Abstract
All organisms must solve the same fundamental problem: they must acquire rewards and avoid danger in order to survive. A key challenge for the nervous system is therefore to connect motivationally salient sensory stimuli to neural circuits that engage appropriate valence-specific behavioral responses. Anatomical, behavioral, and electrophysiological data have long suggested that the amygdala plays a central role in this process. Here we review experimental efforts leveraging recent technological advances to provide previously unattainable insights into the functional, anatomical, and genetic identity of neural populations within the amygdala that connect sensory stimuli to valence-specific behavioral responses.
The amygdala and valence-specific behavior
Sensory stimuli can be described by both their salience (how arousing a stimulus is) and their valence (how good or bad a stimulus is). Salient sensory stimuli elicit behavioral responses that can be either specific to the valence of a stimulus, such as approach towards a reward, or valence non-specific, such as increases in autonomic reactivity in the presence of both rewarding and aversive stimuli. The notion that the amygdala plays a central role in connecting motivationally salient sensory stimuli to behavioral output was first suggested by the observation that removal of the temporal lobes caused profound motivational disturbances in primates [1–3]. This finding inspired many other groups to investigate the role of the amygdala in motivated behavior, typically by employing a Pavlovian fear-conditioning paradigm. In this paradigm, an aversive stimulus (unconditioned stimulus, US) is contingently paired with a neutral stimulus (conditioned stimulus, CS) such that an animal learns that the CS predicts the US [4]. Subsequent presentation of the CS alone elicits defensive responses similar to those evoked by the US. Lesions of either the basolateral or central nuclei of the amygdala prevent the acquisition and expression of learned defensive responses to a CS, while lesions of the basolateral amygdala impair innate responses to an aversive US [5–9]. These data have profoundly influenced the study of the neurobiology of motivated behavior, and have firmly established the importance of the amygdala in mediating behavioral and physiological responses to aversive stimuli.
In addition to the amygdala’s well-established role in defensive behaviors, converging evidence has implicated the amygdala in specific forms of appetitive behavior. Lesions of the basolateral nucleus of the amygdala impair behaviors that depend on both the sensory qualities and the value of a stimulus (such as reinforcer devaluation, second-order conditioning, and outcome-specific Pavlovian-instrumental transfer) [10–13], while lesions of the central nucleus of the amygdala produce deficits in behaviors that depend on the more general motivational properties of a stimulus (such as Pavlovian conditioned approach and generalized Pavlovian-instrumental transfer) [11, 14–16]. Since appetitive and aversive behavioral responses are often distinct, and even opposing, it follows that rewarding and aversive stimuli may engage distinct amygdala circuits to elicit valence-specific behavioral responses. In accord with this premise, converging lines of evidence have identified functionally distinct neural circuits in the amygdala that mediate appetitive and aversive behavioral responses, and recent studies have begun to delineate how these circuits might be defined at the anatomical and genetic levels.
Evidence for distinct positive and negative networks in the amygdala
Electrophysiological recordings of neural activity have consistently identified neural representations of both appetitive and aversive stimuli in the amygdala [17–36]. Some neurons have been shown to respond to CSs [17, 18, 25, 31, 33, 35, 37–39], others to USs [18, 19, 21, 29, 38, 40], and others to both CSs and USs [20–22, 24, 30, 32, 41]. Notably, neural responses to a CS change as a function of learning, with some neurons increasing their firing rate, and other neurons decreasing their firing rate, an observation recently confirmed by using calcium imaging to assess neural activity during fear learning [22–25, 30, 32, 33, 41]. The specific neurons that remain CS-responsive after learning may be dictated by their relative excitability at the time of conditioning [36, 42, 43].
Electrophysiological recordings in monkeys performing a trace conditioning task have shown that CSs of each valence elicit higher levels of activity in largely distinct, but anatomically intermingled, populations of neurons; this signal about CS valence is distinct from a neural signal that represents CS identity [32]. In these studies “valence preference” is termed “value coding”, and is defined by the differential activity of a neuron in response to a CS associated with either rewarding or aversive reinforcement. Neurons that exhibit higher activity in response to a CS when it predicts a rewarding US than to the same CS when it predicts an aversive US are classified as ‘positive value-coding’, and neurons with higher activity in response to a CS when it predicts an aversive US than when it predicts a rewarding US are classified as ‘negative value-coding’ (Figure 1A). Notably, simultaneously recorded neurons that prefer CSs with the same valence have been shown to exhibit short latency peaks in cross-correlograms, consistent with direct connectivity [44] and have been observed to update their responses to a CS upon a reversal in reinforcement contingencies at different rates depending upon the valence-preference of recorded neurons [45]. More recent studies in rodents have observed that CSs and USs of each valence differentially engage largely distinct yet anatomically intermingled neuronal populations[35, 41, 46–48]. Taken together, these results suggest that neurons within the amygdala display valence selectivity and exist in functionally discrete appetitive and aversive circuits [29, 30, 32, 44, 45]
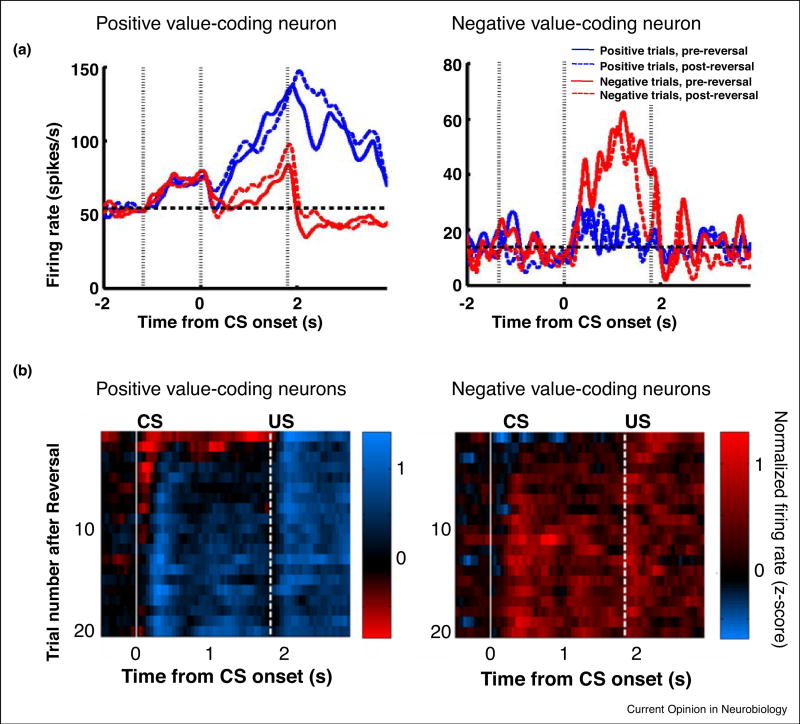
Figure 1. Positive and negative value-coding neurons in the amygdala.
A. Response profiles of two representative value-coding neurons in the primate amygdala recorded during a reversal learning task. A positive value-coding neuron (left) increases its firing rate to a CS that predicts an appetitive US (blue lines) while a negative value-coding neuron (right) responds to a CS that predicts an aversive US (red lines). Note that these neurons reflect the value of a CS rather than its identity, as they maintain value-coding after reinforcement contingencies are switched (solid lines, pre-reversal, dashed lines, postreversal). For details, see [44]. B. CS and US response profiles in a population of positive value-coding (left) or negative value-coding (right) neurons across 20 trials following a reversal in reinforcement contingencies. The color code plots the average normalized difference in activity on rewarding and aversive trials where a z-score of 0 means there is no difference in response to an appetitive vs aversive CS. Immediately after reversal (trial 1), there is discordance between CS and US selectivity (different colors after CS and US appearance on trial 1 for both populations). With learning, CS responses, at the population level, come to match US responses (same color after CS and US). For details, see [29]. Reproduced with permission from [29] and [44].
Through learning, neural representations of appetitive and aversive CSs becomes more similar to the representation of appetitive and aversive USs that entrained learning, at both the population and single-cell levels (Figure 1B) [29, 30, 41, 46] (but note that the response to USs themselves change as a function of learning, since amygdala neurons modulate their responses to a CS as a function of expectation [29, 49]
In principle, the valence-specific neural responses evoked by a learned CS in the amygdala may represent information about the sensory qualities of the US associated with a CS, rather than its valence per se. However, positive value-coding neurons have been shown to fire more in response to multiple stimuli of the same valence, including to a fixation point (itself a mildly positive CS), CSs predicting large rewards, and to multiple distinct rewarding USs [30, 47]. Negative value-coding neurons have been shown to exhibit the opposite response profile, decreasing their firing rate to a fixation point, and increasing their firing rate to CSs predicting aversive stimuli, and to multiple distinct aversive USs [30, 41]. Strikingly, on average positive value-coding neurons not only increase their firing in response to rewarding stimuli, but also decrease their firing rate to a CS predicting an aversive air-puff; negative value-coding neurons exhibit the opposite response profile [30]. In addition, while negative value coding neurons respond most strongly to a CS predicting an aversive air-puff, these cells also tend to respond more strongly to a CS predicting a small reward compared to a CS predicting a large reward [30]. These data indicate that individual amygdala neurons are capable of integrating information about different types of associated USs. Taken together, these findings demonstrate that amygdala neurons do not merely represent the sensory quality of an associated US; instead, they track the moment-to-moment value of stimuli that can span sensory modality and valence. This observation has led to the proposition that the amygdala represents state value; how good or bad a stimulus is at any given moment in time, given an animal’s internal and external situation.
Additional support for the proposition that the amygdala represents state value has been provided by a recent report utilizing a contrast revaluation procedure to demonstrate that amygdala neurons encode the relative value of a stimulus compared to other stimuli presented to an animal in the same experimental session [50]. In these experiments, the amount of reward associated with a CS remained constant, but its relative value was manipulated by changing the amount of reward associated with a second CS. Monkeys and amygdala neurons tracked the change in relative value of the first CS, indicating that even when a CS-US contingency does not change, an internal evaluative process can alter state value and the representation in the amygdala [50]. Similar observations have been made regarding amygdala neural responses to USs [19].
The extensive evidence linking the amygdala to valence-specific processing does not imply that the amygdala lacks neural signals related to other aspects of motivation. In addition to encoding the perceived value of motivationally salient stimuli, the amygdala also encodes signals related to motivational salience, irrespective of valence. Electrophysiological studies have demonstrated that neural responses in the amygdala are enhanced when unconditioned stimuli are unexpected [29, 49]; some studies have also reported amygdala neurons that respond to CSs that predict either rewarding or aversive USs [35]. Neurons activated by stimuli of either valence may play a role in orchestrating valence non-specific responses to motivationally salient stimuli, such as increases in arousal or attention [51]
The identification of discrete neural circuits that respond to rewarding and aversive stimuli in the amygdala suggests that these circuits may play a central role in connecting sensory stimuli to valence-specific behavioral output. To explicitly test this hypothesis requires asking whether activating amygdala neurons that respond to either a rewarding or an aversive stimulus is capable of eliciting valence-specific behavioral responses. Unfortunately, the absence of obvious gross anatomical segregation between positive and negative networks within the amygdala (but see [52]) has made it difficult to perform causal manipulations to assay the function of each network independently using classical lesion, pharmacological, electrical, or even optical techniques to manipulate neural activity. To circumvent this, rodent researchers have recently developed several different approaches that utilize immediate early gene (IEG) promoters to drive expression of reporter proteins in an activity-dependent manner, facilitating genetic access to neurons that are activated by an array of different stimuli [46, 53–64].
Two recent studies utilized this approach to induce expression of the photoactivatable cation channel, channelrhodopsin, in cells that respond to either rewarding or aversive stimuli in the basolateral amygdala (BLA) [46, 64]. It should be noted that since neurons in the amygdala display mixed-selectivity [39], it is unlikely that these neurons respond exclusively to rewarding or aversive stimuli, but instead are likely to respond to multiple stimuli, potentially even of opposing valence. In support of this, an electrophysiology study in monkeys showed that “positive” valence preferring neurons often respond to both rewarding and aversive USs, an observation also made for “negative” valence preferring neurons[32].This notwithstanding, in both studies, subsequent photoactivation of neurons in appetitive networks elicited reward-related behaviors, and photoactivation of neurons in aversive networks elicited defensive behavioral responses. In addition, photoactivation of US representations was capable of driving valence-specific learning, while photoinhibition of an aversive US representation prevented the expression of previously learned defensive responses [46]. These data suggest that CSs generate learned behavioral responses through the activity of the entraining US representation. Finally, consistent with prior electrophysiological studies [29, 30, 32, 44, 45], the activity of neurons from appetitive and aversive networks in the amygdala could not be entrained to the opposite valence [64], indicating that valence-specific networks in the amygdala may be fixed. This observation raises the interesting possibility that appetitive and aversive networks in the amygdala are predetermined to elicit valence-specific behavioral responses by virtue of their anatomical connectivity, their genetic identity, or both.
Defining positive and negative networks
Anatomical Connections
One mechanism by which the distinct appetitive and aversive networks within the amygdala might coordinate opposing behavioral responses is through differential connectivity to downstream brain regions. Anatomical tracing studies have demonstrated that the amygdala sends projections to numerous cortical and subcortical structures that have been implicated in the generation of an array of different motivated behaviors [65, 66]. The advent of optogenetics, a strategy with which to express light-sensitive channels and pumps in genetically defined populations of neurons in order to make their activity controllable by light [67], has enabled researchers to ask whether the activity of specific efferent projections from the amygdala can drive different motivated behaviors. Photoactivation of BLA axon terminals in the prelimbic cortex, ventral hippocampus, and central amygdala elicits defensive and anxiety-related behaviors [68–71], while photoactivation of BLA axon terminals in the nucleus accumbens elicits appetitive behaviors, and supports instrumental responding [70, 72]. These studies demonstrate that distinct anatomical projections from the amygdala are capable of eliciting valence-specific behavioral responses. Yet, the extent to which these projections are engaged during natural behavior has remained somewhat unclear.
Recent studies have begun to examine the physiological function of amygdala neurons that project to distinct brain regions. In one study, investigators employed retrograde labeling strategies to identify BLA neurons that project to either the nucleus accumbens or the central nucleus of the amygdala [70]. The authors demonstrated that photoactivation of these neurons could serve as a positive or negative reinforcer, respectively. Moreover, electrophysiological recordings in ex vivo slice preparations revealed that these neural populations undergo opposite changes in synaptic plasticity following reward or fear learning. In a subsequent study, the same group conducted in vivo electrophysiological recordings of BLA neurons that project to either the nucleus accumbens or central nucleus of the amygdala during the performance of an appetitive or aversive conditioning paradigm [48]. BLA neurons projecting to the nucleus accumbens preferentially responded to an auditory cue predicting an appetitive sucrose US; by contrast, BLA neurons that project to the central nucleus of the amygdala preferentially responded to an auditory cue predicting an aversive quinine US. These data suggest that positive and negative networks in the BLA may be segregated in their outputs to the nucleus accumbens and central amygdala (Figure 2). However, the use of tastants as unconditioned stimuli means that the ‘correct’ behavioral response to the quinine predictive cue was to stop licking in order to avoid the quinine. As a consequence, avoidance of the quinine punishment may itself be rewarding, complicating interpretation of neural responses to the ‘aversive CS’. In addition, the authors observed substantial collateralization of BLA projection neurons to both the nucleus accumbens and central amygdala [48]. This collateralization may help to explain why a recent study reported that BLA neurons that respond to either reward or punishment project to both the nucleus accumbens and central nucleus of the amygdala [52]. Furthermore, photoactivation of the nucleus accumbens projections from a genetically defined subset of BLA neurons can elicit defensive behaviors [52]. It is therefore possible that appetitive and aversive networks in the BLA send projections to both the nucleus accumbens and central amygdala to mediate different elements of valence-specific behavioral responses (Figure 2).
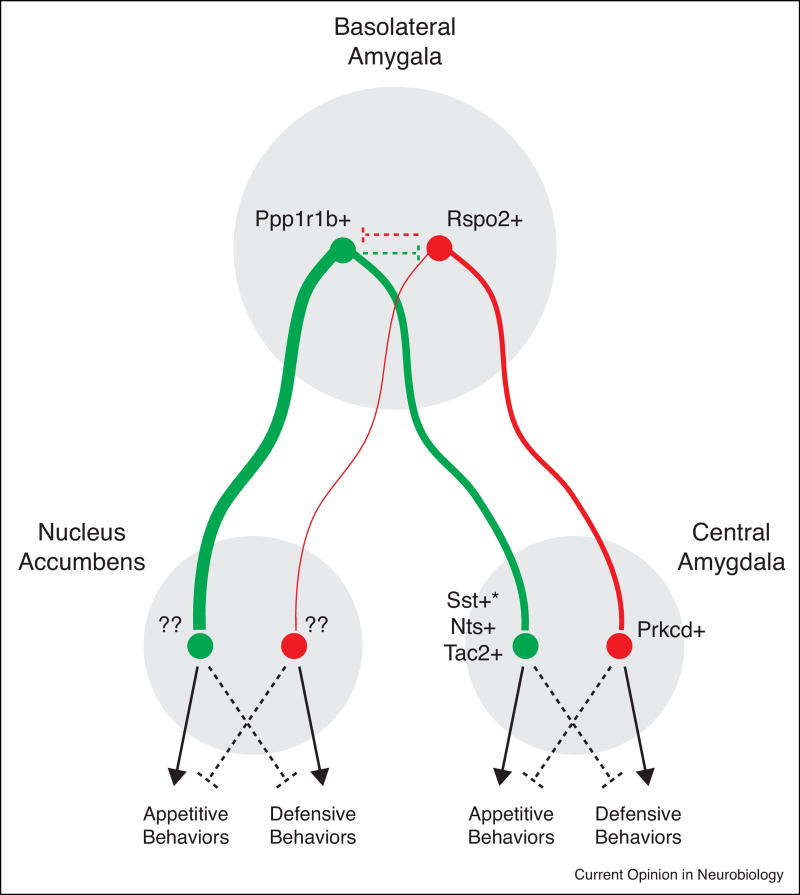
Figure 2. Anatomical connectivity and genetic identity of positive and negative value-coding BLA neurons.
A model, based on the integration of findings from [48, 52, 70, 74], in which genetic markers and anatomical connections from the BLA to the nucleus accumbens and central amygdala determine valence-specific behaviors. Green = neurons activated by rewarding stimuli. Red = neurons activated by aversive stimuli. Solid line = excitatory connection. Dashed line = inhibitory connection. Ppp1rlb = protein phosphatase 1 regulatory inhibitor subunit 1B. Rspo2 = R-spondin 2. Sst = somatostatin. Nts = neurotensin. Tac2 = tachykinin 2. *Note that the activity of somatostatin-expressing neurons in the central amygdala has also been shown to be able to elicit defensive behaviors [75].
Although the activity of amygdala axonal projections to specific brain regions is capable of eliciting valence-specific behavioral responses, the biological function of many of these projections may be more nuanced. Indeed, some behavioral responses to rewarding and aversive stimuli are clearly distinct; liquid rewards induce licking, whereas the presence of a predator may induce micturition. These behavioral responses are likely mediated through specific neural circuitry mediating distinct functions. However some behavioral responses to rewarding and aversive stimuli are less distinct: approach to a reward and avoidance of an aversive stimulus likely both engage motor circuits involved in action planning and execution. This might explain why the BLA projection to the nucleus accumbens, which has extensive connectivity with motor circuits [73], is capable of eliciting both appetitive and aversive behavioral responses. Overall, specific anatomical connectivity must underlie the expression of appetitive and aversive behavior, but the anatomical scale – whether at the local circuit or brain area level – may vary depending upon the behavioral or physiological response.
Genetic Identity
Recent advances in activity-dependent labeling of neurons and genetic sequencing technologies permit rapid and comprehensive genetic characterization of neurons that respond to different stimuli. One group combined this approach with screening on the Allen Mouse Brain Atlas to identify two genes, protein phosphatase 1 regulatory inhibitor subunit 1B (Ppp1r1b) and R-spondin 2 (Rspo2), that are selectively enriched in amygdala neurons that respond to rewarding and aversive stimuli, respectively (Figure 2) [52]. In contrast to prior studies demonstrating that neurons responding to rewarding and aversive stimuli are anatomically intermingled, the researchers observed an anterior to posterior segregation of Ppp1r1b+ and Rspo2+ neurons, with Ppp1r1b expressed predominantly in the posterior BLA, and Rspo2 expressed predominantly in the anterior BLA. The authors replicated this finding using c-Fos immunohistochemistry to demonstrate that other rewarding stimuli, including sucrose and water, primarily activate the posterior BLA, while other aversive stimuli, such as quinine and trimethylthiazoline (TMT), primarily activate the anterior BLA. The discrepancy between this finding and prior reports may be due to the fact that prior studies have mainly sampled from the medial portion of the BLA, where both rewarding and aversive stimuli induce comparable levels of c-Fos expression.
In the same set of studies, the group then examined the functional role of Ppp1r1b+ and Rspo2+ neurons in the generation of valence-specific behavioral responses. Optogenetic activation and inactivation experiments demonstrated that the activity of Ppp1r1b+ and Rspo2+ neurons was both necessary and sufficient for appetitive and aversive behavioral responding, respectively. Moreover, photoactivation of Ppp1r1b+ neurons inhibited Rspo2+ neurons and disrupted aversive behavior, while activation of Rspo2+ neurons inhibited Ppp1r1b+ neurons and disrupted reward-seeking behavior. These data suggest that in addition to eliciting valence-specific responses directly, these genetically defined subsets of neurons also mutually inhibit each other, potentially favoring binary behavioral responses. In addition, subsequent analyses in the central nucleus of the amygdala have suggested that Ppp1r1b+ and Rspo2+ BLA neurons may have genetically distinct post-synaptic targets capable of facilitating and suppressing appetitive responding [74].
While the behavioral results of this study are compelling, it should be noted that only a small fraction of either Rspo2+ or Ppp1r1b+ neurons are activated (based on c-fos expression) by either footshock or female presentation, respectively. It therefore remains to be determined whether neurons within each genetically defined population that are not activated by these reinforcers respond to other stimuli of the same valence, or not. Future experiments conducting single cell RNA sequencing of neurons activated by a range of rewarding or aversive stimuli or events are therefore critical to furthering our understanding of the extent to which appetitive and aversive networks are defined by genetic identity.
Conclusions and Future Directions
The amygdala plays a central role in connecting sensory stimuli to appropriate valence-specific behavioral output. The studies outlined in this review suggest that inherently reinforcing unconditioned stimuli (USs) activate populations of amygdala neurons predetermined to elicit appetitive or aversive behavioral responses via a combination of their genetic identity and anatomical connectivity. Since neutral sensory stimuli only acquire valence through experience, it follows that absent learning, neutral CSs do not robustly activate valence-specific behavioral outputs [48, 70]. However, after learning a CS can drive valence-specific behavior, likely through mechanisms that require reactivation of US representations in the BLA [46]. The capacity of a CS to drive valence-specific behavior, however, often depends upon rapidly evolving contextual information; in blackjack the presentation of a king can be highly rewarding or highly aversive depending upon the other cards in a player’s hand. Recent recordings in monkeys have shown that both behavioral and neural responses to a CS can switch when subjects use inference to adjust predictions of reinforcement outcomes [39]. This flexible adjustment of responses to a CS cannot be attributed to learning-related plasticity but instead must be due to the implementation of a rule. How rule-based tasks rapidly engage appropriate appetitive and aversive networks in the amygdala is unclear. It therefore remains to be established how appetitive and aversive networks are dynamically regulated to mediate complex goal-directed behaviors.
Highlights.
Neurons in the amygdala respond to rewarding and aversive stimuli and provide a representation of state value
Neurophysiological, anatomical and genetic methods have characterized distinct neural circuits in the amygdala that preferentially process appetitive and aversive stimuli
Valence-specific behavioral responses to reinforcing stimuli are mediated by these distinct neural circuits in the amygdala
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have a conflict of interest.
References
- 1.Brown S, Schafer E. An investigation into the functions of the occipital and temporal lobes of the monkey's brain. Philosophical Transactions of the Royal Society. 1888;179:303–327. [Google Scholar]
- 2.Klüver H, Bucy P. 'Psychic blindness' and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. American Journal of Physiology. 1937;119:352–353. [Google Scholar]
- 3.Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49(4):381–91. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 4.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81(2):281–90. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10(4):1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15(3 Pt 2):2301–11. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110(4):718–26. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 9.Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680–5. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16(16):5256–65. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25(4):962–70. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci. 2002;15(11):1841–53. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- 13.Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci. 2001;21(22):9018–26. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10(6):1906–11. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13(10):1984–92. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 16.Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17(8):1680–94. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- 17.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1(2):155–9. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 18.Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68(2):339–61. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 19.Bermudez MA, Schultz W. Reward magnitude coding in primate amygdala neurons. J Neurophysiol. 2010;104(6):3424–32. doi: 10.1152/jn.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52(3):621–36. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- 21.Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107(3):444–50. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- 22.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15(5):1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 23.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19(3):613–24. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417(6886):282–7. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 25.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453(7199):1253–7. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23(35):11054–64. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59(4):648–61. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishijo H, Ono T, Nishino H. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J Neurosci. 1988;8(10):3570–83. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55(6):970–84. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28(40):10023–30. doi: 10.1523/JNEUROSCI.1400-08.2008. ** The authors conducted single unit electrophysiological recordings while monkeys performed a trace conditioning task. Amygdala neurons were shown to respond to stimuli of the same valence irrespective of sensory quality; positive value-coding cells exhibited higher firing rates in response to large liquid reward, visual stimuli that predict reward and stimuli long associated with reward (a fixation point). Moreover, these same neurons responded less to air puff and smaller magnitude rewards. These experiments led to the hypothesis that the amygdala tracks state value. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cain DP, Bindra D. Responses of amygdala single units to odors in the rat. Exp Neurol. 1972;35(1):98–110. doi: 10.1016/0014-4886(72)90062-3. [DOI] [PubMed] [Google Scholar]
- 32.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–70. doi: 10.1038/nature04490. ** The authors recorded the activity of amygdala neurons as monkeys were trained to associate visual stimuli with either a liquid reward or an aversive air puff in the same session. Individual neurons were found to encode the positive or negative value of conditioned stimuli, independent of stimulus identity. Specifically, cells classified as ‘positive value-coding’ continued to respond to the reward-predictive CS even after reversal of CS-US contingencies, while cells classified as ‘negative value-coding’ continued to respond to the CS that predicted an aversive stimulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4(7):724–31. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 34.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390(6660):604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 35.Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc Natl Acad Sci U S A. 2009;106(35):15031–6. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83(3):722–35. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–6. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 38.Livneh U, Paz R. Aversive-bias and stage-selectivity in neurons of the primate amygdala during acquisition, extinction, and overnight retention. J Neurosci. 2012;32(25):8598–610. doi: 10.1523/JNEUROSCI.0323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saez A, Rigotti M, Ostojic S, Fusi S, Salzman CD. Abstract Context Representations in Primate Amygdala and Prefrontal Cortex. Neuron. 2015;87(4):869–81. doi: 10.1016/j.neuron.2015.07.024. *The authors used a serial-reversal task in which stimulus-reinforcement contingencies of two CSs were reversed in a block-wise fashion. Behaviorally, monkeys displayed inference; they updated the meaning of one CS even though they had only been previously presented with the other reversed CS. Remarkably, appetitive and aversive networks in the amygdala also displayed inference, responding appropriately to both CSs after exposure to just one reversed CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509(7501):453–8. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- 41.Grewe BF, Grundemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, et al. Neural ensemble dynamics underlying a long-term associative memory. Nature. 2017;543(7647):670–675. doi: 10.1038/nature21682. * The authors utilized recently developed head-mounted miniature microsopes in mice to stably record calcium dynamics in a population of BLA neurons across several days of fear conditioning. This work bolsters previous electrophysiological evidence for the convergence of CS and US representations during learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rashid AJ, Yan C, Mercaldo V, Hsiang HL, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, et al. Competition between engrams influences fear memory formation and recall. Science. 2016;353(6297):383–7. doi: 10.1126/science.aaf0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiang HL, Epp JR, van den Oever MC, Yan C, Rashid AJ, Insel N, Ye L, Niibori Y, Deisseroth K, Frankland PW, et al. Manipulating a "cocaine engram" in mice. J Neurosci. 2014;34(42):14115–27. doi: 10.1523/JNEUROSCI.3327-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Schneider DM, Belova MA, Morrison SE, Paton JJ, Salzman CD. Functional circuits and anatomical distribution of response properties in the primate amygdala. J Neurosci. 2013;33(2):722–33. doi: 10.1523/JNEUROSCI.2970-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison SE, Saez A, Lau B, Salzman CD. Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron. 2011;71(6):1127–40. doi: 10.1016/j.neuron.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gore F, Schwartz EC, Brangers BC, Aladi S, Stujenske JM, Likhtik E, Russo MJ, Gordon JA, Salzman CD, Axel R. Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses. Cell. 2015;162(1):134–45. doi: 10.1016/j.cell.2015.06.027. ** The authors used activity-dependent gene expression to label neurons in the mouse basolateral amygdala that responded to a rewarding (nicotine) or an aversive (foot shock) stimulus. In support of electrophysiological reports in monkeys, they found that rewarding and aversive stimuli activated distinct but anatomically intermingled populations of neurons. Moreover, photoactivation of these neurons was able to elicit valence-specific behavior, while photoinhibition of shock-responsive neurons prevented expression of learned defensive responses indicating that activity of the entraining US ensemble is necessary for a CS to elicit learned behavioral responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiu J, Zhang Q, Zhou T, Zhou TT, Chen Y, Hu H. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat Neurosci. 2014;17(11):1552–9. doi: 10.1038/nn.3813. [DOI] [PubMed] [Google Scholar]
- 48.Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, Tye KM. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron. 2016;90(2):348–361. doi: 10.1016/j.neuron.2016.03.004. ** This study utilized retrograde labeling to examine how basolateral amygdala neurons that project to either the nucleus accumbens or the central nucleus of the amygdala are engaged during appetitive and aversive learned behavior. The authors demonstrated that the majority of neurons that project to the nucleus accumbens were excited by a sucrose-predictive cue, whereas a small majority of neurons that projected to the central nucleus of the amygdala were excited by a quinine-predictive cue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci. 2010;13(8):979–86. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saez RA, Saez A, Paton JJ, Lau B, Salzman CD. Distinct Roles for the Amygdala and Orbitofrontal Cortex in Representing the Relative Amount of Expected Reward. Neuron. 2017;95(1):70–77. e3. doi: 10.1016/j.neuron.2017.06.012. * This study demonstrated that neural responses to a CS in the amygdala can reflect changes in state value induced by an internal process, rather than by changes in reinforcement prediction. Monkeys were trained on a ‘contrast revaluation task’ in which the amount of liquid reward associated with one CS remained constant while its relative value was varied by altering the amount of reward associated with another CS. Neurons in the amygdala (and orbitofrontal cortex) tracked the change in relative value of the CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peck CJ, Lau B, Salzman CD. The primate amygdala combines information about space and value. Nat Neurosci. 2013;16(3):340–8. doi: 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016;19(12):1636–1646. doi: 10.1038/nn.4414. ** The authors conducted genetic sequencing of neurons that were activated by either a rewarding or aversive event. Using this approach, they identified two genetically distinct populations of amygdala neurons that were activated by either rewarding or aversive stimuli, and that mediated valence-specific behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341(6144):387–91. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 54.Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78(5):773–84. doi: 10.1016/j.neuron.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317(5842):1230–3. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 56.Sorensen AT, Cooper YA, Baratta MV, Weng FJ, Zhang Y, Ramamoorthi K, Fropf R, LaVerriere E, Xue J, Young A, et al. A robust activity marking system for exploring active neuronal ensembles. Elife. 2016;5 doi: 10.7554/eLife.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Root CM, Denny CA, Hen R, Axel R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515(7526):269–73. doi: 10.1038/nature13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawashima T, Kitamura K, Suzuki K, Nonaka M, Kamijo S, Takemoto-Kimura S, Kano M, Okuno H, Ohki K, Bito H. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat Methods. 2013;10(9):889–95. doi: 10.1038/nmeth.2559. [DOI] [PubMed] [Google Scholar]
- 59.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12(8):1069–73. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye L, Allen WE, Thompson KR, Tian Q, Hsueh B, Ramakrishnan C, Wang AC, Jennings JH, Adhikari A, Halpern CH, et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell. 2016;165(7):1776–1788. doi: 10.1016/j.cell.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD, Fu M, Han BX, Wang F. Capturing and Manipulating Activated Neuronal Ensembles with CANE Delineates a Hypothalamic Social-Fear Circuit. Neuron. 2016;92(4):739–753. doi: 10.1016/j.neuron.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim CK, Ye L, Jennings JH, Pichamoorthy N, Tang DD, Yoo AW, Ramakrishnan C, Deisseroth K. Molecular and Circuit-Dynamical Identification of Top-Down Neural Mechanisms for Restraint of Reward Seeking. Cell. 2017;170(5):1013–1027. e14. doi: 10.1016/j.cell.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W, Wildes CP, Pattarabanjird T, Sanchez MI, Glober GF, Matthews GA, Tye KM, Ting AY. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat Biotechnol. 2017 doi: 10.1038/nbt.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513(7518):426–30. doi: 10.1038/nature13725. ** The authors utilized activity-dependent expression of channelrhodopsin to label basolateral amygdala neurons that were activated by either a rewarding (exposure to female) or an aversive (contextual fear conditioning) event. Photoactivation of appetitive or aversive ensembles elicited reward-related or defensive behaviors, respectively. Notably, in contrast to event-activated representations in the dentate gyrus, valence-specific representations in the amygdala could not be entrained to the opposing valence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amaral DG, Price J, Pitkanen A, Carmichael S. In: In the amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J, editor. Wiley-Liss; New York: 1992. [Google Scholar]
- 66.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83(3):803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 67.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, Luthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81(2):428–37. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci. 2017;20(6):824–835. doi: 10.1038/nn.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520(7549):675–8. doi: 10.1038/nature14366. * This work suggests that valence specific behaviors may be mediated through projection specific BLA populations. The authors used retrograde labeling methods to identify BLA neurons that project to the nucleus accumbens or central nucleus of the amygdala. Photoactivation of accumbens projecting or central nucleus projecting neurons could drive appetitive or aversive learning, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79(4):658–64. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–80. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293(2):282–98. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- 74.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017;93(6):1464–1479. e5. doi: 10.1016/j.neuron.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience dependent modification of a central amygdala fear circuit. Nat. Neurosci. 2013;16(3):332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]