ABSTRACT
Fasting in general causes several metabolic changes. In the present study, we examined the possible changes of several types of nociception during the food deprivation were investigated in mice. After the mice were forced into the fasting for 12, 24, or 48 h, the changes of nociception were measured by the tail-flick, writhing, formalin or von-frey tests. We found that the nociceptive behavior induced by intraperitoneally (i.p.) administered acetic acid (writhing response) or intraplantar injection of 5% formalin into the hind-paw were reduced in fasted group. In addition, the tail-flick response and threshold for nociception in mechanical von-frey test were also elevated in fasted group. Moreover, the p-CREB and p-ERK levels in the dorsal root ganglia (DRG) and the spinal cord were reduced in food-deprived group. Furthermore, p-AMPKα1 expressions in DRG and the spinal cord were up-regulated, whereas p-mTOR in DRG and the spinal cord was down-regulated in food-deprived group. Our results suggest that the chemical, mechanical, and thermal nociceptions appear to be reduced in a food-deprived mouse group. Additionally, reduction of nociception in food-deprived group appears to be closely associated with the expressions of several signal transduction molecules such as ERK, CREB, AMPKα1 and mTOR proteins in DRG and the spinal cord.
KEYWORDS: Nociception, pain, food deprivation, signal transduction, dorsal root ganglia
Introduction
The nociception can be regulated by fasting. Earlier studies have demonstrated that the analgesia is produced by food deprivation (Hamm et al. 1985; Hamm and Knisely 1986). In addition, Davidson et al. (1992) have previously reported that the analgesia is developed by fasting for 24 h as revealed in the tail-flick test.
Both CREB and ERK proteins have been implicated in various types of pain transmission. For example, when nociceptive pain is stimulated, both CREB and ERK proteins expressions are elevated in dorsal root ganglia (DRG) and the spinal cord levels. The study (Crown et al. 2006) have demonstrated that the expression of spinal ERK and CREB proteins are well correlated with the allodynia development in spinal cord injury animal model. Han et al. (2011) have previously reported that ERK protein plays an important role in the production of neuropathic pain. In addition, both CREB and ERK protein expressions are elevated in neuropathic pain model, diabetic neuropathy model or capsaicin treated pain model (Miyabe and Miletic 2005; Song et al. 2005; Wu et al. 2005; Dang et al. 2014). However, the possible roles of CREB and ERK proteins in DRG and the spinal cord in the regulation of nociceptive changes in a food depravation animal model are not clear yet.
Several lines of evidence have demonstrated that the AMPK and mTOR systems are also closely related with nociception. For example, the expression of mTOR protein in DRG or the spinal cord are altered in several types pain models such as neuropathic pain (Zhang et al. 2013; Cui et al. 2014), diabetic neuropathy (He et al. 2016), and inflammation-induced pain (Asante et al. 2009; Xu et al. 2011) models. In addition, the activation of AMPK signal in general reduces nociception, allodynia or hyperalgesia observed in several types of pain models (Russe et al. 2013; Ma et al. 2015; Song et al. 2015; Hasanvand et al. 2016), suggesting that AMPK is an ultimate target molecules for relieving pain (Melemedjian et al. 2011; Asiedu et al. 2016; Price et al. 2016). However, the relationship between nociception and AMPK and mTOR systems during the food deprivation is still uncertain.
Although previous studies have demonstrated that pain perception during the fasting state is changed, the exact alteration of pain behavior and the mechanisms involved in the regulation of nociception during food deprivation have not been well characterized yet. Thus, the present study was designed to assess the possible pain behavior changes during food deprivation in several mouse pain models. Especially, the possible roles of ERK, CREB, AMPK, and mTOR proteins in the regulation of nociception during the food deprivation were investigated.
Materials and methods
Experimental animals
Male ICR mice (6 week of age) weighing 25–30 g at the beginning of experiments (Koatech, Seoul, Korea) were used in the experiments. The mice were housed 5 per cage in a room maintained at 22 ± 1°C with an alternating 12 h light-dark cycles. Food and water were available ad libitum. The animals were allowed to adapt to the laboratory for at least 2 hr before testing and were only used once. To reduce variation, all experiments were performed during the light phase of the cycle (10:00∼17:00).
Nociceptive models and pain behavior measurements
For the visceral pain model (Koster et al. 1959), 1% acetic acid was injected intraperitoneally. The number of writhing response was counted for 30 min after acetic acid injection. For the formalin pain model (Hunskaar et al. 1985; Hunskaar and Hole 1987), 10 μl of 5% formalin was injected subcutaneously under the plantar surface of the left hind-paw. The pain behaviors such as shaking and licking the hind-paws were counted during the first (0–5 min) and the second (20–40 min) phases using a stop watch. The mechanical allodynia was assessed by von-frey tests (Bonin et al. 2014). For the von-frey test, mice were individually placed in a clear glass cells with a metal mesh floor allowed to adapt to the testing environment for 30 min, and then von-frey filaments (North Coast Medical, Inc., Gilroy, CA, USA) were applied to the plantar surface using an up and down paradigm. The number of animal used in the experiment was 8 in each group.
Protein extraction and western blotting
The DRG and spinal cord of mice was dissected. Tissue was washed two times with cold Tris-buffered saline (20 mmol/L Trizma base and 137 mmol/L NaCl, pH 7.5). Immediately after washing, tissues were lysed with sodium dodecyl sulfate lysis buffer (62.5 mmol/L Trizma base, 2% w/v sodium dodecyl sulfate, 10% glycerol) containing 0.1 mmol/L Na3VO4, 3 mg/mL aprotonin, and 20mmolL NaF. After brief sonication, the concentration of protein was determined with a detergent-compatible protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA) using bovine serum albumin as the standard. After adding bromophenol blue (0.1% w/v), the proteins were boiled, separated by electrophoresis in 6%–10% polyacrylamide gels, and transferred onto the polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membranes were immunoblotted with antibodies p-AMPKα1 (Santa Cruz, 1:1000), p-mTOR (Abcam, 1:1000), p-ERK1/2 (Abcam, 1:1000), p-CREB (Abcam, 1:1000) and β-actin (Cell Signaling Technology, 1:1000) in blocking buffer for overnight. Membranes were then washed 4 times with Tris-buffered saline containing 20% Tween-20 (TBST; 10 mM Trizma base, pH8.0, 150 mM NaCl, and 20% Tween 20) for 20 min and then incubated with the anti-rabbit IgG-horseradish peroxidase conjugate (1:4000) in blocking buffer at room temperature for 1 h. After washing the membranes with TBST for 20 min (4 times), ECL-plus solution (Millipore, Billerica, MA, USA) was added. The membranes were then exposed to Luminescent Image Analyzer (LAS-4000, Fuji Film Co., Japan) for the detection of light emission. The specific signals were quantified with the Multi-Gauge Version 3.1 (Fuji Film, Japan) and expressed as the percentage of the control.
Statistics
Statistical analysis was carried out by one-way analysis of variance (ANOVA) with a Bonferroni post-hoc test using GraphPad Prism Version 6.0 for Windows (GraphPad Software, USA). P values less than 0.05 were considered to indicate statistical significance. All values were expressed as the mean ± S.E.M.
Results
Changes of pain behaviors during food deprivation in several pain models
ICR mice were forced into fasting state for 12, 24, or 48 h, and the response to various types of pain stimulation was measured. In the writhing test, 1% acetic acid was administered i.p. and the number of writhing was counted for 30 min. As shown in Figure 1(A), the number of writhing response induced by acetic acid was reduced by food deprivation. In the formalin test, 5% formalin was injected into the hind-paw. As shown in Figure 1(B), the pain behaviors during both 1st and 2nd phases were reduced by food deprivation. Moreover, the tail-flick response was lengthened in food deprivation (Figure 1(C)). Furthermore, as shown in Figure 1(D), an elevation of threshold to mechanical stimulation was elevated as manifested by Von-frey filaments test.
Figure 1.
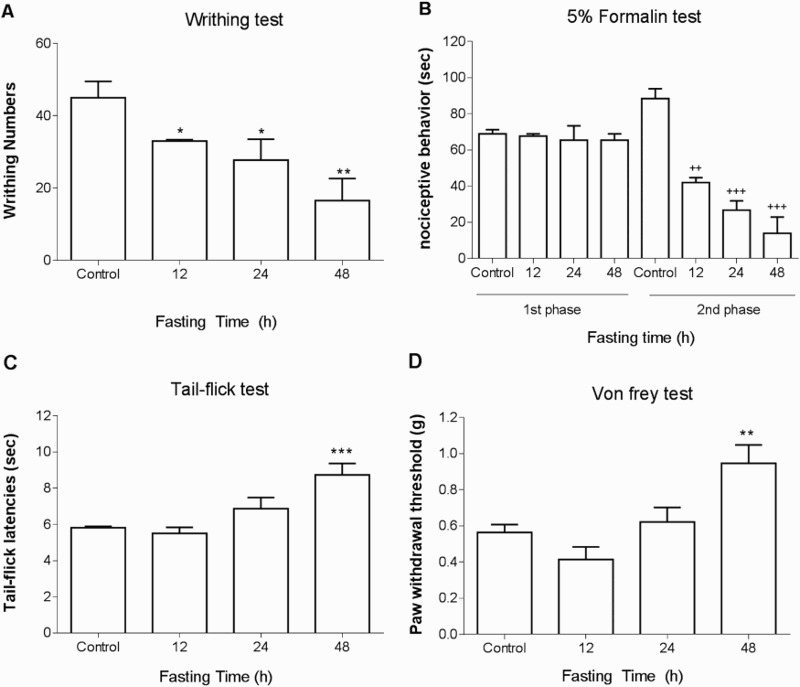
Nociceptive behavioral changes in food deprived group in various types of pain models. The mice were forced into food deprivation for 12, 24, or 48 h. Then, alteration of pain behaviors induced by (A) 1% acetic acid (i.p.), (B) 5% formalin (intraplantar injection into the hind-paw), (C) tail-flick or (D) mechanical pain stimulation by von-frey were assessed. The number of writhing response was counted for 30 min after acetic acid injection. In the formalin pain test, the pain behaviors such as vigorous licking and shaking paws were counted during the first (0–5 min) and the second (20–40 min) phases using a stop watch. The response time of tail-flick to radiant heat was measured. The mechanical pain threshold was measured by von-frey. The vertical bars indicate the standard error of mean (*P < 0.05, **p < 0.01, ***p < 0.001 compared to Control group. ++P < 0.001, +++P < 0.0001; compared to Control group). The mice number of animal used in each group was 8.
Changes of phosphorylated CREB, ERK, AMPK, and mTOR proteins in the dorsal root ganglia and the spinal cord during food deprivation
To detect changes of CREB, ERK, AMPK, or mTOR expression in DRG and the spinal cord during food deprivation, the proteins were extracted from dissected DRG or lumbar spinal cord at 12, 24, or 48 h after food deprivation for Western blot analysis. As shown in Figure 2(A) and 2(B), food deprivation caused down-regulation of p-CREB and p-ERK protein levels in both DRG and the spinal cord. In addition, p-AMPKα1 expression in DRG and the spinal cord was up-regulated, whereas p-mTOR in DRG and the spinal cord was down-regulated in food deprivation group. (Figure 3(A) and 3(B)).
Figure 2.
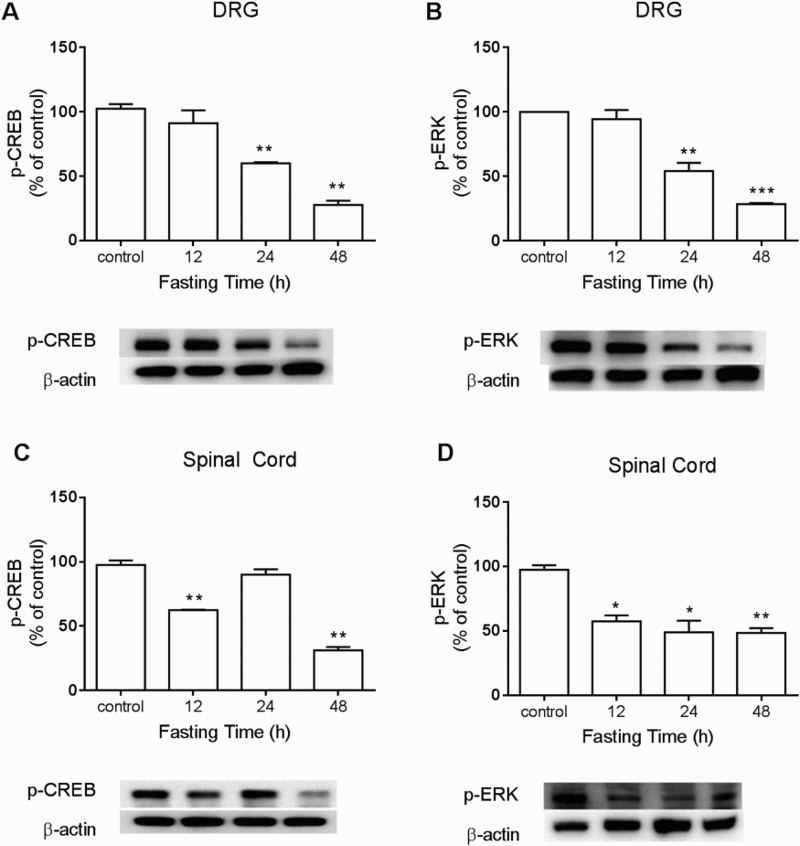
The changes of p-CREB and p-ERK expressions during food deprivation in the dorsal horn and the spinal cord. The mice were forced into food deprivation for 12, 24, or 48 h. Then, DRG and the lumbar region of the spinal cord were dissected. p-CREB and p-ERK expressions in the spinal cord and the DRG were analyzed by Western blot. The number of animals in each group was 6. β-Actin (1:1000 dilution) was used as an internal loading control. Signals were quantified with the use of laser scanning densitometry and expressed as a percentage of the control. Values are mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001; compared to Control group).
Figure 3.
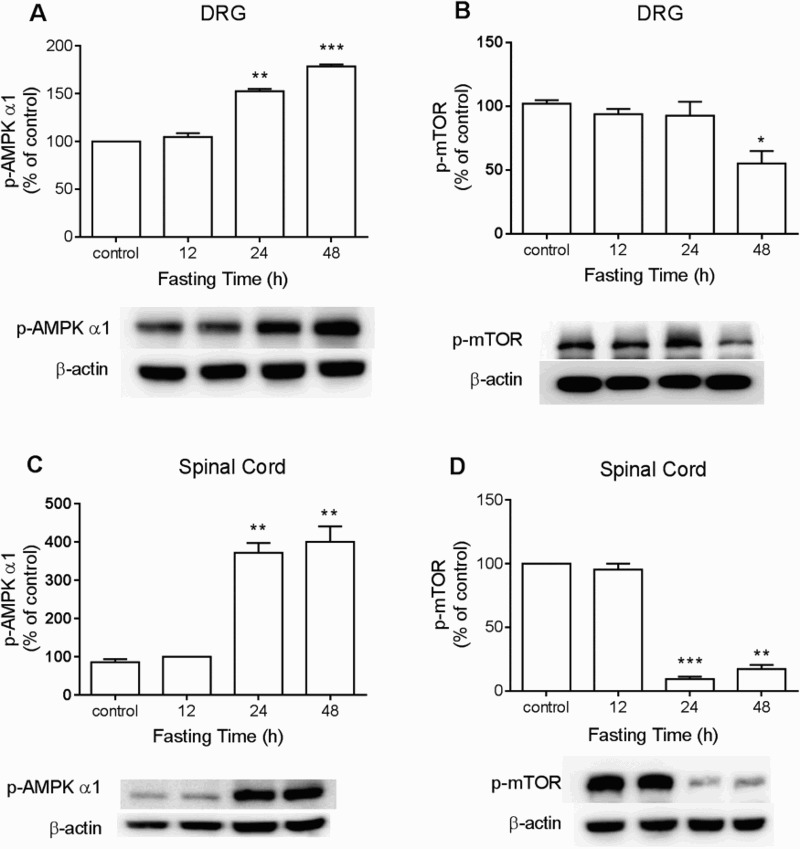
The changes of p-mTOR and p-AMPKα1 expressions during food deprivation in the dorsal horn and the spinal cord. The mice were forced into food deprivation for 12, 24, or 48 h. Then, DRG and the lumbar region of the spinal cord were dissected. p-mTOR and p-AMPKα1 expressions in the spinal cord and the DRG were analyzed by Western blot. The number of animals in each group was 6. β-Actin (1:1000 dilution) was used as an internal loading control. Signals were quantified with the use of laser scanning densitometry and expressed as a percentage of the control. Values are mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001; compared to Control group)
Discussion
In the present study we found that the nociceptive behaviors were changed in several types of pain models in food deprived mouse group, such as writhing (chemical and visceral), formalin (chemical-induced inflammation), tail-flick (thermal), and von-frey (mechanical) pain models. The pain behaviors were reduced in food deprivation group in writhing and formalin pain models. In addition, the tail-flick response to radiant heat and threshold for mechanical pain were elevated in food deprived group, as revealed in tail-flick and von-frey stimulation tests, respectively, suggesting that the fasting may be closely related with the regulation of nociception. Our present findings are in line with several previous studies which demonstrated the food deprivation causes the analgesia in several pain models deprivation (Hamm et al. 1985; Hamm and Knisely 1986; Davidson et al. 1992). Furthermore, in the present study, we found the threshold to mechanical pain is also elevated in food deprivation group for the first time, suggesting that the mechanical nociception is also altered during fasting status.
Several lines of evidence have demonstrated that CREB and ERK proteins are closely associated with pain transmission. Both p-CREB and p-ERK expressions in the spinal cord or dorsal root ganglia are up-regulated in various types of chronic pain models, such as neuropathic pain and neuropathy (Miyabe and Miletic 2005; Song et al. 2005). Furthermore, p-CREB and p-ERK expressions in the spinal cord or brain regions are up-regulated in an acute inflammatory pain model such as formalin pain model (Hermanson and Blomqvist 1997; Seo et al. 2008; Hagiwara et al. 2009; Mao et al. 2013; Tochiki et al. 2015). In the present study, we found that down-regulation of p-CREB and p-ERK expressions in DRG and spinal cord were observed in food-deprived group, suggesting that the reduction of nociception in food-deprived group at least due to the reductions of p-CREB and p-ERK levels in the DRG and the spinal cord. In contrast to our results, several previous studies have reported that the phosphorylation of ERK and CREB are up-regulated in hypothalamic arcuate nucleus during fasting, while the expression of ERK in hypothalamic paraventricular nucleus is down-regulated during fasting (Shimizu-Albergine et al. 2001; Morikawa et al. 2004). However, in db/db mouse, phosphorylation of ERK and CREB are down-regulated in hypothalamic arcuate nucleus during fasting (Morikawa et al. 2004), indicating that the CREB or ERK proteins are differentially regulated in distinct regions in the central nervous system, depending on the energy balance.
In addition to p-CREB and p-ERK proteins, we also found that p-mTOR expressions in DRG and the spinal cord are down-regulated during the food deprivation up to 48 h, suggesting that the reduction of phosphorylation of mTOR might be responsible for the production of antinociception in food deprivation. Our findings are consistent with previous studies in the mTOR system in the activation of chronic pain models such as chronic inflammatory pain (Liang et al. 2013) or neuropathic pain (Asante et al. 2010), suggesting that mTOR system is activated during the elevation of pain perception. In addition, the strategy of reduction of mTOR system is one of new target for relieving the pain.
In contrast to the mTOR, we found that p-AMPKα1 expression in DRG and the spinal cord are up-regulated, suggesting that the increase of phosphorylation of AMPKα1 might be also responsible for the production of antinociception observed in food-deprived group. The activation of AMPK signal is generally associated with the regulation of nociception, allodynia or hyperalgesia observed in several types of pain models (Yamada et al. 1967; Russe et al. 2013; Song et al. 2015; Hasanvand et al. 2016). To support of these findings, the AMPK activators such as resveratrol and metformin exert an antinociceptive effect in chronic pain models (Tillu et al. 2012; Ma et al. 2015).
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [grant number 2015R1D1A1A01060442] and Hallym University Fund [grant number HRF-201707-014].
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2015R1D1A1A01060442) and Hallym University Fund (HRF-201707-014).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Asante CO, Wallace VC, Dickenson AH.. 2009. Formalin-induced behavioural hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol Pain. 5:27. doi: 10.1186/1744-8069-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante CO, Wallace VC, Dickenson AH.. 2010. Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat. J Pain. 11:1356–1367. doi: 10.1016/j.jpain.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu MN, Dussor G, Price TJ.. 2016. Targeting AMPK for the alleviation of pathological pain. EXS. 107:257–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Bories C, De Koninck Y.. 2014. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 10:26. doi: 10.1186/1744-8069-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE.. 2006. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 199:397–407. doi: 10.1016/j.expneurol.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Cui J, He W, Yi B, Zhao H, Lu K, Ruan H, Ma D.. 2014. mTOR pathway is involved in ADP-evoked astrocyte activation and ATP release in the spinal dorsal horn in a rat neuropathic pain model. Neuroscience. 275:395–403. doi: 10.1016/j.neuroscience.2014.06.030 [DOI] [PubMed] [Google Scholar]
- Dang JK, Wu Y, Cao H, Meng B, Huang CC, Chen G, Li J, Song XJ, Lian QQ.. 2014. Establishment of a rat model of type II diabetic neuropathic pain. Pain Med. 15:637–646. doi: 10.1111/pme.12387_1 [DOI] [PubMed] [Google Scholar]
- Davidson TL, McKenzie BR, Tujo CJ, Bish CK.. 1992. Development of tolerance to endogenous opiates activated by 24-h food deprivation. Appetite. 19:1–13. doi: 10.1016/0195-6663(92)90232-U [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Ishida M, Arita J, Mitsushima D, Takahashi T, Kimura F, Funabashi T.. 2009. The cAMP response element-binding protein in the bed nucleus of the stria terminalis modulates the formalin-induced pain behavior in the female rat. Eur J Neurosci. 30:2379–2386. doi: 10.1111/j.1460-9568.2009.07002.x [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Knisely JS.. 1986. The analgesia produced by food deprivation in 4-month old, 14-month old, and 24-month old rats. Life Sci. 39:1509–1515. doi: 10.1016/0024-3205(86)90380-2 [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Knisely JS, Watson A, Lyeth BG, Bossut DF.. 1985. Hormonal mediation of the analgesia produced by food deprivation. Physiol Behav. 35:879–882. doi: 10.1016/0031-9384(85)90254-9 [DOI] [PubMed] [Google Scholar]
- Han M, Huang RY, Du YM, Zhao ZQ, Zhang YQ.. 2011. Early intervention of ERK activation in the spinal cord can block initiation of peripheral nerve injury-induced neuropathic pain in rats. Sheng Li Xue Bao. 63:106–114. [PubMed] [Google Scholar]
- Hasanvand A, Amini-Khoei H, Hadian MR, Abdollahi A, Tavangar SM, Dehpour AR, Semiei E, Mehr SE.. 2016. Anti-inflammatory effect of AMPK signaling pathway in rat model of diabetic neuropathy. Inflammopharmacology. 24:207–219. doi: 10.1007/s10787-016-0275-2 [DOI] [PubMed] [Google Scholar]
- He WY, Zhang B, Xiong QM, Yang CX, Zhao WC, He J, Zhou J, Wang HB.. 2016. Intrathecal administration of rapamycin inhibits the phosphorylation of DRG Nav1.8 and attenuates STZ-induced painful diabetic neuropathy in rats. Neurosci Lett. 619:21–28. doi: 10.1016/j.neulet.2016.02.064 [DOI] [PubMed] [Google Scholar]
- Hermanson O, Blomqvist A.. 1997. Differential expression of the AP-1/CRE-binding proteins FOS and CREB in preproenkephalin mRNA-expressing neurons of the rat parabrachial nucleus after nociceptive stimulation. Brain Res Mol Brain Res. 51:188–196. doi: 10.1016/S0169-328X(97)00236-2 [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Fasmer OB, Hole K.. 1985. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 14:69–76. doi: 10.1016/0165-0270(85)90116-5 [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Hole K.. 1987. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 30:103–114. doi: 10.1016/0304-3959(87)90088-1 [DOI] [PubMed] [Google Scholar]
- Koster R, Anderson M, De Beer E.. 1959. Acetic acid-induced analgesic screening; 1959: FEDERATION PROCEEDINGS.
- Liang L, Tao B, Fan L, Yaster M, Zhang Y, Tao YX.. 2013. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res. 1513:17–25. doi: 10.1016/j.brainres.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L.. 2015. Metformin attenuates hyperalgesia and allodynia in rats with painful diabetic neuropathy induced by streptozotocin. Eur J Pharmacol. 764:599–606. doi: 10.1016/j.ejphar.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Mao Q, Ruan J, Cai X, Lu W, Ye J, Yang J, Yang Y, Sun X, Cao J, Cao P.. 2013. Antinociceptive effects of analgesic-antitumor peptide (AGAP), a neurotoxin from the scorpion buthus martensii karsch, on formalin-induced inflammatory pain through a mitogen-activated protein kinases-dependent mechanism in mice. PLoS One. 8:e78239. doi: 10.1371/journal.pone.0078239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Sanoja R, Yan J, Lark A, Khoutorsky A, Johnson J, Peebles KA, Lepow T, et al. 2011. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain. 7:70. doi: 10.1186/1744-8069-7-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe T, Miletic V.. 2005. Multiple kinase pathways mediate the early sciatic ligation-associated activation of CREB in the rat spinal dorsal horn. Neurosci Lett. 381:80–85. doi: 10.1016/j.neulet.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Ueyama E, Senba E.. 2004. Fasting-induced activation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus. J Neuroendocrinol. 16:105–112. doi: 10.1111/j.0953-8194.2004.01135.x [DOI] [PubMed] [Google Scholar]
- Price TJ, Das V, Dussor G.. 2016. Adenosine monophosphate-activated protein kinase (AMPK) activators for the prevention, treatment and potential reversal of pathological pain. Curr Drug Targets. 17:908–920. doi: 10.2174/1389450116666151102095046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russe OQ, Moser CV, Kynast KL, King TS, Stephan H, Geisslinger G, Niederberger E.. 2013. Activation of the AMP-activated protein kinase reduces inflammatory nociception. J Pain. 14:1330–1340. doi: 10.1016/j.jpain.2013.05.012 [DOI] [PubMed] [Google Scholar]
- Seo YJ, Kwon MS, Choi HW, Choi SM, Kim YW, Lee JK, Park SH, Jung JS, Suh HW.. 2008. Differential expression of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse hippocampus induced by various nociceptive stimuli. Neuroscience. 156:436–449. doi: 10.1016/j.neuroscience.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Shimizu-Albergine M, Ippolito DL, Beavo JA.. 2001. Downregulation of fasting-induced cAMP response element-mediated gene induction by leptin in neuropeptide Y neurons of the arcuate nucleus. J Neurosci. 21:1238–1246. doi: 10.1523/JNEUROSCI.21-04-01238.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Han Y, Pan C, Deng X, Dai W, Hu L, Jiang C, Yang Y, Cheng Z, Li F, et al. 2015. Activation of adenosine monophosphate-activated protein kinase suppresses neuroinflammation and ameliorates bone cancer pain: involvement of inhibition on mitogen-activated protein kinase. Anesthesiology. 123:1170–1185. doi: 10.1097/ALN.0000000000000856 [DOI] [PubMed] [Google Scholar]
- Song XS, Cao JL, Xu YB, He JH, Zhang LC, Zeng YM.. 2005. Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin. 26:789–798. doi: 10.1111/j.1745-7254.2005.00123.x [DOI] [PubMed] [Google Scholar]
- Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, Price TJ.. 2012. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain. 8:5. doi: 10.1186/1744-8069-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochiki KK, Maiaru M, Miller JR, Hunt SP, Geranton SM.. 2015. Short-term anesthesia inhibits formalin-induced extracellular signal-regulated kinase (ERK) activation in the rostral anterior cingulate cortex but not in the spinal cord. Mol Pain. 11:49. doi: 10.1186/s12990-015-0052-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Su G, Ma L, Zhang X, Lei Y, Li J, Lin Q, Fang L.. 2005. Protein kinases mediate increment of the phosphorylation of cyclic AMP-responsive element binding protein in spinal cord of rats following capsaicin injection. Mol Pain. 1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Fitzsimmons B, Steinauer J, O'Neill A, Newton AC, Hua XY, Yaksh TL.. 2011. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J Neurosci. 31:2113–2124. doi: 10.1523/JNEUROSCI.2139-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada C, Clark AJ, Swendseid ME.. 1967. Actinomycin D effect on amino acid absorption from rat jejunal loops. Science. 158:129–130. doi: 10.1126/science.158.3797.129 [DOI] [PubMed] [Google Scholar]
- Zhang W, Sun XF, Bo JH, Zhang J, Liu XJ, Wu LP, Ma ZL, Gu XP.. 2013. Activation of mTOR in the spinal cord is required for pain hypersensitivity induced by chronic constriction injury in mice. Pharmacol Biochem Behav. 111:64–70. doi: 10.1016/j.pbb.2013.07.017 [DOI] [PubMed] [Google Scholar]


