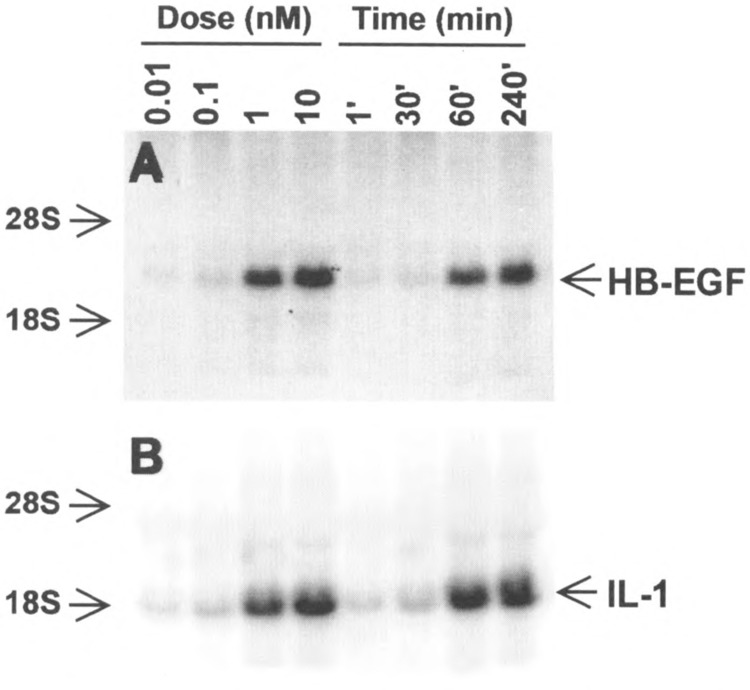
Accumulation of cytoplasmic RNA in PAF-stimulated monocytes. Total RNA from monocytes was prepared after stimulation with PAF (100 nM) for various times or for 1 h at different concentrations. Twenty micrograms of the total RNA was analyzed by Northern blot and probed with cDNA for HB-EGF (A) or IL-10 (B).
FIG. 2.
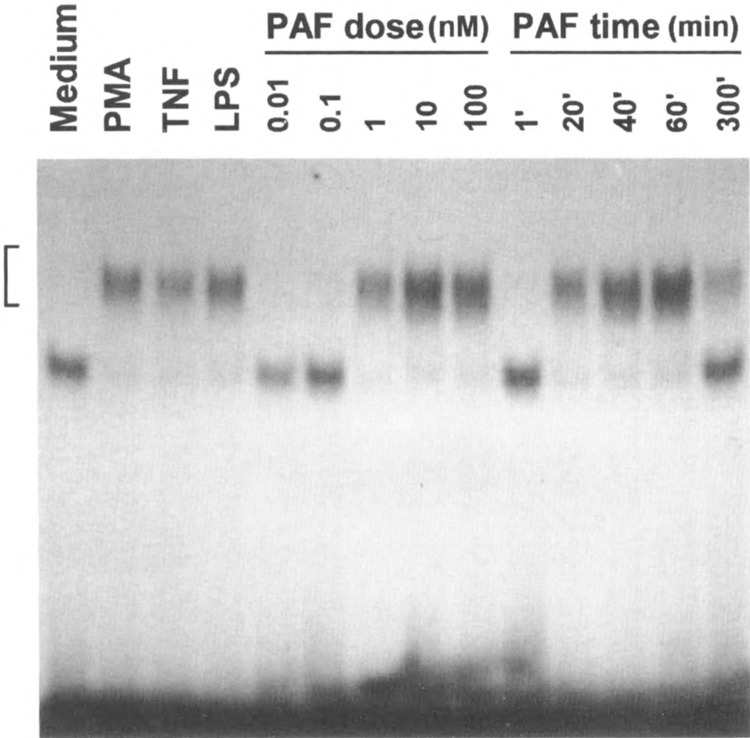
Induction of κB binding activity in PAF-stimulated monocytes. Monocytes were either unstimulated or stimulated with PAF (0.01–100 nM), PMA (100 nM), TNF-α (40 ng/ ml), and LPS (0.1 μg/ml) for 40 min or as indicated, before preparation of nuclear extracts. A 32P-labeled 21 mer, containing the consensus κB site (GGGACTTTCC), was used for EMSA. An autoradiograph of EMSA data is shown. The gel-retarded DNA-protein complex is indicated by a bracket.
FIG. 3.
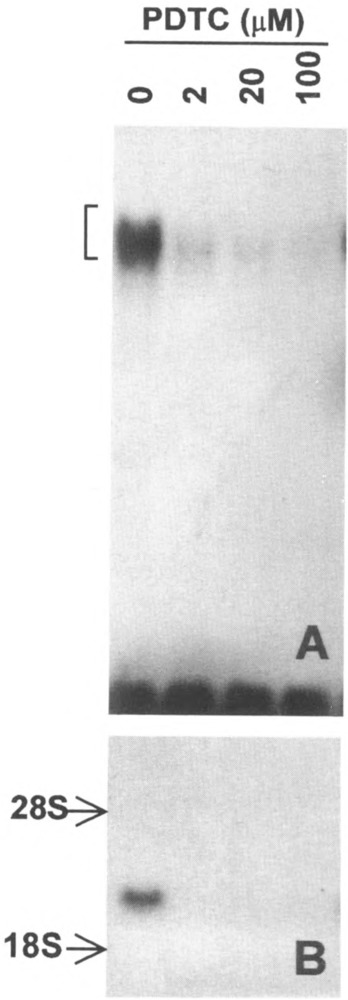
Inhibition of PAF-induced κB binding activity and HB-EGF gene expression by PDTC. (A) PDTC inhibits PAF-induced κB binding activity. Nuclear protein extracts were prepared from monocytes preincubated for 40 min with PDTC at the indicated concentrations, followed by PAF (100 nM) stimulation for 60 min. The autoradiograph of an EMSA result is shown. (B) PDTC inhibits PAF-induced HB-EGF gene expression. Cells pretreated with PDTC, as indicated above, were stimulated with PAF (100 nM) for 60 min. Total cellular RNA was extracted for Northern blot as described. The autoradiograph of a Northern blot is shown with positions of the ribosomal RNAs indicated
A stably transfected Chinese hamster ovary cell line expressing the PAF receptor (CHO-PAFR cell) was employed for further characterization of PAF-induced NFκB activation. The results indicate that PAF stimulation of NFκB activation requires the binding of a specific PAF receptor, which can be blocked by two PAF antagonists tested (not shown). In addition, PAF-induced NFκB activation involves nuclear translocation of p50/p65, but not c-rel, as shown by UV cross-linking experiments (Fig. 4). Similar to other induces of NFκB, PAF-induced NFκB activation in CHO cells is preceded by IκB degradation followed by resynthesis (Fig. 5). PAF stimulated not only a DNA binding activity but also transcription activation in CHO cells as detected by the expression of a CAT reporter gene (6) with a κB site from the IκB promoter (Fig. 6). These experiments provided detailed information about NFκ B activation induced by a G-protein-coupled chemoattractant receptor. Using gel mobility shift assays with radiolabeled probe containing the AP-1 binding site, it was observed that PAF induced AP-1 activity in the CHO-PAFR cells, although with a different response time compared to that of the activated NFκB (Fig. 7).
FIG. 4.
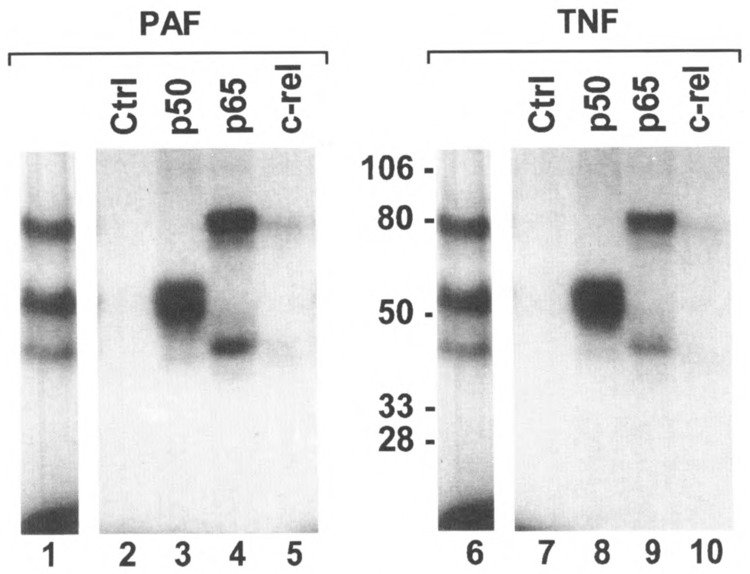
Identification of p50 and p65 in the DNA-protein complexes induced by PAF. A 32P-labeled probe containing BrdU was incubated with nuclear extracts from PAF- and TNF-stimulated CHO-PAFR cells (lanes 1 and 6, respectively). DNA-protein complexes in EMSA gel were UV cross-linked, excised, eluted, and resolved by SDS-PAGE. In separate experiments, UV cross-linked samples were immunoprecipitated with nonspecific (Ctrl, shown in lanes 2 and 7) or specific antibodies against three members of the NFκB/Rel family of proteins as noted at the top of each lane and analyzed by SDS-PAGE.
FIG. 5.
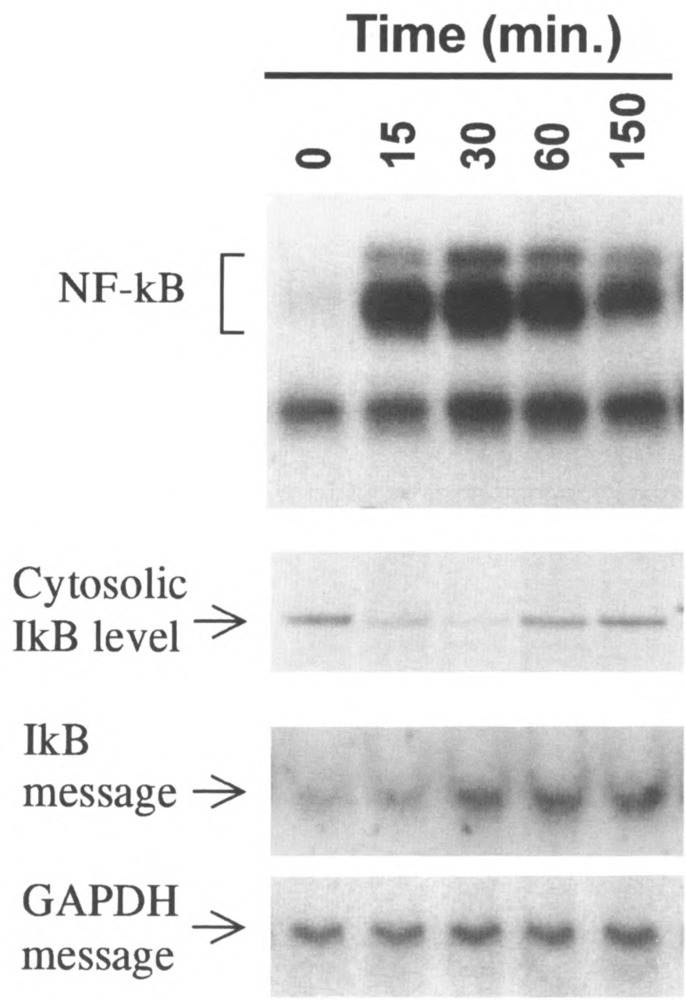
Time-dependent stimulation of κB binding activity and degradation of IκBα. Shown are simultaneous measurements of NFκB activation by EMSA (top panel), cytosolic IκB content by Western blot (second panel), and the message for IκB by Northern blot (third panel), as a function of time after stimulation with 10 nM PAF. GAPDH was used as a mRNA control.
FIG. 6.
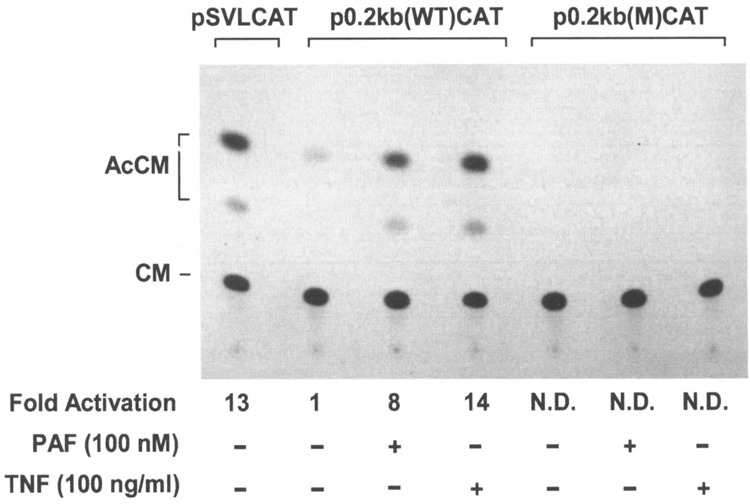
PAF- and TNF-α-induced IκBα promoter-directed CAT gene expression in transiently transfected CHO-PAFR cells. CHO-PAFR cells were transfected with 5 μg of IκBα promoter-CAT reporter plasmids (specified at the top) or 5 μg of pSVLCAT plasmid, together with 1 μg of pCMVβ plasmid. CAT activity was determined in these cells after stimulation of PAF or TNF-α. The results shown here are representative of three CAT assays. N.D.: not detectable.
FIG. 7.
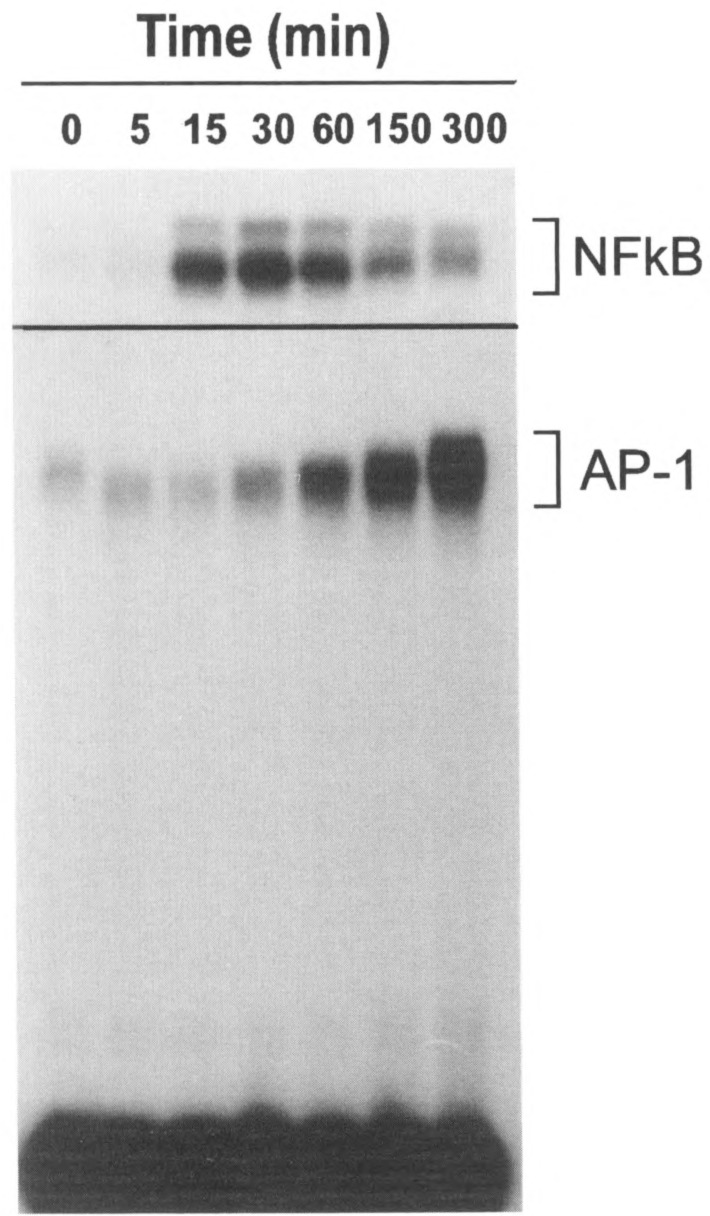
Time course of PAF-induced NFκB (top panel) and AP-1 binding activity in CHO-PAFR cells. The cells were stimulated with 100 nM PAF and nuclear extracts were prepared. Radiolabeled probes with the KB site and AP-1 site were incubated separately with the nuclear extracts and analyzed by SDS gel. Note the difference in the time course for the induced KB and AP-1 binding activities, which is consistent with previous reports by others.
Results derived from the above studies prompted us to investigate the capability of other chemoattractants to regulate gene transcription. Selected chemoattractants were employed in a study with freshly isolated peripheral blood mononuclear cells. The cells are known to express receptors for a large number of chemoattractants, in addition to the ones used for the study. As shown in Fig. 8, mononuclear cells responded to three classical chemoattractants and two chemokines with the formation of DNA-protein complexes detected by gel mobility shift assays using radiolabeled κ B probe. Further studies with selected chemoattractants produced dose and time response data (not shown) similar to those obtained previously with PAF. These results support the notion that induction of gene transcription may be a general property of chemoattractants.
FIG. 8.
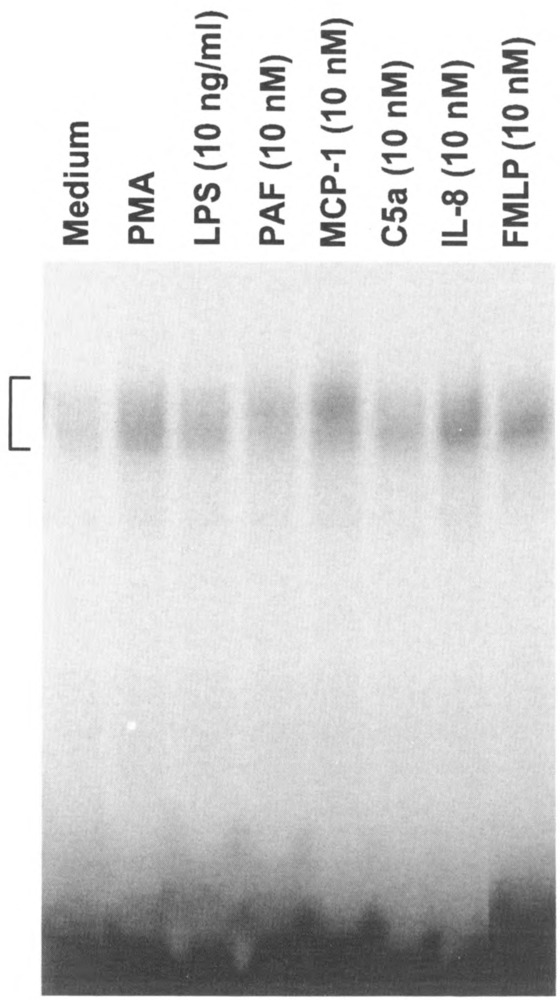
Induction of κB binding activity by several chemoattractants. Peripheral blood mononuclear cells were stimulated for 45 min with five chemoattractants, at the indicated concentrations. As controls, the cells were also treated with PMA (100 nM) and LPS. Shown is an autoradiograph of the EMSA result.
The two chemokines (IL-8 and MCP-1) tested in the above experiments belong to the CXC and CC chemokine subgroups, respectively. Biosynthesis of these chemokines can be induced by a number of proinflammatory stimuli, including LPS, IL-1β, and TNF-α (2). It has been reported recently that expression of IL-8 and MCP-1 is regulated mainly at the transcription level (18). NFκB sites or similar sequences have been identified in the promoter regions of the genes encoding IL-8 and MCP-1, and studies using CAT reporter constructs have shown that these sites contribute to the transcription of the IL-8 and MCP-1 genes. The finding that IL-8 and MCP-1 themselves are capable of inducing NFκB activation suggests a potential autocrine or positive regulatory mechanism for the biosynthesis of these chemokines, as depicted schematically in Fig. 9. Indeed, our preliminary results indicate potentiation of the IL-8 message in IL-8-stimulated mononuclear cells (Fig. 9, right panel). It has yet to be determined whether this message upregulation is accompanied by IL-8 protein synthesis.
FIG. 9.
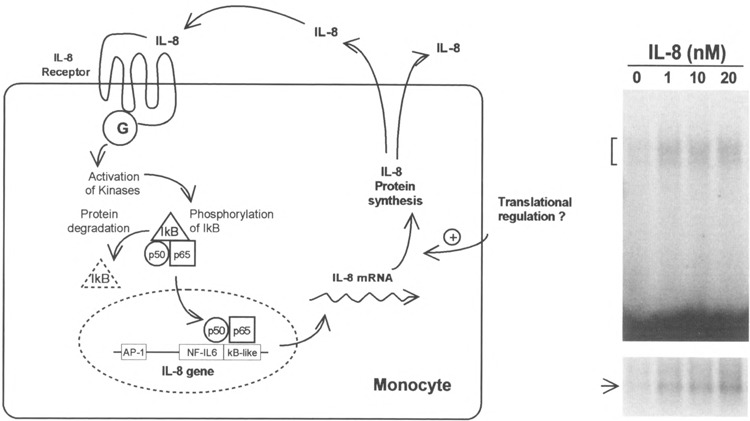
Potential autocrine regulation of IL-8 synthesis. Left: schematic representation of a possible autocrine mechanism for IL-8-stimulated IL-8 production. Right: autoradiographs of an EMSA (top) showing IL-8 induced KB binding activity, and of a Northern blot (bottom) depicting the accumulation of cytoplasmic IL-8 message in the same cells. Freshly isolated blood mononuclear cells were treated with IL-8 at the indicated concentrations for 1 h before harvesting for the preparation of total RNA and nuclear extracts, as described in Materials and Methods.
Chemoattractant receptors are G-protein-coupled serpentine receptors and, as such, they may utilize signaling mechanisms different from those employed by other inducers for transcription activation. PAF treatment of transfected CHO cells induces NFκB activation that requires the functional coupling of the receptor with G-proteins, as cholera toxin effectively blocks this effect of PAF (Fig. 10). However, PAF-stimulated responses in mononuclear cells were refractory to cholera toxin treatment. Instead, pertussis toxin can block PAF-stimulated HB-EGF gene expression (23) and NFκB activation (not shown). These observations suggest cell-specific coupling of the PAF receptor to different G-proteins, consistent with previous results from other studies (1,28).
FIG. 10.
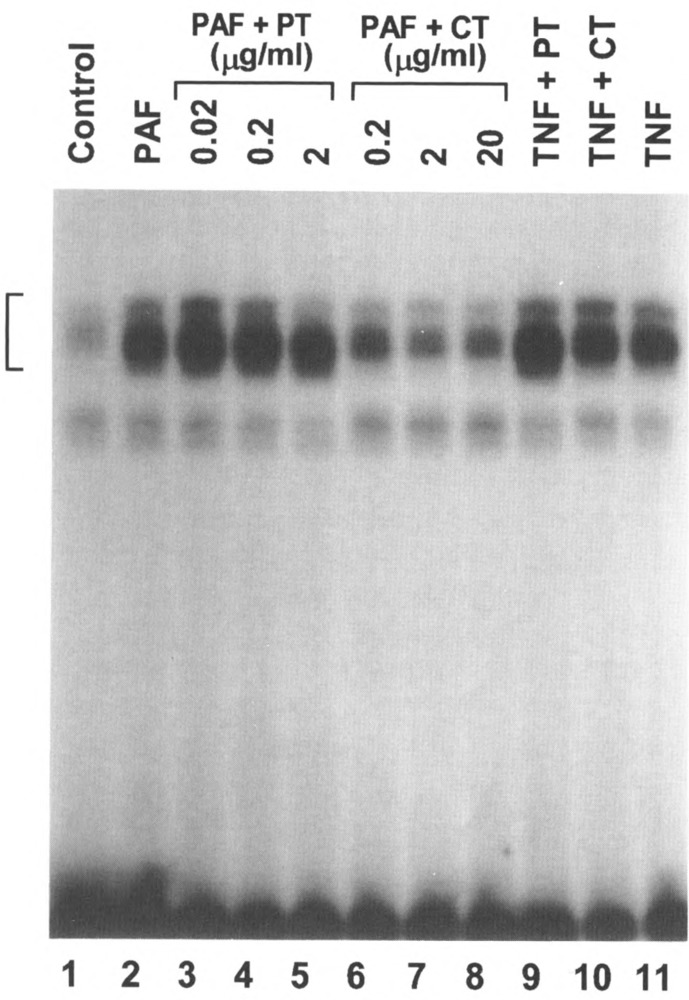
Effect of bacterial toxins on PAF-induced κB binding activity. The CHO-PAFR cells were pretreated with either cholera toxin (CT) or pertussis toxin (PT) at the indicated concentrations for 4 h in the culture medium. The cells were then stimulated with ligands for 40 min and nuclear extracts were analyzed by EMSA. The concentrations of the toxins used with TNF-α were 2 μg/ml (PT) and 20 μg /ml (CT). The ligand concentrations were 10 nM (PAF) and 40 ng/ml (TNF-α). Control: without ligand stimulation and toxin treatment.
The potential signaling pathways employed by chemoattractants were compared with those utilized by two other NFκB inducers, TNF-α and PMA. Treatment of mononuclear cells with the protein kinase inhibitors herbimycin A (HA) and staurosporine (STSP) blocked PAF-induced NFκB activation, while having no effect on TNF-α- and PMA-induced NFκB activation (Fig. 11 A). Similarly, two inhibitors for the phosphoinositol 3-kinase (PI3K), wortmannin and LY294002, selectively blocked PAF-induced NFκB activation but had no inhibitory effect on the TNF-α- and PMA-stimulated responses (Fig. 11B). These findings suggest that PAF regulates NFκB activity through the activation of G-proteins, protein tyrosine kinases, and PI3K.
FIG.11.
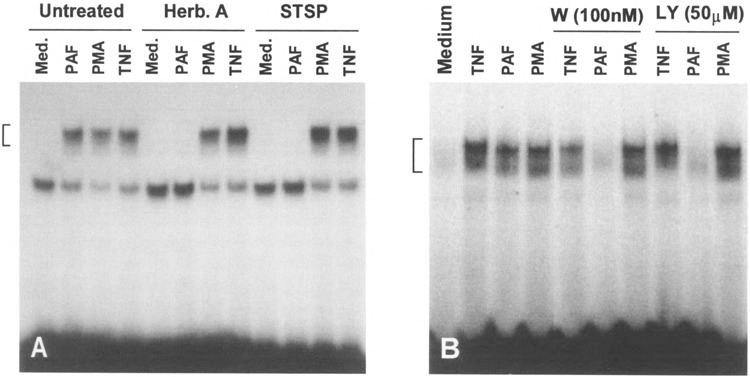
Selective inhibition of PAF-induced κB binding activity by protein kinase inhibitors. (A) Autoradiograph of EMSA showing the differential effects of herbimycin A (Herb. A, 1 μM) and staurosporine (STSP, 200 nM) on κB binding activity induced by PAF (100 nM), PMA (100 nM), and TNF-α (40 ng/ml). Mononuclear cells were treated with the inhibitors for 30 min followed by agonist stimulation for 1 h. (B) Similar to (A), but mononuclear cells were treated with wortmannin or LY294002 for 5 min before agonist stimulation.
DISCUSSION
A large number of proinflammatory factors are synthesized as a result of leukocyte activation.
Understanding the regulatory mechanism for the gene expression process, therefore, is crucial to the development of effective therapeutic agents. NFκB and AP-1 are major transcription activators that control the expression of a variety of immediate-early genes (14,16). The protein products of some of these genes, including TNF-α and IL-1β, in turn stimulate NFκB activation (2). Data from the current study expand the list to include several chemoattractants. These are agents derived from various sources and are activators of leukocytes during inflammation. Thus, migrating leukocytes may be exposed to chemoattractants that induce the synthesis of proinflammatory cytokines before the cells reach the inflammatory sites. Previously published data support the notion that chemoattractants stimulate the production of proinflammatory cytokines (5,10,13,27,32), but the transcription mechanisms adopted by chemoattractant have not been understood.
Chemoattractants may regulate gene transcription with different modes of action. Apart from the known paracrine and the potential autocrine mechanisms discussed above, juxtacrine stimulation is used by PAF. Vascular endothelial cells produce cell-associated PAF upon stimulation with inflammatory agents (24,35). By contacting leukocytes through their cell surface proteins such as P-selectin, the endothelial cell-associated PAF may interact with and stimulate the PAF receptor on leukocytes (36). This function of PAF, combined with the capability of PAF to induce transcription activators, is likely to contribute to gene expression essential for the development of several pathological conditions, including atherosclerosis. PAF stimulates monocytes to express HB-EGF, a potent mitogen for smooth muscle cells, and IL-1β, an inflammatory mediator. Both proteins are believed to be contributing factors for atherogenesis (20,26).
Chemoattractant receptors constitute a subgroup within the G-protein-coupled receptor superfamily (19). Several G-protein-coupled receptors are known to stimulate gene transcription (17,25). With these receptors, the signaling pathways leading to the activation of AP-1 has been partially delineated (34). PAF-induced AP-1 activity is likely to employ a similar mechanism. With respect to NFκB activation, current data support a prototypic model that involves IκB phosphorylation and degradation, followed by nuclear translocation of the p50/p65 heterodimer (12,16). The protein kinases that phosphorylate IκB, and the upstream signaling molecules, have not been completely identified. In this regard, an understanding of the chemoattractant receptor signal transduction pathway may provide complementary information to studies with other receptor systems. The observed differences between PAF and two other NFκB inducers suggest the existence of alternative mechanisms leading to IκB phosphorylation and degradation. Future experiments will focus on the identification of upstream signaling molecules utilizing reporter gene constructs and constitutively activated and dominant negative signaling molecules.
ACKNOWLEDGEMENTS
This work was done during the tenure of an Established Investigatorship (to R.D.Y.) from the American Heart Association, and was supported in part by NIH grants GM46572 and AI33503. This is publication 9768-IMM from the Scripps Research Institute. Figures 2, 3, 4, 6, and 10 were reproduced from Pan et al. (23) and Kravchenko et al. (15) with permission from The Journal of Biological Chemistry.
REFERENCES
- 1. Avodonin P. V.; Svitina-Ulitina I. V.; Kulikov V. I. Stimulation of high-affinity hormone-sensitive GTPase of human platelets by platelet activating factor. Biochem. Biophys. Res. Commun. 131:307–313; 1985. [DOI] [PubMed] [Google Scholar]
- 2. Baeuerle P. A.; Henkel T. Function and activation of NF-kB in the immune system. Annu. Rev. Immunol. 32:141–179; 1994. [DOI] [PubMed] [Google Scholar]
- 3. Baggiolini M.; Dewald B.; Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 55:97–179; 1994. [PubMed] [Google Scholar]
- 4. Beg A. A.; Baldwin A. S. Jr. Activation of multiple NF-kappa B/Rel DNA-binding complexes by tumor necrosis factor. Oncogene 9:1487–1492; 1994. [PubMed] [Google Scholar]
- 5. Cassatella M. A.; Bazzoni F.; Ceska M.; Ferro I.; Baggiolini M.; Berton G. IL-8 production by human polymorphonuclear leukocytes: The chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J. Immunol. 148:3216–3220; 1992. [PubMed] [Google Scholar]
- 6. Chiao P. J.; Miyamoto S.; Verma I. M. Autoregulation of IkBα activity. Proc. Natl. Acad. Sci. USA 91:28–32; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chomczynski P.; Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Anal. Biochem. 162:156–159; 1987. [DOI] [PubMed] [Google Scholar]
- 8. Cogswell J. P.; Godlevski M. M.; Wisely G. B.; Clay W.C.; Leesnitzer L.M.; Ways J. P.; Gray J. G. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J. Immunol. 153:712–723; 1994. [PubMed] [Google Scholar]
- 9. Dignam J. D.; Lebovitz R. M.; Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475–1489; 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ember J. A.; Sanderson S. D.; Hugli T. E.; Morgan E. L. Induction of interleukin-8 synthesis from monocytes by human C5a anaphylatoxin. Am. J. Pathol. 144:393–403; 1994. [PMC free article] [PubMed] [Google Scholar]
- 11. Fen Z.; Dhadly M. S.; Yoshizumi M.; Hilkert R. J.; Quertermous T.; Eddy R. L.; Shows T. B.; Lee M.-E. Structural organization and chromosomal assignment of the gene encoding the human heparin-binding epidermal growth factor-like growth factor/diptheria toxin receptor. Biochemistry 32:7932–7938; 1993. [DOI] [PubMed] [Google Scholar]
- 12. Finco T. S.; Baldwin A. S. Jr. Mechanistic aspects of NF-kappa B regulation: The emerging role of phosphorylation and proteolysis. Immunity 3:263–272; 1995. [DOI] [PubMed] [Google Scholar]
- 13. Goodman M. G.; Chenoweth D. E.; Weigle W. O. Induction of interleukin 1 1ecretion and enhancement of humoral immunity by binding of human C5a to macrophage surface C5a receptors. J. Exp. Med. 1156:912–917; 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483–16486; 1995. [DOI] [PubMed] [Google Scholar]
- 15. Kravchenko V. V.; Pan Z.; Han J.; Herbert J.-M.; Ulevitch R. J.; Ye R. D. Platelet-activating factor induces NF-kappa β activation through a G protein-coupled pathway. J. Biol. Chem. 270:14928–14934; 1995. [DOI] [PubMed] [Google Scholar]
- 16. Liou H. C.; Baltimore D. Regulation of the NF-kB/rel transcription factor and IkB inhibitor system. Curr. Biol. 5:477–487; 1993. [DOI] [PubMed] [Google Scholar]
- 17. Mari B.; Imbert V.; Belhacene N.; Far D. F.; Peyron J. F.; Pouyssegur J.; Van Obberghen Schilling E.; Rossi B.; Auberger P. Thrombin and thrombin receptor agonist peptide induce early events of T cell activation and synergize with TCR cross-linking for CD69 expression and interleukin 2 production. J. Biol. Chem. 269:8517–8523; 1994. [PubMed] [Google Scholar]
- 18. Mukaida N.; Okamoto S.; Ishikawa Y.; Matsushima K. Molecular mechanism of interleukin-8 gene expression. J. Leukocyte Biol. 56:554–558; 1994. [PubMed] [Google Scholar]
- 19. Murphy P. M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12:593–633; 1994. [DOI] [PubMed] [Google Scholar]
- 20. Nakano T.; Raines E. W.; Abraham J. A.; Klagsbrun M.; Ross R. Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc. Natl. Acad. Sci. USA 91:1069–1073; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oeth P. A.; Parry G. C.; Kunsch C.; Nantermet P.; Rosen C. A.; Mackman N. Lipopolysaccharide induction of tissue factor gene expression in monocytic cells is mediated by binding of c-Rel/p65 heterodimers to a kappa B-like site. Mol. Cell. Biol. 14:3772–3781; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oppenheim J. J.; Zachariae C. O. C. ; Mukaida N.; Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu. Rev. Immunol. 9:617–648; 1991. [DOI] [PubMed] [Google Scholar]
- 23. Pan Z.; Kravchenko V. V.; Ye R. D. Platelet-activating factor stimulates transcription of the heparin-binding epidermal growth factor-like growth factor in monocyte. Correlation with an increased kappa B binding activity. J. Biol. Chem 270:7787–7790; 1995. [DOI] [PubMed] [Google Scholar]
- 24. Prescott S. M.; Zimmerman G. A.; McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (l-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated by thrombin. Proc. Natl. Acad. Sci. USA 81:3534–3538; 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramirez M. T.; Post G. R.; Sulakhe P. V.; Brown J. H. Ml muscarinic receptors heterologously expressed in cardiac myocytes mediate Ras-dependent changes in gene expression. J. Biol. Chem. 270:8446–8451; 1995. [DOI] [PubMed] [Google Scholar]
- 26. Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362:801–809; 1993. [DOI] [PubMed] [Google Scholar]
- 27. Schindler R.; Gelfand J. A.; Dinarello C. A. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: Translational signal provided by lipopolysaccharide or IL-1 itself. Blood 76:1631–1638; 1990. [PubMed] [Google Scholar]
- 28. Schlondorff D.; Singhal P.; Hassid A.; Satriano J. A.; DeCandido S. Relationship of GTP-binding proteins, phospholipase C, and PGE2 synthesis in rat glomerular mesangial cells. Am. J. Physiol. 256: F171–F178; 1989. [DOI] [PubMed] [Google Scholar]
- 29. Sen R.; Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46:705–716; 1986. [DOI] [PubMed] [Google Scholar]
- 30. Snyderman R.; Uhing R. J. Phagocytic cells: Stimulus-response coupling mechanisms. In: Gallin J. I.; Goldstein I. M.; Snyderman R., eds. Inflammation: Basic principles and clinical correlates. 2nd ed. New York: Raven Press; 1992:421–439. [Google Scholar]
- 31. Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 76:301–314; 1994. [DOI] [PubMed] [Google Scholar]
- 32. Strieter R. M.; Kasahara K.; Allen R. M.; Standiford T. J.; Rolfe M. W.; Becker F. S.; Chensue S. W.; Kunkel S. L. Cytokine-induced deutrophils-derived interleukin-8. Am. J. Pathol. 141:397–407; 1992. [PMC free article] [PubMed] [Google Scholar]
- 33. Sun S. C.; Ganchi P. A.; Ballard D. W.; Greene W. C. NF-kB controls expression of inhibitor IkB: Evidence for an inducible autoregulatory pathway. Science 259:1912–1915; 1993. [DOI] [PubMed] [Google Scholar]
- 34. Van Obberghen Schilling E.; Pouyssegur J. Signaling pathways of the thrombin receptor. Thromb. Haemost. 70:163–167; 1993. [PubMed] [Google Scholar]
- 35. Whatley R. E.; Zimmerman G. A.; McIntyre T. M. Endothelium from diverse vascoular sources synthesizes platelet-activating factor. Arteriosclerosis 8:321–331; 1988. [DOI] [PubMed] [Google Scholar]
- 36. Zimmerman G. A.; McIntyre T. M.; Mehra M.; Prescott S. M. Endothelial cell-associated platelet-activating factor: A novel mechanism for signaling intercellular adhesion. J. Cell Biol. 110:529–540; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


