Abstract
Background
Osteonectin (OSN) plays a pivotal role in cardiac remodeling, but predictive value for OSN in ischemic chronic heart failure (CHF) has not been defined. The aim of the study was to evaluate the prognostic value of OSN for cumulative survival and hospitalization among patients with ischemic-induced CHF.
Methods
A total of 154 patients with ischemic symptomatic moderate-to-severe CHF were enrolled in the study at discharge from the hospital. Observation period was up to 3 years (156 weeks). Blood samples for biomarkers measurements were collected at baseline prior to study entry. ELISA methods for measurements of circulating level of OSN were used.
Results
During a median follow-up of 2.18 years, 21 participants died and 106 subjects were re-admitted. Medians of circulating levels of OSN in survival and died patient cohorts were 670.96 ng/mL (95% confidence interval [CI] = 636.53–705.35 ng/mL) and 907.84 ng/mL (95% CI = 878.02–937.60 ng/mL). Receiver operation characteristic curve analysis has shown that cut off point of OSN concentration for cumulative survival function was 845.15 ng/mL. It has been found a significant divergence of Kaplan–Meier survival curves in patients with high (>845.15 ng/mL) and low (<845.15 ng/mL) concentrations of OSN. Circulating OSN independently predicted all-cause mortality (odds ratio [OR] = 1.23; 95% CI = 1.10–1.36; p < 0.001), CHF-related death (OR = 1.46; 95% CI = 1.22–1.80; p < 0.001), and also CHF-related re-admission (OR = 1.92; 95% CI = 1.77–2.45; p < 0.001) within 3 years of observation period.
Conclusion
Increased circulating secreted protein acidic and rich in cysteine family member OSN associates with increased 3-year CHF-related death, all-cause mortality, and risk for recurrent hospitalization due to CHF.
Keywords: Chronic heart failure, Hospitalization, Osteonectin, Prognosis, Survival
At a glance commentary
Scientific background on the subject
Osteonectin plays a pivotal role in extracellular matrix remodeling in various diseases, including chronic heart failure. Elevation of osteonectin associates with cardiac hypertrophy and impaired heart function. However, the independent prediction of elevated serum osteonectin in heart failure patients is still not completely clear.
What this study adds to the field
This study adds new information regarding the predictive role of elevated serum osteonectin in patients with chronic heart failure. Osteonectin is posed as an inflammatory biomarker with possible predictive value. Results of the study reported being of an independent association of elevated osteonectin with poor 3-year prognosis in chronic heart failure patients.
Matrix cellular proteins, that is, secreted protein acidic and rich in cysteine (SPARC), play a key role in postsynthetic procollagen processing in heart failure myocardium and regulate cell adhesion, growth factor activity, and cell cycle [1]. It has been found that SPARC family member osteonectin (OSN) causes myocardial hypertrophy, increased fibrillar collagen content, stimulates cell signaling, adhesion, survival, proliferation, and migration in several cell types, mediates calcification of the vascular wall, coagulation, and endothelial dysfunction [2]. Moreover, OSN increases collagen deposition in response to myocardial infarction (MI) or in some types of cardiac hypertrophy can impair heart function [3]. Recent animal studies have been revealed that increased circulating OSN level strongly associates with a higher incidence of mortality following MI. Therefore, clinical studies have shown that serum OSN level relates to increased rates of cardiac wall rupture and newly, heart failure in MI subjects [4], [5]. However, the role of OSN in ischemic-induced chronic heart failure (CHF) development and progression has not been defined. The aim of the study was to evaluate the prognostic value of OSN for cumulative survival and hospitalization among patients with ischemic-induced CHF.
Methods
The study prospectively evolved 154 patients (86 male, 68 females) aged 48–62 years with ischemic symptomatic CHF with II–IV New York Heart Association (NYHA) class. CHF was diagnosed according to current European Society of Cardiology clinical guidelines [6]. The exclusion criteria are: Q-wave and non-Q-wave MI within 3 months before study entry; severe kidney and liver diseases that may affect clinical outcomes; malignancy; creatinine plasma level above 440 μmol/L; estimated glomerular filtration rate (GFR) < 35 mL/min/m2; brain injury within 3 months before the enrollment; body mass index (BMI) above 30 kg/m2; pulmonary edema; tachyarrhythmia; valvular heart disease; thyrotoxicosis; ischemic stroke; intracranial hemorrhage; acute infections; surgery; trauma; all the ischemic events within three previous months; inflammations within a previous month; neoplasm; pregnancy; implanted pacemaker, any disorder that may discontinue patient's participation in the study according to investigators; and patient's refusal to participate in the study or to give his consent for it. Observation period was up to 3 years (156 weeks). We analyzed cumulative survival related to CHF, and additionally all-cause mortality was examined.
Methods for visualization of coronary arteries
Multi-spiral computed contrast enhanced tomography angiography and/or angiographic study have been carried out to verify the ischemic nature of the disease prior to the study entry. Angiographic procedure was used when ischemic signs were presented at baseline, and no MI was found previously. Multi-spiral computed contrast enhanced tomography angiography was performed when no current ischemic signs/previously documented old MI at baseline were detected, but signs and symptoms of CHF were presented. Multi-spiral computed tomography angiography has been carried out for all the patients prior to their inclusion in the study. When atherosclerotic lesions of the coronary arteries were verified, patients were subjected to the conventional angiographic examination provided indications for re-vascularization were available. Coronary artery disease (CAD) was considered to be diagnosed upon availability of previous angiographic examinations carried out not later than 6 months ago provided no new cardiovascular events occurred for this period, and the procedure is available for assay. The coronary artery wall structure was measured by means of contrast spiral computed tomography angiography on Somatom Volume Zoom Scanner (Siemens, Erlangen, Germany) with two detector rows when holding patients breathe at the end of breathing in [7]. After preliminary native scanning, nonionic contrast Omnipaque (Amersham Health, Ireland) was administered for the optimal image of the coronary arteries. To reconstruct the image, 0.6-mm width axial tomographic slices were used. When >50% of the diameter of three and more coronary arteries were found, the multi-vessel disease was determined.
Transthoracic echocardiography
Transthoracic echocardiography was performed according to a conventional procedure on ACUSON apparatus, Siemens, Germany, in В-mode regimen and tissue Doppler echocardiography regimen from parasternal, subcostal, and apical positions over the short and long axis with phased transducer of 5 МHz. Left ventricular end-diastolic and end-systolic volumes were measured by modified Simpson's method. Left ventricular ejection fraction (LVEF) was assessed in compliance with the requirements of American Society of Echocardiography [8]. Tissue Doppler echocardiography was carried out in 4-, 3- and 2-chamber projections in each of 16 segments of the left ventricle and in 4 spots of the mitral annulus: At the base of posterior septal, lateral, inferior, and anterior left ventricular walls [9]. Peak systolic (Sm), early diastolic (Em), and late diastolic (Аm) myocardial velocities were measured in the mitral annulus area, followed by calculating velocity of early diastolic left ventricular filling (E) to Аm (Е/Аm) ratio and to Em (Е/Em) ratio.
Calculation of glomerular filtration rate
GFR was calculated by modification of diet in the renal disease-6 formula [10].
Measurement of biomarkers
Blood samples were collected at baseline in the morning (at 7–8 am) into cooled silicone test tubes, when patients were discharged from the hospital with stable clinical status. Samples were processed according to the recommendations of the manufacturer of the analytical technique used. They were centrifuged upon permanent cooling at 6000 rpm for 3 min. Then, plasma samples were stored at a temperature ≤−200 °C.
Circulating OSN level was determined by ELISA method (Bender MedSystems GmbH, Vienna, Austria). N-terminal pro-brain natriuretic peptide (NT-pro-BNP) concentration was measured by immunoelectrochemoluminescent assay using commercial kits produced by Roche (Mannheim, Germany) on Elecsys 1010 analyzer (Roche, Mannheim, Germany).
Concentrations of total cholesterol (TC) and cholesterol of high-density lipoproteins were measured by fermentation method.
Ethical principles
The investigators followed strictly all the requirements to clinical trials in conformity with the World Medical Association declaration of Helsinki, 1964, good clinical practice provided by International Conference on Harmonization, Council of Europe Convention for the Protection of Human rights and dignity of the human being in view of using achievements in biology and medicine, convention on human rights and biomedicine, including additional protocol to the convention on human rights and biomedicine, concerning Biomedical Research, and legislation of Ukraine. The study was approved by local IRB (State Medical University, Zaporozhye, Ukraine). The IRB approval number is 02/21.02.2012. All the patients have given their written informed consent for participation in the study.
Statistical analysis
Statistical analysis of the results obtained was carried out in SPSS system for Windows, Version 20 (SPSS Inc., Chicago, IL, USA). The data were presented as mean (М) and standard error (±SE) or 95% confidence interval (CI); median (Ме); and interquartile range. Design of the study was nonrandomized prospective open cohort study. The sample size was computed on http://www.sealedenvelope.com/power/binary-superiority/. Our sample size (17 cases in died cohort and 89 cases in survival cohort) allowed us to detect large effect sizes (>0.80) for systemic inflammatory marker OSN between survived and died patients with a power of 80%, taking a two-sided type I error of 5%. The hypothesis of the normal distribution of the parameters analyzed was checked by means of Kolmogorov–Smirnov test. To compare the main parameters of patients' groups (subject to the type of distribution of the parameters analyzed), two-tailed Student's t-test or Mann–Whitney U-test were used. To compare categorical variables between groups, Chi-square test (χ2) and Fisher exact test were used. The circulating OSN and NT-pro-BNP level in the blood failed to have a normal distribution, while distribution of the TC and cholesterol fractions had a normal character (estimated by means of Kolmogorov–Smirnov test) and was not subjected to any mathematical transformation. Receiver operation characteristic curve (ROC) analysis was performed to identify the well-balanced cutoff points of the OSN concentration with the predicted value. To compare survival rate between two groups Log-rank test was used. The difference between the areas under ROC curves was calculated with the method of DeLong et al. and reclassification measures (index discrimination improvement [IDI]) [11], [12]. Odds ratio (OR) and 95% CI were calculated for all the independent predictors of survival of the patients. A calculated difference of p < 0.05 was considered significant.
Results
General characteristics of study patient population
During a median follow-up of 2.18 years, 21 participants died, and CHF-related death was defined in 18 patients. Additionally, 106 subjects were hospitalized repetitively due to advance CHF (17 cases in died cohort and 89 cases in survival cohort). Table 1 shows a general characteristic of the patients included in the study. As one can see from Table 1, no substantial age and gender differences were found among persons who died and survived, as well as differences in BMI, GFR, glycated hemoglobin, fasting blood glucose level, blood creatinine level, TC, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol, and numerous of coronary vessels were damaged. No difference was found between the two cohorts in systemic office blood pressure and heart rate. Documented incidence of type 2 diabetes mellitus (T2DM) in patients of the two cohorts was 38.1% and 33.8% (p = 0.06). Note that there was not a statistically significant change in Е/Аm and Е/Em between the two cohorts, while a decrease in the LVEF value was quite anticipated in the setting in dead patients. At the same time, the level of circulating NT-pro-BNP was statistically significantly higher in dead patients than in survived persons. When analyzing details of pharmacotherapy, no substantial differences were found between the two cohorts with regard to the administration of the majority of drugs.
Table 1.
General characteristic of patients participating in the study.
| Variables | All patients (n = 154) (%) | Subjects who were died (n = 21) (%) | Subjects who were survived (n = 133) (%) |
|---|---|---|---|
| Age, years (mean ± SD) | 58.50 ± 6.10 | 57.20 ± 6.70 | 59.50 ± 7.30 |
| Males, n (%) | 79 (51.3) | 12 (57.1) | 67 (50.3) |
| Hypertension, n (%) | 73 (47.4) | 12 (57.1) | 61 (45.9) |
| Hyperlipidemia, n (%) | 61 (39.6) | 9 (42.8) | 52 (39.1) |
| T2DM, n (%) | 53 (34.4) | 8 (38.1) | 45 (33.8) |
| Adherence to smoking, n (%) | 31 (20.1) | 7 (33.3) | 24 (29.3) |
| II class NYHA, n (%) | 41 (26.6) | 6 (28.6) | 35 (26.3) |
| III class NYHA, n (%) | 74 (48.1) | 9 (42.8) | 65 (48.9) |
| IV class NYHA, n (%) | 39 (25.3) | 6 (28.6) | 33 (24.8) |
| BMI, kg/m2 (mean, 95% CI) | 23.9 (22.8–26.1) | 23.7 (22.5–27.3) | 24.2 (22.0–27.9) |
| GFR, mL/min/1.73 m2 (mean, 95% CI) | 83.4 (70.2–91.3) | 82.1 (69.9–93.1) | 85.2 (70.3–112.5) |
| HbA1c (%) | 6.5 (4.7–8.6) | 6.3 (4.4–9.0) | 7.0 (4.3–9.2) |
| Fasting blood glucose, mmol/L (mean, 95% CI) | 4.95 (3.8–8.0) | 4.80 (3.6–8.5) | 5.40 (3.4–9.1) |
| Creatinine, μmol/L (mean, 95% CI) | 72.6 (61.3–82.5) | 70.5 (59.6–88.3) | 74.9 (65.1–90.3) |
| TC, mmol/L (mean, 95% CI) | 5.1 (4.7–5.6) | 5.3 (4.6–6.0) | 5.0 (4.2–5.8) |
| LDL-C, mmol/L (mean, 95% CI) | 3.35 (3.16–4.02) | 3.60 (3.20–4.18) | 3.02 (2.80–3.90) |
| HDL-C, mmol/L (mean, 95% CI) | 0.92 (0.90–1.02) | 0.94 (0.92–1.06) | 0.88 (0.82–0.97) |
| NT-pro-BNP, pg/mL (mean, 95% CI) | 1266.1 (811.5–2220.7) | 1533.6 (644.5–2560.6) | 1031.2 (704.8–1560.7)* |
| Osteonectin, ng/mL (mean, 95% CI) | 788.54 (665.12–912.30) | 907.84 (878.02–937.60) | 670.96 (636.53–705.35)* |
| Systolic BP, mm Hg (mean, 95% CI) | 131 (127–135) | 129 (126–133) | 135 (130–139) |
| Diastolic BP, mm Hg (mean, 95% CI) | 77 (73–82) | 77 (72–82) | 78 (73–82) |
| HR, beats per 1 min (mean, 95% CI) | 71 (68–75) | 76 (72–79) | 68 (63–74) |
| LVEF, % (mean, 95% CI) | 47.60 (42.5–54.3) | 42.80 (40.1–44.6) | 52.40 (46.1–55.4)* |
| Е/Аm, U (mean, 95% CI) | 16.6 (15.9–17.2) | 16.6 (16.0–17.3) | 16.5 (15.6–17.9) |
| Е/Em, U (mean, 95% CI) | 16.6 (15.8–17.5) | 16.6 (15.7–17.5) | 16.6 (15.5–17.8) |
| One coronary artery lesion, n (%) | 29 (18.8) | 5 (23.8) | 24 (18.0) |
| Two coronary arteries lesion, n (%) | 64 (41.6) | 8 (38.1) | 54 (40.6) |
| Three-and multi-vessel lesion of coronary arteries, n (%) | 63 (40.9) | 8 (38.1) | 55 (41.4) |
| ACEI/ARAs, n (%) | 154 (100) | 21 (100) | 133 (100) |
| Acetylsalicylic acid, n (%) | 130 (84.4) | 19 (90.5) | 121 (91.0) |
| Other antiplatelets, n (%) | 14 (9.1) | 2 (9.5) | 12 (9.0) |
| Statins, n (%) | 94 (61.0) | 14 (66.7) | 80 (60.2) |
| Metformin, n (%) | 53 (34.4) | 8 (38.1) | 45 (33.8) |
| Diuretics, n (%) | 139 (90.3) | 18 (85.7) | 121 (91.0) |
| Mineralocorticoid receptor antagonist, n (%) | 79 (51.3) | 9 (42.9) | 70 (52.6) |
Data are presented as mean and 95% CI; categorical variables are expressed as numerous (n) and percentages (%). P value is a comparison of mean or median variables between both cohorts. *Statistically differences between parameters (P < 0.05). Abbreviations: CI: Confidence interval; CAD: Coronary artery disease, T2DM: type 2 diabetes mellitus; GFR: glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; BMI: body mass index; LVEF: left ventricular ejection fraction; U: unit; Em: early diastolic myocardial velocity; Аm: late diastolic myocardial velocity; E: peak velocity of early diastolic left ventricular filling; ACEI: angiotensin-converting enzyme inhibitor; ARAs: angiotensin-2 receptors antagonists; SD: standard deviation; NYHA: New York Heart Association; HbA1c: glycated hemoglobin; BP: blood pressure; TC: total cholesterol; NT-pro-BNP: N-terminal pro-brain natriuretic peptide; HR: heart rate.
Circulating osteonectin level in survived and dead patients
Medians of circulating levels of OSN in survived and dead patient cohort were 670.96 ng/mL (95% CI = 636.53–705.35 ng/mL) and 907.84 ng/mL (95% CI = 878.02–937.60 ng/mL) (p < 0.001).
The predictive value of osteonectin concentration in study patient population
Multivariate logistic regression was used to assess whether any combination of assays was able to better discriminate between survived and dead patients. In the logistic regression analysis, the main factors independently related with cumulative mortality and CHF-related readmission were some biomarkers (OSN alone, NT-pro-BNP alone), LVEF, T2DM, and three- and multi-vessel lesion. Circulating OSN independently predicted all-cause mortality (OR = 1.23; 95% CI = 1.10–1.36; p < 0.001), CHF-related death (OR = 1.46; 95% CI = 1.22–1.80; p < 0.001), and also CHF-related readmission (OR = 1.92; 95% CI = 1.77–2.45; p < 0.001) within 3 years of observation period [Table 2]. NT-pro-BNP and LVEF remained statistically significant for all categories: All-cause mortality, CHF-related death, and CHF-related readmission, whereas T2DM and three- and multi-vessel lesion for all variables were not.
Table 2.
Independent variables related to 3-years all-cause mortality, CHF-related death, and CHF-related readmission.
| Variables | All-cause mortality |
CHF-related death |
CHF-related readmission |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| OSN (<845.15 ng/mL vs. >845.15 ng/mL) | 1.23 | 1.10–1.36 | 0.006 | 1.46 | 1.22–1.80 | 0.004 | 1.92 | 1.77–2.45 | 0.001 |
| NT-pro-BNP (>1250.4 pg/mL vs. <1250.4 pg/mL) | 1.09 | 1.02–1.16 | 0.002 | 1.42 | 1.22–1.73 | 0.006 | 1.44 | 1.28–1.67 | 0.002 |
| LVEF (<45% vs. >45%) | 1.06 | 1.01–1.12 | 0.001 | 1.15 | 1.12–1.18 | 0.014 | 1.22 | 1.07–1.45 | 0.016 |
| T2DM (present vs. absent) | 1.05 | 1.01–1.11 | 0.001 | 1.03 | 0.93–1.10 | 0.32 | 1.04 | 0.97–1.06 | 0.42 |
| Three-and multi-vessel lesion of coronary arteries (present vs. absent) | 1.02 | 0.88–1.09 | 0.56 | 1.01 | 0.92–1.07 | 0.27 | 1.14 | 1.03–1.26 | 0.012 |
Abbreviations: OR: odds ratio; CI: confidence interval; LVEF: left ventricular ejection fraction; BNP: brain natriuretic peptide; T2DM: type 2 diabetes mellitus; CHF: chronic heart failure; OSN: osteonectin; NT-pro-BNP: N-terminal pro-brain natriuretic peptide.
Using a stepwise model selection method for multi-variable prediction model we have been investigated the summary effect of any combinations of OSN, NT-pro-BNP, LVEF on all-cause mortality, CHF-related death, and CHF-related readmissions. We found that OSN alone (Model 1) and combination of OSN with NT-pro-BNP (Model 2) remained statistically significant predictors for all-cause mortality (B-coefficient = 1.14, p = 0.001 and B-coefficient = 1,14, p = 0.001, respectively), CHF-related death (B-coefficient = 2.24, p = 0.003 and B-coefficient = 2,76, p = 0.008, respectively), and CHF-related recurrent hospitalizations (B-coefficient = 2.06, p = 0.003 and B-coefficient = 2,11, p = 0.004, respectively), whereas combination of OSN with both NT-pro-BNP and LVEF (Model 4) did not (B-coefficient = 0.014, p = 0.543 and B-coefficient = 0.016, p = 0.528 and B-coefficient = 0.012, p = 0.448, respectively). NT-pro-BNP alone (Model 3) predicted CHF-related recurrent admissions (B-coefficient = 1.88, p = 0.001) only, whereas all-cause mortality (B-coefficient = 0.025, p = 0.68) and CHF-related death (B-coefficient = 0.036, p = 0.62) did not. In fact, a stepwise model selection method demonstrated that LVEF, T2DM and three- and multi-vessel lesion of coronary arteries added to combination of OSN and NT-pro-BNP do not offer any additional information to discriminate between survived and dead patients with CHF (B-coefficient of 0.012, 0.067, and 0.023, respectively; P values of 0.277, 0.300, and 0.522, respectively).
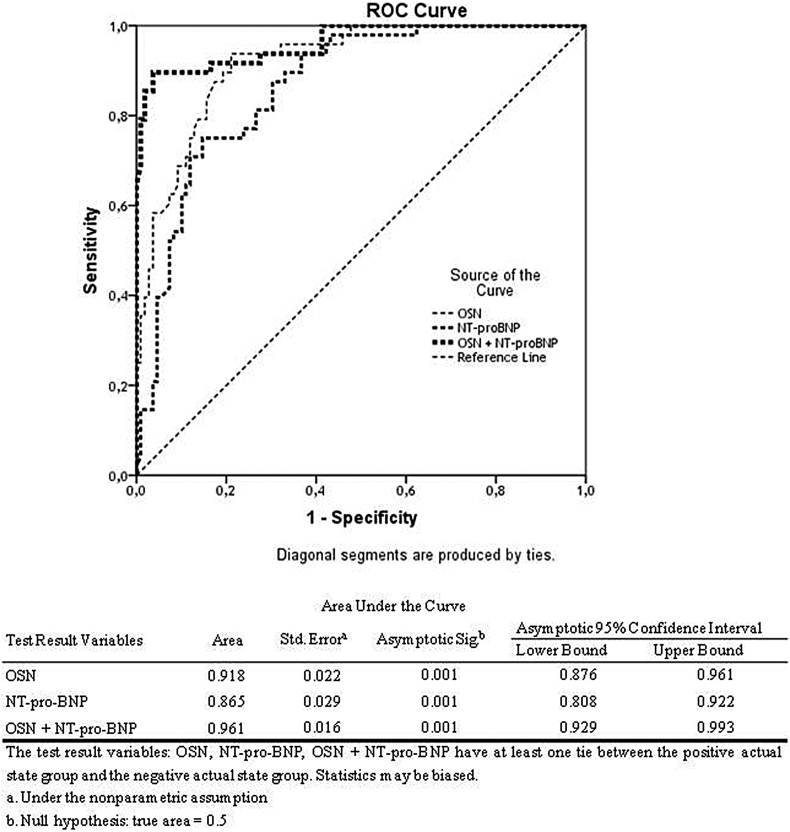
The optimum cut-off point for OSN is determined by the relative importance of the sensitivity and specificity of the test. ROC curve analysis has shown that cut-off point of OSN concentration for cumulative cardiovascular events was 845.15 ng/mL [Fig. 1]. Area under curve was 0.918 (SE = 0.022; 95% CI = 0.876–0.961), sensitivity and specificity were 79.2% and 84.4%, respectively. Derived from ROC curve, a NT-pro-BNP cut-off of 1250.4 pg/mL showed the best-balanced sensitivity and specificity for predicting mortality and hospital readmission (72.3% sensitivity and 81.6% specificity). Using cut-off points for OSN and NT-pro-BNP over mentioned above we found that the discriminative ability for both biomarkers showed a small trend to increase only. Combination of both biomarkers leads increased sensitivity up 88.6% and specificity up 92.4%. Model discrimination was excellent for OSN alone, NT-pro-BNP alone, and both in combination. For determination of the difference between the ROC curves, we used DeLong method and reclassification measures (IDI). Using DeLong method we found a statistical difference between areas under curves that are suitable for OSN alone, NT-pro-BNP alone and its combination were found (p < 0.001 for all cases). Integrated discrimination improvement after the inclusion of NT-pro-BNP into the model was calculated. However, we found that the inclusion of NT-pro-BNP does not significantly improved model discrimination based on OSN alone (AUC 0.961 vs. AUC 0.918, p = 0.22; and IDI = 0.035, p = 0.42). The difference between discrimination for OSN alone and NT-pro-BNP alone was not significant to the IDI (IDI = 0.031; p = 0.38) [Table 3].
Fig. 1.
Results of the receiver operation characteristic analysis: The graphical plot illustrates the predictive discriminations for osteonectin alone, NT-pro-BNP alone, and Osteonectin with N-terminal pro-brain natriuretic peptide for chronic heart failure patients.
Table 3.
C-statistic calculated for predictive models based on biomarker measurement.
| Model | AUC | IDI | p |
|---|---|---|---|
| Model 1 (OSN alone) | 0.918 | – | – |
| Model 2 (NT-pro-BNP alone) versus Model 1 | 0.865 | 0.031 | 0.38 |
| Model 3 (OSN + NT-pro-BNP) versus Model 1 | 0.961 | 0.035 | 0.42 |
Abbreviations: IDI: index discrimination improvement; CI: confidence interval; AUC: area under curve; OSN: osteonectin; NT-pro-BNP: N-terminal pro-brain natriuretic peptide.
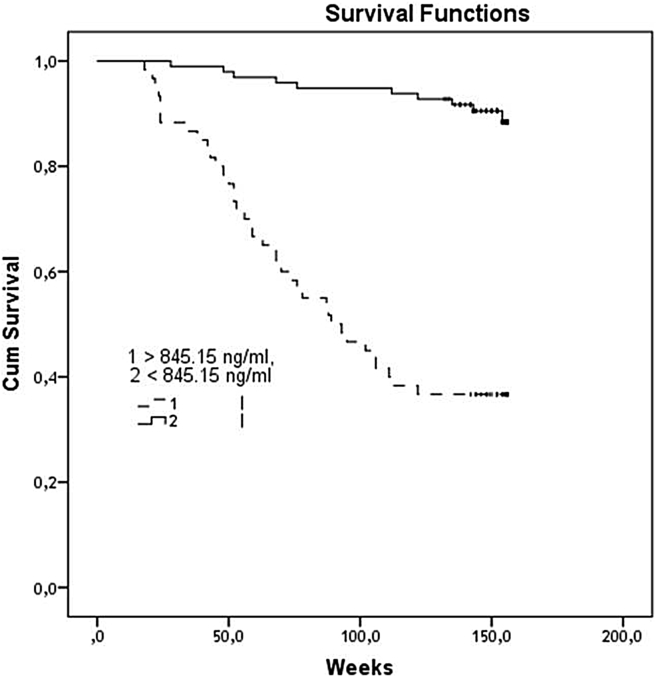
It has been found a significantly divergence of Kaplan–Meier survival curves in patients with high (>845.15 ng/mL) and low (<845.15 ng/mL) concentrations of OSN [Fig. 2]. The curves divergence of events accumulation reached a statistical significance in 26 weeks of the observation period (p < 0.001).
Fig. 2.
The Kaplan–Meier survival plot shows the significant difference in cumulative survival between patient groups with low (<845.15 ng/mL) and high (>845.15 ng/mL) circulating osteonectin.
Discussion
The results of the study clarify being of positive association between circulating level of SPARC family member OSN and increased 3-year combined cardiovascular events (CHF-related death, all-cause mortality, and readmission due to CHF) among patient population with ischemic-induced symptomatic cardiac failure. Recently, OSN was not defined as a biological marker with possible predictive value in heart failure subjects, although its role as a surrogate biomarker of the atherosclerotic lesion and cardiovascular remodeling was well-established.
It is well-known that extracellular matrix (ECM) proteins may modulate cell-matrix interactions and cell functions, and does not seem to have a direct structural role and mediates left ventricular remodeling. Several members of the matrix cellular protein family, like OSN, are up-regulated in CHF. OSN (also known as SPARC) is synthesized by wide spectrum cells, such as osteoblasts, fibroblasts, and activated macrophages at sites of wound repair and platelet degranulation [3]. It is found in the serum of patients with CHF predominantly reflected a positive pro-inflammatory response and alterations in protein metabolism that leads to biomechanical stress [2], [3], [4]. OSN also regulates the proliferation of some cells, especially endothelial cells, mediated by its ability to bind to cytokines and growth factor [5]. Excess degradation and disruption of the cardiac ECM network structure thereby, OSN overexpression is the formation of fibrotic lesions. Because myocardial fibrosis is also a well-known cause of diastolic dysfunction and CHF, remodeling of ECM is considered as a key aspect of the myocardial response to biomechanical stress and advanced heart failure [13].
Recent studies have been suggested that SPARC, such as OSN, osteopontin, and osteoprotegerin, presumably can play an important role in not only CHF, but in atherogenesis also [14], [15]. The animal models give evidence that OSN levels directly correlate with increased mortality post-MI due to increased rupture rate [16]. Probably, this effect may be related to OSN inhibition ability of mitogenesis of the vascular endothelial growth factor on microvascular endothelial cells [17]. Because OSN may induce deadhesion, through loss of actin fibers and focal adhesion plaques, cell migration and cell infiltration of the vulnerable zone of plaque may facilitate. Therefore, OSN may regulate MMP activity involving matrix rearrangement that leads to remodeling of the vessels [17], [18]. Both these processes are considered a pivotal mechanism for triggering plaque instability as well as heart wall rupture [19]. Thus, OSN is multi-functional matrix protein with powerful ability to inhibition of tissue response to injury and probably, to the mediation of low-intensity inflammation. As a fact, the predictive role of OSN in cardiovascular diseases is uncertain. Taken together the data mentioned above, clarify that OSN may be considered as a biological marker with high predictive value for CHF evolution, especially for patients with ischemic causes of myocardial dysfunction.
Currently, there are no data on the utility or discriminatory ability of OSN in determining the mortality from CHF. It is predisposed that increased OSN concentration would be a powerful indicator of not only CHF-related events, but also all-cause mortality. We found that circulating OSN level was really increased in CHF patients with poor short-term prognosis. Indeed, OSN concentration independently predicted combined cardiovascular events. On the one hand, OSN is secreted by activated macrophages due to pro-inflammatory activation and leads profound ECM reposition, exaggerated left ventricular remodeling, endothelial dysfunction, vascular calcification, and procoagulation [1], [3]. On the other hand, given results of recent investigations, the absence of tissue over-expression of OSN is associated with increased cardiac rupture and dysfunction after acute MI due to worsening postsynthetic procollagen processing [4], [20]. There are predispositions that these mechanisms, which are involved in reparation processes of heart and vessels, are under the control of mineralocorticoid receptors. It has been postulated that beneficial effects of mineralocorticoid receptor antagonist eplerenone in CHF were associated with normalization of matrix cellular protein expression, such as OSN [21]. Decreased expression of OSN improves cardiac structural and functional parameters, delaying the progression of heart failure that was found in the small animal study [21]. Thus, the role of OSN in cardiac outcomes is probably controversial.
Probably, treatment strategy might particularly explain the interrelation of OSN with outcomes in the patient population. We enrolled in our study, patients with ischemic-induced CHF treated with eplerenone also. At least 42.9% and 52.6% patients of the both cohorts (dead persons and survived subjects) are given high selective mineralocorticoid receptor antagonist eplerenone. Despite similar co-administration of eplerenone with standard heart failure therapy, no significant effect of medication for a survival rate of the patients with CHF was found. Probably, collagen deposition as functions in the extracellular processing that is controlled by OSN is a key protective event for acute MI, whereas for advance CHF similar process is considered the potential harm and prognostically negative [2], [3], [5]. Obviously, structural changes of the ECM are significantly modulated by OSN associated several signaling pathways, which are differed on their ability to induce repair changes on the several stages on cardiovascular continuum [22], [23]. It may have important value for reclassification of the patients at high risk of cardiovascular outcomes related to several causes, which are suitable for ischemic CHF. A variety of biomarkers have been investigated on their values to predict cardiovascular outcomes, but OSN was not [24]. We determined that predictive value of circulating OSN was superior when compared with NT-pro-BNP alone. Taken into consideration that a significant divergence of Kaplan–Meier survival curves in patients with high (>845.15 ng/mL) and low (<845.15 ng/mL) concentrations of OSN within 26 weeks was found. The convergence of Kaplan–Meier survival curves at the end of the study was not found. There are preexisted data about age-related increasing of OSN, but we found no substantial age and gender differences of OSN among persons who were experienced combined cardiovascular events and who were not [15]. Because the weak association between echocardiographic features and NYHA class was previously determined, we added circulating OSN level to conventional prognostic model, which included NT-pro-BNP and LVEF. In fact, large prospective studies are required to provide robust evidence of the prognostic role of combination OSN and NT-pro-BNP on the mortality rate among CHF persons.
In fact, long-term prospective studies are required to provide robust evidence of the prognostic role of combination OSN and NT-pro-BNP in the associated mortality from CHF. However, some limitation of our study, such as small size, absence of randomization, and probably, conventional treatment of CHF without biological markers-guided control, may limit real-time prognostic value of OSN leading increased statistical power. In this study, OSN level was measured at baseline after discharge of the patients from the hospital with stable CAD and without clinical signs and symptoms of acute decompensated CHF. It can introduce some limitations to the interpretation of the results of the investigation, especially around OSN level in CHF subjects independently before readmission. Needed new studies are required for determination whether OSN may predict cardiovascular outcomes in ischemic CHF patients. Therefore, understanding the mechanisms that contribute to the cardiac remodeling may help to design new studies aimed at determination of OSN role in CHF.
Conclusion
We found that increased circulating SPARC family member OSN closely associates with increased 3-year combined cardiovascular events (CHF-related death, all-cause mortality, and recurrent hospitalization due to CHF). Using a combination of OSN and NT-pro-BNP showed the best-balanced sensitivity and specificity for predicting mortality and hospital readmission in ischemic CHF subjects.
Limitations of the study
This study has some limitations. We believed that a greater cohort would be desirable to improve the power of the study. Therefore, serial measurements of OSN will be desirable for determination of the biomarker level in patients at readmission and after discharge from the hospital. Dynamic of OSN may have powerful value for prediction of cardiovascular outcomes and it is able to be considered a part of guided therapy of CHF in perspective. We also relied on clinical data to rule out infection and other inflammatory diseases before sampling, but we could not exclude that some patients had unrecognized the conditions responsible for the elevated OSN levels observed. We supposed to mean that these limitations would not have a significant influence to study data interpretation.
Source of support
Nil.
Conflicts of interest
None declared.
Acknowledgment
We thank all patients for their participation in the investigation, staff of the Regional Zaporozhye Hospital (Ukraine) and the doctors, nurses, and administrative staff in City hospital # 6 (Zaporozhye, Ukraine), general practices, and site-managed organizations that assisted with the study.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Harris B.S., Zhang Y., Card L., Rivera L.B., Brekken R.A., Bradshaw A.D. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am J Physiol Heart Circ Physiol. 2011;301:H841–H847. doi: 10.1152/ajpheart.01247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCurdy S.M., Dai Q., Zhang J., Zamilpa R., Ramirez T.A., Dayah T. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;301:H497–H505. doi: 10.1152/ajpheart.01070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsey M.L., Mann D.L., Entman M.L., Spinale F.G. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35:316–326. doi: 10.1080/07853890310001285. [DOI] [PubMed] [Google Scholar]
- 4.Schellings M.W., Vanhoutte D., Swinnen M., Cleutjens J.P., Debets J., van Leeuwen R.E. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCurdy S., Baicu C.F., Heymans S., Bradshaw A.D. Cardiac extracellular matrix remodeling: fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC) J Mol Cell Cardiol. 2010;48:544–549. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray J.J., Adamopoulos S., Anker S.D., Auricchio A., Böhm M., Dickstein K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 7.Bluemke D.A., Achenbach S., Budoff M., Gerber T.C., Gersh B., Hillis L.D. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation. 2008;118:586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. [DOI] [PubMed] [Google Scholar]
- 8.Schiller N.B., Shah P.M., Crawford M., DeMaria A., Devereux R., Feigenbaum H. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 9.Pellerin D., Sharma R., Elliott P., Veyrat C. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart. 2003;89:iii9–iii17. doi: 10.1136/heart.89.suppl_3.iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., Feldman H.I. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 12.Pencina M.J., D'Agostino R.B., D'Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 13.Kandalam V., Basu R., Moore L., Fan D., Wang X., Jaworski D.M. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation. 2011;124:2094–2105. doi: 10.1161/CIRCULATIONAHA.111.030338. [DOI] [PubMed] [Google Scholar]
- 14.Gabbasov Z.A., Agapov A.A., Saburova O.S., Komlev A.E., Soboleva E.L., Akchurin R.S. Circulating stromal osteonectin-positive progenitor cells and stenotic coronary atherosclerosis. Can J Physiol Pharmacol. 2007;85:295–300. doi: 10.1139/y07-001. [DOI] [PubMed] [Google Scholar]
- 15.Horn M.A., Graham H.K., Richards M.A., Clarke J.D., Greensmith D.J., Briston S.J. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J Mol Cell Cardiol. 2012;53:82–90. doi: 10.1016/j.yjmcc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Komatsubara I., Murakami T., Kusachi S., Nakamura K., Hirohata S., Hayashi J. Spatially and temporally different expression of osteonectin and osteopontin in the infarct zone of experimentally induced myocardial infarction in rats. Cardiovasc Pathol. 2003;12:186–194. doi: 10.1016/s1054-8807(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 17.Kupprion C., Motamed K., Sage E.H. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P., Collen D. Transgenic mouse models in angiogenesis and cardiovascular disease. J Pathol. 2000;190:387–405. doi: 10.1002/(SICI)1096-9896(200002)190:3<387::AID-PATH595>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Bradshaw A.D., Sage E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradshaw A.D., Baicu C.F., Rentz T.J., Van Laer A.O., Bonnema D.D., Zile M.R. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol. 2010;298:H614–H622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Pacheco P., Ortega-Hernández A., Caro-Vadillo A., Casanueva-Eliceiry S., Aragoncillo P., Egido J. Eplerenone enhances cardioprotective effects of standard heart failure therapy through matricellular proteins in hypertensive heart failure. J Hypertens. 2013;31:2309–2318. doi: 10.1097/HJH.0b013e328364abd6. [DOI] [PubMed] [Google Scholar]
- 22.Dobaczewski M., Bujak M., Zymek P., Ren G., Entman M.L., Frangogiannis N.G. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 23.Wu R.X., Laser M., Han H., Varadarajulu J., Schuh K., Hallhuber M. Fibroblast migration after myocardial infarction is regulated by transient SPARC expression. J Mol Med (Berl) 2006;84:241–252. doi: 10.1007/s00109-005-0026-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen W.S., Chen S.J., Lee C.C., Cherng W.J., Liu M.H., Wang C.H. Plasma P-selectin predicts long-term cardiovascular events in hospitalized patients with suspected coronary artery disease and preserved left ventricular function: a 10-year follow-up study. Biomed J. 2013;36:137–143. doi: 10.4103/2319-4170.113231. [DOI] [PubMed] [Google Scholar]