ABSTRACT
Myelin formation during peripheral nervous system development, as well as myelin repair after injury and in disease, requires multiple intrinsic and extrinsic signals. Neurotrophin-4 (NT-4) is a member of the neurotrophin family, which regulates the development of neuronal networks by participating in the growth of neuronal processes, synaptic development and plasticity, neuronal survival, and differentiation. However, the intracellular signaling pathways by which NT-4 participates in myelination by Schwann cells remain elusive. In this study, we examined the effects of NT-4 on the expression of compact myelin proteins in cultured Schwann cells. Using real-time quantitative RT-PCR and western blotting, we found that NT-4 could significantly enhance the expression of myelin protein zero (MPZ) but not the expression of myelin basic protein or peripheral myelin protein 22. Further, knockdown of truncated TrkB with small interfering RNA could eliminate the effect of NT-4 on MPZ expression. Moreover, we demonstrated that the NT-4-enhanced MPZ expression depended on Akt and mTORC1 signaling. Taken together, these results suggest that NT-4 binds TrkB to enhance the expression of MPZ in Schwann cells, probably through the PI3K/Akt/mTORC1 signaling pathway, thus contributing to myelination.
KEYWORDS: neurotrophin-4, Schwann cells, myelin, myelin protein zero, peripheral nerve
Introduction
Myelinated nerve fibers are essential for the rapid propagation of action potentials by saltatory conduction, a crucial feature of the vertebrate nervous system. Myelin synthesis by Schwann cells is an important process in the regeneration of injured peripheral nerves as well as in the normal development of the peripheral nervous system (PNS) (Taveggia 2016). Impairment of the myelinated Schwann cell-axon unit due to gene mutations, altered metabolism, or inflammation is a frequent cause of disease, attesting to the crucial importance of PNS myelination (England & Asbury 2004). It is well known that myelination by Schwann cells is regulated by various endogenous modulators such as extracellular matrix proteins, neurotrophins, and cytokines (Richner et al. 2014). Of these, neurotrophins are particularly important. The neurotrophin family consists of nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 (NT-4). The neurotrophins signal in a spatially and temporally complex manner via two structurally unrelated types of receptors, p75 neurotrophin receptor (p75NTR) and the tropomyosin receptor kinase receptors (TrkA, TrkB, and TrkC) (Richner et al. 2014).
NT-4 and other neurotrophins regulate the development of neuronal networks by participating in the growth of neuronal processes, synaptic development and plasticity, neuronal survival, and differentiation (D'Angelo et al. 2016). In mammals, several features distinguish NT-4 from other members of the neurotrophin family. NT-4 mRNA is expressed at much lower levels than those of any other neurotrophins, but NT-4 expression is ubiquitous and less influenced by environmental signals (Timmusk et al. 1993; Ibanez 1996). Peripheral nerve injury has been reported to result in down-regulation of the NT-4 mRNA level shortly after injury. However, 2 weeks post-injury, the NT-4 mRNA level has been found to be upregulated in the distal nerve stump of the injured rodent sciatic nerve (Omura et al. 2005). Previous studies have shown that NT-4 acts as a potent survival factor for a subpopulation of motor neurons, and mice lacking NT-4 display enlarged and fragmented neuromuscular junctions with disassembled postsynaptic acetylcholine receptor (AChR) clusters, reduced AChR binding, and decreased acetylcholinesterase activity (Henderson et al. 1993; Belluardo et al. 2001). Studies performed using grafts from homozygous or heterozygous NT-4 knockout mice incorporated into wild-type sciatic nerves have shown that even a small reduction in endogenous NT-4 results in a significant reduction in the growth of regenerating motor neuron axons. In line with these results, application of NT-4 to heterozygous NT-4 knockout mice at the time of surgical repair of a cut nerve has been found to restore motor axon growth (English et al. 2005). Moreover, application of NT-4 to repaired rat sciatic nerves has been reported to result in enhanced axon regeneration, as observed via an increase in the number of regenerated axons, and to increase axonal diameter and myelin thickness (Yin et al. 2001). Although the various biological functions of NT-4 mentioned above are known, its roles in myelination have not been fully elucidated.
To further clarify the role of NT-4 in the synthesis of peripheral myelin, we examined the effects of NT-4 on compact myelin proteins, including myelin protein zero (MPZ), myelin basic protein (MBP), and peripheral myelin protein 22 (PMP22), using cultured Schwann cells. We also investigated the NT-4-mediated signaling pathways involved in the myelination process.
Materials and methods
Reagents
Materials used for cell culture included DMEM/F12 (Hyclone, SH30023.01B), laminin (Sigma, L2020), fetal bovine serum (FBS, Gibco, 10099-141), collagenase II (Sigma, C6885), recombinant human neurotrophin 4 protein (Abcam, ab200241), ANA-12 (Sigma), LM11A (Sigma), PD98059 (Abcam, ab120234), LY294002 (Abcam, ab120243), rapamycin (Abcam, ab120224), and SB216763 (Sigma, S3442). Other reagents included Trizol (Invitrogen, 10296010), protease and phosphatase inhibitors (Thermo Scientific™, 78441), the GoScript™ Reverse Transcription System (Promega, A5000), and GoTaq® qPCR Master Mix (Promega, A6001). Primary antibodies included rabbit anti-s100β (Abcam, ab52642), rabbit anti-MBP (CST, 78896), rabbit anti-MPZ (Abcam, ab31851), rabbit anti-PMP22 (Abcam, ab15506), rabbit anti-Akt (Abcam, ab179463), rabbit anti-pAkt (T308; Abcam, ab38449), and rabbit anti-GAPDH (Abcam, ab9485). Secondary antibodies included horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Bioworld, BS10043) and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (JR, 111-545-144). Transfections were performed using Lipofectamine 3000 RNAi Max (Invitrogen, L3000015). Other chemicals and reagents used in this study were of analytical grade.
Isolation, purification, and enrichment of Schwann cells
All animal care and experiments were undertaken in strict compliance with the approval of the Institutional Animal Ethics Committee of Tianjin Medical University, which follows the NIH guidelines for care and use of experimental animals. Adult Wistar rats (150–200 g) were sacrificed by cervical dislocation and immersed in 75% ethanol for 10 min for sterilization. Schwann cells were obtained from sciatic nerves. The nerve segments were freed of fat and connective tissue and rinsed with cold phosphate-buffered saline (PBS, pH 7.4). Then, nerves were cut into lengths of 1 mm and treated with 0.2% collagenase II at 37°C under 5% CO2 for 40 min. Nerve fascicles were mechanically dissociated and then centrifuged at 1000 rpm for 5 min, after which the supernatant was discarded. After the cells were washed in DMEM/F12, they were centrifuged at 1000 rpm for 5 min again and then resuspended in DMEM/F12 with 10% FBS containing 1% antibiotics (penicillin and streptomycin) and seeded at a density of 2.1 × 105 cells/ml in 25 cm2 culture flasks previously coated with laminin. Previous studies demonstrated that Schwann cells detach more quickly than fibroblasts under treatment with collagenase, allowing Schwann cells to be isolated (Jin et al. 2008). After 48 h of in vitro culture, tissue debris including myelin components was discarded, leaving the adherent cells behind, and the culture medium was replaced with 2.5 ml (0.1 ml/cm2) of 0.05% collagenase II. After incubation for 35–45 min at 37°C, the flask was shaken horizontally for 1–3 min to detach the Schwann cells. Then, the suspended cells were collected and centrifuged. After the supernatant was discarded, the pellet was resuspended in DMEM/F12 with 10% FBS and seeded in a flask at a density of 2.0 × 105 cells/ml. We conducted the same method to purify the Schwann cells again 48 h later. In all of the experiments, cells were used between passages 3 and 5.
Immunocytochemistry
Schwann cells were characterized using immunofluorescence of an antibody directed against S100β protein. The immunostaining was performed after two rounds of purification. Briefly, cells cultured on coverslips were fixed with 4% paraformaldehyde for 30 min, and then the membranes were permeabilized with 0.2% Triton X-100. Nonspecific sites were blocked with goat serum at 37°C for 30 min. Then, the cells were incubated with rabbit anti-S100β monoclonal antibody (1:200) overnight at 4°C, washed with PBS three times for 5 min each, and incubated with the corresponding secondary antibody (1:300) for 2 h at room temperature. After washing three times for 5 min with PBS, the cell nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI). Samples were examined, and images were captured under a confocal microscope.
Small interfering RNA transfection
Four individual small interfering RNAs (siRNAs) targeting truncated TrkB and four individual siRNAs targeting p75NTR were obtained from Ribobio; the siRNA sequences are provided in supplementary table 1. Confluent SCs were seeded at a density of 3 × 105 cells/well in a BD Falcon six-well plate. Then, 24 h later, cells were transfected with siRNA using Lipofectamine 3000 RNAi Max as recommended by the manufacturer.
Real-time quantitative RT-PCR
Cells were kept in DMEM/F12 with 0.1% FBS for 12 h before each experiment, and then NT-4 was applied at the indicated concentrations in fresh DMEM/F12 medium containing 0.1% FBS for 12 h. Total mRNA was extracted from cultured Schwann cells by using Trizol, and cDNA was produced using the GoScript™ Reverse Transcription System. qRT-PCR analysis was performed on a Roche LightCycler 480 for real-time PCR, using GoTaq® qPCR Master Mix with the following primers: truncated TrkB, F: 5′-AATGGAGACTACACCCTAATGGC-3′, R: 5′-GCAGGCAGAATCCTACCACAG-3′; p75NTR, F: 5′-ACATTCTCCGATGTGGTGAGC-3′, R: 5′-CCTCGTCCTGGTAGTAGCCATA-3′; MBP, F: 5′-AGAGTCCGACGAGCTTCAGA-3′, R: 5′-CAGGTACTTGGATCGCTGTG-3′; MPZ, F: 5′-TCTCAGGTCACGCTCTATGTC-3′, R: 5′-GCCAGCAGTACCGAATCAG-3′; PMP22, F: 5′-CCCAACTCCCAGCCACCATG-3′, R: 5′-TCATTCGCGTTTCCGCAGGATC-3′; and GAPDH, F: 5′-GTATGTCGTGGAGTCTACTGGCGT-3′, R: 5′-TACTCCTTGGAGGCCATGTAGGCC-3′. We set 40 cycles as a cycle cutoff point for detectability. Relative expression values for each mRNA were obtained by normalizing them to GAPDH expression, and differences between samples were calculated using the 2(−ΔΔC(T)) method (Livak & Schmittgen 2001).
Western blotting
Schwann cells were serum starved with 0.1% FBS DMEM/F12 for 12 h, and then the medium was changed to DMEM/F12 with 0.1% FBS containing NT-4 for 12 h at the indicated concentration. Cultured cells were homogenized in lysis buffer (0.25 M Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 10% mercaptoethanol) supplemented with protease and phosphatase inhibitors. Samples containing equal amounts of protein (10 µg) were electrophoresed on SDS–polyacrylamide gels. Proteins were then transferred to polyvinylidene fluoride membranes. Then, the membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) at room temperature for 1 h, and the membranes were incubated overnight at 4°C with primary antibodies diluted (1:3000) in prepared TBST containing 5% nonfat milk. The secondary antibodies were used at a dilution of 1:6000 at room temperature for 1 h, and the immunoblots were developed by using an electrochemiluminescence (ECL) system. The pictures were analyzed using ImageJ software to obtain the gray value for each band. Then, the relative gray value of each marker (gray value of the specific marker/gray value of GAPDH) was calculated.
Statistics
Each experiment was repeated at least three independent times, and the Schwann cells in each experiment were harvested from a single isolation. The results of real-time quantitative RT-PCR and the gray values from western blotting are shown as the mean ± SEM. Statistical significance was determined using a two-tailed Student’s t-test (SPSS 22.0). Significance was set at *p < .05, **p < .01. NS, not significant.
Results
NT-4 upregulated the level of MPZ mRNA and increased the expression of MPZ protein in Schwann cells
Figure 1 shows Schwann cells purified from adult rat sciatic nerve. At least 95% of the cells were positive for S100β, a Schwann cell-specific marker (Liu et al. 2015). Using these cultured Schwann cells, we examined the effect of NT-4 on the mRNA and protein levels of MBP, MPZ, and PMP22. As shown in Figure 2(A), using real-time quantitative RT-PCR analysis, we found that the MBP mRNA level was not significantly increased after treatment with NT-4 at concentrations of 50, 150, or 300 ng/ml compared with that obtained in the absence of NT-4. The mRNA level of PMP22 was also not significantly changed. However, treatment with NT-4 at 150 ng/ml or 300 ng/ml significantly increased the level of MPZ mRNA compared with that in cells cultured without NT-4 (150 ng/ml, n = 5, p = .002; 300 ng/ml, n = 5, p = .010). Western blotting analysis demonstrated that treatment with NT-4 at 150 ng/ml or 300 ng/ml also increased the protein expression of MPZ compared with that in cells cultured without NT-4 (150 ng/ml, n = 5, p = .001; 300 ng/ml, n = 5, p = .001), which is consistent with the results from real-time quantitative RT-PCR (Figure 2(B)).
Figure 1.
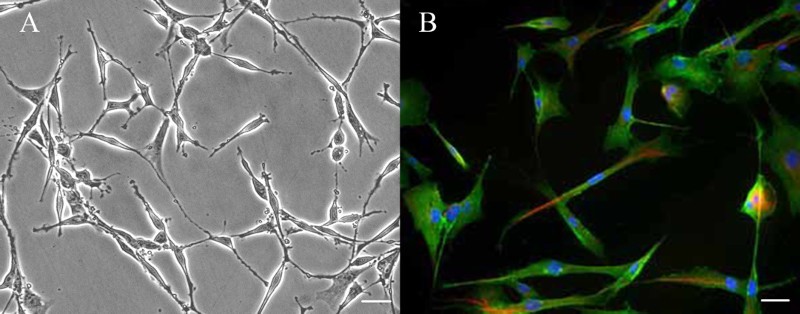
Ιmmunofluorescence staining of Schwann cells cultured from adult rat sciatic nerve. (A) Phase-contrast picture of cultured Schwann cells. Scale bar: 50 µm. (B) Schwann cells were stained with anti-s100β antibody (green) and anti-p75NTR antibody (red), nuclei were labeled with DAPI (blue), noted that the purity of cultured Schwann cells was more than 95%. Scale bar: 10 µm.
Figure 2.
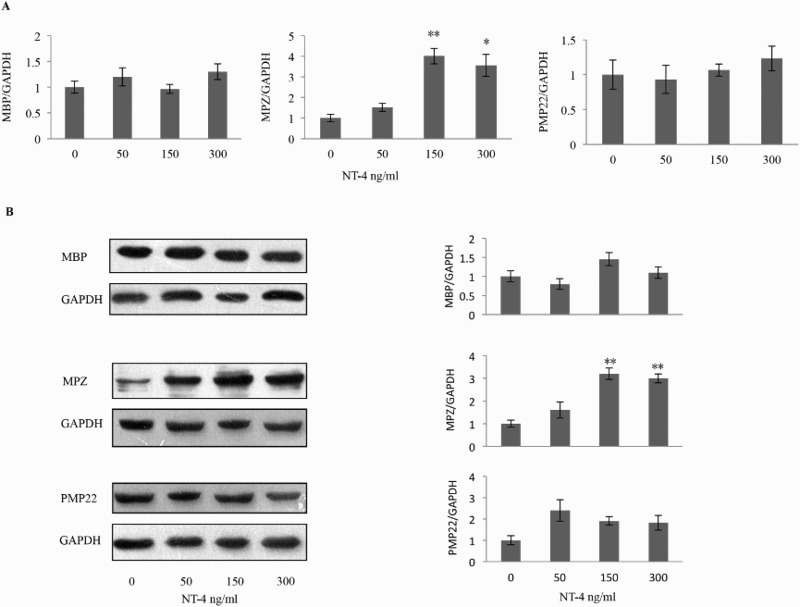
Effects of NT-4 on the mRNA levels of compact myelin proteins. (A) Schwann cells were exposed to NT-4 at the indicated concentration, and the mRNA level of MBP, MPZ, PMP22, and GAPDH was analyzed by real-time quantitative RT-PCR 12 h later. NT-4 significantly increased the MPZ mRNA level at the concentration of 150 ng/ml (n = 5, p = 0.002) and 300 ng/ml (n = 5, p = 0.013). (B) Expression of MBP, MPZ, and PMP22 was examined by western blotting analysis 12 h after NT-4 stimulation at the indicated concentration. A bar graph represents band intensities normalized to those for GAPDH. Each bar represents the average ± SEM of independent experiments. The MPZ protein level increased at the concentration of 150 ng/ml (n = 5, p = .001) and 300 ng/ml (n = 5, p = .001). *p < .05, **p < .01, compared with the control.
The efficiency of siRNA transfection
As shown in Figure 3(A), qRT-PCR and western blotting results indicated that knockdown of truncated TrkB with si-r-TrkB-3 could significantly decrease the expression of truncated TrkB in cultured SCs. Further, transfection of siRNAs targeting p75NTR led to efficient knockdown of p75NTR in SCs, with si-r-p75NTR-2 giving the best knockdown efficiency (Figure 3(B)).
Figure 3.
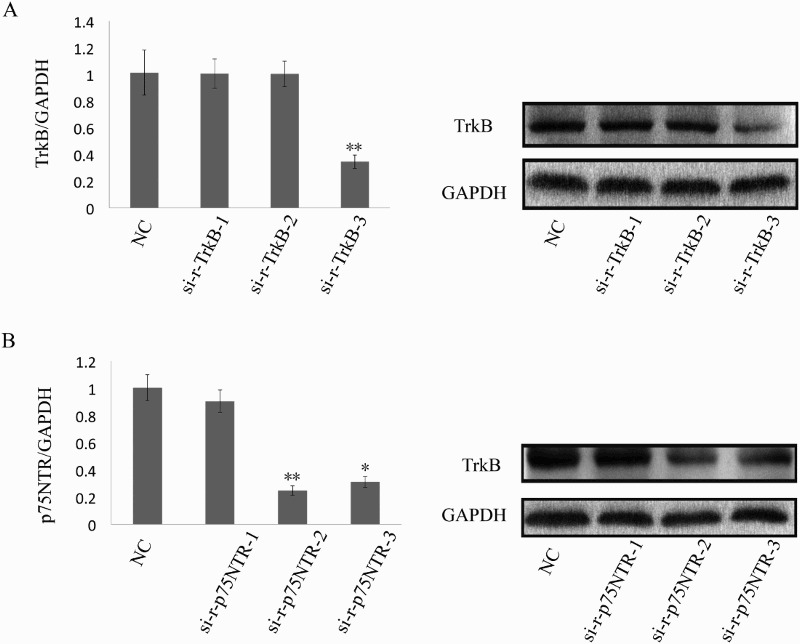
The efficiency of siRNA transfection. (A) qRT-PCR (left) and western blotting (right) indicated that knockdown of truncated TrkB with si-r-TrkB-3 could significantly decrease the expression of truncated TrkB in cultured SCs; (B) qRT-PCR (left) and western blotting (right) demonstrated that transfection of siRNAs targeting p75NTR led to efficient knockdown of p75NTR in SCs cells with si-r-p75NTR-2 giving the best knockdown efficiency. *p < .05; **p < .01.
The effect of TrkB on the enhanced expression of MPZ
NT-4 interacts with two entirely distinct classes of receptors exhibited on the membranes of Schwann cells (Kidd et al. 2013; Richner et al. 2014). The first receptor to be discovered, named p75NTR, has been identified as a low-affinity receptor for all neurotrophins; the other receptor, which interacts with NT-4 specifically and with high affinity, is truncated TrkB (Bothwell 2014). Therefore, we separately knocked down p75NTR and truncated TrkB in order to observe any alterations in NT-4-induced MPZ expression in cultured Schwann cells. As shown in Figure 4 with western blotting analysis, knockdown of p75NTR expression did not change the expression of MPZ in SCs; in contrast, knockdown of truncated TrkB expression markedly decreased the expression of MPZ. These results demonstrate that NT-4 elevated the expression of MPZ by binding to truncated TrkB.
Figure 4.
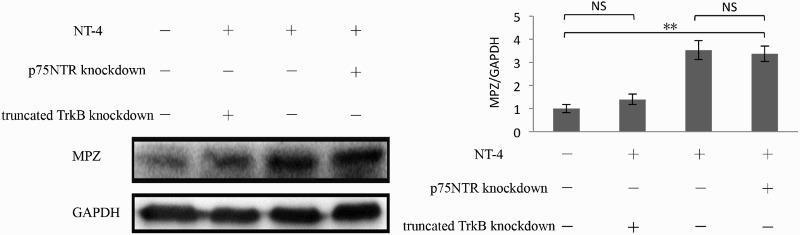
Effects of p75NTR and truncated TrkB knockdown on NT-4-induced MPZ expression. Knockdown of truncated TrkB expression significantly reduced the NT-4-induced MPZ expression (n = 5, p = 0.001), while knockdown of p75NTR expression did not change the expression of MPZ induced by NT-4 in SCs. A bar graph represents band intensities normalized to those for GAPDH. Each bar represents the mean ± SEM of five independent experiments. *p < .05; **p < .01, NS, not significant.
Essential role of the PI3K/Akt pathway in NT-4-mediated MPZ expression
TrkB is classified as one of the receptor-type tyrosine kinases, which transmit intracellular signals mainly through mitogen-activated protein kinase (MAPK) pathways and phosphatidylinositol-3 kinase (PI3K) pathways (Deinhardt & Chao 2014). Therefore, we examined the intracellular signaling pathways involved in the increase in MPZ expression by pretreating Schwann cells with an MAPKK or PI3K inhibitor. As shown in Figure 5(A), pretreatment with 25 µM PD98059 (MAPKK inhibitor) did not significantly inhibit the increase in MPZ expression compared with the intensities obtained without pretreatment. In contrast, pretreatment with 40 µM LY294002 (PI3K inhibitor) markedly inhibited the increase in the expression of MPZ, indicating the involvement of PI3K in the up-regulation of MPZ expression. These findings imply that the NT-4-enhanced expression of MPZ in Schwann cells may be related to the activation of the PI3K/Akt pathway.
Figure 5.
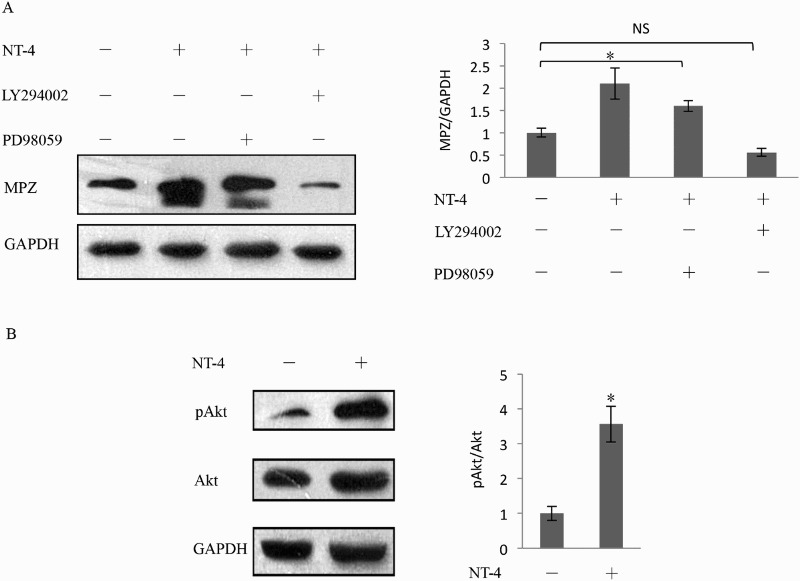
Regulation of MPZ expression by the PI3K/Akt pathway. As shown in (A) by western blotting analysis, before stimulated with 150 ng/ml recombinant NT-4, Schwann cells were pretreated with 25 μM PD98059 for 1 h and 40 μM LY294002 for 2 h. PD98059 did not show inhabitation to the elevation of MPZ expression. In contrast, LY294002 markedly inhibited the increase in the expression of MPZ (n = 5, p = .001). (B) The Akt phosphorylation was examined by western blotting analysis. The level of Akt phosphorylation was significantly increased in the presence of NT-4 stimulation compared with the absence of NT-4. Each bar represents the mean ± SEM of five independent experiments. *p < .05; **p < .01, NS, not significant.
To further confirm the downstream effectors of PI3K pathways, we used western blotting to examine the levels of phosphorylated Akt in the absence and presence of NT-4 stimulation. As shown in Figure 5(B), the level of Akt phosphorylation was significantly increased in the presence of NT-4 stimulation compared with the absence of NT-4. Because NT-4 promotes the MPZ expression accompanied by increased phosphorylation of Akt, these results indicate that NT-4 exerts its effect on MPZ expression in Schwann cells via the TrkB/PI3K/Akt pathway.
Possible participation of mTORC1 signaling in MPZ expression
To further investigate the signaling downstream of Akt, we assessed the involvement of glycogen synthase kinase 3β (GSK-3β) and mammalian target of rapamycin (mTOR), both of which are well-known downstream molecules in PI3K/Akt pathways (Aksamitiene et al. 2012). The activity of GSK-3β is at least partly regulated by the phosphorylation and dephosphorylation of its N-terminal serine residue (serine-9), and GSK-3β usually suppresses effector molecules when it is in a dephosphorylated state. When GSK-3β is phosphorylated by Akt, it becomes inactive, and its effector molecules become active (Beurel et al. 2015). SB216763 is a small molecule that potently inhibits the activity GSK-3β (Miller et al. 2014). mTOR, the other major target of Akt, transmits signals from various growth factors. Rapamycin specifically inhibits mTORC1 pathways by forming an inhibitory complex with the immunophilin known as FK506 binding protein 12 (Hidalgo & Rowinsky 2000). Much of the knowledge on mTORC1 signaling has come from studies using rapamycin. As shown in Figure 6 via western blotting analysis, pretreatment of Schwann cells with rapamycin rapidly reduced the NT-4-induced expression of MPZ. However, SB216763 did not enhance MPZ expression in these cells. Moreover, the level of GSK-3β phosphorylation was not changed after stimulation with NT-4 (Figure 7). These data implied that mTORC1, rather than GSK-3β, is involved in NT-4-mediated MPZ up-regulation.
Figure 6.
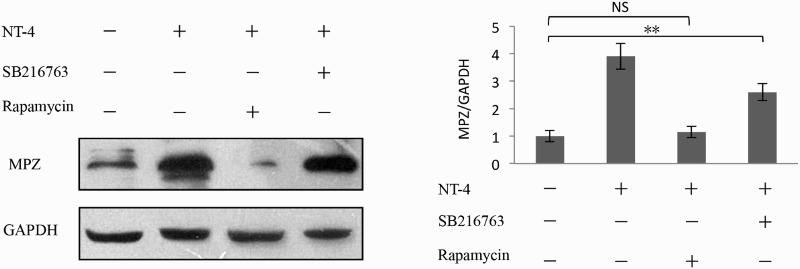
Involvement of mTORC1 signaling in MPZ expression. Impact of rapamycin on MPZ expression in Schwann cells was determined by western blotting analysis. Rapamycin (50 μM, 2 h) pretreatment Schwann cells rapidly reduced the expression of MPZ induced by NT-4; however, SB216763 (40 μm, 2 h) did not affect MPZ expression in these cells. A bar graph represents band intensities normalized to those for GAPDH. Each bar represents the average ± SEM of independent experiments. *p < .05; **p < .01, NS, not significant.
Figure 7.
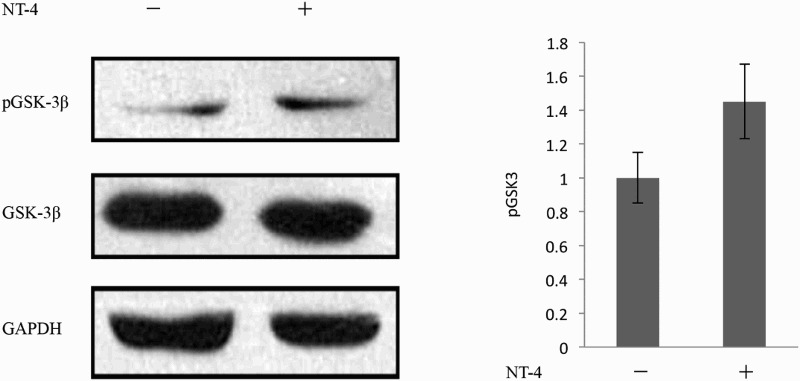
The level of GSK-3β phosphorylation was not changed after the stimulation of NT-4.
Discussion
Schwann cell myelination is essential not only for the development of the PNS but also for its regeneration after nerve injury. MPZ is a type I transmembrane glycoprotein with a single extracellular immunoglobulin-like domain, a single transmembrane domain, and a cytoplasmic carboxy terminus (Patzig et al. 2016). When transfected into non-adherent cells in vitro, the extracellular domain of MPZ protein mediated homophilic plasma membrane cell adhesion (D'Urso et al. 1990). During normal myelination, MPZ has been detected in compact myelin but not in mesaxon membranes. MPZ missense mutations cause a variety of clinically defined human peripheral neuropathies including Charcot-Marie-Tooth type 1B, Dejerine and Sottas syndrome, and congenital hypomyelination, and these diseases are thought to result from impeded interaction between the extracellular domains (Sanmaneechai et al. 2015). Increased MPZ gene dosage resulted in developmental mistargeting to all Schwann cell membranes and in the accumulation of MPZ, particularly in the inner mesaxon, blocking spiral mesaxon growth and preventing myelin formation (Yin et al. 2000, 2015). Although the regulation of MPZ expression is evidently essential for myelination, the underlying molecular mechanisms of this process have not been fully elucidated.
It is well known that NT-4 binds and activates two different receptors, TrkB and p75NTR, both of which are present on Schwann cell membranes (Kidd et al. 2013; Richner et al. 2014). At the cellular level, NT-4 binding to p75NTR results in the activation of several intracellular signaling pathways, including stimulation of NF-κB, Jun kinase, and acidic sphingomyelinase, as well as suppression of RhoA activation. The NF-κB pathway appears to promote survival, whereas the Jun kinase pathway and sphingomyelin hydrolysis promote apoptosis (Meeker & Williams 2014). TrkB, the other receptor for NT-4, is involved in many signal transduction pathways and is associated with cell survival, proliferation, the fate of neural precursors, axon and dendrite growth and patterning, and the expression and activity of proteins important for myelin function (Deinhardt & Chao 2014). However, the roles of p75NTR and truncated TrkB in myelination remain unclear. Previous research demonstrated that truncated TrkB is capable of binding NT-4 and thus localizing it on the cell surface of Schwann cells; however, this receptor–ligand complex is not static but is endocytosed. In this study, the NT-4 might accumulate intracellularly and represent a potential biologically active material to activate the PI3K/Akt pathway. As shown in Figure 4 via western blotting analysis, knockdown of truncated TrkB could significantly reduce the effect of NT-4 on the expression of MPZ, whereas p75NTR knockdown did not inhibit the effect of NT-4 on MPZ expression. Taking all these data together, our research demonstrated that the truncated TrkB might be the main receptor mediating the NT-4 signal for regulating MPZ expression. However, the details of the mechanism by which truncated TrkB and the PI3K/Akt pathway interact remain unclear.
It is now established that MAPK and PI3K pathways are major downstream signaling pathways of Trks and that PI3K acts as a docking phospholipid, binding and recruiting Akt and phosphorylating Akt at T308 (Norrmen & Suter 2013). Akt belongs to the AGC family of kinases, and it phosphorylates numerous substrates involved in cell growth, survival, metabolism and proliferation, among functions, with many of those targets likely to be involved in several different cellular functions (Li et al. 2016). Norrmen and Suter (2013) have revealed that Akt may be a major player controlling myelination, but it remains unclear which processes Akt critically regulates. Moreover, studies have indicated that silencing Akt in Schwann cells resulted in decreased thickness of the myelin sheath (Cotter et al. 2010), and transgenic mice overexpressing a constitutively active form of Akt in oligodendrocytes and Schwann cells showed enhanced myelination and expression of myelin proteins (Flores et al. 2008). In the present study, our results demonstrate that NT-4 mediates MPZ expression via the PI3K/Akt pathway rather than via the MAPK pathway.
As discussed above, the main downstream molecules of Akt are GSK-3β and mTORC1. In our experiment, we found that Akt promotes the expression of MPZ by activating mTORC1 rather than GSK-3β, which suggests an important role for mTORC1 in peripheral nerve myelination. These findings are also consistent with previous research that identified a crucial role for mTORC1 in the development of peripheral nerve myelination (Norrmen et al. 2014). However, previous studies have reported that GSK-3β signaling could also participate in the myelination of peripheral nerves (Ogata et al. 2004). The relationship between these regulatory processes and the present results is currently unclear.
In conclusion, we have demonstrated that NT-4 can enhance the expression of MPZ via the truncated TrkB/PI3K/Akt/mTORC1 pathway in Schwann cells. However, the details of the mechanism by which truncated TrkB interacts with the PI3K/Akt pathway remain unclear. Further study is necessary to clarify the detailed mechanism of Schwann cell myelination downstream of truncated TrkB/PI3K/Akt/mTORC1 signaling. If this pathway is critical for in vivo myelin synthesis, such studies would be useful for the development of therapeutic procedures after nerve injury and for the elucidation of the early process of myelination by Schwann cells.
Supplementary Material
Funding Statement
This work was supported by the National Nature Science Foundation of China (State Key Program; 81330042), the Ministry of Science and Technology, China (Special Program for Sino-Russian Joint Research; 2014DFR31210), and the Tianjin Science and Technology Committee, China (Key Program; 13RCGFSY19000, 14ZCZDSY00044).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aksamitiene E, Kiyatkin A, Kholodenko BN. 2012. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 40:139–146. doi: 10.1042/BST20110609 [DOI] [PubMed] [Google Scholar]
- Belluardo N, Westerblad H, Mudo G, Casabona A, Bruton J, Caniglia G, Pastoris O, Grassi F, Ibanez CF. 2001. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol Cell Neurosci. 18:56–67. doi: 10.1006/mcne.2001.1001 [DOI] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Jope RS. 2015. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 148:114–131. doi: 10.1016/j.pharmthera.2014.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. 2014. NGF, BDNF, NT3, and NT4. Handb Exp Pharmacol. 220:3–15. doi: 10.1007/978-3-642-45106-5_1 [DOI] [PubMed] [Google Scholar]
- Cotter L, Ozcelik M, Jacob C, Pereira JA, Locher V, Baumann R, Relvas JB, Suter U, Tricaud N. 2010. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science. 328:1415–1418. doi: 10.1126/science.1187735 [DOI] [PubMed] [Google Scholar]
- D’Angelo L, Avallone L, Cellerino A, de Girolamo P, Paolucci M, Varricchio E, Lucini C. 2016. Neurotrophin-4 in the brain of adult Nothobranchius furzeri. Ann Anat. 207:47–54. [DOI] [PubMed] [Google Scholar]
- D’Urso D, Brophy PJ, Staugaitis SM, Gillespie CS, Frey AB, Stempak JG, Colman DR. 1990. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron. 4:449–460. doi: 10.1016/0896-6273(90)90057-M [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Chao MV. 2014. Trk receptors. Handb Exp Pharmacol. 220:103–119. doi: 10.1007/978-3-642-45106-5_5 [DOI] [PubMed] [Google Scholar]
- England JD, Asbury AK. 2004. Peripheral neuropathy. Lancet. 363:2151–2161. doi: 10.1016/S0140-6736(04)16508-2 [DOI] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. 2005. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x [DOI] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. 2008. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, et al. 1993. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 363:266–270. doi: 10.1038/363266a0 [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Rowinsky EK. 2000. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 19:6680–6686. doi: 10.1038/sj.onc.1204091 [DOI] [PubMed] [Google Scholar]
- Ibanez CF. 1996. Neurotrophin-4: the odd one out in the neurotrophin family. Neurochem Res. 21:787–793. doi: 10.1007/BF02532301 [DOI] [PubMed] [Google Scholar]
- Jin YQ, Liu W, Hong TH, Cao Y. 2008. Efficient Schwann cell purification by differential cell detachment using multiplex collagenase treatment. J Neurosci Methods. 170:140–148. doi: 10.1016/j.jneumeth.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Kidd GJ, Ohno N, Trapp BD. 2013. Biology of Schwann cells. Handb Clin Neurol. 115:55–79. doi: 10.1016/B978-0-444-52902-2.00005-9 [DOI] [PubMed] [Google Scholar]
- Li X, Wu C, Chen N, Gu H, Yen A, Cao L, Wang E, Wang L. 2016. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 7(22):33440–33450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Jin YQ, Chen L, Wang Y, Yang X, Cheng J, Wu W, Qi Z, Shen Z, Vinci MC. 2015. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS One. 10:e0123278. doi: 10.1371/journal.pone.0123278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Meeker R, Williams K. 2014. Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J Neuroimmune Pharmacol. 9:615–628. doi: 10.1007/s11481-014-9566-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Barr JL, Harper LJ, Poole RL, Gould TJ, Unterwald EM, Vrana KE. 2014. The GSK3 signaling pathway is activated by cocaine and is critical for cocaine conditioned reward in mice. PLoS One. 9:e88026. doi: 10.1371/journal.pone.0088026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Figlia G, Lebrun-Julien F, Pereira JA, Trotzmuller M, Kofeler HC, Rantanen V, Wessig C, van Deijk AL, Smit AB, et al. 2014. mTORC1 controls PNS myelination along the mTORC1-RXRgamma-SREBP-lipid biosynthesis axis in Schwann cells. Cell Rep. 9:646–660. doi: 10.1016/j.celrep.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Norrmen C, Suter U. 2013. Akt/mTOR signalling in myelination. Biochem Soc Trans. 41:944–950. doi: 10.1042/BST20130046 [DOI] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. 2004. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T, Sano M, Omura K, Hasegawa T, Doi M, Sawada T, Nagano A. 2005. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J Peripher Nerv Syst. 10:293–300. doi: 10.1111/j.1085-9489.2005.10307.x [DOI] [PubMed] [Google Scholar]
- Patzig J, Kusch K, Fledrich R, Eichel MA, Luders KA, Mobius W, Sereda MW, Nave KA, Martini R, Werner HB. 2016. Proteolipid protein modulates preservation of peripheral axons and premature death when myelin protein zero is lacking. Glia. 64:155–174. doi: 10.1002/glia.22922 [DOI] [PubMed] [Google Scholar]
- Richner M, Ulrichsen M, Elmegaard SL, Dieu R, Pallesen LT, Vaegter CB. 2014. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol. 50:945–970. doi: 10.1007/s12035-014-8706-9 [DOI] [PubMed] [Google Scholar]
- Sanmaneechai O, Feely S, Scherer SS, Herrmann DN, Burns J, Muntoni F, Li J, Siskind CE, Day JW, Laura M, et al. 2015. Genotype-phenotype characteristics and baseline natural history of heritable neuropathies caused by mutations in the MPZ gene. Brain. 138:3180–3192. doi: 10.1093/brain/awv241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C. 2016. Schwann cells-axon interaction in myelination. Curr Opin Neurobiol. 39:24–29. doi: 10.1016/j.conb.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Timmusk T, Belluardo N, Metsis M, Persson H. 1993. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x [DOI] [PubMed] [Google Scholar]
- Yin Q, Kemp GJ, Yu LG, Wagstaff SC, Frostick SP. 2001. Neurotrophin-4 delivered by fibrin glue promotes peripheral nerve regeneration. Muscle Nerve. 24:345–351. doi: [DOI] [PubMed] [Google Scholar]
- Yin X, Kidd GJ, Wrabetz L, Feltri ML, Messing A, Trapp BD. 2000. Schwann cell myelination requires timely and precise targeting of P(0) protein. J Cell Biol. 148:1009–1020. doi: 10.1083/jcb.148.5.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Kiryu-Seo S, Kidd GJ, Feltri ML, Wrabetz L, Trapp BD. 2015. Proteolipid protein cannot replace P0 protein as the major structural protein of peripheral nervous system myelin. Glia. 63:66–77. doi: 10.1002/glia.22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


