Abstract
Key Points
It is unknown if a sex difference exists in cardiac apamin‐sensitive small conductance Ca2+‐activated K+ (SK) current (I KAS).
There is no sex difference in I KAS in the basal condition. However, there is larger I KAS in female rabbit ventricles than in male during isoproterenol infusion.
I KAS activation by isoproterenol leads to action potential triangulation in females, indicating its abundant activation at early phases of repolarization.
I KAS activation in females induces negative Ca2+–voltage coupling and promotes electromechanically discordant phase 2 repolarization alternans.
I KAS is important in the mechanisms of ventricular fibrillation in females during sympathetic stimulation.
Abstract
Sex has a large influence on cardiac electrophysiological properties. Whether sex differences exist in apamin‐sensitive small conductance Ca2+‐activated K+ (SK) current (I KAS) remains unknown. We performed optical mapping, transmembrane potential, patch clamp, western blot and immunostaining in 62 normal rabbit ventricles, including 32 females and 30 males. I KAS blockade by apamin only minimally prolonged action potential (AP) duration (APD) in the basal condition for both sexes, but significantly prolonged APD in the presence of isoproterenol in females. Apamin prolonged APD at the level of 25% repolarization (APD25) more prominently than APD at the level of 80% repolarization (APD80), consequently reversing isoproterenol‐induced AP triangulation in females. In comparison, apamin prolonged APD to a significantly lesser extent in males and failed to restore the AP plateau during isoproterenol infusion. I KAS in males did not respond to the L‐type calcium current agonist BayK8644, but was amplified by the casein kinase 2 (CK2) inhibitor 4,5,6,7‐tetrabromobenzotriazole. In addition, whole‐cell outward I KAS densities in ventricular cardiomyocytes were significantly larger in females than in males. SK channel subtype 2 (SK2) protein expression was higher and the CK2/SK2 ratio was lower in females than in males. I KAS activation in females induced negative intracellular Ca2+–voltage coupling, promoted electromechanically discordant phase 2 repolarization alternans and facilitated ventricular fibrillation (VF). Apamin eliminated the negative Ca2+–voltage coupling, attenuated alternans and reduced VF inducibility, phase singularities and dominant frequencies in females, but not in males. We conclude that β‐adrenergic stimulation activates ventricular I KAS in females to a much greater extent than in males. I KAS activation plays an important role in ventricular arrhythmogenesis in females during sympathetic stimulation.
Keywords: ventricular arrhythmias, Ca2+ activated potassium channel, sex dimorphism
Key Points
It is unknown if a sex difference exists in cardiac apamin‐sensitive small conductance Ca2+‐activated K+ (SK) current (I KAS).
There is no sex difference in I KAS in the basal condition. However, there is larger I KAS in female rabbit ventricles than in male during isoproterenol infusion.
I KAS activation by isoproterenol leads to action potential triangulation in females, indicating its abundant activation at early phases of repolarization.
I KAS activation in females induces negative Ca2+–voltage coupling and promotes electromechanically discordant phase 2 repolarization alternans.
I KAS is important in the mechanisms of ventricular fibrillation in females during sympathetic stimulation.
Introduction
Sex is an important biological variable in cardiac electrophysiology. Females and males exhibit differences in electrocardiographic characteristics, morbidities, clinical manifestations and prognosis to cardiac electrical diseases, and drug sensitivities (Salama & Bett, 2014). For instance, women have longer corrected QT interval (QTc) and higher incidence of Torsade de Pointes than men, while males account for the vast majority of patients with J wave syndrome (Merri et al. 1989; Antzelevitch et al. 2016). Rabbits have highly matched sex‐linked QT prolongation and arrhythmogenic phenotypes as in humans and are commonly used for studying the sex differences in cardiac ion channels (Salama & Bett, 2014). Previous studies in rabbits reported sex differences in several cardiac ionic currents, including L‐type calcium currents (I Ca,L) (Sims et al. 2008), inward rectifier potassium currents (I K1) (Liu et al. 1998) and rapidly (I Kr) (Liu et al. 1998) and slowly (I Ks) (Zhu et al. 2013) activating delayed rectifier potassium currents. The apamin‐sensitive small conductance Ca2+‐activated K+ (SK) current (I KAS) is abundant in atria (Xu et al. 2003; Lu et al. 2007; Tuteja et al. 2010), sinoatrial node (Torrente et al. 2017), atrioventricular node (Zhang et al. 2008) and Purkinje systems (Reher et al. 2017). Although SK channels are less abundantly expressed in ventricles, previous studies have demonstrated that I KAS exerted important influences on repolarization and arrhythmogenicity of ventricles under pathological conditions such as heart failure (Chua et al. 2011; Chang et al. 2013b), myocardial infarction (Lee et al. 2013; Zhang et al. 2013), atrioventricular block and hypokalaemia (Chan et al. 2015). Due to the fact that I KAS is only weakly activated under basal conditions in normal ventricles, whether or not sex differences exist in ventricular I KAS activation is unknown. Sympathetic activity plays important roles in modulation of the physiological fight‐or‐flight response as well as in cardiac arrhythmogenesis (Chen et al. 2014; Shen & Zipes, 2014). Since β‐adrenergic stimulation could enhance I Ca,L and increase intracellular Ca2+ (Cai), we hypothesized that the Ca2+‐dependent I KAS may be activated by isoproterenol, thus allowing us to distinguish the potential sex differences. The purpose of the present study is to test the hypotheses that there are sex differences in I KAS activation during β‐adrenergic stimulation, and that I KAS activation plays important roles in ventricular action potential (AP) triangulation and arrhythmogenesis.
Methods
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine (IACUC no. 10961), and conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
Optical mapping
A total of 50 adult (5–6 months old) New Zealand White rabbits (26 females and 24 males) were used for optical mapping studies. We placed the rabbits in a restrainer and euthanized them by intravenous sodium pentobarbitone overdose (160 mg kg−1, i.v.) before heart removal. Hearts were harvested and Langendorff perfused with Tyrode solution (in mmol L−1: 128.3 NaCl, 4.7 KCl, 20.2 NaHCO3, 0.4 NaH2PO4, 1.8 CaCl2,1.2 MgSO4, 11.1 glucose and bovine serum albumin 40 mg L−1) that was bubbled with 95% O2–5% CO2 to maintain a pH of 7.40. All chemicals were purchased from Sigma‐Aldrich (St Louis, MO, USA).
Simultaneous optical mapping of the membrane potential (V m) and Cai was performed using techniques similar to that reported elsewhere (Chang et al. 2013a). The hearts were stained with RH237 (10 μmol L−1, from Thermo Fisher Scientific, Waltham, MA, USA) for V m mapping and Rhod‐2 AM (1.4 μmol L−1, from Thermo Fisher Scientific) for Cai mapping. Blebbistatin (15∼20 μmol L−1, from Tocris Bioscience, Minneapolis, MN, USA) was used to inhibit contraction. The epicardial surfaces of right and left ventricles were excited with a laser (Verdi G5, Coherent Inc., Santa Clara, CA, USA) at a wavelength 532 nm, and the emitted fluorescence was filtered at 715 nm long pass. The fluorescence was recorded with 100 × 100 pixels for a spatial resolution of 0.35 × 0.35 mm2 per pixel at 2 ms frame−1 sampling rate. Optical signals were processed both spatially (3 × 3 pixels Gaussian filter) and temporally (3 frames moving average).
Seven different protocols were performed as listed below. Optical mapping data were collected at baseline and after each treatment. Protocol I was performed to test ventricular I KAS at basal condition. Protocols II–IV aimed to examine if I KAS is differentially activated by isoproterenol (100 nmol L−1) in females and males and to determine the effects of I KAS blockade on AP duration (APD) and Cai transient duration (CaiTD). Apamin (100 nmol L−1) blocked I KAS conducted by all SK channel isoforms while Lei‐Dab7 (20 nmol L−1), a highly specific SK channel subtype 2 (SK2) blocker, differentiated I KAS conducted by SK2 from that conducted by other isoforms. Chromanol 293B (I Ks blocker, 10 μmol L−1) was used to exclude the effects of I Ks activation during β‐adrenergic stimulation. Protocols V and VI aimed to investigate the mechanisms underlying sex differences of I KAS. Previous studies found that casein kinase‐2 (CK2) interacted with SK2 channels and diminished I KAS in neurons (Pachuau et al. 2014). Therefore, we used a CK2 specific inhibitor, 4,5,6,7‐tetrabromobenzotriazole (TBB, 10 μmol L−1), to determine if CK2 inhibition can increase I KAS in males. BayK8644 (I Ca,L agonist, 1 μmol L−1) was applied to examine the effects of I Ca,L on I KAS activation. Protocol VII was performed to determine the effects of I KAS activation and blockade on ventricular arrhythmogenicity and ventricular fibrillation (VF) dynamics.
Since ventricular pacing and atrioventricular node ablation could activate I KAS and induce short‐term cardiac memory in normal rabbit ventricles (Chan et al. 2015), we avoided ventricular pacing in Protocols I–IV and VI for APD measurement. In Protocol I, we optically mapped the ventricles with right atrial (RA) pacing at cycle length (PCL) 200, 250 and 300 ms. Since isoproterenol markedly accelerated the sinus rhythm to about 240–290 beats min−1, in Protocols II–IV, we optically mapped the ventricles at a fixed atrial PCL 200 ms in order to reach 1:1 capture. Optical mapping data in Protocols V and VII were collected under ventricular pacing. However, to minimize the ventricular pacing‐induced I KAS activation, we left the ventricles unpaced except during induction of cardiac alternans and VF. In all protocols, the agents were sequentially added to the perfusate and recirculated until washout.
Protocol I
Baseline–apamin–washout in three females and three males. After baseline mapping, apamin was added to the perfusate. We then waited 15 min to collect post‐apamin optical mapping data.
Protocol II
Baseline–isoproterenol–apamin–washout in eight females and eight males. After baseline mapping, the heart was perfused with isoproterenol for 10 min before the data collection. Apamin was then added to the perfusate while isoproterenol was kept in recirculation. During the washout, isoproterenol can be completely washed out while apamin cannot be fully washed out.
Protocol III
Baseline–isoproterenol–Lei‐Dab7–apamin–washout in four females. Lei‐Dab was administered while isoproterenol was recirculated in the perfusate. Data collection was performed 10 min after Lei‐Dab administration.
Protocol IV
Baseline–chromanol 293B–isoproterenol–apamin–washout in four females and three males. Chromanol 293B was pretreated for 30 min before isoproterenol administration and kept in the perfusate until washout.
Protocol V
Baseline–TBB–apamin–washout in three males. TBB was perfused for 15 min before data collection and was recirculated until washout.
Protocol VI
Baseline–BayK8644–apamin–washout in three females and three males. BayK8644 was perfused for 15 min before data collection and kept in perfusate until washout.
Protocol VII
Baseline–isoproterenol–apamin–washout with ventricular pacing in four females and four males. In this protocol, RV pacing at PCL 150 ms was performed to induce cardiac alternans. RV burst pacing (PCL 50 ms, duration 10 s) was used to induce VF. VF induction was attempted 10 times at baseline and after each treatment. Any VF occurrence was allowed to continue for 2 min before electrical defibrillation. We optically captured the first 100 ms at the initiation of VF. Mapping of phase singularities (PSs) and dominant frequency was also performed. The PS number was manually counted at each time point separated by 10 frames (20 ms) of data (Hayashi et al. 2007). Pseudo‐electrocardiograms (pECG) were simultaneously monitored throughout the entire experiment with widely spaced electrodes in the tissue bath.
Transmembrane potential recording
To verify the optical mapping findings at the cellular level, we performed transmembrane potential (TMP) recording of cardiomyocytes from the epicardium of LV base using the protocol of baseline–isoproterenol–apamin–washout in four hearts (2 females and 2 males). After immobilization of the Langendorff‐perfused hearts by blebbistatin, standard glass capillary microelectrodes filled with 3 mol L−1 KCl with a tip resistance of ∼20 MΩ at the digitization rate of 10 kHz was used to record TMP. The data were stored with Axoscope 10.2 (Molecular Devices, Sunnyvale, CA, USA). Experiments were performed at 38.3°C.
Ventricular cardiomyocytes isolation
Ventricular cardiomyocytes were isolated from an additional eight rabbits (4 females and 4 males) for use in patch clamp, western blot and immunostaining studies. After intravenous sodium pentobarbitone overdose, hearts were quickly excised by thoracotomy and Langendorff perfused for 5 min with Tyrode solution followed by perfusion with an oxygenated buffer containing (in mmol L−1): 138 NaCl, 5.4 KCl, 0.3 NaH2PO4, 1.2 MgCl2, 10 HEPES, 10 taurine and 10 glucose (pH 7.4 with NaOH at 37°C). This was followed by a 15 min perfusion with the same buffer containing 1 mg mL−1 collagenase (Type II, 270 U mg−1; Worthington Biochemical Corp., Lakewood, NJ, USA) and 0.1 mg mL−1 protease (Type XIV, ≥3.5 U mg−1; Sigma‐Aldrich). The heart was removed from the Langendorff apparatus. Cardiomyocytes from the base of the left ventricles were dissociated from digested ventricles by gentle mechanical dissociation.
I KAS densities determined by voltage‐clamp techniques
Voltage‐clamp experiments were conducted at room temperature. An Axopatch 200B amplifier and pCLAMP 10 software (Molecular Devices) were used to generate and record all patch experiments. Pipette electrodes were fabricated using Corning 7056 glass capillaries (Warner Instruments, Hamden, CT, USA).
For whole‐cell I KAS measurements, the pipette solution contained (in mmol L−1): 144 potassium gluconate; 1.15 MgCl2, 5 EGTA, 10 HEPES and CaCl2 yielding a free (unchelated) [Ca2+] of 1 μm (pH 7.25 using KOH). The extracellular solution contained (in mm): 140 N‐methylglucamine, 4 KCl, 1 MgCl2, 5 glucose and 10 HEPES (pH 7.4 using HCl). The voltage‐clamp protocol consisted of a ramp‐pulse protocol (test pulse: between +40 and −120 mV, holding potential: −70 mV; pulse frequency: every 3 s). Pipette resistance ranged from 1 to 2 MΩ. Series resistance was electronically compensated by 70–80%. Currents were recorded at baseline and after the exposure to 100 nmol L−1 apamin. We also recorded the current in the presence of 100 nmol L−1 isoproterenol and after apamin. To obtain whole‐cell I KAS, currents recorded in the presence of 100 nmol L−1 apamin were digitally subtracted from those recorded in its absence.
To verify the selectivity of Lei‐Dab7 for I KAS conducted by SK2 but not SK3, we tested the effects of Lei‐Dab7 on human embryonic kidney (HEK) 293 cells transfected with KCNN2 and KCNN3, respectively. HEK 293 cell were cultured in Iscove's modified Dulbecco's medium (Thermo Fisher Scientific) with 10% fetal bovine serum and 1% penicillin/streptomycin in 5% CO2 at 37°C; 35 mm dishes of HEK 293 cells were transiently transfected using Effectene Transfection Reagent (Qiagen, Germantown, MD, USA) according to the manufacturer's protocol and were harvested for patch clamp experiment 48∼72 h later. The SK2 and SK3 clones were developed in our laboratory. HEK 293 cells were transfected with 2.0 μg of KCNN2/pIRES‐EGFP2 or 2.0 μg of KCNN3/pIRES‐EGFP2 plasmids, respectively. Single cells were picked and propagated in selection medium containing hygromycin 200 μg mL−1. Currents were recorded at baseline and after exposure to 20 nmol L−1 Lei‐Dab 7, followed by addition of 100 nmol L−1 apamin. The LeiDab7‐sensitive current and apamin‐sensitive current (in the presence of LeiDab7) were calculated, respectively.
Western blot
Western blot was performed in isolated rabbit left ventricular cardiomyocytes. A Lowry protein assay was performed before western blot to guarantee that the protein loadings were identical among lanes. Samples were loaded on SDS‐PAGE and transferred to a nitrocellulose membrane. The blot was probed with the following antibodies: anti‐KCa2.2 (SK2) antibody (1:500, APC‐028, Alomone, Jerusalem, Israel), anti‐CK2α antibody (1:500, ab181734, Abcam, Cambridge, UK), anti‐CK2β antibody (1:500, ab201990, Abcam) and anti‐SERCA antibody 2A7‐A1 (1:1000). Antibody‐binding protein bands were quantified with a Bio‐Rad (Hercules, CA, USA) Personal Fx phosphorimager. The control sample for SK2 was the heterologously expressed human isoform of SK2 in cultured HEK 293 cells.
Immunofluorescence confocal microscopy
Both isolated myocytes and ventricular tissues were used for immunofluorescence staining and confocal microscopy. Isolated rabbit ventricular myocytes were fixed with 1% paraformaldehyde for 30 min and seeded on the laminin‐coated glass slides for at least 1 h to allow cell attachment. In addition, ventricular tissue samples were fixed with 4% paraformaldehyde for 45 min, followed by storage in 70% alcohol for at least 48 h. The tissue samples were then processed routinely and embedded in paraffin. Tissue sections (5 μm in thickness) were deparaffinized and hydrated by multiple xylene and ethanol washes.
Slides with either isolated myocytes or ventricular tissue were washed with water and PBS in Tween 20 at least 3 times. Samples were then permeabilized and blocked in PBS with 3% BSA and 0.2% Triton X‐100 for 1 h. After blocking, slides were incubated with anti‐KCa2.2 (SK2) antibody (APC‐028, Alomone) 1:200 diluted in PBS (BSA 1%) at 4°C overnight. Subsequently, slides were washed 3 times for 5 min with PBS and incubated with protein A conjugated with fluorescent dyes (Alexa 488, Thermo Fisher Scientific, 1:2000) and 4′,6‐diamidino‐2‐phenylindole for 1 h. After this step, the slides were again washed 3 times for 5 min with PBS followed by mounting with coverslips. The slides were allowed to dry at room temperature and then sealed with nail polish overnight. For comparison, samples from females and males were stained simultaneously.
Confocal images were obtained through a DMI6000 adaptive focus automated inverted microscope, Leica TCS SP8 FSU (argon ion laser) spectral confocal system with HyD supersensitivity detection. For comparison of the fluorescence intensities, slides from females and males were microscopically detected on the same day using identical microscopy settings.
Statistics and data analysis
APD at the level of 25% (APD25) and 80% (APD80) repolarization of the AP was optically measured. We used APD25 to characterize the early phases (phases 1 and 2) of repolarization and APD80 to represent the entire repolarization (phase 1, 2 and 3). Thus, the differences between APD25 and APD80 (APD80 − APD25) were used to characterize the repolarization at phase 3. The APD25/APD80 ratio was used as a quantitative measurement of the AP morphology, with a smaller APD25/APD80 ratio reflecting greater AP triangulation and a larger APD25/APD80 ratio representing AP squaring. Intracellular calcium transient duration at 25% and 80% recovery (CaiTD25 and CaiTD80) were used as measures of early and total Cai duration, respectively. We also calculated the differences between CaiTD and APD (CaiTD25 − APD25 and CaiTD80 − APD80) to characterize the Cai–V m coupling. We averaged the APD in the regions of interest and presented them in the summary data.
Continuous variables were expressed as mean ± SEM. Student's paired t test was used to compare two variables from the same rabbit (such as APD25 before and after apamin). Unpaired t tests were used to compare variables between two groups (such as ΔAPD25 between females and males). Multiple t tests were used to compare I KAS current densities between sexes at different membrane potentials. One‐way or two‐way ANOVA with the appropriate post hoc test, as indicated in each experiment, was used to compare the means among three or more different variables. A two‐sided P value of <0.05 was considered statistically significant.
Results
Isoproterenol activates I KAS in female rabbit ventricles
Consistent with previous studies (Chua et al. 2011), I KAS blockade by apamin only minimally prolonged APD (by <5%) in ventricles from females and males at baseline (Protocol I, Fig. 1). The magnitudes of APD prolongation was not significantly different between males and females. We then tested the effects of I KAS blockade in the presence of isoproterenol in female rabbit ventricles (Fig. 2 A, Protocol II). The AP exhibited a prominent phase 2 plateau at baseline. Isoproterenol shortened and triangulated the AP. Subsequent apamin administration (with isoproterenol in the recirculation) significantly prolonged APD25 (from 29 ± 2 to 40 ± 2 ms, P < 0.001) and APD80 (from 92 ± 4 to 101 ± 3 ms, P < 0.001, Fig. 2 B and C), indicating I KAS activation during isoproterenol perfusion. In addition to APD prolongation, apamin also affected the AP morphology, i.e. reversing isoproterenol‐induced AP triangulation and restoring the phase 2 plateau of repolarization, by lengthening APD25 (11 ± 1 ms and 41 ± 6%) more prominently than APD80 (9 ± 1 ms and 10 ± 1%, P = 0.018 for absolute value and P < 0.001 for percentage, Fig. 2 D). As a result, the ratio APD25/APD80 was significantly increased (from 32 ± 2% to 40 ± 2%, P < 0.001, Fig. 2 E). Apamin significantly abbreviated differences between APD80 and APD25 from 63 ± 2 to 60 ± 3 ms (P = 0.043, Fig. 2 F), indicating a slight acceleration of phase 3 repolarization. Furthermore, as shown in Fig. 2 G, the prolongation of the entire repolarization (positive ΔAPD80) was associated with the abbreviation of phase 3 repolarization (negative Δ(APD80 − APD25)) in 7/8 females (circles in quadrant IV), indicating the APD prolongation was completely attributable to the prolongation of phase 2 repolarization. After isoproterenol washout, APD was further prolonged towards the baseline level. As shown in Fig. 2 H, TMP recording in cardiomyocytes of female rabbits also verified the optical mapping findings. These results indicate that I KAS is activated during β‐adrenergic stimulation in female rabbit ventricles.
Figure 1. Effects of I KAS blockade on APD at basal condition.
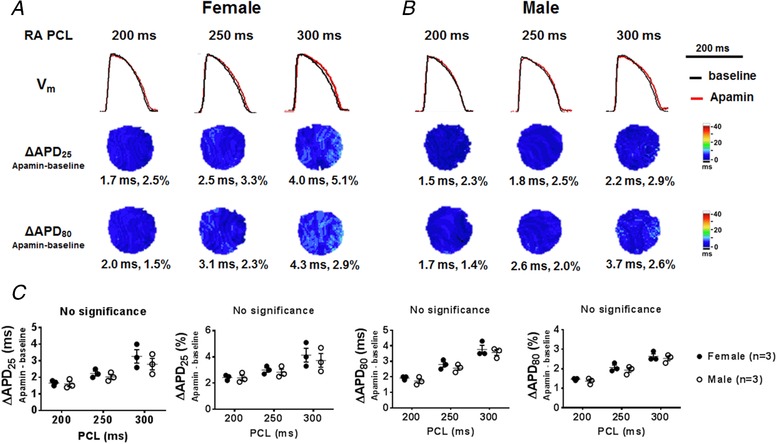
A and B, representative V m traces, ΔAPD25 and ΔAPD80 maps in female (A) and male (B) rabbit ventricles at RA PCL 200, 250 and 300 ms under Protocol I. The apamin‐induced APD prolongation was less than 5% in the absence of β‐adrenergic stimulation. Representative V m traces were obtained at the LV base. C, summary data showed that no significant difference existed in ΔAPD25 and ΔAPD80 between females and males at baseline (by two‐way ANOVA with Sidak's post hoc test). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2. Effects of I KAS blockade on APD during isoproterenol infusion in female rabbit ventricles.
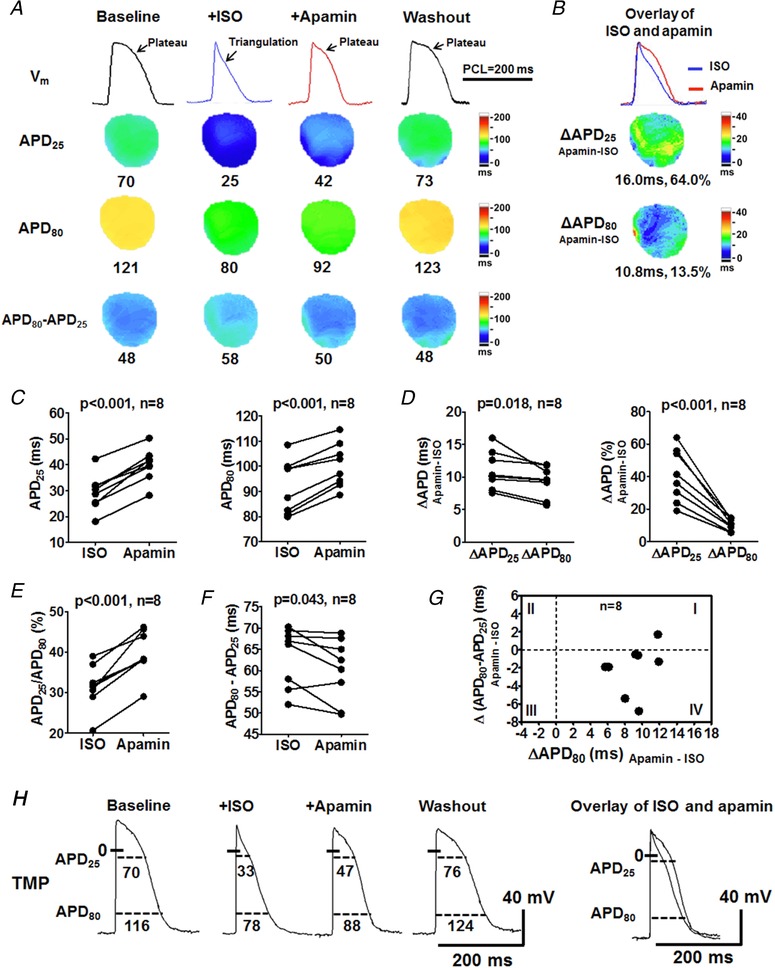
A, representative V m traces, APD25, APD80 and APD80 − APD25 maps at baseline, during isoproterenol, after apamin and after washout (Protocol II). At baseline, the AP exhibited a prominent phase 2 plateau. Isoproterenol markedly shortened APD and more prominently APD25 than APD80, leading to a short and triangular AP. Representative V m traces were obtained at the LV base. B, C and D, apamin significantly prolonged both APD25 and APD80, but more prominently APD25 (D), consequently reversing the AP triangulation and restoring the AP plateau. After washout, the APD was further prolonged to a level similar to the baseline. E, apamin significantly increased the ratio of APD25/APD80, resulting in AP squaring and plateau restoration. F, apamin significantly abbreviated APD80 − APD25, indicating an acceleration of phase 3 repolarization. Student's paired t tests were performed in C–F. G, the prolongation of the total repolarization by apamin (represented by ΔAPD80) corresponded to the shortening of phase 3 repolarization (Δ(APD80 − APD25)) in 7 out of 8 females (circles in quadrant IV). Therefore, the apamin predominantly lengthened APD25. H, representative TMP recording in ventricular cardiomyocytes from a female rabbit. ISO, isoproterenol. [Color figure can be viewed at http://wileyonlinelibrary.com]
Cai mapping was simultaneously performed in females under Protocol II (Fig. 3). Compared with baseline, isoproterenol markedly shortened CaiTD25 and CaiTD80 (Fig. 3 A) but increased the peak Cai (Fig. 3 B). These findings indicate higher Cai at phase 2 repolarization. In addition, isoproterenol markedly increased the differences between CaiTD and APD (CaiTD − APD) and more drastically for CaiTD25 − APD25 than for CaiTD80 − APD80 (30 ± 2 vs. 23 ± 3, P = 0.041, Fig. 3 C and F), suggesting an early activated outward current in compensation for the amplified I Ca,L during phase 2 repolarization. Apamin had minimal effect on CaiTD and peak Cai (Fig. 3 B and D), but produced significant abbreviation of CaiTD25 − APD25 (from 30 ± 2 to 20 ± 2 ms, P < 0.001, Fig. 3 E) and elimination of the isoproterenol‐induced differences CaiTD25 − APD25 and CaiTD80 − APD80 (P = 0.563, Fig. 3 G). Taken together, isoproterenol increases Cai, which in turn activates I KAS to shorten the APD. Therefore, isoproterenol increases the differences between CaiTD25 and APD25 during phase 2 (negative Cai–APD coupling).
Figure 3. Effects of I KAS blockade on Cai and Cai–V m coupling during isoproterenol infusion in female rabbit ventricles.
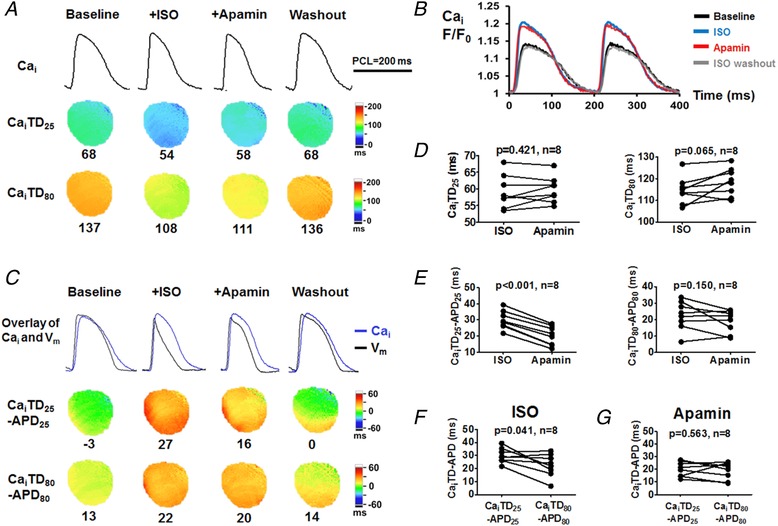
A, representative Cai traces, CaiTD25 and CaiTD80 maps at baseline, during isoproterenol, after apamin and after washout (Protocol II). Compared with baseline, isoproterenol markedly shortened CaiTD25 and CaiTD80. Apamin only slightly prolonged CaiTD. After washout, the CaiTD was prolonged towards the baseline level. Representative Cai traces were obtained at the LV base. B, Cai F/F 0 showed that isoproterenol markedly increased peak Cai F/F 0 and accelerated Cai upstroke and decay. Apamin had minimal effect on peak Cai F/F 0 and Cai transient. C, overlapped Cai and V m traces and CaiTD − APD maps showed that isoproterenol enlarged the differences between Cai and APD compared with baseline, especially CaiTD25 − APD25. Apamin markedly attenuated the CaiTD25 − APD25 attributable to the remarkably prolonged APD25. CaiTD80 − APD80 remained similar before and after apamin. Washout further abbreviated CaiTD − APD towards baseline. D, apamin had insignificant effect on CaiTD25 and CaiTD80. E, apamin significantly decreased CaiTD25 − APD25 while having an insignificant effect on CaiTD80 − APD80. F, during isoproterenol, CaiTD25 − APD25 was significantly larger than CaiTD80 − APD80. G, after apamin, the differences between CaiTD25 − APD25 and CaiTD80 − APD80 were eliminated. Student's paired t test was performed in D–G. [Color figure can be viewed at http://wileyonlinelibrary.com]
SK2, but not SK3 or I KS, is responsible for isoproterenol‐induced AP triangulation in females
Among subtypes of SK channels which generate I KAS, SK2 and SK3 are important to ventricular repolarization and are completely blocked by 100 nmol L−1 apamin (Yu et al. 2014). Here, we took advantage of the specific SK2 blocker Lei‐Dab7 (20 nmol L−1) (Shakkottai et al. 2001) to distinguish the contributions of I KAS conducted by different SK subtypes. Firstly, we verified the selectivity of Lei‐Dab7 on SK2 over SK3 (Fig. 4 A and B). Lei‐Dab7 at 20 nmol L−1 inhibited the majority of whole‐cell currents in KCNN2‐transfected HEK293 cells but had only minimal effect on KCNN3‐transfected cells. These results indicate Lei‐Dab7 at 20 nmol L−1 only blocks SK2 channels.
Figure 4. SK2, but not SK3, is responsible for AP triangulation in females.
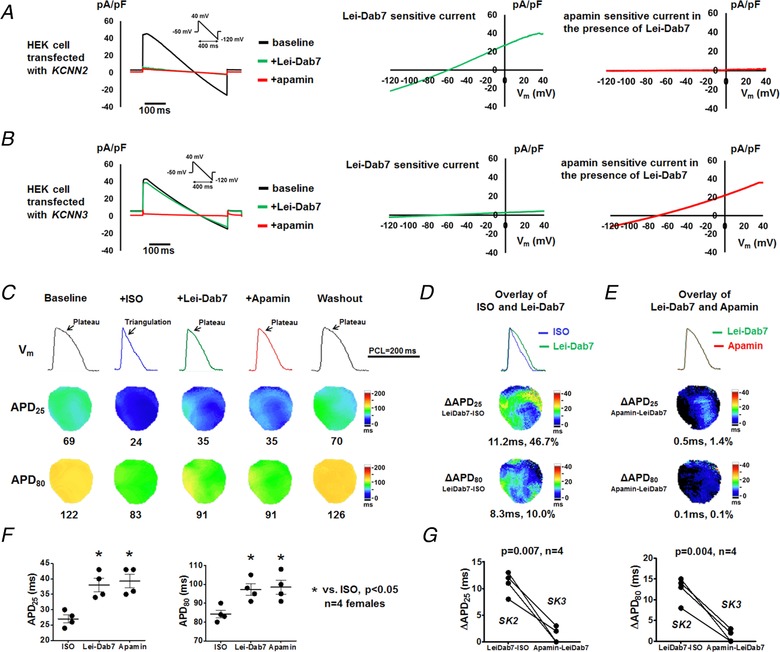
A and B, representative whole cell currents sequentially recorded from KCNN2‐ (A) or KCNN3‐ (B) transfected HEK293 cells at baseline and in the presence of Lei‐Dab7 (20 nmol L−1) and subsequently apamin (100 nmol L−1). Lei‐Dab‐sensitive current was calculated as the difference between baseline and Lei‐Dab7. Apamin‐sensitive current in the presence of Lei‐Dab7 was calculated as the difference between Lei‐Dab7 and apamin. C, representative optical V m traces, APD25 and APD80 maps at baseline, during isoproterenol, after Lei‐Dab7, after apamin and after washout at PCL 200 ms (Protocol III). Representative V m traces were obtained at the LV base. D, compared with isoproterenol, Lei‐Dab7 prolonged both APD25 and APD80, but more prominently APD25, thus leading to the phase 2 plateau restoration. E, compared with Lei‐Dab7, subsequently administered apamin did not further prolong APD. F, summary data showed that APD25 and APD80 were significantly longer after Lei‐Dab7 and after apamin, both in comparison with those at baseline. APD were similar between Lei‐Dab7 and apamin. *P < 0.05 by one‐way ANOVA with Tukey's post hoc test. G, I KAS conducted by SK2 (represented by ΔAPDLeiDab7−isoproterenol) was significantly more activated than I KAS by SK3 (represented by ΔAPDApamin−LeiDab7) measured at both APD25 and APD80 (by Student's paired t test). ISO, isoproterenol. [Color figure can be viewed at http://wileyonlinelibrary.com]
We further tested the effects of Lei‐Dab7 (20 nmol L−1) in females under Protocol III (Fig. 4). In the presence of isoproterenol, SK2 blockade by Lei‐Dab7 significantly prolonged APD25 and to a smaller extent APD80 (Fig. 4 C and D). Subsequently administered apamin, which only blocked SK3 with Lei‐Dab7 pretreatment, failed to further lengthen APD (Fig. 4 E and F). In other words, SK2 blockade (ΔAPDLeiDab7−isoproterenol) had significantly larger effects than SK3 blockade (ΔAPDApamin−LeiDab7) on both APD25 (11 ± 1 vs. 1 ± 1 ms, P = 0.007) and APD80 (13 ± 2 vs. 1 ± 1 ms, P = 0.004, Fig. 4 G). These results suggest that SK2, rather than SK3, is the predominant channel isoform underlying I KAS activation during β‐adrenergic stimulation.
Similar to I KAS, I Ks is also calcium sensitive and is enhanced during sympathetic stimulation (Aflaki et al. 2014; Banyasz et al. 2014; Bartos et al. 2017). To exclude I Ks as a cause of AP triangulation in females, we pretreated ventricles with the I Ks blocker chromanol 293B (10 μmol L−1) in Protocol IV (Fig. 5). With I Ks blockade, isoproterenol still shortened and triangulated AP. Subsequent apamin administration significantly prolonged APD25 and to a lesser extent APD80, indicating that I KAS contributes to APD shortening and AP triangulation independently of I Ks.
Figure 5. I Ks is not responsible for AP triangulation in females.
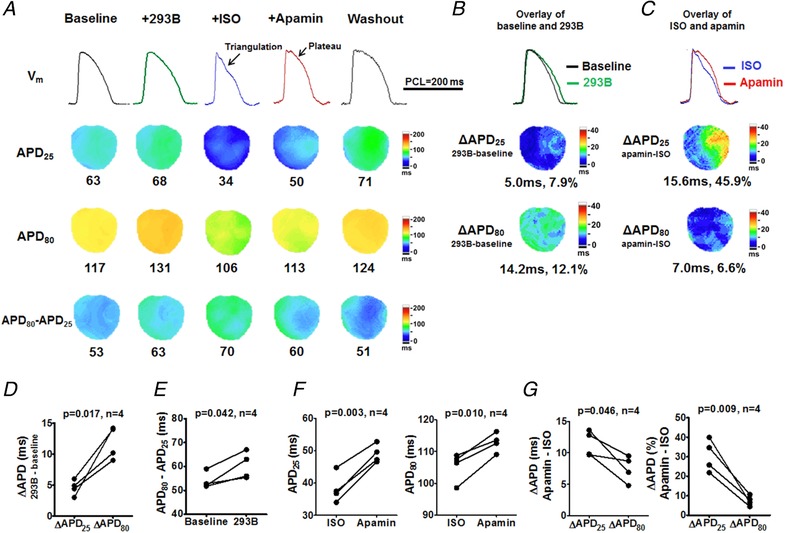
A, representative V m traces, APD25, APD80 and APD80 − APD25 maps at baseline, after pretreatment of I Ks blocker chromanol 293B, after isoproterenol, after apamin and after washout (Protocol III). Representative V m traces were obtained at the LV base. B, D and E, compared with baseline, chromanol 293B prolonged APD25 and APD80 (B), but more prominently APD80 (D), by markedly slowing repolarization at phase 3 (APD80 − APD25; E). With chromanol 293B pretreatment, isoproterenol still shortened and triangulated AP. C, F and G, apamin prolonged APD25 and APD80 (C and F) but more prominently APD25 than APD80 (G). Student's paired t tests were performed in D–G. [Color figure can be viewed at http://wileyonlinelibrary.com]
I KAS is minimally activated by isoproterenol in male rabbit ventricles
To test sex differences in I KAS activation, we repeated Protocol II in male rabbit ventricles (Fig. 6 A). In contrast to females, apamin prolonged APD very slightly after isoproterenol infusion, although the increment was statistically significant (for APD25, from 30 ± 2 to 33 ± 2 ms and for APD80, from 93 ± 3 to 98 ± 4 ms, P = 0.001 for both, Fig. 6 B and C). The prolongations of APD25 and APD80 were similar (for absolute value: 3 ± 0 ms vs. 4 ± 0 ms, P = 0.154; for percentage: 11 ± 0% and 6 ± 1%, P = 0.060; Fig. 6 D) and barely altered the APD25/APD80 ratio (32 ± 2 vs. 33 ± 2, P = 0.101, Fig. 6 E), indicating no major AP morphology change by apamin. Apamin had little effect on phase 3 repolarization (represented by APD80 − APD25, 64 ± 3 ms vs. 65 ± 4 ms, P = 0.180, Fig. 6 F). Figure 6 G shows that apamin abbreviated phase 3 repolarization but prolonged the APD80 in 4/8 male rabbit ventricles (positive ΔAPD80 with negative Δ(APD80 − APD25), circles in quadrant IV). These data indicate that the APD prolongation, although only slight, was completely attributable to phase 2 prolongation in these four rabbits. For the other four males, however, the APD80 prolongation relied on the phase 3 prolongation (positive ΔAPD80 with positive Δ(APD80 − APD25), circles in quadrant I), which was a different apamin responses from females. As shown in Fig. 6 H, we further verified the findings of optical mapping at the cellular level by TMP recording in cardiomyocytes from male rabbits. Taken together, in contrast to females, males only exhibit minimal I KAS activation both at baseline and during isoproterenol infusion. I KAS activation could shorten either phase 2 or phase 3 repolarization of the AP in males.
Figure 6. Effects of I KAS blockade on APD during isoproterenol in male rabbit ventricles.
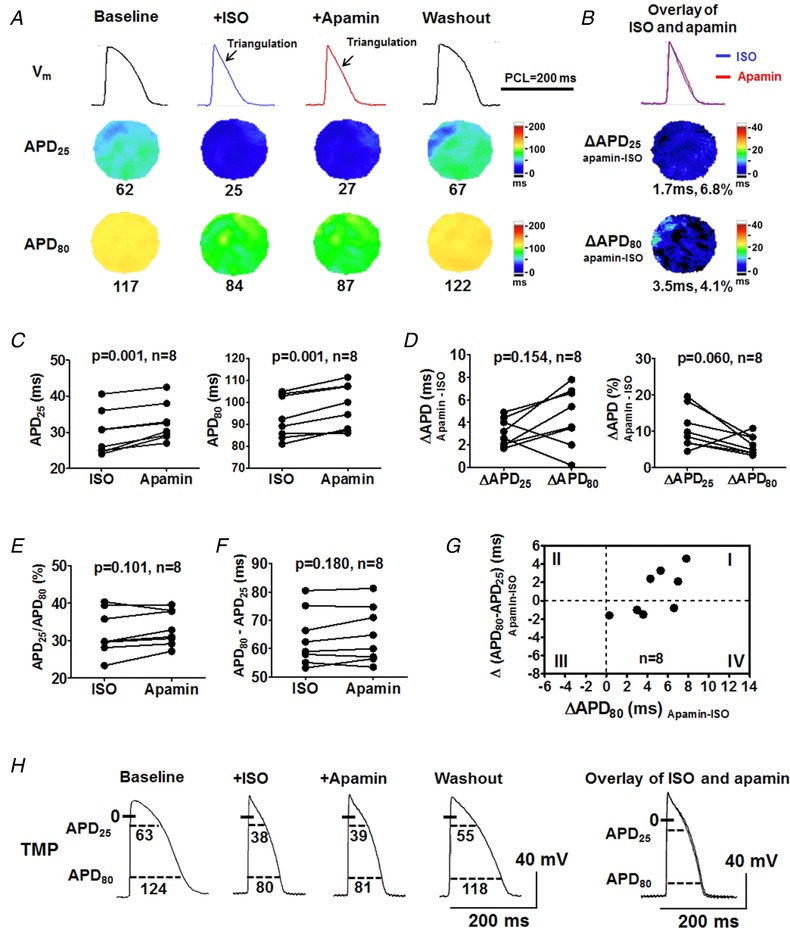
A, representative V m traces, APD25 and APD80 maps at baseline, during isoproterenol, after apamin and after isoproterenol washout (Protocol II). After isoproterenol, APD was markedly shortened and triangulated. Apamin slightly prolonged APD but did not reverse the AP triangulation. After washout, AP plateau was restored and APD was lengthened towards baseline. Representative V m traces were obtained at the LV base. B and C, apamin prolonged APD25 and APD80 significantly but only slightly. D, apamin prolonged APD25 and APD80 to a similar extent. E, APD25/APD80 ratios were similar before and after apamin. F, APD80 − APD25 was similar before and after apamin. Student's paired t test was performed in C–F. G, APD80 prolongation by apamin was partly attributed to phase 3 prolongation in 4/8 ventricles (circles in quadrant I). While in the other 4 ventricles, the APD80 prolongation corresponded to slight phase 3 acceleration (circles in quadrant IV). H, representative TMP recording in ventricular cardiomyocytes from a male rabbit. [Color figure can be viewed at http://wileyonlinelibrary.com]
To evaluate the mechanism of isoproterenol‐induced AP shortening and triangulation that is independent of I KAS activation in males, we pretreated male rabbits with chromanol 293B to inhibit I Ks, which is activated by isoproterenol and exhibits stronger activation in males than females (Zhu et al. 2013). As shown in Fig. 7, chromanol 293B prolonged both APD25 and APD80 and more prominently APD80. Compared with females, males had significantly larger APD prolongation, suggesting stronger I Ks activation in males than females. However, isoproterenol still shortened and triangulated APD even with I Ks and subsequent I KAS blockade. These results indicate that isoproterenol‐induced APD shortening and triangulation in males cannot be explained only by I Ks and I KAS activation.
Figure 7. I Ks is not responsible for AP triangulation in males.
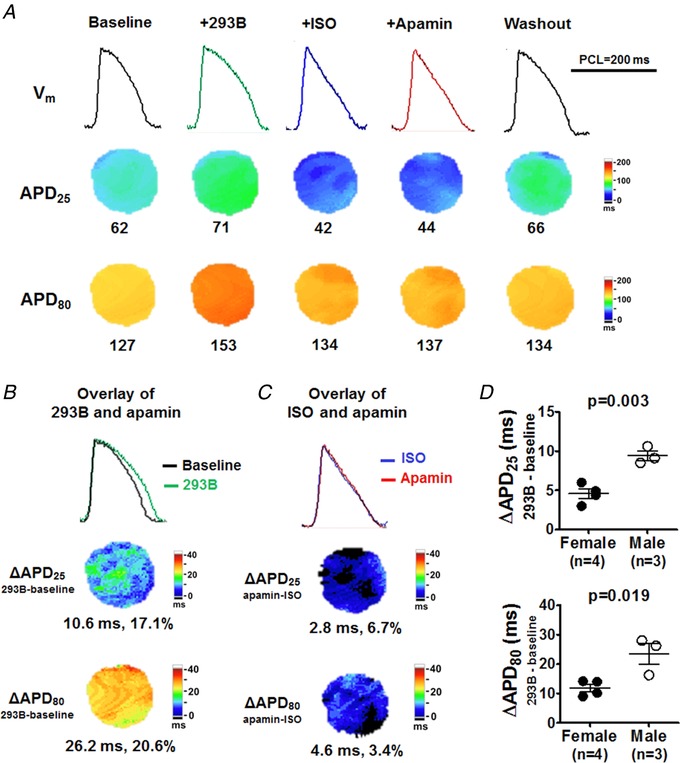
A, representative V m traces, APD25, APD80 and APD80 − APD25 maps at baseline, after pretreatment with I Ks blocker chromanol 293B, after isoproterenol, after apamin and after washout (Protocol III). Representative V m traces were obtained at the LV base. B, compared with baseline, chromanol 293B markedly prolonged APD25 and APD80 but more prominently APD80. With chromanol 293B pretreatment, isoproterenol still shortened and triangulated AP. C, apamin only minimally prolonged APD25 and APD80. D, compared with females, males had significant larger response in chromanol 293B‐induced APD prolongation (by unpaired t test). [Color figure can be viewed at http://wileyonlinelibrary.com]
The differential I KAS activation after isoproterenol infusion between sexes is summarized in Fig. 8. Females had significantly greater responses to I KAS blockade than males in APD25 (absolute value: 11 ± 1 vs. 3 ± 0 ms, P < 0.001; percentage: 39 ± 5% vs. 11 ± 2%, P < 0.001; Fig. 8 A) and in APD80 (absolute value: 9 ± 1 vs. 5 ± 1 ms, P = 0.003; percentage: 10 ± 1% vs. 5 ± 1%, P = 0.006; Fig. 8 B). Apamin slightly accelerated phase 3 repolarization in females but not in males (Δ(APD80 − APD25): −2 ± 1 vs. 0 ± 1 ms, P = 0.035, Fig. 8 C). The APD25/APD80 ratio was significantly larger in females than in males (40 ± 2% vs. 34 ± 2%, P = 0.037, Fig. 8 D), indicating differential AP morphology changes by I KAS blockade between sexes.
Figure 8. Sex differences of the apamin effects (Protocol II).
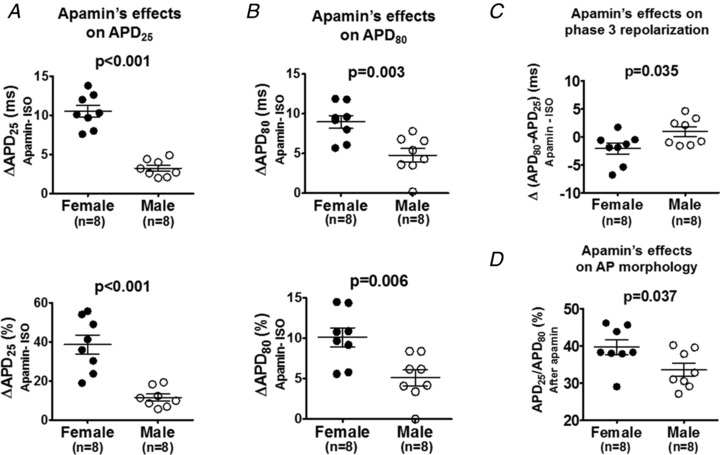
A and B, in the presence of isoproterenol, female rabbit ventricles exhibited significantly larger apamin‐induced prolongation than male rabbit ventricles for both (A) APD25 and (B) APD80. C, females and males responded to apamin significantly differently on phase 3 repolarization (Δ(APD80 − APD25)). Female rabbit ventricles exhibited an abbreviation of Δ(APD80 − APD25) while male rabbit ventricles did not. D, APD25/APD80 ratio after apamin was significantly larger in females than in males. Unpaired t tests were performed in comparison between sexes.
I KAS current densities are higher in females than males
To better understand the mechanisms of the sex differential ventricular I KAS activation, we examined I KAS at cellular levels by performing patch clamp studies in isolated ventricular cardiomyocytes. As shown in Fig. 9 A, with free Ca2+ concentration of 1 μm, mean I KAS densities were significantly larger in cells from females than males measured at a potential >0 mV (at +10mV: 0.35 ± 0.03 vs. 0.19 ± 0.03 pA pF−1; at +20 mV: 0.47 ± 0.04 vs. 0.21 ± 0.03 pA pF−1; at +30 mV: 0.75 ± 0.05 vs. 0.28 ± 0.05 pA pF−1; at +40 mV: 0.98 ± 0.09 vs. 0.34 ± 0.06 pA pF−1; P < 0.05). We further tested I KAS densities with pretreatment of 100 nmol L−1 isoproterenol (Fig. 9 B). In the presence of isoproterenol, I KAS densities were significantly larger in myocytes from female than male rabbits at a potential >0 mV (at +10 mV: 0.42 ± 0.08 vs. 0.13 ± 0.03 pA pF−1; at +20 mV: 0.70 ± 0.09 vs. 0.24 ± 0.02 pA pF−1; at +30 mV: 0.99 ± 0.10 vs. 0.38 ± 0.03 pA pF−1; at +40 mV: 1.30 ± 0.12 vs. 0.51 ± 0.03 pA pF−1; P < 0.05). These patch clamp results supported our observations in optical mapping.
Figure 9. I KAS current density is larger in females than males.
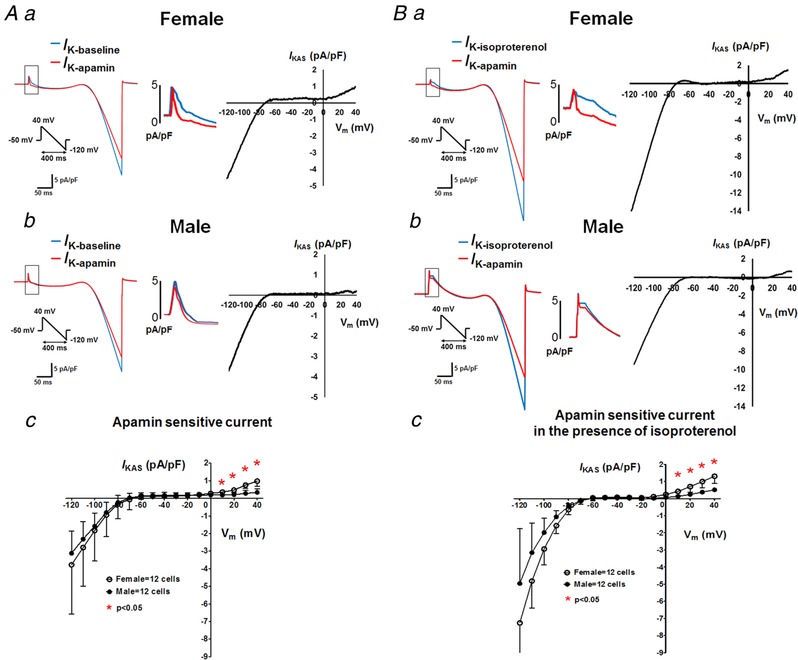
A and B, I KAS densities of isolated ventricular myocytes by patch clamp using ramp‐pulse protocol (test pulse: between +40 and −120 mV; holding potential −70 mV; pulse frequency: every 3 s) in the absence (A) or presence (B) of isoproterenol. Left: representative membrane current traces obtained from female (a) and male (b) rabbit ventricular myocytes. Currents were recorded with an intrapipette free Ca2+ of 1 μmol L−1 in the absence (I K‐baseline or I K‐isoproterenol, blue) and the presence (I K‐apamin, red) of 100 nmol L−1 apamin. Middle: amplified images. Right: I KAS was calculated as the difference between I K‐baseline/I K‐isoproterenol and I K‐apamin. Ac and Bc, I KAS density–voltage relationships from female and male rabbit ventricular cardiomyocytes. *P < 0.05 by multiple t tests. [Color figure can be viewed at http://wileyonlinelibrary.com]
SK2 channel protein expression is higher in females than males
Since SK2 carried the enhanced I KAS by isoproterenol in females, we examined the SK2 channel expression by western blot and confocal immunofluorescence microscopy in both sexes. Western blotting in Fig. 10 A showed that SK2 protein expression was significantly higher in female rabbit cardiomyocytes than in males (SK2 normalized to SERCA: 0.075 ± 0.005 vs. 0.043 ± 0.004, P = 0.003). As further shown in Fig. 10 B and C, confocal immunofluorescence microscopy detected the expression of SK2 along the Z‐line of plasma membrane at higher fluorescence intensities in females than that in males from both isolated cardiomyocytes and ventricular tissue sections. These results indicate that sex differences exist in SK2 protein expression, which contribute to sex differences in I KAS.
Figure 10. SK2 western blotting and immunostaining of rabbit ventricles.
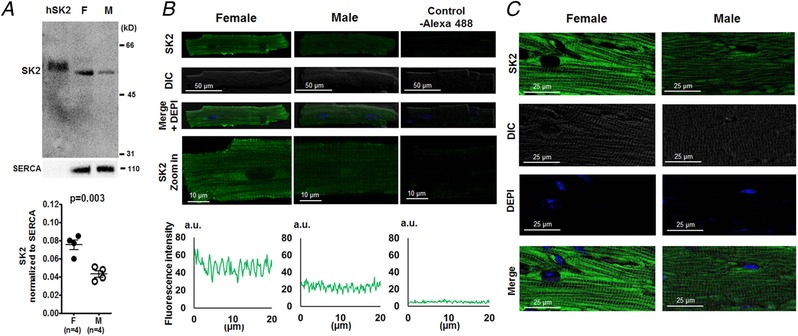
A, western blot showing that SK2 channel protein expression (normalized to SERCA) was significantly higher in females than males (by unpaired t test). hSK2 is heterologously expressed human isoform of SK2 in HEK 293 cells, which serves as the positive control. B, representative confocal immunofluorescence microscopy of SK2 staining in isolated ventricular myocytes. The fluorescence intensity was higher in female than in male ventricular myocytes. Protein A conjugated with Alexa 488 without pretreatment of anti‐SK2 antibody was used as a negative control. The fluorescence signals were detected under an identical confocal microscopy setting. a.u., arbitrary units. C, representative confocal immunofluorescence microscopy of SK2 in left ventricular tissues. The fluorescence signals were stronger in female rabbit ventricular tissues than in males. The fluorescence signals were detected under an identical confocal microscopy setting. [Color figure can be viewed at http://wileyonlinelibrary.com]
CK2 activity correlates with I KAS suppression in males
In its participation in a broad range of cellular signalling pathways, CK2 phosphorylates calmodulin, thus lowing the Ca2+ affinity of SK2 channels and reducing I KAS in neurons (Pachuau et al. 2014). To evaluate if CK2 activity contributed to I KAS suppression in male rabbit ventricles, we tested the effect of a CK2‐specific blocker, TBB, on APD (Protocol V). As shown in Fig. 11 A, TBB drastically abbreviated and triangulated APD. Subsequently, apamin significantly prolonged both APD25 (from 15 ± 2 to 22 ± 1 ms, P = 0.034) and APD80 (from 39 ± 2 to 64 ± 4 ms, P = 0.045, Fig. 11 B and C), indicating that CK2 inhibition in males can increase the I KAS activation. Although western blotting showed CK2α and CK2β had similar expression levels between sexes (Fig. 11 D), the significantly higher CK2/SK2 ratio in males than in females (CK2α/SK2: 12 ± 2 vs. 21 ± 2, P = 0.012; CK2β/SK2 ratio: 11 ± 1 vs. 18 ± 2, P = 0.031, Fig. 10 E) may correlate with smaller I KAS in males.
Figure 11. Effects of I KAS blockade during CK2 inhibition in rabbit ventricles.
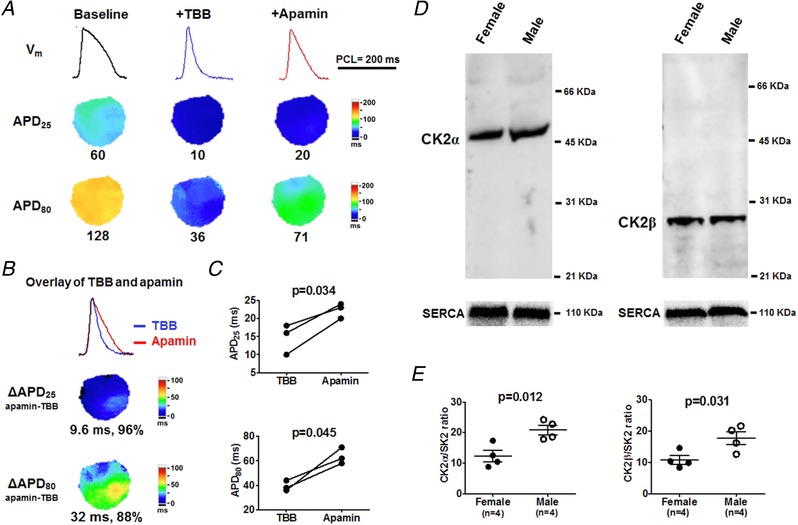
A, representative V m traces, APD25 and APD80 maps at baseline, after CK2 inhibitor TBB and after apamin (Protocol V). Compared with baseline, TBB prominently abbreviated APD25 and APD80, leading to a short and triangular AP. B and C, apamin significantly prolonged APD25 and APD80. Representative V m traces were obtained at the LV base. Student's paired t test was performed in C. D, western blot showing similar protein expression of CK2α and CK2β between cardiomyocytes from female and male rabbit ventricles. E, males had significantly higher CK2α/SK2 and CK2β/SK2 ratios than females (by unpaired t test). [Color figure can be viewed at http://wileyonlinelibrary.com]
Sex differences in I KAS are not secondary to sex differences in I Ca,L
SK2 is molecularly coupled with L‐type calcium channels (LTCCs) (Lu et al. 2007). The magnitude of I Ca,L density is known to be smaller in males than in females (Vizgirda et al. 2002). It is therefore possible that the weaker I Ca,L in males partially explains the lower of I KAS. To test this hypothesis, BayK8644 was used to amplify I Ca,L (Protocol VI, Fig. 12). In males, compared with baseline, BayK8644 led to markedly enlarged CaiTD, drastically increased peak Cai and prolonged APD. However, subsequently administered apamin failed to further prolong APD, indicating that BayK8644 failed to activate I KAS in males. As a comparison, apamin prolonged APD in the presence of BayK8644 in females. These results are inconsistent with the hypothesis that the sex differences in I KAS are simply secondary to the sex differences in I Ca,L.
Figure 12. Effects of I KAS blockade during BayK8644 in male (A–F) and female (G and H) rabbit ventricles.
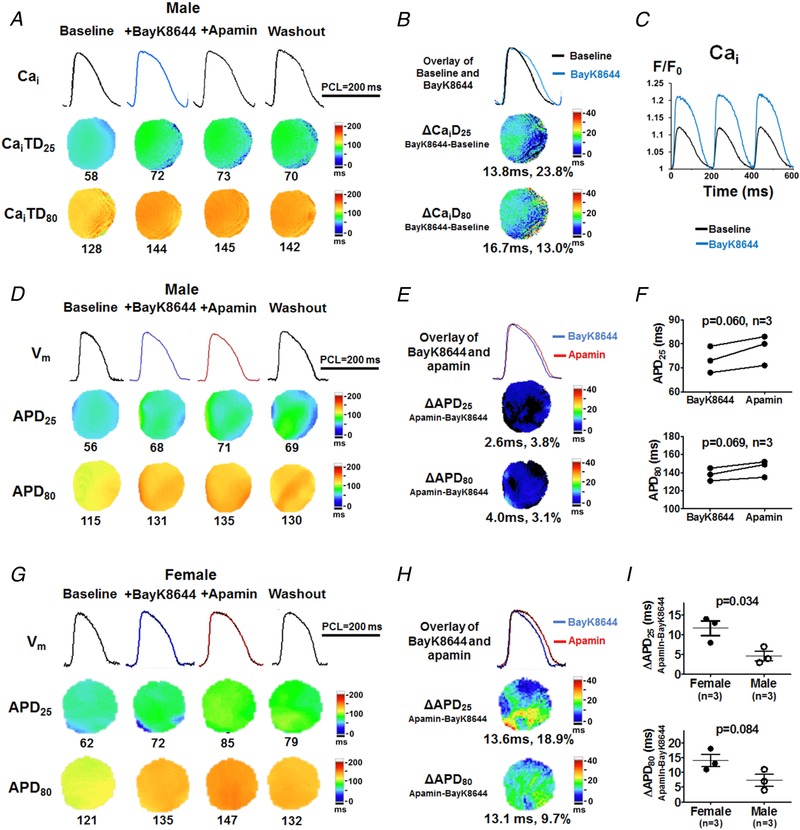
A, representative Cai traces, CaiTD25 and CaiTD80 maps at baseline, after BayK8644, after apamin and after washout in a male rabbit (Protocol VI). BayK8644 markedly prolonged CaiTD25 and CaiTD80 while apamin had minimal effect on CaiTD, B, overlapped Cai traces and ΔCaiTD maps showed BayK8644 markedly prolonged CaiTD compared with baseline. C, BayK8644 markedly increased the peak Cai F/F 0. D, corresponding V m traces, APD25 and APD80 maps. Compared with baseline, BayK8644 markedly prolonged APD25 and APD80. E, apamin only slightly prolonged APD25 and APD80. Representative V m and Cai traces were obtained at the LV base. F, with BayK8644 pretreatment, apamin prolonged APD25 and APD80, both insignificantly (by Student's paired t test). G and H, representative V m traces, APD25 and APD80 maps in a female rabbit (Protocol VI). Compared with baseline, BayK8644 markedly prolonged APD25 and APD80. Subsequent apamin administration further prolonged APD25 and APD80, but more prominently APD25. I, female rabbits had significant larger response in apamin‐induced APD prolongation than male rabbits with BayK8644 pretreatment (by unpaired t test). [Color figure can be viewed at http://wileyonlinelibrary.com]
I KAS blockade eliminates negative Cai–V m coupling at phase 2 and attenuates APD alternans
Cardiac repolarization alternans often precedes life‐threatening ventricular arrhythmias. In alternans, Cai and V m are coupled either positively (a large Cai transient prolongs APD of the same beat) or negatively (a large Cai transient shortens APD of the same beat) (Kennedy et al. 2017). To test the effects of I KAS on alternans, rapid pacing was performed in females (Protocol VII, Fig. 13). At basal condition, Cai–V m coupling was positive such that alternans was electromechanically concordant. During isoproterenol (Fig. 13 A), the Cai–V m coupling was still positive and the alternans remained electromechanically concordant at the level of APD80. However, at early repolarization phases at the APD25 level, Cai–V m coupling became negative such that alternans became electromechanically discordant. In other words, during phase 2, a larger Cai transient (beat 1) led to a shorter and more triangular AP, while a smaller Cai transient (beat 2) corresponded to a longer and less triangular AP. As shown in Fig. 13 B, I KAS blockade by apamin eliminated the negative Cai–V m coupling and APD25 alternans (represented by ΔAPD25,beat1−beat2) and also markedly attenuated APD80 alternans (represented by ΔAPD80,beat1−beat2). Figure 13 C shows that apamin prolonged APD25 of beat 1 more prominently than that of beat 2 (13 ± 1 vs. 6 ± 2 ms, P = 0.004) while APD80 prolongations were similar between the two adjacent beats (5 ± 1 vs. 7 ± 1 ms, P = 0.250). Figure 13 D shows that apamin eliminated alternans of APD25 (from −7 ± 0 to 0 ± 1 ms, P = 0.002) and attenuated alternans of APD80 (from 21 ± 1 to 17 ± 1, P = 0.010). These results indicate that β‐adrenergic stimulation elicits more abundant I KAS activation at phase 2 than at phase 3 repolarization and during beats with a larger Cai transient than those with smaller Cai transient, leading to negative Cai–V m coupling and electromechanically discordant alternans at early repolarization phases. I KAS blockade mainly prolonged phase 2 repolarization in the beats with larger Cai transients and had less effect on the beats with smaller Cai transients, resulting in the elimination of negative Cai–V m coupling and electromechanically discordant alternans. As a comparison (Fig. 13 E and F), in male rabbits, isoproterenol induced Cai alternans at PCL 150 ms with less prominent APD alternans. Both Cai transient and V m had little response to apamin. Therefore, the elimination of phase 2 repolarization alternans by I KAS blockade in female rabbits suggests its potential antiarrhythmic effects during β‐adrenergic stimulation.
Figure 13. I KAS blockade eliminates negative Cai–V m coupling at phase 2 and attenuates APD alternans in female (A‐‐D) but not male (E and F) rabbit ventricles (Protocol VII).
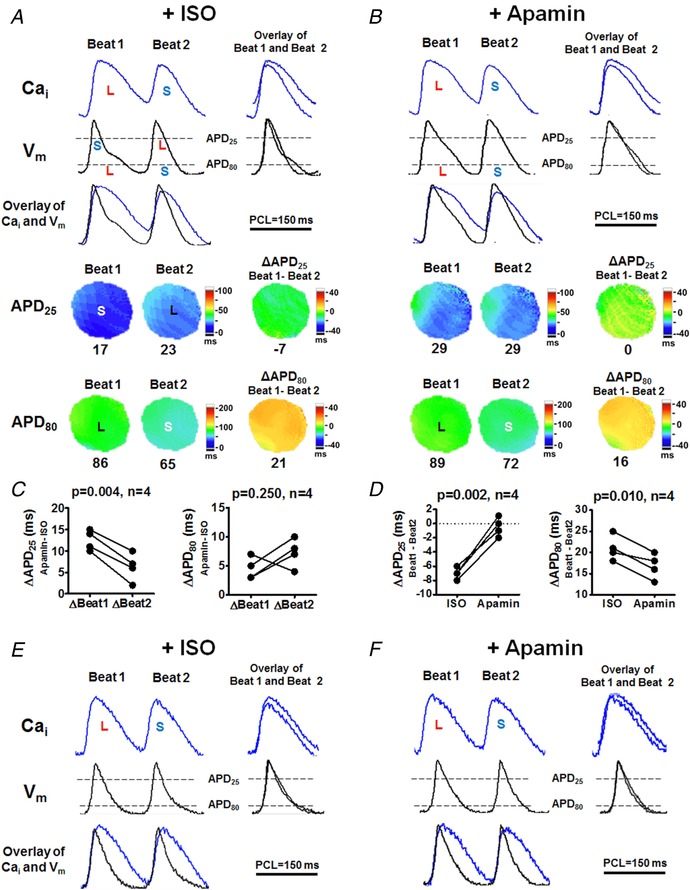
A exhibited electromechanically discordant phase 2 alternans after isoproterenol. Despite the positive Cai–APD80 coupling, Cai and APD25 became negatively coupled, i.e. larger Cai transient (beat 1) shortened APD25 while the smaller Cai transient (beat 2) corresponded to longer APD25. Notice a more triangular AP (smaller APD25/APD80) in beat 1. B, apamin eliminated APD25 alternans and Cai–APD25 negative coupling (ΔAPD25,beat1−beat2, from −7 ms to 0 ms). In addition, apamin did not eliminate but markedly attenuated APD80 alternans (ΔAPD80,beat1−beat2, from 21 ms to 16 ms). C, apamin prolonged APD25 of beat 1 more prominently than that of beat 2. The prolongation of APD80 was similar between beat 1 and beat 2. D, apamin eliminated APD25 alternans (ΔAPD25,beat1−beat2) and attenuated APD80 alternans (ΔAPD80,beat1−beat2) both significantly (by Student's paired t test). E and F, in male rabbits, isoproterenol induced Cai alternans at PCL 150 ms without prominent APD alternans. Both Cai transient and V m had little response to apamin. L, larger Cai transient or longer APD; S, smaller Cai or shorter APD. [Color figure can be viewed at http://wileyonlinelibrary.com]
Antiarrhythmic effects of I KAS blockade during isoproterenol infusion
To further assess the antiarrhythmic effects of I KAS blockade during sympathetic stimulation, we compared VF vulnerabilities and characteristics between females and males (Protocol VII, Fig. 14). None of these ventricles developed spontaneous VF. Ventricular fast pacing was therefore performed to induce VF. As shown in Fig. 14 A, VF inducibility (the number of induced VF episode) was similar between sexes at baseline, during isoproterenol and after washout. However, VF inducibility after apamin became significantly lower in females than in males. In addition, the first 100 ms at the onset of each episode of VF was optically captured. As shown in the phase map of VF (Fig. 14 B), isoproterenol markedly increased the number of PSs in both groups compared with baseline. Apamin prominently decreased the number of PSs in females, but less prominently in males. As summarized in Fig. 14 C, the numbers of PSs were similar between sexes at baseline, during isoproterenol and after washout. However, apamin elicited significant lower PS numbers in females than in males. The dominant frequencies were also similar between sexes at baseline. Isoproterenol infusion led to significantly higher dominant frequencies in females than in males. The differences were eliminated by apamin (Fig. 14 D). These results indicate that I KAS activation plays important roles in proarrhythmia and VF dynamics during sympathetic stimulation, especially in females.
Figure 14. Effects of I KAS blockade on VF inducibility and VF dynamics.
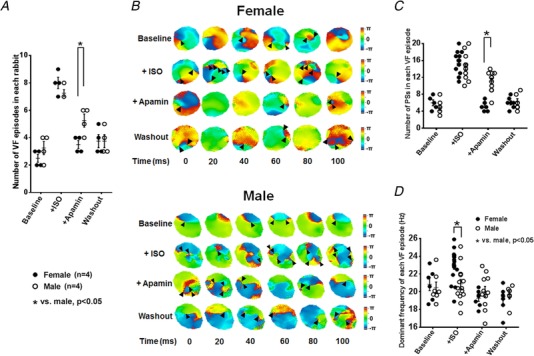
A, the numbers of induced VF episodes were similar between females and males at baseline, during isoproterenol and after washout. After apamin, however, the numbers of induced VF episodes became significantly lower in females than in males. VF inductions were attempted 10 times at baseline and after each treatment. B, representative phase maps of the first 100 ms of VF after the onset at baseline, after isoproterenol, after apamin and after washout in females (upper panel) and males (bottom panel) (Protocol VII). Compared with baseline, isoproterenol markedly increased the numbers of phase singularities (PSs, black arrowhead) in both groups. Apamin prominently decreased PSs in females, but the effect was less prominent in males. After washout, the numbers of PSs in all three groups were similar to those at baseline. C, the numbers of PSs were similar between sexes at baseline, after isoproterenol and after washout. After apamin, however, the numbers of PSs became significantly lower in females than in males. D, VF dominant frequencies were similar between sexes at baseline. After isoproterenol, dominant frequency became significantly higher in females than in males. Apamin eliminated the isoproterenol‐induced differences in dominant frequency between sexes. After washout, the dominant frequencies remained similar in females and males. *P < 0.05 by two‐way ANOVA with Sidak's post hoc test. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
We discovered that β‐adrenergic stimulation activates ventricular I KAS. Because sympathetic tone varies throughout the day, the sympathetic sensitivities indicate that I KAS plays a role in modulating ventricular repolarization of normal ventricles. This new finding suggests that I KAS is physiologically important. In addition, sympathetic stimulation unmasks the different I KAS activation profiles between females and males. I KAS activation promotes AP triangulation and ventricular arrhythmias during β‐adrenergic stimulation in females while I KAS blockade is antiarrhythmic. Because of the importance of both sympathetic tone and sex differences in cardiac arrhythmogenesis, these findings may be clinically important.
I KAS and sympathetic stimulation
Even in patients without apparent heart diseases, abnormal adrenergic regulation increases the susceptibility to ventricular arrhythmias (Jouven et al. 2005). β‐Adrenergic activation is known to regulate cardiac repolarization directly and indirectly via a number of currents including I Ca,L (Reuter, 1983), I KATP (Maruyama et al. 2014), I Ks (Zhu et al. 2013; Aflaki et al. 2014; Bartos et al. 2017), and possibly I Kr (Karle et al. 2002; Harmati et al. 2011) and I K1 (Fauconnier et al. 2005; Banyasz et al. 2014). Although increased I Ca,L tends to prolong APD, isoproterenol results in net shortening of APD by activating multiple compensatory outward currents at different phases of repolarization (Harmati et al. 2011; Zhu et al. 2013; Aflaki et al. 2014; Banyasz et al. 2014; Maruyama et al. 2014; Bartos et al. 2017). In addition, as observed in this study and by others (Banyasz et al. 2014), isoproterenol also gave rise to prominent AP morphology changes. While guinea pigs basally exhibited I Kr > I K1 > I Ks, isoproterenol shaped the AP morphology by reversing the dominance pattern to I Ks > I K1 > I Kr (Banyasz et al. 2014). However, the isoproterenol‐induced AP triangulation, i.e. preferentially shortening at phase 2 rather than phase 3 repolarization, is unlikely to rely on I Kr, I K1 or I Ks because these voltage‐dependent currents exhibit limited activation at early repolarization phases under adrenergic stimulation. As an example, in the absence of adrenergic stimulation, I Ks activates slowly during phase 1 and 2 repolarization and reaches its peak at the end of phase 2 (around 0 mV), followed by a rapid decline at phase 3 repolarization. However, in the presence of adrenergic stimulation, the peak of I Ks is postponed from mid plateau to phase 3 repolarization (Banyasz et al. 2014). This was supported by the result that I Ks blockade failed to reverse isoproterenol‐induced AP triangulation in this study (Figs 5 and 7).
Although weak under basal conditions, I KAS in females was markedly amplified during β‐adrenergic stimulation. The I KAS activation happens robustly at the early repolarization phases when I Ca,L activity, sarcoplasmic Ca2+ release and Cai are all high, resulting in AP triangulation due to more prominent shortening of APD25 than APD80. I KAS blockade preferentially prolonged phase 2 repolarization to restore the AP plateau and, surprisingly, even slightly shortened phase 3 repolarization, resulting in a squared AP morphology. The AP squaring after I KAS blockade may be attributed to the further I Ks activation facilitated by the longer or perhaps more positive plateau, which subsequently accelerated phase 3 repolarization. These findings indicate possible synergistic action between I KAS and I Ks in AP repolarization during adrenergic stimulation, especially in females. However, due to the results that isoproterenol still shortened and triangulated AP with both I KAS and I Ks blockade in male rabbits, other mechanisms might also contribute in the AP shortening and triangulation, such as the activation of sodium–calcium exchanger (I NCX) and cAMP‐dependent Cl− current (I CFTR‐Cl) (Perchenet et al. 2000; Zhang et al. 2001; Lin et al. 2006).
Sex differences in SK2 channels
Sex differences are widely present in a variety of cardiac ion currents and exert large influences on cardiac electrophysiological properties and arrhythmogenesis (Liu et al. 1998; Vizgirda et al. 2002; Sims et al. 2008; Zhu et al. 2013). In this study, we found that I KAS also exhibited sex differences but they were only unmasked during isoproterenol infusion. The activation of I KAS could be attributed to the increased currents conducted by either SK2 or SK3, or both. Our experiments with the selective SK2 blocker Lei‐Dab7 suggest that SK2, rather than SK3, is the dominant isoform mediating the enhanced I KAS during sympathetic stimulation. SK2 channel protein had significantly higher expression in females than in males, thus contributing to the majority of the sex differences in I KAS activation. However, it is known that SK2 and SK3 proteins can form heteromeric channels in atria (Tuteja et al. 2010; Hancock et al. 2015). Therefore, we cannot exclude the possibility that the SK2‐specific blocker also inhibits I KAS conducted by the heteromerically assembled SK2–SK3 channels in ventricles.
The sex differences in I KAS were also verified by voltage clamp studies showing that outward I KAS densities were larger in isolated ventricular myocytes from females than males with 1 μmol L−1 intracellular Ca2+ at basal condition or in the presence of isoproterenol. Western blot and immunostaining suggested that SK2 protein expression was higher in females than males. However, similar I KAS densities between sexes were detected at potentials ≤0 mV. Since SK2 channels are known for their versatile function and plasticity, the significant increase in outward I KAS occurring only at potentials >0 mV suggests the mechanism is unlikely to simply result from differences in SK2 protein expression. The sex‐specific differences in outward I KAS could be due to differential sensitivities of SK channels to voltage‐dependent block by internal Mg2+ or other ions. Another explanation is due to the fixed intracellular Ca2+ in the patch clamp study. In intact cardiomyocytes, Ca2+ influx and subsequent release through LTCC and ryanodine receptor (RyR) might increase the local subsarcolemmal Ca2+ concentration up to 100 μmol L−1, while intracellular Ca2+ concentration might only reach 1 μmol L−1. Since I KAS is very sensitive to local Ca2+ between the SK channel and LTCC/RyR (Zhang et al. 2018), it is possible that I KAS recorded at a fixed global Ca2+ concentration was not high enough to differentially activate I KAS between sexes. Further studies with dynamical Ca2+ fluctuations are needed.
The Ca2+ sensitivity of SK2 channels is strongly modulated by CK2 in neurons (Adelman et al. 2012). Heart failure decreases the interaction between CK2 and SK2 and consequently enhances the sensitivity of I KAS to Ca2+ (Yang et al. 2015). Consistent with those studies, our results further suggested the potential participation of CK2 inhibition in cardiac I KAS activation. While male rabbit ventricles expressed lower SK2 channels than those of females, the equally expressed CK2 between sexes resulted in a higher CK2/SK2 ratio in males. While these results might in part contribute to the findings of the present study, more work will be needed to prove a causal relationship between the CK2/SK2 ratio and the sex differences of I KAS densities.
Notably, the function of SK channels not only relies on the total protein expression, but also depends critically on the subcellular distribution and the cell‐surface membrane expression. Interacting proteins, such as α‐actinin and filamin A, regulate SK channel anterograde trafficking to the surface membrane and recycling (Lu et al. 2009; Rafizadeh et al. 2014; Zhang et al. 2017). It is possible that differential channel trafficking properties exist between sexes and contribute to different I KAS responses to sympathetic stimulation.
Antiarrhythmic effects of I KAS blockade
AP triangulation, with either APD abbreviation or APD prolongation, predicts serious proarrhythmia (Hondeghem et al. 2001). Agents that prolong phase 3 repolarization (triangulation) are proarrhythmic, while agents that prolong phase 2 without slowing phase 3 (squaring) are generally antiarrhythmic (Hondeghem et al. 2001). Thus, it is important to develop repolarization‐delaying agents that lengthen APD in a safe fashion. In females, I KAS blockade prolongs phase 2 repolarization without prolonging phase 3 repolarization, reversing AP triangulation and leading to AP squaring, thereby predicting a potential antiarrhythmic effect. Therefore, I KAS could be a promising target for the development of antiarrhythmic agents, especially for females.
Cardiac alternans is a precursor to VF. APD and Cai transient alternans are caused by instabilities in both V m and Cai cycling dynamics and their coupling (Weiss et al. 2006). V m and Cai are coupled via calcium‐sensitive currents, such as I Ca,L (Weiss et al. 2006), I NCX (Weiss et al. 2006), I Ks (Kennedy et al. 2017) and I KAS (Kennedy et al. 2017). In silico models in the absence of I KAS, Cai and V m exhibit positive coupling and electromechanically concordant alternans (Kennedy et al. 2017). After introducing I KAS with a relatively low calcium affinity, APD is shortened when the Cai transient is large, resulting in negative Cai–V m coupling and electromechanically discordant alternans (Kennedy et al. 2017). In the present study, we have experimentally validated this prediction by demonstrating that I KAS activation by isoproterenol caused Cai–V m coupling to shift from positive to negative, resulting in electromechanically discordant alternans, especially at phase 2 repolarization. I KAS blockade attenuated negative Cai–V m coupling and phase 2 repolarization alternans. In addition, I KAS blockade reduced the VF vulnerability and altered VF dynamics by reducing the number of PSs and dominant frequency, also suggesting its potential antiarrhythmic effects in females.
Study limitations
The optical mapping was performed only on the epicardium. These findings may not be applicable to mid and endocardium due to the transmurally heterogeneous distribution of I KAS (Yu et al. 2015). Due to the unavailability of normal human cardiac tissue, the sex differences of I KAS in human ventricles remain unknown. However, the rabbit is considered as a good animal model for studying sex differences in cardiac ion currents relevant to humans (Salama & Bett, 2014). Isoproterenol also induces AP triangulation in male rabbit ventricles, but apamin failed to normalize the AP morphology. The mechanism of AP triangulation in males remains unclear.
Conclusions and implications
A variety of cardiac electrical diseases exhibit sexual dimorphism (Salama & Bett, 2014; Antzelevitch et al. 2016). Understanding the arrhythmogenic mechanisms may therefore be important for generating sex‐specific therapies. Although ventricular I KAS is relatively small, it still plays important roles in regulating APs under a variety of physiological and pathological conditions. In the present study, we show that adrenergic stimulation causes greater activation of I KAS in females than males. The sex differences of I KAS are attributable to the differences in the expression and biophysical properties of SK2 channels and the CK2/SK2 ratios. Activation of I KAS is more abundant during phase 2 than during phase 3 repolarization, leading to AP triangulation, negative Cai–V m coupling, electromechanically discordant phase 2 alternans and increased VF vulnerability. I KAS blockade is potentially antiarrhythmic in females. Drugs specifically targeting cardiac I KAS should be effective and safe. Amiodarone, a commonly used antiarrhythmic drug, is known to suppress I KAS (Turker et al. 2013). More specific I KAS blockers have also been tested in animal models (Diness et al. 2017; Ko et al. 2018). I KAS blockers may prove to be a new class of antiarrhythmic drugs with sex‐specific clinical applications.
Additional information
Competing interests
None
Author contributions
M.C.: design, data acquisition and analysis/interpretation; drafting and revision. D.Y., S.G., D.Z.X. and Z.W.: data acquisition and analysis. Z.C., M.R.L., S.F.L., T.H.E. and J.N.W.: design, interpretation and revision. P.S.C.: conception, design, interpretation, drafting and revision. All authors approved the final version of the manuscript. All authors agree to be accountable for the data integrity and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by NIH R01HL139829, P01HL78931, R56HL71140, R42DA043391 and TR0022080, a Charles Fisch Cardiovascular Research Award endowed by Dr Suzanne B. Knoebel of the Krannert Institute of Cardiology, a Medtronic‐Zipes Endowment and the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative.
Acknowledgements
We thank Nicole Courtney, Jin Guo, Christopher Corr, David Adams, David Wagner and Jian Tan for their assistance.
Biography
Mu Chen received his MD from Shanghai Jiao Tong University and did resident training at Xinhua Hospital Shanghai. He now works as a Postdoctoral Fellow under the supervision of Dr Peng‐Sheng Chen in Krannert Institute of Cardiology, Indiana University. His studies focus on the cardiac small conductance calcium‐activated potassium channel and its regulation on ventricular repolarization and arrhythmias.

Edited by: Kim Barrett & Colleen Clancy
[Corrections made on August 16, 2018 after first online publication: Figures 1–14 were replaced.]
This is an Editor's Choice article from the 15 September 2018 issue.
Linked articles This article is highlighted in a Perspectives article by Dobrev. To read this article, visit http://doi.org/10.1113/JP276663.
References
- Adelman JP, Maylie J & Sah P (2012). Small‐conductance Ca2+‐activated K+ channels: Form and function. Annu Rev Physiol 74, 245–269. [DOI] [PubMed] [Google Scholar]
- Aflaki M, Qi XY, Xiao L, Ordog B, Tadevosyan A, Luo X, Maguy A, Shi Y, Tardif JC & Nattel S (2014). Exchange protein directly activated by cAMP mediates slow delayed‐rectifier current remodeling by sustained β‐adrenergic activation in guinea pig hearts. Circ Res 114, 993–1003. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, Ma C, Morita H, Nam GB, Sacher F, Shimizu W, Viskin S & Wilde AA (2016). J‐Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm 13, e295–e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyasz T, Jian Z, Horvath B, Khabbaz S, Izu LT & Chen‐Izu Y (2014). Beta‐adrenergic stimulation reverses the I Kr–I Ks dominant pattern during cardiac action potential. Pflugers Arch 466, 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos DC, Morotti S, Ginsburg KS, Grandi E & Bers DM (2017). Quantitative analysis of the Ca2+‐dependent regulation of delayed rectifier K+ current I Ks in rabbit ventricular myocytes. J Physiol 595, 2253–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y‐H, Tsai W‐C, Ko J‐S, Yin D, Chang P‐C, Rubart M, Weiss JN, Everett T, Lin S‐F & Chen P‐S (2015). Small conductance calcium‐activated potassium current is activated during hypokalemia and masks short term cardiac memory induced by ventricular pacing. Circulation 132, 1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Hsieh YC, Hsueh CH, Weiss JN, Lin SF & Chen PS (2013a). Apamin induces early afterdepolarizations and Torsades de Pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm 10, 1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Turker I, Lopshire JC, Masroor S, Nguyen BL, Tao W, Rubart M, Chen PS, Chen Z & Ai T (2013b). Heterogeneous upregulation of apamin‐sensitive potassium currents in failing human ventricles. J Am Heart Assoc 2, e004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Chen LS, Fishbein MC, Lin SF & Nattel S (2014). Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 114, 1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart‐von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T & Chen PS (2011). Small‐conductance calcium‐activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 108, 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diness JG, Skibsbye L, Simo‐Vicens R, Santos JL, Lundegaard P, Citerni C, Sauter DRP, Bomholtz SH, Svendsen JH, Olesen SP, Sorensen US, Jespersen T, Grunnet M & Bentzen BH (2017). Termination of vernakalant‐resistant atrial fibrillation by inhibition of small‐conductance Ca2+‐activated K+ channels in pigs. Circ Arrhythm Electrophysiol 10, e005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconnier J, Lacampagne A, Rauzier JM, Vassort G & Richard S (2005). Ca2+‐dependent reduction of I K1 in rat ventricular cells: a novel paradigm for arrhythmia in heart failure? Cardiovasc Res 68, 204–212. [DOI] [PubMed] [Google Scholar]
- Hancock JM, Weatherall KL, Choisy SC, James AF, Hancox JC & Marrion NV (2015). Selective activation of heteromeric SK channels contributes to action potential repolarization in mouse atrial myocytes. Heart Rhythm 12, 1003–1015. [DOI] [PubMed] [Google Scholar]
- Harmati G, Banyasz T, Barandi L, Szentandrassy N, Horvath B, Szabo G, Szentmiklosi JA, Szenasi G, Nanasi PP & Magyar J (2011). Effects of β‐adrenoceptor stimulation on delayed rectifier K+ currents in canine ventricular cardiomyocytes. Br J Pharmacol 162, 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Lin SF & Chen PS (2007). Preshock phase singularity and the outcome of ventricular defibrillation. Heart Rhythm 4, 927–934. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Dujardin K & De Clerck F (2001). Phase 2 prolongation, in the absence of instability and triangulation, antagonizes class III proarrhythmia. Cardiovasc Res 50, 345–353. [DOI] [PubMed] [Google Scholar]
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D & Ducimetiere P (2005). Heart‐rate profile during exercise as a predictor of sudden death. N Envgl J Med 352, 1951–1958. [DOI] [PubMed] [Google Scholar]
- Karle CA, Zitron E, Zhang W, Kathofer S, Schoels W & Kiehn J (2002). Rapid component I Kr of the guinea‐pig cardiac delayed rectifier K+ current is inhibited by β1‐adrenoreceptor activation, via cAMP/protein kinase A‐dependent pathways. Cardiovasc Res 53, 355–362. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Bers DM, Chiamvimonvat N & Sato D (2017). Dynamical effects of calcium‐sensitive potassium currents on voltage and calcium alternans. J Physiol 595, 2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JS, Guo S, Hassel J, Celestino‐Soper P, Lynnes TC, Tisdale JE, Zheng JJ, Taylor SE, Foroud T, Murray MD, Kovacs RJ, Li X, Lin SF, Chen Z, Vatta M, Chen PS & Rubart M (2018). Ondansetron blocks wildtype and p.F503L variant small conductance calcium activated potassium channels. Am J Physiol Heart Circ Physiol 315, H375–H388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Chang PC, Hsueh CH, Maruyama M, Park HW, Rhee KS, Hsieh YC, Shen C, Weiss JN, Chen Z, Lin SF & Chen PS (2013). Apamin‐sensitive calcium‐activated potassium currents in rabbit ventricles with chronic myocardial infarction. J Cardiovasc Electrophysiol 24, 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Jo H, Sakakibara Y, Tambara K, Kim B, Komeda M & Matsuoka S (2006). β‐Adrenergic stimulation does not activate Na+/Ca2+ exchange current in guinea pig, mouse, and rat ventricular myocytes. Am J Physiol Cell Physiol 290, C601–C608. [DOI] [PubMed] [Google Scholar]
- Liu XK, Katchman A, Drici MD, Ebert SN, Ducic I, Morad M & Woosley RL (1998). Gender difference in the cycle length‐dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther 285, 672–679. [PubMed] [Google Scholar]
- Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR & Chiamvimonvat N (2009). α‐Actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+‐activated K+ channel (SK2 channel). Proc Natl Acad Sci U S A 106, 18402–18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA & Chiamvimonvat N (2007). Molecular coupling of a Ca2+‐activated K+ channel to L‐type Ca2+ channels via α‐actinin2. Circ Res 100, 112–120. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Ai T, Chua SK, Park HW, Lee YS, Shen MJ, Chang PC, Lin SF & Chen PS (2014). Hypokalemia promotes late phase 3 early afterdepolarization and recurrent ventricular fibrillation during isoproterenol infusion in Langendorff perfused rabbit ventricles. Heart Rhythm 11, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merri M, Benhorin J, Alberti M, Locati E & Moss AJ (1989). Electrocardiographic quantitation of ventricular repolarization. Circulation 80, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Pachuau J, Li DP, Chen SR, Lee HA & Pan HL (2014). Protein kinase CK2 contributes to diminished small conductance Ca2+‐activated K+ channel activity of hypothalamic pre‐sympathetic neurons in hypertension. J Neurochem 130, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchenet L, Hinde AK, Patel KC, Hancox JC & Levi AJ (2000). Stimulation of Na/Ca exchange by the β‐adrenergic/protein kinase A pathway in guinea‐pig ventricular myocytes at 37°C. Pflugers Arch 439, 822–828. [DOI] [PubMed] [Google Scholar]
- Rafizadeh S, Zhang Z, Woltz RL, Kim HJ, Myers RE, Lu L, Tuteja D, Singapuri A, Bigdeli AA, Harchache SB, Knowlton AA, Yarov‐Yarovoy V, Yamoah EN & Chiamvimonvat N (2014). Functional interaction with filamin A and intracellular Ca2+ enhance the surface membrane expression of a small‐conductance Ca2+‐activated K+ (SK2) channel. Proc Natl Acad Sci U S A 111, 9989–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reher TA, Wang Z, Hsueh CH, Chang PC, Pan Z, Kumar M, Patel J, Tan J, Shen C, Chen Z, Fishbein MC, Rubart M, Boyden P & Chen PS (2017). Small‐conductance calcium‐activated potassium current in normal rabbit cardiac purkinje cells. J Am Heart Assoc 6, e005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H ( 1983). Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature 301, 569–574. [DOI] [PubMed] [Google Scholar]
- Salama G & Bett GC (2014). Sex differences in the mechanisms underlying long QT syndrome. Am J Physiol Heart Circ Physiol 307, H640–H648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakkottai VG, Regaya I, Wulff H, Fajloun Z, Tomita H, Fathallah M, Cahalan MD, Gargus JJ, Sabatier JM & Chandy KG (2001). Design and characterization of a highly selective peptide inhibitor of the small conductance calcium‐activated K+ channel, SkCa2. J Biol Chem 276, 43145–43151. [DOI] [PubMed] [Google Scholar]
- Shen MJ & Zipes DP (2014). Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114, 1004–1021. [DOI] [PubMed] [Google Scholar]
- Sims C, Reisenweber S, Viswanathan PC, Choi BR, Walker WH & Salama G (2008). Sex, age, and regional differences in L‐type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug‐induced long QT type 2. Circ Res 102, e86–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrente AG, Zhang R, Wang H, Zaini A, Kim B, Yue X, Philipson KD & Goldhaber JI (2017). Contribution of small conductance K+ channels to sinoatrial node pacemaker activity: insights from atrial‐specific Na+/Ca2+ exchange knockout mice. J Physiol 595, 3847–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker I, Yu C‐C, Chang P, Chen Z, Sohma Y, Lin S‐F, Chen P‐S & Ai T (2013). Amiodarone inhibits apamin‐sensitive potassium currents. PLoS One 8, e70450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja D, Rafizadeh S, Timofeyev V, Wang S, Zhang Z, Li N, Mateo RK, Singapuri A, Young JN, Knowlton AA & Chiamvimonvat N (2010). Cardiac small conductance Ca2+‐activated K+ channel subunits form heteromultimers via the coiled‐coil domains in the C termini of the channels. Circ Res 107, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT & Schwertz DW (2002). Mechanisms of sex differences in rat cardiac myocyte response to β‐adrenergic stimulation. Am J Physiol Heart Circ Physiol 282, H256–H263. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A & Qu Z (2006). From pulsus to pulseless: the saga of cardiac alternans. Circ Res 98, 1244–1253. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN & Chiamvimonvat N (2003). Molecular identification and functional roles of a Ca2+‐activated K+ channel in human and mouse hearts. J Biol Chem 278, 49085–49094. [DOI] [PubMed] [Google Scholar]
- Yang D, Wang T, Ni Y, Song B, Ning F, Hu P, Luo L, Wang Y & Ma A (2015). Apamin‐sensitive K+ current upregulation in volume‐overload heart failure is associated with the decreased interaction of CK2 with SK2. J Membr Biol 248, 1181–1189. [DOI] [PubMed] [Google Scholar]
- Yu CC, Ai T, Weiss JN & Chen PS (2014). Apamin does not inhibit human cardiac Na+ current, L‐type Ca2+ current or other major K+ currents. PLoS One 9, e96691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CC, Corr C, Shen C, Shelton R, Yadava M, Rhea I, Straka S, Fishbein MC, Chen Z, Lin SF, Lopshire JC & Chen PS (2015). Small conductance calcium‐activated potassium current is important in transmural repolarization of failing human ventricles. Circ Arrhythm Electrophysiol 8, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HX, Silva JR, Lin YW, Verbsky JW, Lee US, Kanter EM, Yamada KA, Schuessler RB & Nichols CG (2013). Heterogeneity and function of KATP channels in canine hearts. Heart Rhythm 10, 1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, Bond CT, Adelman JP & Chiamvimonvat N (2008). Functional roles of a Ca2+‐activated K+ channel in atrioventricular nodes. Circ Res 102, 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Coulibaly ZA, Chen WC, Ledford HA, Lee JH, Sirish P, Dai G, Jian Z, Chuang F, Brust‐Mascher I, Yamoah EN, Chen‐Izu Y, Izu LT & Chiamvimonvat N (2018). Coupling of SK channels, L‐type Ca2+ channels, and ryanodine receptors in cardiomyocytes. Sci Rep 8, 4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Hinde AK & Hancox JC (2001). Anti‐adrenergic effect of adenosine on Na+‐Ca2+ exchange current recorded from guinea‐pig ventricular myocytes. Cell Calcium 29, 347–358. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ledford HA, Park S, Wang W, Rafizadeh S, Kim HJ, Xu W, Lu L, Lau VC, Knowlton AA, Zhang XD, Yamoah EN & Chiamvimonvat N (2017). Distinct subcellular mechanisms for the enhancement of the surface membrane expression of SK2 channel by its interacting proteins, α‐actinin2 and filamin A. J Physiol 595, 2271–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ai X, Oster RA, Bers DM & Pogwizd SM (2013). Sex differences in repolarization and slow delayed rectifier potassium current and their regulation by sympathetic stimulation in rabbits. Pflugers Arch 465, 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]


