Abstract
Cerebral arterial gas embolism (CAGE) shows various manifestations according to the quantity of gas and the brain areas affected. The symptoms range from minor motor weakness, headache, and confusion to disorientation, convulsions, hemiparesis, unconsciousness, and coma. A 46-year-old man was transferred to our emergency department due to altered sensorium. Immediately after a controlled ascent from 33 m of seawater, he complained of shortness of breath and rigid extremities, lapsing into unconsciousness. He was intubated at another medical center, where a brain computerized axial tomography scan showed no definitive abnormal findings. Pneumothorax and obstructing lesions were apparent in the left thorax of the computed tomography scan. Following closed thoracostomy, we provided hyperbaric oxygen therapy (HBOT) using U.S. Navy Treatment Table (USN TT) 6A. A brain magnetic resonance imaging diffusion image taken after HBOT showed acute infarction in both middle and posterior cerebral arteries. We implemented targeted temperature management (TTM) to prevent worsening of cerebral function in the intensive care unit. After completing TTM, we repeated HBOT using USN TT5 and started rehabilitation therapy. He fully recovered from the neurological deficits. This is the first case of CAGE treated with TTM and consecutive HBOTs suggesting that TTM might facilitate salvage of the penumbra in severe CAGE.
Keywords: : cerebral arterial gas embolism, hyperbaric oxygen therapy, targeted temperature management
Introduction
Decompression illness (DCI) is caused by bubbles in the blood. Bubbles exist in the blood or tissue during or after a reduction in environmental pressure (decompression). DCI includes two pathophysiological syndromes: arterial gas embolism (AGE) and the more common decompression sickness (DCS) (Vann et al., 2011; Weaver, 2014; Pollock and Buteau, 2017). AGE occurs when alveolar capillaries rupture secondary to pulmonary barotrauma, allowing alveolar gas to enter the arterial circulation (Vann et al., 2011). AGE is usually precipitated by rapid ascent, breath holding, or the presence of lung disease in divers (Vann et al., 2011; Weaver, 2014; Pollock and Buteau, 2017).
Cerebral arterial gas embolism (CAGE) shows various manifestations according to the quantity of gas and the areas of the brain affected, from minor motor weakness, headache, and confusion to disorientation, convulsions, hemiparesis, unconsciousness, and coma. The treatment of choice for DCI is hyperbaric oxygen therapy (HBOT) within 6 hours after the development of symptoms. DCS results from uncontrolled release of gas from tissues during or after surfacing with inadequate time for equilibration (Weaver, 2014). Type 1 DCS is usually characterized by musculoskeletal pain and mild cutaneous symptoms. More serious type 2 DCS falls into three categories: neurological, inner ear, and cardiopulmonary (Pollock and Buteau, 2017). Neurological symptoms of type 2 DCS include numbness; paresthesia or tingling; muscle weakness; impaired gait, physical coordination, or bladder control; paralysis; or altered mental status.
Targeted temperature management (TTM) is an established neuroprotective therapy for hypoxic/ischemic brain injury, particularly in patients after cardiac arrest (Callaway et al., 2015). However, the role of TTM in acute ischemic stroke is not clearly understood, despite ongoing feasibility and large clinical trials (Callaway et al., 2015; Marehbian and Greer, 2017). Furthermore, TTM is clinically used in various diseases such as traumatic brain injury, sepsis, cerebral air embolism, and acute carbon monoxide poisoning (Deng et al., 2003; Rim et al., 2012; Inoue et al., 2013; Perman et al., 2014; Oh et al., 2016).
We report a severe case of CAGE, presenting with acute change in mental status after diving. The patient received emergent HBOT and TTM for 24 hours. After completion of TTM, the patient recovered consciousness. We describe the clinical course of the patient and the possible mechanism of action of HBOT and TTM in a patient with CAGE.
Case
A 46-year-old man, a previously healthy and experienced scuba diver and diving instructor, was transferred to our emergency department. At the scene of the accident, immediately after controlled ascent from 33 m of seawater (bottom time 25 minutes), the patient became unconscious after complaining of shortness of breath and rigidity of extremities. The diver was taken to the nearest medical center. He showed semicomatose mentality, bilateral slurred movements on exposure to noxious stimuli, pupil 5 mm/5 mm with prompt light reflex, and SpO2 89%. He was intubated for airway protection and appropriate oxygenation. A computed tomography (CT) scan of the brain showed no abnormalities. Three hours after the accident, he was transferred to our hospital for HBO2 treatment. He was sedated with lorazepam IV 4 mg before transportation. On arrival, the patient's examination was notable for stupor with withdrawal response to painful stimuli in all extremities. Vital signs were as follows: blood pressure, 111/90 mmHg; heart rate, 127/min; respiratory rate, 20 breaths/min; and body temperature, 36.3°C. Initial arterial blood gas showed the following: paO2, 90 mmHg; paCO2, 40 mmHg; bicarbonate, 18.8 mEq/L; base excess, −7.5 mEq/L; and O2 saturation, 96%. Serum creatine kinase was 602 U/L (normal range 50–250). No medical history and diving profile could be obtained because of his mental status. A chest CT scan taken before recompression treatment showed a small left pneumothorax and obstruction of the left upper lobe bronchus with obstructive pneumonia (Fig. 1). After performing a closed thoracostomy, we provided emergent HBOT using U.S. Navy Treatment Table (USN TT) 6A (United States, 2005). A magnetic resonance imaging (MRI) scan of the brain was performed after the HBOT showed multiple high signal intensities on diffusion-weighted imaging (DWI) in widespread areas of the frontal, parietal, and occipital lobes. Both cortical gray matter and subcortical white matters were involved in the majority of brain lesions (Fig. 2). The MR diffusion images were compatible with the findings of acute air embolism in both middle cerebral artery (MCA) and posterior cerebral artery (PCA) territory. The patient showed a GCS score 8 (E 2, V 2, M 4) with spontaneous movement requiring restraints of all extremities. The neurological examination was not correlated with lesions seen on the MR imaging. The neurologist suspected that his symptoms were secondary to hypoxic injury due to multiple gas embolisms. Due to his continuous unresponsiveness as well as bilateral multiple cerebral infarcts on DWI, the patient was transferred to the intensive care unit. We began TTM over a period of 24 hours. He was cooled to 33°C ± 1°C for 24 hours using a surface cooling method and then rewarmed at the rate of 0.25°C/h, followed by normothermia (37°C) under ventilator care using sedatives and muscle relaxants. After completion of TTM, the patient regained consciousness and was extubated. His muscle power increased gradually. 99mTc-labeled hexamethylpropyleneamine oxime (HMPAO) single-photon emission computed tomography (SPECT) of the brain was performed to evaluate the progress of the treatments on hospital day 7. It revealed segmental perfusion defects in the right parieto-occipital region and the left upper parietal region (Fig. 3A). We repeated 20 sessions of HBO using USN TT55 in conjunction with rehabilitation therapy for 1 month. F/U 99mTc-labeled HMPAO SPECT on hospital day 18 showed that the previous perfusion defect was remarkably improved in the right parieto-occipital region and the left upper parietal region. Perfusion seemed almost normal (Fig. 3B). On hospital day 31, the patient fully recovered from the neurological deficits, and could carry out all usual duties and activities.
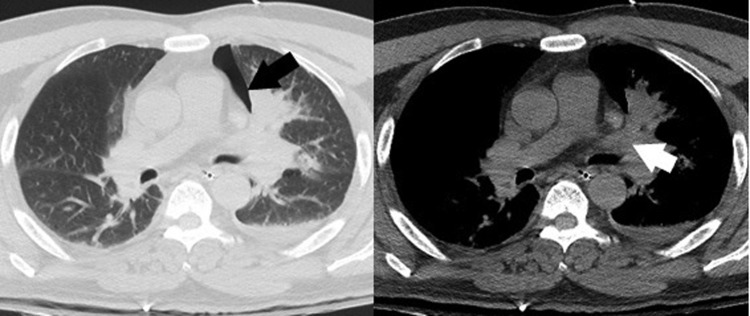
FIG. 1.
Pneumothorax on chest CT. Chest CT scan showed pneumothorax (black arrow) and obstruction of left upper lobe bronchus with obstructive pneumonia (white arrow). CT, computed tomography.
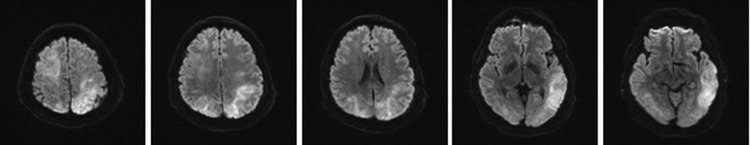
FIG. 2.
MR diffusion images of the brain after HBOT. Multiple high signal lesions on initial MR diffusion images of the brain after HBOT were shown in widespread areas of frontal, parietal, and occipital lobes. Both cortical gray matter and subcortical white matter were involved in the brain lesions. The MR diffusion images were compatible with the findings of acute air embolism in both MCA and PCA territories. HBOT, hyperbaric oxygen therapy; MCA, middle cerebral artery; MR, magnetic resonance; PCA, posterior cerebral artery.
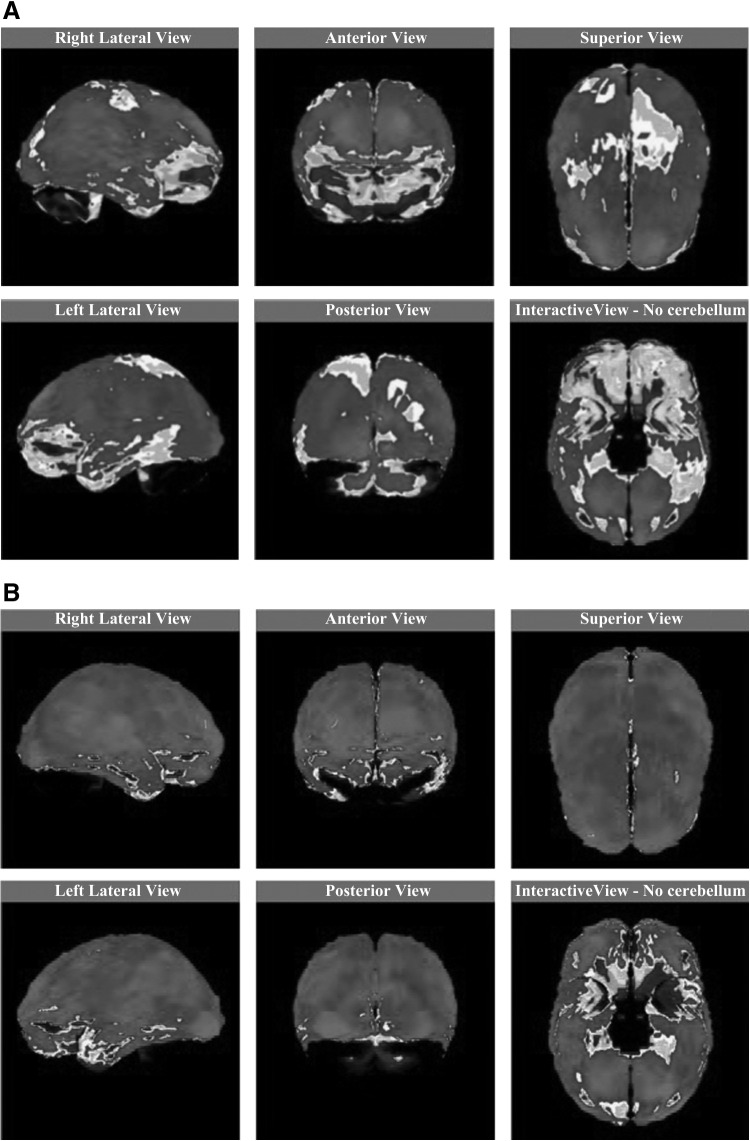
FIG. 3.
99mTc-labeled HMPAO SPECT (A) on hospital day 7 and (B) follow-up on hospital day 18. (A) 99mTc-labeled HMPAO SPECT on hospital day 7 revealed segmental perfusion defects in the right parieto-occipital region and the left upper parietal region. Medium gray color means normal perfusion. (B) F/U 99mTc-labeled HMPAO SPECT on hospital day 18 showed that the previous perfusion defects were remarkably improved in the right parieto-occipital region and the left upper parietal region. Perfusion seemed almost normal. HMPAO, hexamethylpropyleneamine oxime; SPECT, single-photon emission computed tomography.
Fiberoptic bronchoscopy was performed to determine the cause of the obstructing lesion seen on chest CT. Endobronchial biopsy revealed squamous cell carcinoma. As the cancer was far advanced (T4N1M0) on the staging workup, adjuvant chemotherapy and radiotherapy were administered before thoracic surgery. After left pneumonectomy, the patient was in recovery mode and undergoing respiratory rehabilitation.
Discussion
This is the first case of CAGE treated with TTM and consecutive HBOTs. Until now, HBOT was the only effective treatment available for CAGE (Vann et al., 2011). The rationale for HBOT was to reduce the size of air bubble in the vessel by increasing the gradient of dissolved oxygen between the blood and air bubble to favor bubble denitrogenation (Vann et al., 2011; Weaver, 2014; Pollock and Buteau, 2017). CAGE may result in severe neurological sequelae in the absence of prompt and rapid intervention, including HBOT (Vann et al., 2011). Adjunctive therapies for isolated AGE include the following: oxygen administered as a first aid; lidocaine; NSAIDs; anticoagulants; corticosteroids; intravenous fluids; and colloid solution (Moon, 2014). Delays frequently occurred due to the absence of timely HBOT facility (Lippmann et al., 2011).
Chang and Marshall (2012) reported a case of acute cerebral air embolism following CT-guided lung biopsy, and treated with therapeutic hypothermia alone due to pneumothorax. However, our case underwent TTM and consecutive HBOTs together. We believe that our treatment was better than TTM alone, because the treatment of choice for CAGE was HBOT. TTM is recommended in comatose adult patients with return of spontaneous circulation after cardiac arrest, but the role of induced hypothermia or TTM in acute ischemic stroke is controversial (Jauch et al., 2013; Marehbian and Greer, 2017). Our patient immediately underwent a single session of HBOT. However, there remained still short of improvement in neurological status, and MRI images showed multiple ischemic strokes on both hemispheres. We suspected a global hypoxic injury with no specific treatment available. The optimal treatment for severe DCS remains still lacking, while the outcome of HBOT varies with complete resolution reported in 13–63% of patients with severe DCS (Amir et al., 2015). Theoretically, neuroprotection by TTM was considered to be efficacious during the period of ischemia/reperfusion injury due to thromboembolism. Therefore, we treated the patient with TTM after obtaining informed consent, expecting neuroprotective anti-inflammatory and immune-related responses against postischemia reperfusion. !During a period of TTM therapy, there was no specific adverse event. The extubation was conducted on the seventh day of hospitalization, and the follow-up brain MRI showed nearly normal findings. The patient's neurological and physical functions recovered gradually with consecutive HBOT and rehabilitation therapy. TTM may represent an adjunctive therapy for CAGE but should not in any way be viewed as a replacement or reason to delay hyperbaric treatment, which is most efficacious when conducted immediately (Lippmann et al., 2011).
Pulmonary barotrauma is one of the mechanisms associated with CAGE occurrence (Clarke et al., 2002). AGE in scuba divers results from inadequate lung ventilation secondary to inadvertent breath holding or rapid buoyant ascent. It rapidly induces a critical state of pulmonary overpressurization, which may also occur as a consequence of acute or chronic pulmonary pathologies. The associated barotrauma frequently causes structural failure within the terminal airway, resulting in alveolar rupture. Respiratory gas embolism occurs in the systemic circulation via pulmonary vasculature and the left heart. Our patient had no history of known pulmonary disease and experienced no respiratory symptoms in his daily life, even during previous frequent scuba dives. Considered the endobronchial lesions, which were identified through lung biopsy, the small-sized endobronchial lesions might not occlude the airway in normobaric respiration but play a role in overinflation of the distal airway in decompression scenarios such as ascent. Eventually, alveolar rupture occurs and small bubbles entering pulmonary vasculature may act as cerebral emboli.
The differential diagnosis between AGE and DCS is frequently difficult in the initial stages (Trout et al., 2015). In the absence of local manifestations of lung injury, and significant gas loading during the preceding dive, it is difficult or even impossible to distinguish an AGE from DCS. On the contrary, even in obvious cases of AGE, the untrained physician may miss the diagnosis or fail to treat it properly. Brain MRI might facilitate the differentiation of AGE from DCS. High signal intensities on multiple vessel territories in the patient's brain MR diffusion images with a low value on the apparent diffusion coefficient (ADC) map suggest multiple small gas emboli even though the brain CT scan reveals no gas bubbles (Moon, 2014). Kamtchum Tatuene et al. (2014) described the following MR findings supporting the arterial occlusion theory of pathophysiology of DCI: restricted diffusion appearing as hyperintensity on DWI with low values on the ADC map, high signal on T2-weighted magnetic resonance images (T2WI), and usually without contrast enhancement.
In conclusion, this is the first case of CAGE treated with TTM and consecutive HBOTs in an experienced scuba diver. HBOT is the only effective treatment available for CAGE. However, in cases of suspected ischemia after HBOT, TTM may have been helpful to neurological protection in some patients with CAGE.
Author Disclosure Statement
No competing financial interests exist.
References
- Amir H, Gregori F, Yair B, Jacob B, Mony F, Amit M, Shai E. Delayed recompression for decompression sickness: retrospective analysis. PLoS One 2015;10:e0124919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. . Part 8: post-cardiac arrest care: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(18 Suppl 2):S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Marshall J. Therapeutic hypothermia for acute air embolic stroke. West J Emerg Med 2012;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D, Gerard W, Norris T. Pulmonary barotrauma-induced cerebral arterial gas embolism with spontaneous recovery: commentary on the rationale for therapeutic compression. Aviat Space Environ Med 2002;73:139–146 [PubMed] [Google Scholar]
- Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke 2003;34:2495–2501 [DOI] [PubMed] [Google Scholar]
- Inoue S, Takizawa H, Yamamoto Y, Tangoku A. Therapeutic hypothermia for severe cerebral air embolism complicating pleural lavage for empyema. Interact Cardiovasc Thorac Surg 2013;17:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947 [DOI] [PubMed] [Google Scholar]
- Kamtchum Tatuene J, Pignel R, Pollak P, Lovblad KO, Kleinschmidt A, Vargas MI. Neuroimaging of diving-related decompression illness: current knowledge and perspectives. AJNR Am J Neuroradiol 2014;35:2039–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann J, Fock A, Arulanandam S. Cerebral arterial gas embolism with delayed treatment and a fatal outcome in a 14-year-old diver. Diving Hyperb Med 2011;41:31–34 [PubMed] [Google Scholar]
- Marehbian J, Greer DM. Normothermia and stroke. Curr Treat Options Neurol 2017;19:4. [DOI] [PubMed] [Google Scholar]
- Moon RE. Hyperbaric oxygen treatment for air or gas embolism. Undersea Hyperb Med 2014;41:159–166 [PubMed] [Google Scholar]
- Oh B, Im Y, Park E, Min Y, Choi S. Treatment of acute carbon monoxide poisoning with induced hypothermia. Clin Exp Emerg Med 2016;3:100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perman SM, Goyal M, Neumar RW, Topjian AA, Gaieski DF. Clinical applications of targeted temperature management. Chest 2014;145:386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock NW, Buteau D. Updates in Decompression Illness. Emerg Med Clin North Am 2017;35:301–319 [DOI] [PubMed] [Google Scholar]
- Rim KP, Kim K, Jo YH, Lee JH, Rhee JE, Kang KW, et al. . Effect of therapeutic hypothermia according to severity of sepsis in a septic rat model. Cytokine 2012;60:755–761 [DOI] [PubMed] [Google Scholar]
- Trout BM, Caruso JL, Nelson C, Denoble PJ, Nord DA, Chimiak J, et al. In: DAN Annual Diving Report 2012–2015 Edition: A Report on 2010–2013 Data on Diving Fatalities, Injuries, and Incidents. Buzzacott P, ed. Durham, NC: Divers Alert Network, 2015 [PubMed] [Google Scholar]
- United States. Naval Sea Systems Command, United States. Navy Department, United States. Naval Sea Systems Command. Supervisor of Diving, 2005. Accessed at Best Publishing Co at http://everyspec.com/USN/NAVSEA/SS521-AG-PRO-010_4104 on September 4, 2017
- Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness. Lancet 2011;377:153–164 [DOI] [PubMed] [Google Scholar]
- Weaver LK, Undersea and Hyperbaric Medical Society (UHMS), Hyperbaric Oxygen Committee. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report, 13th ed North Palm Beach, FL: Best Publishing Company, 2014. [Google Scholar]