Abstract
Introduction:
Diabetes mellitus (DM) affects peripheral nerves inducing diabetic polyneuropathy (DPN). Mitochondrial dysfunction and oxidative stress are potential causes of DPN.
Methods:
Nerve conduction studies were performed in 20 type 2 DM patients (11 with DPN) and 15 healthy controls. Perception threshold values of cold, warm and vibration were measured by quantitative sensory testing. Variants of a superoxide dismutase 2 (SOD2) gene single nucleotide polymorphism (SNP) (rs5746136) were determined by polymerase chain reaction (PCR) and following NexteraXT DNA Library.
Results:
DPN patients showed significantly increased threshold values. DM patients without DPN and healthy controls showed comparable values. TT variant of the SOD2 SNP was more prevalent in DM and DPN patients. DM patients with the TT variant displayed increased perception threshold values.
Conclusions:
Quantitative sensory testing is not superior to standard nerve conduction studies in DPN. Carriers of SOD2 SNP manifested increased sensory threshold levels which is important in further corroborating the link between oxidative stress and DPN.
Keywords: Diabetes mellitus, polyneuropathy, superoxide dismutase, quantitative sensory testing, polymorphism
INTRODUCTION
Peripheral neuropathy is a frequently encountered complication of diabetes mellitus (DM). Nerve conduction study is a reliable and sensitive method for diagnosis and progression follow-up of diabetic polyneuropathy (DPN). Nevertheless, many DM patients with evident neuropathic signs and symptoms may display normal nerve conduction studies prompting development of more sensitive methods (1). In this context, quantitative sensory testing (QST) has been widely used to improve the diagnostic accuracy of DPN (2, 3).
A plethora of potential underlying factors including altered Na/K ATPase activity, reduced anaerobic glycolysis, polyol accumulation, altered protein glycation, nerve microangiopathy and mitochondrial dysfunction have been proposed for the physiopathology of DPN. Mitochondrial dysfunction leads to oxidative stress and increased levels of reactive oxygen species, which in due course cause axonal dysfunction (4).
Superoxide dismutase 2 (SOD2) is an antioxidant defense enzyme in which dismutate two superoxide radicals, converting to H2O2 and O2 in mitochondria (5). Mn-SOD (SOD2) gene is located on chromosome 6q25 which has five exons and four introns. We aimed to investigate C/T polymorphism (rs5746136) located at 5th intron of SOD2 gene, the variations of which have a very important role in micro- and macrovascular complications in diabetes (6). Genetic variants of several genes involved in oxidative stress, including the superoxide dismutase (SOD) 2 gene, have been associated with DPN (7, 8).
In this study, we assessed the diagnostic value of QST in DPN and investigated the association between electrophysiological findings of DM patients and a well-established SOD2 single nucleotide polymorphism (SNP) rs5746136 (9–12).
METHODS
Patients
Consecutive 20 type 2 DM patients (average age ± standard deviation, 57.5±11.1; 15 men, 5 women) and 15 age-gender matched healthy controls (average age ± standard deviation, 59.1±8.5; 11 men, 4 women) were included in the study. DM patients had an average of 13.3±5.5 years of disease duration. All patients and healthy controls filled out a questionnaire for neuropathic symptoms, underwent a detailed neurological examination, blood biochemistry, and total blood count tests. DM patients with less than 1 year of disease duration, additional coexisting disorders including peripheral nerve trauma, peripheral vascular disease, infections and malignancy, a history of alcohol or substance abuse, thyroid dysfunction, vitamin B12, folate deficiency, and a history of exposure to any medication or toxic substance that can potentially cause polyneuropathy were excluded. The local ethical committee approved the study, and all subjects gave written consent.
Electromyography and QST
Motor nerve conduction velocity, compound muscle action potential, sensory nerve conduction velocity, and sensory nerve action potential were measured unilaterally in motor (median, ulnar, tibial and peroneal) and sensory (median, ulnar, sural and medial plantar) nerves using standard methods. DPN was diagnosed using previously published criteria based on presence of neuropathic symptoms, decreased/absent ankle reflexes, abnormal sensory and motor findings, and abnormal nerve conduction studies (1).
Cold, warm, and vibration perception thresholds were assessed by a computer aided sensory evaluator (CASE IV, WR Medical Electronics, MN, USA). The thresholds were measured using the 4, 2 and single stepping algorithm with null stimuli as described (13). The measurements were conducted at the dorsum of foot and hand of the same side. For each test, the computer calculated the just-noticeable difference (JND) for discriminating two levels of stimuli, using a set of 25 standard vibratory and thermal stimulation levels. The JND threshold at which the patient failed to discriminate stimuli was recorded and expressed as computed threshold (CT) value for a given modality of sensory stimulus. A value of 26 was given to JNDs higher than the maximum which is 25. Higher CT levels corresponded to a relatively worse sensory perception level, and thus a potential damage of the peripheral nerves.
Polymerase chain reaction (PCR)
Venous blood samples were collected from EDTA-containing tubes, and DNA extraction was performed using blood DNA isolation kit (Norgen Inc., Canada). Extracted DNA samples were prepared, and then 17.5 kB DNA fragments were amplified with PCR using forward 5’ACATTTACGCCTCATGCCCT3’, reverse 5’TAGGTCCCAAGGTCGGCTTA3’, forward 5’GCTATGACTAGGGCAGGCAC3’ and reverse 5’GGAGGGCATGAGGCGTAAAT3’ primers under following PCR conditions; 1 min 94°C, 10 sec 98°C (30 cycles), 15 min 68°C (30 cycles), and 10 min 72°C in T100 Thermal Cycler (Bio-Rad, USA). Amplified products were controlled and electrophoresed in 3.5% agarose gel under UV trans-illuminator.
NexteraXT DNA Library
Library preparation of all PCR Amplicons were carried out using NexteraXT DNA Library Preparation Kit and NexteraXT Index Kit according to the producer’s instructions. The prepared libraries were sequenced in MiSeq instrument (Illumina, San Diego, CA, USA).
Statistics
Clinical, demographic, and QST parameters of DM patient subgroups were compared with Student’s t-test or chi-square test, as required. CT values of healthy controls and DM patients with and without polyneuropathy were compared with ANOVA and Tukey’s post-hoc test. A p value lower than 0.05 was accepted as statistically significant.
RESULTS
Electrophysiological studies
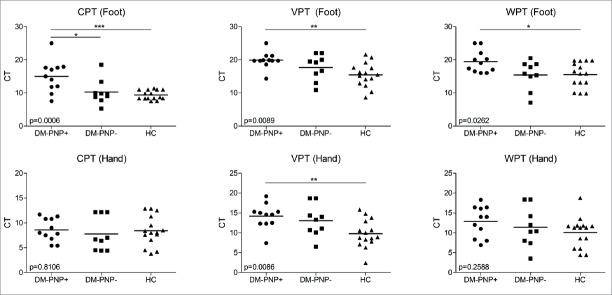
Electromyography investigations detected DPN in 11 DM patients, and none of the healthy controls. QST showed significantly increased cold (p=0.0006), vibration (p=0.0089), and warm (p=0.0262) perception threshold values in foot recordings of DM patients with polyneuropathy. In hand recordings, statistically significant differences could only be obtained in vibration perception threshold values (p=0.0086), whereas cold (p=0.8106) and warm (p=0.2588) perception threshold values were comparable among study groups. In two-group comparisons, DM patients with polyneuropathy displayed significantly increased computed threshold values than healthy controls in cold, vibration, and warm perception threshold values of the feet, and vibration perception threshold values of the hands. When DM patients with and without polyneuropathy was compared, a significant difference was obtained only in cold perception threshold values of the feet (Figure 1).
Figure 1.
Computed threshold (CT) values of healthy controls (HC) and diabetes mellitus (DM) patients with and without polyneuropathy (PNP+ vs PNP-). Horizontal bars show mean values. p values obtained by ANOVA test are indicated in the lower left corner of each panel. Lines on the top of the panels indicate significant p values in two-group comparisons (by Tukey’s post-hoc test). *, p<0.05; **, p<0.01; ***, p<0.001. CPT, cold perception threshold; VPT, vibration perception threshold; WPT, warm perception threshold.
SOD2 SNP and associated features
Fourteen out of 15 (93.3%) healthy controls showed the CT genotype of the SOD2 gene (rs5746136), while one healthy control (6.7%) had the TT genotype. In contrast, CT and TT genotypes were found in 14 (70%), and 6 of 20 (30%) DM patients, respectively. None of the patients or controls showed the CC genotype. Thus, DM patients had a higher prevalence of the TT variant of SOD2 (rs5746136) than healthy controls [p=0.044 by chi-square test; odds ratio (95% confidence interval), 0.2 (0.1–1.5)]. DM patients with CT and TT genotypes had comparable age, gender, blood, and triglyceride levels. No significant difference was found between DM patients with CT and TT genotypes by means of DPN prevalence [p=0.246 by chi-square test; odds ratio (95% confidence interval), 0.5 (0.1–3.7)]. Although DM patients with TT genotype showed higher fasting blood glucose and total cholesterol levels, these differences did not attain statistical significance. While DM patients with the TT genotype generally displayed higher computed threshold values, only vibration perception thresholds of the feet, and cold and warm perception thresholds of the hands were significantly higher than those of DM patients with the CT genotype (Table 1).
Table 1.
Demographic, clinical and quantitative sensory testing features of diabetes mellitus (DM) patients with CT and TT variants of SOD2 (rs5746136)
| CT (n=14) | TT (n=6) | p value* | |
|---|---|---|---|
| Age | 56.7±9.5 | 59.3±15.3 | 0.355 |
| Gender | 11 men/ 3 women | 4 men/ 2 women | 0.287 |
| Disease duration (years) | 12.5±5.8 | 15.3±4.5 | 0.131 |
| Fasting blood glucose (mg/dl) | 142.4±46.1 | 202.0±73.5 | 0.057 |
| Hemoglobin A1 c (%) | 6.9±2.2 | 7.7±2.7 | 0.295 |
| Total cholesterol (mg/dl) | 172.2±43.9 | 199.0±23.5 | 0.052 |
| Triglyceride (mg/dl) | 168.5±30.6 | 179.3±78.7 | 0.252 |
| Polyneuropathy (+/-) | 7/7 | 4/2 | 0.246 |
| Cold perception CT** (foot) | 12.3±6.0 | 13.7±4.5 | 0.315 |
| Vibration perception CT (foot) | 18.0±3.5 | 21.0±0.9 | 0.004 |
| Warm perception CT (foot) | 16.5±4.9 | 18.7±2.1 | 0.084 |
| Cold perception CT (hand) | 7.3±2.8 | 11.5±0.8 | <0.001 |
| Vibration perception CT (hand) | 12.9±3.5 | 15.4±2.9 | 0.061 |
| Warm perception CT (hand) | 10.1±4.4 | 17.4±1.2 | <0.001 |
Numeric values were denoted as mean ± standard deviation. Significant p values were denoted with bold characters.
p values were calculated with chi-square test (gender and polyneuropathy prevalence) or Student’s t-test.
CT, computed threshold values.
DISCUSSION
SOD2, also known as manganese-dependent SOD, is a mitochondrial enzyme encoded by genomic DNA, and is involved in detoxification of free radicals produced by mitochondrial respiration. The SOD2 gene is upregulated under oxidative stress, and protects the cells against the deleterious effects of reactive oxygen species (14). As an oxidative stress-related gene, the effects of the SOD2 variant rs5746136 have been investigated in asthma, primary open angle glaucoma, and prostate cancer (9–11). It has also been associated with increased risk of lower gestational age, and birth weight (12). However, to our knowledge, the rs5746136 variant has never been studied in DM or polyneuropathy patients.
In our study, individuals carrying the TT genotype of rs5746136 manifested a modestly increased risk of DM in comparison with those carrying the CT genotype. Moreover, TT genotype carriers showed worse vibration and thermal perception threshold levels than non-TT carriers. These results suggest that this SOD2 SNP might potentially contribute to increased DPN risk, presumably through oxidative stress-related disease mechanisms. An intriguing finding was absence of the CC genotype in our diabetic and healthy participants, although this genotype has been previously demonstrated in several cohorts (9, 11). This might be due to small number of patients in our study, and/or disparate genetic features of our local population.
Standard nerve conduction studies predominantly evaluate the large myelinated fibers, whereas QST examines the functions of both large myelinated nerve fibers (vibration), and small A-delta and C nerve fibers (cold and warm) (15). Since DPN often affects small nerve fibers, QST is expected to yield positive results. However, in our study, only the diabetic patients with abnormal nerve conduction studies showed increased CT values. Although diabetic patients with normal nerve conduction studies showed trends towards higher CT values than healthy controls, these differences did not attain statistical significance. Our results are consistent with a previous study using the same method (16), and thus overall this QST method does not appear to be superior to standard nerve conduction studies in diagnosis of DPN.
Although the SOD2 rs5746136 TT genotype carriers did not exhibit a significantly enhanced polyneuropathy risk, they showed increased temperature and vibration perception threshold levels. This might suggest that our QST method is sensitive to the deleterious effects of oxidative stress-mediated peripheral nerve damage caused by the SOD2 SNP, and thus might be a useful tool in future exploration of oxidative stress related neuropathy mechanisms.
In conclusion, DM patients with TT variant SOD2 gene were shown to exhibit increased perception threshold values for the first time. A limitation of our study was the small number of included DM patients. Further studies with larger case numbers are required for the confirmation of our results.
Footnotes
Ethics Committee Approval: The study was approved by Istanbul Medical Faculty Clinical Research Ethics Committee.
Informed Consent: Written informed consent was obtained from patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - İS, AEÖ, CİK, ET, EÖ; Design - CİK, ET, EÖ, AI; Supervision - AEÖ, İS, ET, EÖ, ZM; Resource - CİK, EÖ, İS, AEÖ; Materials - AI, AKÜ, İS, ZM, SE; Data Collection and/ or Processing - AI, AKÜ, İS, ZM, ET, EÖS; Analysis and/or Interpretation - EÖ, ZM, AKÜ, ET; Literature Search - AI, ET, EÖ; Writing - CİK, ET, EÖ, AKÜ; Critical Reviews - İS, ET, EÖ, AEÖ, ZM, AKÜ.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was supported by İstanbul University Research Funds, Project No: 17429.
REFERENCES
- 1.England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. American Academy of Neurology;American Association of Electrodiagnostic Medicine;American Academy of Physical Medicine and Rehabilitation Distal symmetric polyneuropathy:a definition for clinical research:report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64:199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- 2.Rinkel WD, Castro Cabezas M, Setyo JH, Van Neck JW, Coert JH. Traditional Methods versus Quantitative Sensory Testing of the Feet at Risk:Results from the Rotterdam Diabetic Foot Study. Plast Reconstr Surg. 2017;139:752e–763e. doi: 10.1097/PRS.0000000000003047. [DOI] [PubMed] [Google Scholar]
- 3.Mythili A, Kumar KD, Subrahmanyam KA, Venkateswarlu K, Butchi RG. A Comparative study of examination scores and quantitative sensory testing in diagnosis of diabetic polyneuropathy. Int J Diabetes Dev Ctries. 2010;30:43–48. doi: 10.4103/0973-3930.60007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy:Mechanisms, Bioenergetics, and Pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Yu M, Li M, Zhao R, Zhu Q, Zhou W, Lu M, Lu Y, Zheng T, Jiang J, Zhao W, Xiang K, Jia W, Liu L. Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase-1(GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Mol Cell Biochem. 2012;363:85–91. doi: 10.1007/s11010-011-1160-3. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee M, Vats P. Reactive metabolites and antioxidant gene polymorphisms in type 2 diabetes mellitus. Indian J Hum Genet. 2014;20:10–19. doi: 10.4103/0971-6866.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasznicki J, Sliwinska A, Kosmalski M, Merecz A, Majsterek I, Drzewoski J. Genetic polymorphisms (Pro197Leu of Gpx1, +35A/C of SOD1, -262C/T of CAT), the level of antioxidant proteins (GPx1, SOD1, CAT) and the risk of distal symmetric polyneuropathy in Polish patients with type 2 diabetes mellitus. Adv Med Sci. 2016;61:123–129. doi: 10.1016/j.advms.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Tian C, Fang S, Du X, Jia C. Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications:a meta-analysis. Diabetologia. 2011;54:803–811. doi: 10.1007/s00125-010-2004-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang IJ, Karmaus WJ. Oxidative Stress-Related Genetic Variants May Modify Associations of Phthalate Exposures with Asthma. Int J Environ Res Public Health. 2017;14:E162. doi: 10.3390/ijerph14020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Shuai P, Li X, Liu X, Wang J, Yang Y, Hao F, Lin H, Zhang D, Gong B. Association of SOD2 polymorphisms with primary open angle glaucoma in a Chinese population. Ophthalmic Genet. 2015;36:43–49. doi: 10.3109/13816810.2014.985844. [DOI] [PubMed] [Google Scholar]
- 11.Ding G, Liu F, Shen B, Feng C, Xu J, Ding Q. The association between polymorphisms in prooxidant or antioxidant enzymes (myeloperoxidase, SOD2, and CAT) and genes and prostate cancer risk in the Chinese population of Han nationality. Clin Genitourin Cancer. 2012;10:251–255. doi: 10.1016/j.clgc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Poggi C, Giusti B, Vestri A, Pasquini E, Abbate R, Dani C. Genetic polymorphisms of antioxidant enzymes in preterm infants. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):131–134. doi: 10.3109/14767058.2012.714976. [DOI] [PubMed] [Google Scholar]
- 13.Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43:1508–1512. doi: 10.1212/wnl.43.8.1508. [DOI] [PubMed] [Google Scholar]
- 14.Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal. 2014;20:1599–1617. doi: 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Duarte A, Lem-Carrillo M, Guerrero-Torres L. Normative values of quantitative sensory testing in Hispanic Latino population. Brain Behav. 2016;6:e00466. doi: 10.1002/brb3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan ST, Rayman G. The LDIflare:a novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care. 2004;27:2930–2935. doi: 10.2337/diacare.27.12.2930. [DOI] [PubMed] [Google Scholar]