Abstract
Prostate cancer (PCA) is one of the most common cancer types in men, with cancer progression being linked to hypoxia and the induction of hypoxia-inducible factor (HIF).We investigated the expression of pyruvate kinase M2 (PKM2), its regulation by HIF isoforms 1α and 2α, and its role in HIF stabilization. We additionally examined cell survival in the prostate cancer cell lines PC3 and LNCaP under severe hypoxic (0.1% O2) and normoxic (20% O2) conditions. qRT-PCR showed higher up-regulation of PKM2 mRNA expression in LNCaP cells than in PC3 cells, while western blotting showed that PKM2 protein levels were up-regulated only in LNCaP cells. Inhibition of HIF-1α and HIF-2α by small interfering RNA (si-RNA) demonstrated HIF-1α dependent up-regulation of PKM2 at the mRNA and protein levels in LNCaP cells. PKM2 inhibition by si-RNA significantly decreased hypoxia-response element (HRE) activation in a gene reporter assay and down-regulated HIF-1α target vascular endothelial growth factor (VEGF) mRNA expression in PC3 cells, whereas HIF-1α protein levels were not significantly reduced. Additionally, PKM2 inhibition significantly reduced clonogenic survival in both cell lines in a colony formation assay. Prolyl hydroxylase 3 (PHD3) mRNA expression was up-regulated in both cell lines. It has been shown that PKM2 expression is regulated by HIF-1α and that PKM2 favors HIF-1α transactivation under mild (1% O2) but not severe (0.1% O2) hypoxic conditions, and some of our findings are consistent with these previous results. However, this mechanism was not fully observed in our studied cell lines, as PKM2 regulation and HIF-1α stabilization at the transactivation level occurred under severe hypoxic conditions. This discrepancy suggests that tumor tissue origin and cell type influence this model. Our findings expand the current knowledge of the mechanisms of PCA regulation, and would be important in developing novel therapeutic strategies.
Introduction
Prostate cancer (PCA) is one of the most common cancer types in men, with an annual estimated 1.1 million cases and 307,000 deaths worldwide [1]. Low oxygen concentration, or hypoxia, has been shown to correlate with resistance to chemotherapy in vitro and radiotherapy in vivo, as well as to promote malignancy progression [2]. The degree of hypoxia ranges within tumors between 0.3% and 4.2% O2, with a median level of <2% depending on the tissue of origin [3]. In addition, there is a notable diversity of oxygen levels within individual tumors [4], depending on the efficiency and proximity of local blood vessels [5]. This reflects the fact that tumor cells exhibit differing hypoxia-inducible factor 1α (HIF-1α) expression and regulation under mild 1% O2 and severe 0.1% O2 hypoxic conditions [6]. Invasive prostate adenocarcinoma shows higher HIF-1α expression than does the normal epithelium, stromal cells, and benign prostatic hyperplasia [7]. Overexpression of HIF-1α in PCA is associated with shorter relapse time in patients receiving surgery or radio therapy in addition to chemo/ castration resistance and metastasis [8].
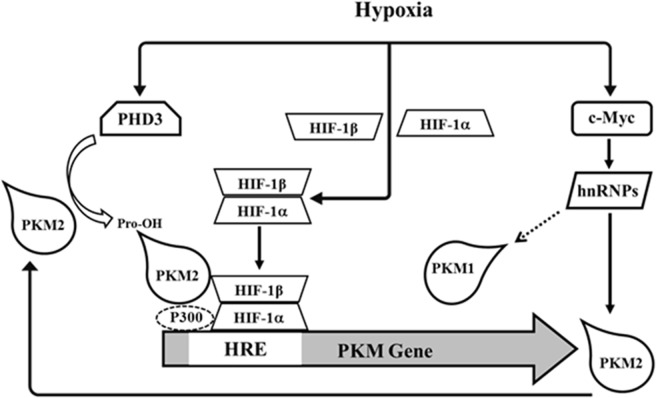
HIF-1α regulates pyruvate kinase M2 (PKM2) expression by binding to the hypoxia-response elements (HREs) located within the first intron of the PKM2 gene [9] (Fig 1), thus regulating cell metabolism. PKM2 catalyzes the conversion of phosphoenolpyruvate into pyruvate, which is last step of the glycolytic pathway [10]. PKM2 expression has been associated with esophageal squamous cell carcinoma (ESCC) chemoresistance [11], while its knockdown in non-small cell lung cancer (NSCLC) increased the radiosensitivity of resistant cell lines [12]. Moreover, up-regulation and specific modification of PKM2 has also been associated with PCA progression [13].
Fig 1. Schematic representation of regulation of PKM2 and its interaction with HIF-1α.
Activation of HIF-1α occurs due to protein stabilization [14] (Fig 1) by inhibition of the prolyl hydroxylase family (PHD1-3), which hydroxylates specific prolyl residues in the oxygen-dependent degradation (ODD) domain of HIF-1α to enhance its degradation [15, 16]. PHD3 also hydroxylates specific proline residues on PKM2 in mild (1% O2) but not severe (0.1% O2) hypoxic conditions, favoring transactivation of HIF-1α [9] (Fig 1). Additionally, PKM2 increases the binding of HIF-1α and the recruitment of the coactivator p300 to HREs on HIF-1α target genes in a positive feedback loop [9] (Fig 1).
Surgery and radiation therapy are traditional approaches for clinically localized cancers, whereas androgen deprivation associated with chemotherapy is usually used in cases of metastatic disease [17]. Hormone and radiation therapy have good success rates in patients with intermediate-risk factors, however the disease can still recur in those with more advanced tumor burdens [18]. Thus, therapies making the tumor more sensitive to radiation can be beneficial. A better understanding of PCA biological environmental conditions; such as hypoxia and HIF-1α, which play a central role in PCA oncogenesis; growth; and metastasis; is important to decreasing the mortality rate of the disease [19]. Thus, this study was an in-depth investigation of PKM2 and HIF-1α regulation mechanisms and their cross-talk in PCA cell lines under severe hypoxic conditions, rather than the conditions investigated previously [9].
Hypoxia activates HIF-1α dimerization with HIF-1β to form HIF-1α [20], which induces PKM2 at the transcriptional level mediated by its HRE elements [9]. C-myc is relevant to PKM2 since it regulates critical hnRNP proteins [21] that affect differential splicing of PKM1 and PKM2 [22], favoring the latter [23]. Overall, these pathways determine the absolute and relative levels of PKM1 and PKM2. The interaction between PKM2 and HIF-1α involving PHD3 hydroxylation of PKM2 at proline residues is displayed. This modification results in HIF-1α transactivation by interaction with the transcription factor complex [9].
Materials and methods
Cell culture and hypoxic incubation
The human prostate carcinoma cell lines PC3 and LNCaP were used in this study as in previous HIF-1α expression studies [7, 24–26]. PC3 and LNCaP cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured following the manufacturer’s recommendations. Cells were exposed to hypoxia (HOX) in a chamber equilibrated with a water-saturated gas v/v mixture of 0.1% O2, 5% CO2, and the rest nitrogen at 37°C (Innova CO-48; New Brunswick Scientific, Edison, NJ, USA). Control cells were maintained under normoxic conditions (NOX) in water-saturated room air, supplemented with 5% (v/v) CO2 at 37°C.
RNA interference by synthetic si-RNA
PC3 and LNCaP cells were transfected with small interfering RNA (si-RNA) targeting PKM2 and HIF-1α or HIF-2α and control siRNA (sequences shown in S1 Table) synthetized by Biomers (Ulm, Germany). Transient transfection of siRNA was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. Cells were subcultured to 70% confluence in DMEM-F12 supplemented with 10% FBS. Transfection of siRNAs was performed in Opti-MEM medium for 5 h, followed by culturing in DMEM-F12 supplemented with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA).
RNA isolation, reverse transcription and real-time PCR
Total RNA was extracted from the hypoxia-exposed and si-RNA treated cells, and equal amounts (2 μg) were subsequently transcribed into cDNA using M-MuLV reverse transcriptase (Thermo Fisher Scientific). The cDNA was used as a template in real-time PCR performed with the ABI Prism 7300 detection system (Applied Biosystems, Foster City, CA, USA) with SYBR green as a fluorescent dye (Invitrogen). Human-specific primers for PKM2, HIF-1α, HIF-2α, VEGF, and PHD3 (sequences shown in S2 Table) were designed using sequence information from NCBI and were purchased from Biomers (Biomers). Expression was analysed by the ΔCt method. The Ct values of the target genes were normalized to that of the porphobilinogen deaminase (PBGD) gene (endogenous control).
Western blot analysis and quantification
Total protein was extracted by the peqGOLD TriFast procedure (Peqlab Biotechnology GmbH, Erlangen, Germany). Proteins were separated on 10% polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA). After blocking, the membranes were probed with one of the following antibodies: anti-HIF-1α (BD Biosciences, Franklin Lakes, NJ, USA), anti-HIF-2α (LifeSpan BioSciences, Inc., Seattle, WA, USA), anti-PKM2 (Cell Signaling Technologies, Danvers, MA, USA), anti-PKM2 (Y105; Cell Signaling Technologies), and anti-β-actin (Abcam, Cambridge, United Kingdom). All primary antibodies were diluted 1:1000. Then membranes were incubated with secondary antibodies conjugated with horseradish peroxidase (HRP). Antibody complexes were visualized by enhanced chemiluminescence using an ECL kit (GE Healthcare, Little Chalfont, United Kingdom). An image reader (FluorchemTM IS-8900, Alpha Innotech, San Leandro, CA, USA) was used to visualize and quantify western blot bands. Expression was quantified using band intensity values (in arbitrary units), which were normalized to that of β-actin.
HRE reporter gene assay
The pGL3-TK plasmid (Promega, Madison, Wisconsin, USA) with a thymidine kinase minimal promoter (TK-MP) and five repeats of HRE from the phosphoglycerate kinase (PGK) gene were used to construct the HRE-reporter plasmid [27]. The results were normalized against Renilla luciferase, which was co-transfected to correct for variations in transfection efficiency. PC3 and LNCaP cells were grown to 80% confluence in 48-well plates and co-transfected with HRE-reporter plasmid, si-control, and si-PKM2 using Lipofectamine 2000, and then incubated under normoxic or hypoxic conditions for 24 h. The firefly luciferase (FLuc) activity in transfected cells was measured using the luciferase assay system (Promega) and a spectrofluorometer (FL-600 BioTek Instruments, Winooski, VT, USA). Fluorescence values are reported as relative light units (RLUs).
Colony formation assays
PC3 and LNCaP cells were seeded at a density of 1 × 104 cells per 100 mm dish and cultured in 0.35% soft agar in DMEM-F12 plus 10% FBS (Thermo Fisher Scientific) at 37°C for 48 h. Afterwards, cells were transfected with si-PKM2 or si-control once every 3 days a total of two times. After the first transfection, cells were incubated for two weeks in normoxia or hypoxia. Finally, cells were stained with crystal violet, rinsed in water, and air-dried. All visible colonies were counted. The surviving fraction was calculated as follows: (mean colony counts) / (cells inoculated) = plating efficiency (PE), where PE is a measure of the number of colonies originating from single cells.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.03 (GraphPad Software, Inc., La Jolla, CA, USA). All data are represented as means ± standard error (mean ± SEM). Student’s t-test and one-way analysis of variance (ANOVA) were used to determine the levels of statistical differences between two and multiple groups, respectively.
Results
PKM2 expression under normoxic and hypoxic conditions
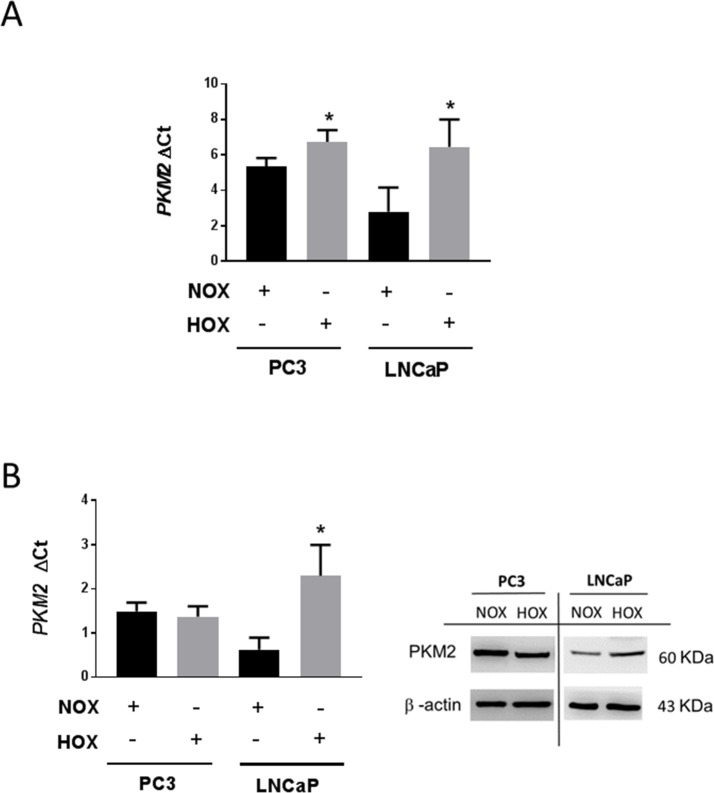
PKM2 mRNA was expressed at a higher level in PC3 cells than in LNCaP cells under normoxic conditions (Fig 2A). Severe hypoxia significantly increased PKM2 mRNA expression in both cell lines, with a more pronounced increment in LNCaP cells (Fig 2A). However, PKM2 protein levels showed significant up-regulation in LNCaP cells, but not in PC3 cells (Fig 2B) in severe hypoxic as compared to normoxic conditions.
Fig 2. PKM2 expression in normoxia and hypoxia in prostate cancer cell lines.
PC3 and LNCaP cells were cultured for 24 h in normoxia 20% O2 or hypoxia 0.1% O2. (A) PKM2 mRNA expression detection and quantification by qRT-PCR (n = 3, Mean ± SEM, * P < 0.05 vs NOX). (B) Densitometric analysis of western blot for PKM2 normalized to β-actin. (n = 3, Mean ± SEM, * P < 0.05).
Inhibition of HIF-1α and HIF-2α by si-RNA and its effect on PKM2
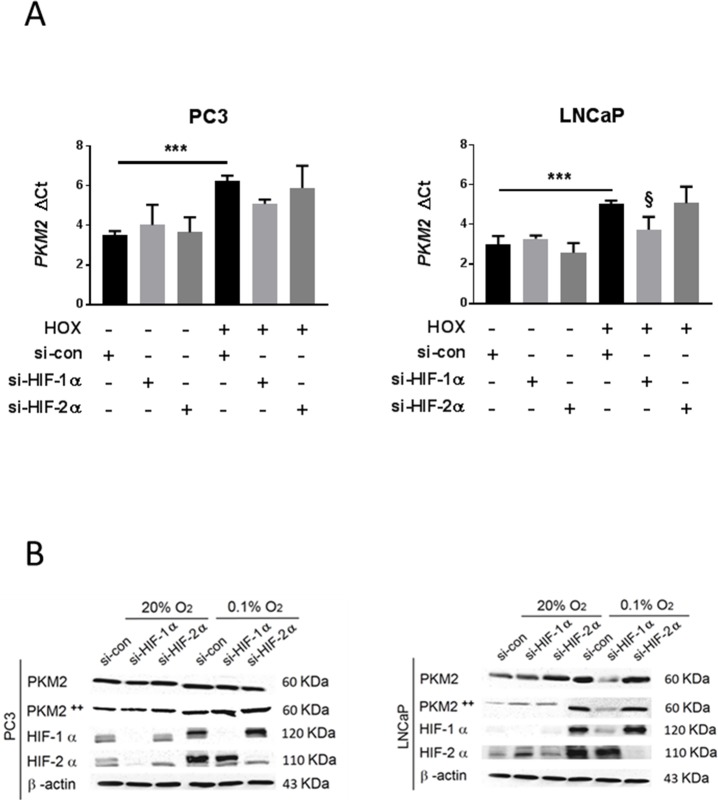
Silencing of HIF-1α and HIF-2α was validated by qRT-PCR and western blotting (S1A Fig and Fig 3B respectively). Since PC3 cells displayed high HIF-1α expression even in normoxia, we further confirmed the efficiency of the silencing by HRE luciferase assay (S1B Fig). qRT-PCR analysis of mRNA extracts from PC3 cells demonstrated no effect on PKM2 mRNA expression from si-HIF-1α or si-HIF-2α as compared to that from si-control in hypoxia. However, LNCaP cells showed a significant down-regulation of PKM2 mRNA expression after si-HIF-1α but not si-HIF-2α transfection as compared to expression after si-control transfection in hypoxia (Fig 3A). No significant changes were observed in normoxia in either cell line. Consistently, western blot analysis demonstrated no changes in PKM2 or phosphor-PKM2 (Tyr105, modified PKM2 with higher activity) protein levels in PC3 cells after si-HIF-1α or si-HIF-2α transfection as compared to levels after si-control transfection in severe hypoxia and normoxia (Fig 3B). However, PKM2 and phospho PKM2 (Tyr105) protein levels were down-regulated in LNCaP cells after si-HIF-1α but not si-HIF-2α transfection as compared to levels after si-control transfection in hypoxia (Fig 3B).
Fig 3. Effects of HIF-1α and HIF-2α inhibition by si-RNA on PKM2 expression.
PC3 and LNCaP cells were transfected and cultured for 24 h in normoxia 20% O2 or hypoxia 0.1% O2. (A) PKM2 mRNA expression detection and quantification by qRT-PCR after treatment of cells with si-HIF-1α or si-HIF-2α (n = 4, Mean ± SEM, *** P < 0.001, § P < 0.05 vs si-con Hox). (B) Western blot analysis of PKM2 and PKM2 ++ (Tyr105) levels after si-HIF-1α or si-HIF-2α transfection and validation of the suppressive effects of si-HIF-1α and si-HIF-2α on HIF-1α and HIF-2α respectively.
PKM2 inhibition by si-RNA and its effect on HIF-1α
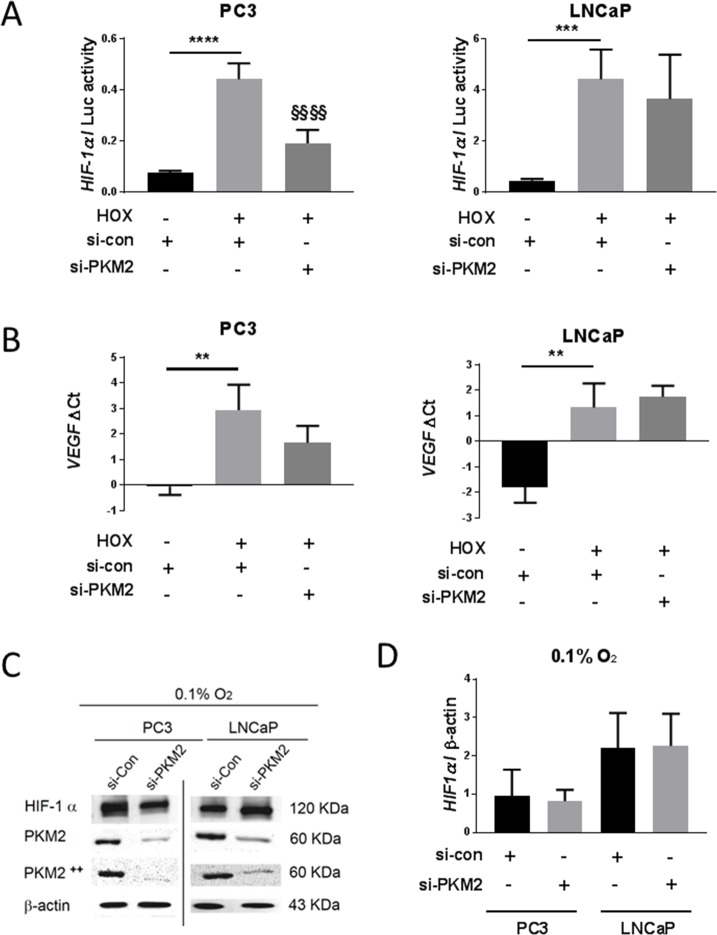
Since silencing HIF-2α did not affect PKM2 expression, we focused on HIF-1α regulation. After confirming the efficiency of PKM2 silencing by qRT-PCR and western blotting (S2 Fig and Fig 4B, respectively), HIF-1α protein levels were analyzed. Densitometric analysis showed no significant difference between the effects of si-PKM2 and si-control transfection on HIF-1α protein levels in PC3 or LNCaP cells in hypoxia (Fig 4C and 4D). Further, HRE luciferase activity was measured. We observed that si-PKM2 significantly down-regulated HRE activity in PC3, but not in LNCaP cells, as compared to si-control under hypoxic conditions (Fig 4A). To further confirm these results, we analyzed mRNA expression of VEGF, a well-known HIF-1α target. Consistently, VEGF mRNA expression was down-regulated by si-PKM2 compared to si-control in PC3 cells but not in LNCaP cells under hypoxic conditions (Fig 4B).
Fig 4. Effect of PKM2 inhibition by si-RNA on HIF-1α.
PC3 and LNCaP cells were transfected and cultured for 24 h in normoxia 20% O2 or hypoxia 0.1% O2. (A) HRE reporter gene assay of transfected cells with si-PKM2 (n = 6, Mean ± SEM, *** P < 0.001, **** P < 0.0001, §§§§ P < 0.0001 vs si-con Hox). (B) VEGF mRNA expression detection and quantification by qRT-PCR after treatment of cells with si-PKM2 (n = 3, Mean ± SEM, ** P < 0.01 vs si-con HoX). (C) Western blot analysis of HIF-1α protein levels after si-PKM2 transfection and validation of the suppressive effects of si-PKM2 on PKM2 and PKM2++ (Tyr105) protein levels. (D) Densitometric analysis of HIF-1α western-blot normalized to β-actin after treatment of cells with si-PKM2.
Expression of PHD3 under normoxic and hypoxic conditions
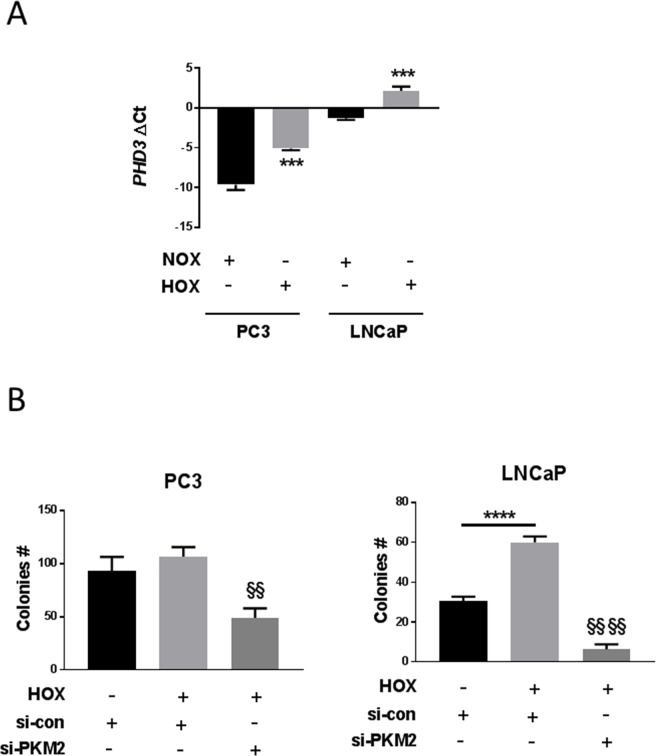
qRT-PCR analysis of mRNA extracts from PC3 and LNCaP cells showed a significant increase in PHD3 mRNA expression in severe hypoxia as compared to that in normoxia in both cell lines, with a more pronounced increase in PC3 cells (Fig 5A).
Fig 5. PHD3 expression and colony survival in prostate cancer cell lines.
PC3 and LNCaP cells were transfected and cultured for 24 h in normoxia 20% O2 or hypoxia 0.1% O2. (A) PHD3 mRNA expression detection and quantification by qRT-PCR (n = 3, Mean ± SEM, *** P < 0.001 vs NOX). (B) Colony survival assay in transfected cells with si-PKM2, (n = 3, Mean ± SEM, ** P < 0.01, **** P < 0.0001, §§§§ P < 0.0001 vs si-con Hox).
Effect of PKM2 inhibition on cell proliferation
To investigate the role of PKM2 on cell proliferation in severe hypoxic conditions, we performed a colony formation assay. PKM2 silencing affected the growth of PC3 and LNCaP cells. There was an 80–90% decrease in LNCaP colony formation and a 50% decrease in PC3 colony formation when the cells were transfected with siRNA directed against PKM2 as compared to si-control in hypoxia (Fig 5B).
Discussion
Oxygen levels within tumors are very heterogeneous, ranging from mild to severe hypoxia [28] or anoxia [29] (0.1% O2). Tumor hypoxia is associated with increased radioresistance and metastatic potential, resulting in poor prognosis. Previous studies have demonstrated the presence of HIF-1α dependent molecular events triggered by severe hypoxia. HIF-1α regulates PKM2 gene expression, and PKM2 in turn interacts with the HIF-1α subunit and stimulates its transactivation in a feedback loop [30]. In the present study, we explored the effects of severe hypoxia on the crosstalk between HIF-1α and PKM2 in the context of prostate cancer.
PKM2 basal mRNA expression was higher in PC3 than in LNCaP cells under normoxic conditions. Its expression was significantly induced by severe hypoxia in both cell lines (Fig 2A). Hypoxic regulation of PKM2 mRNA has already been described in hepatoblastoma cells (HepG2) [31] and mouse embryonic fibroblast (MEFs) [9]. This induction was more pronounced in LNCaP than in PC3 cells. It is further well-known that the transcriptional response of hypoxia target genes varies among different human cancer cells [6], accounting for differences observed in PKM2 up regulation in LNCaP and PC3 cell lines. The increment of PKM2 mRNA expression was coupled with increase in the protein levels in LNCaP but not in PC3 cells (Fig 2B), suggesting the activation of different post-transcriptional and/or post-translational mechanisms. Moreover, previous studies have revealed that this correlation between RNA and protein profiles occurs only in one-third of human cell lines [32].
To analyze the role of HIF transcription factors in regulation of PKM2 in prostate cell lines, knockdowns of both, HIF-1α and HIF-2α were performed. Silencing of HIF-1α, but not HIF-2α, significantly down-regulated severe hypoxia-induced PKM2 mRNA (Fig 3A) and protein levels (Fig 3B) in LNCaP but not in PC3 cells. Our results support the idea of PKM2 regulation by HIF-1α in LNCaP cells, as has been suggested previously [9], but not in PC3 cells. These differences between our studied cell lines could be attributed to the higher basal level of HIF-1α we noticed under normoxia in PC3 cells. Several theories were suggested to explain normoxic HIF-1α up-regulation in PCA, including HIF-1α stabilization relying on tumor hypoxia [33], increased HIF-1α mRNA expression [34], gene amplification [35], and single-nucleotide polymorphisms (SNPs) [36]. A previous study revealed that HIF-1α expression under normoxia in PC3 cells is due to HIF-1α gene amplification [35]. Such a cell behavior of HIF-1α expression and regulation mechanism can influence the PKM2/HIF-1α relationship, and the gene expression response to hypoxic stress in a cell-specific manner.
Further, we assume that the high normoxic PKM2 expression (Fig 2A) in PC3 cells is due to HIF-1α normoxic upregulation (Fig 3B). This could also explain why hypoxic stress failed to intensely increase PKM2 mRNA expression and protein levels (Fig 2B). The lack of any effect of silencing HIF-1α on PKM2 expression in PC3 cells is consistent with this (Fig 3B).
Silencing PKM2 did not affect HIF-1α protein levels in PC3 or LNCaP cells (Fig 4D). However, it significantly reduced HIF-1α activity (Fig 4A) and VEGF mRNA expression (Fig 4B) in PC3 but not LNCaP cells in severe hypoxia. HIF-1α expression is linked to increased expression of VEGF [37], and silencing of HIF-1α resulted in decreased expression of VEGF mRNA [38]. These results suggest that HIF-1α activity is controlled at the transactivation level. It has been demonstrated that PKM2 hydroxylation is crucial to HIF-1α transactivation in VHL-null RCC4 renal carcinoma cells at 1% O2 but not at 0.1% O2 levels [9]. HIF-1α is also regulated by PKM2 via metabolites, including lactate and pyruvate. PKM2 is crucial enzyme to metabolic and glycolytic flux, and its upregulation causes a several-fold increase in glucose consumption and lactate production [39–42].
Hydroxylation of PKM2 by PHD3 in hypoxia is favored by enhanced PHD3 expression, while PHD3 degree of induction varies depending on cell type and pO2 [16] [43–45]. PHD3 expression and contribution to malignancy have been detected in several cancers, including pancreatic biliary tumors [46], gastric cancer [47], NSCLC [48], breast cancer [49], and renal cell carcinoma (RCC) [50]. Severe hypoxia induced PHD3 mRNA levels in both PC3 and LNCaP cell lines (Fig 5A). However, PHD enzyme activity is also affected by succinate and α-ketoglutarate [51–53] and small ubiquitin-related modification (SUMOylation) [54].
Differences in tumor oxygenation can explain the discrepancies between our results and previous ones [9]. Oxygenation is normally reported as a median value [3] and varies among tumors depending on the tissue of origin [3]. The median oxygen value of renal cancer is 1.3%O2 [55], cervical cancer 1.2%O2 [4], and prostate cancer 0.3%O2 [56]. A recent study treated LNCaP xenografts with bicalutamide to keep median oxygen below 0.1%O2 for more than 10 days [57]. Androgens can be another factor to explain our results, since PC3 cells are androgen-independent, while LNCaP cells are androgen dependent. Study shows androgens to stimulate HIF-1α expression via an autocrine loop involving EGF/PI3K/AKT in LNCaP cells [58].
Although the mechanism of PKM2 regulation may differ depending on cell type, there is no doubt that it is essential for tumor progression. In the present study, we reported that severe hypoxia promoted cell proliferation in PC3 androgen-independent and LNCaP androgen-dependent prostate cancer cell lines. Consistent with this, a colony survival assay showed that silencing of PKM2 expression significantly decreased colony formation in both PC3 and LNCaP cells under 0.1% O2 hypoxia (Fig 5B). This may fit functionally with the observation that PKM2 activity shifts glycolytic metabolites away from energy production towards anabolic processes of cellular compounds required for proliferation [59–61].
Several studies have observed excessive HIF-1α expression in PCA, making it a potential therapeutic target. A diverse variety of HIF-1α inhibitors have been developed and investigated clinically [62]. Proper understanding of the hypoxic stress response in correlation with tissue type is critical to understanding the mechanism of tumor cell adaptation to hypoxia and to the development of efficient therapeutic interventions.
Conclusions
According to our study, PKM2 expression is regulated by HIF-1α, but HIF-1α expression is not regulated by PKM2 in LNCaP cells. This mechanism of regulation is reversed in PC3 cells, in which PKM2 expression is not regulated by HIF-1α, but HIF-1α expression is regulated by PKM2. Our results put forward cell-specific differences in hypoxic regulation of HIF-1α and PKM2, which might form an important basis for developing specific targeting strategies in future.
Supporting information
PC3 and LNCaP cells were transfected and cultured for 24 h in normoxia 20% O2 or hypoxia 0.1% O2. (A) HIF-1α and HIF-2α mRNA expression detection and quantification by qRT-PCR after treatment of cells with si-HIF-1α or si-HIF-2α (n = 4, Mean ± SEM, * P < 0.05, ** P < 0.01, *** P < 0.01 vs si-con Nox or Hox, ANOVA). (B) HRE reporter gene assay of transfected cells with si-PKM2 and si-HIF-1α (n = 6, Mean ± SEM, **** P < 0.0001, §§§§ P < 0.0001 vs si-con Hox).
(TIF)
PC3 and LNCaP cells were transfected and cultured for 24 hr in normoxia 20% O2 or hypoxia 0.1% O2. PKM2 mRNA expression detection and quantification by qRT-PCR after treatment of cells with si-PKM2 (n = 4, Mean ± SEM, *** P < 0.001, **** P < 0.0001 vs si-con Hox).
(TIF)
(TIF)
(TIF)
Acknowledgments
We thank PD. Dr. Jörg Hänze and Dr. Swati Dabral for scientific advice and technical orientation during our research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Sierra MS, Soerjomataram I, Forman D. Prostate cancer burden in Central and South America. Cancer Epidemiol. 2016;44 Suppl 1:S131–S40. 10.1016/j.canep.2016.06.010 . [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28(2 Suppl 8):29–35. . [DOI] [PubMed] [Google Scholar]
- 3.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87(1035):20130676 10.1259/bjr.20130676 ; PubMed Central PMCID: PMCPMC4064601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9(8):1221–35. 10.1089/ars.2007.1628 . [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–54. 10.1016/S0076-6879(04)81023-1 . [DOI] [PubMed] [Google Scholar]
- 6.Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS medicine. 2006;3(3):e47 10.1371/journal.pmed.0030047 ; PubMed Central PMCID: PMC1334226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13(3):739–49. 10.1677/erc.1.00728 . [DOI] [PubMed] [Google Scholar]
- 8.Ranasinghe WK, Baldwin GS, Shulkes A, Bolton D, Patel O. Normoxic regulation of HIF-1alpha in prostate cancer. Nat Rev Urol. 2014;11(7):419 10.1038/nrurol.2013.110-c2 . [DOI] [PubMed] [Google Scholar]
- 9.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–44. 10.1016/j.cell.2011.03.054 ; PubMed Central PMCID: PMCPMC3130564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 43(7):969–80. Epub 2010/02/17. doi: S1357-2725(10)00062-2 [pii] 10.1016/j.biocel.2010.02.005 . [DOI] [PubMed] [Google Scholar]
- 11.Fukuda S, Miyata H, Miyazaki Y, Makino T, Takahashi T, Kurokawa Y, et al. Pyruvate Kinase M2 Modulates Esophageal Squamous Cell Carcinoma Chemotherapy Response by Regulating the Pentose Phosphate Pathway. Ann Surg Oncol. 2015;22 Suppl 3:S1461–8. 10.1245/s10434-015-4522-3 . [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Ma Y, Wang P, Song Z, Liu B, Sun X, et al. Knockdown of PKM2 Enhances Radiosensitivity of Non-small cell Lung Cancer. Cell Biochem Biophys. 2015;73(1):21–6. 10.1007/s12013-015-0567-y . [DOI] [PubMed] [Google Scholar]
- 13.Wong N, Yan J, Ojo D, De Melo J, Cutz JC, Tang D. Changes in PKM2 associate with prostate cancer progression. Cancer Invest. 2014;32(7):330–8. 10.3109/07357907.2014.919306 . [DOI] [PubMed] [Google Scholar]
- 14.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–4. Epub 1995/06/06. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–40. Epub 2001/10/13. 10.1126/science.1066373 10663731066373 [pii]. . [DOI] [PubMed] [Google Scholar]
- 16.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. Epub 2001/10/12. doi: S0092-8674(01)00507-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 17.Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017;317(24):2532–42. 10.1001/jama.2017.7248 . [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald TJ, Wang T, Goel HL, Huang J, Stein G, Lian J, et al. Prostate carcinoma and radiation therapy: therapeutic treatment resistance and strategies for targeted therapeutic intervention. Expert Rev Anticancer Ther. 2008;8(6):967–74. 10.1586/14737140.8.6.967 ; PubMed Central PMCID: PMCPMC2764989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deep G, Panigrahi GK. Hypoxia-Induced Signaling Promotes Prostate Cancer Progression: Exosomes Role as Messenger of Hypoxic Response in Tumor Microenvironment. Crit Rev Oncog. 2015;20(5–6):419–34. 10.1615/CritRevOncog.v20.i5-6.130 ; PubMed Central PMCID: PMCPMC5308872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2007;117(4):862–5. 10.1172/JCI31750 ; PubMed Central PMCID: PMCPMC1838952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 463(7279):364–8. Epub 2009/12/17. doi: nature08697 [pii] 10.1038/nature08697 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Zhang J, Manley JL. Turning on a fuel switch of cancer: hnRNP proteins regulate alternative splicing of pyruvate kinase mRNA. Cancer Res. 70(22):8977–80. Epub 2010/10/28. doi: 0008-5472.CAN-10-2513 [pii] 10.1158/0008-5472.CAN-10-2513 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261(29):13807–12. . [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 . [DOI] [PubMed] [Google Scholar]
- 25.Jeong CW, Yoon CY, Jeong SJ, Hong SK, Byun SS, Kwak C, et al. The role of hypoxia-inducible factor-1alpha and -2alpha in androgen insensitive prostate cancer cells. Urologic oncology. 2013;31(8):1448–56. 10.1016/j.urolonc.2012.03.022 . [DOI] [PubMed] [Google Scholar]
- 26.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. American journal of respiratory and critical care medicine. 2011;183(2):152–6. 10.1164/rccm.201009-1393PP ; PubMed Central PMCID: PMC3159088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottschald OR, Malec V, Krasteva G, Hasan D, Kamlah F, Herold S, et al. TIAR and TIA-1 mRNA-binding proteins co-aggregate under conditions of rapid oxygen decline and extreme hypoxia and suppress the HIF-1alpha pathway. J Mol Cell Biol. 2010;2(6):345–56. 10.1093/jmcb/mjq032 . [DOI] [PubMed] [Google Scholar]
- 28.Ameri K, Lewis CE, Raida M, Sowter H, Hai T, Harris AL. Anoxic induction of ATF-4 through HIF-1-independent pathways of protein stabilization in human cancer cells. Blood. 2004;103(5):1876–82. 10.1182/blood-2003-06-1859 . [DOI] [PubMed] [Google Scholar]
- 29.Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22(2):177–80. 10.1016/j.ceb.2009.11.015 . [DOI] [PubMed] [Google Scholar]
- 30.Demaria M, Poli V. PKM2, STAT3 and HIF-1alpha: The Warburg's vicious circle. JAKSTAT. 2012;1(3):194–6. 10.4161/jkst.20662 ; PubMed Central PMCID: PMCPMC3670244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kress S, Stein A, Maurer P, Weber B, Reichert J, Buchmann A, et al. Expression of hypoxia-inducible genes in tumor cells. J Cancer Res Clin Oncol. 1998;124(6):315–20. Epub 1998/08/06. . [DOI] [PubMed] [Google Scholar]
- 32.Gry M, Rimini R, Stromberg S, Asplund A, Ponten F, Uhlen M, et al. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC genomics. 2009;10:365 10.1186/1471-2164-10-365 ; PubMed Central PMCID: PMC2728742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marignol L, Coffey M, Lawler M, Hollywood D. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat Rev. 2008;34(4):313–27. 10.1016/j.ctrv.2008.01.006 . [DOI] [PubMed] [Google Scholar]
- 34.Pipinikas CP, Carter ND, Corbishley CM, Fenske CD. HIF-1alpha mRNA gene expression levels in improved diagnosis of early stages of prostate cancer. Biomarkers. 2008;13(7):680–91. 10.1080/13547500802591992 . [DOI] [PubMed] [Google Scholar]
- 35.Saramaki OR, Savinainen KJ, Nupponen NN, Bratt O, Visakorpi T. Amplification of hypoxia-inducible factor 1alpha gene in prostate cancer. Cancer Genet Cytogenet. 2001;128(1):31–4. . [DOI] [PubMed] [Google Scholar]
- 36.Chau CH, Permenter MG, Steinberg SM, Retter AS, Dahut WL, Price DK, et al. Polymorphism in the hypoxia-inducible factor 1alpha gene may confer susceptibility to androgen-independent prostate cancer. Cancer Biol Ther. 2005;4(11):1222–5. . [DOI] [PubMed] [Google Scholar]
- 37.Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Gittes GK, et al. VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. American journal of physiology Cell physiology. 2000;278(4):C853–60. 10.1152/ajpcell.2000.278.4.C853 . [DOI] [PubMed] [Google Scholar]
- 38.Shi Y. Mammalian RNAi for the masses. Trends in genetics: TIG. 2003;19(1):9–12. . [DOI] [PubMed] [Google Scholar]
- 39.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15(4):300–8. Epub 2005/05/24. doi: S1044-579X(05)00026-X [pii] 10.1016/j.semcancer.2005.04.009 . [DOI] [PubMed] [Google Scholar]
- 40.Marin-Hernandez A, Rodriguez-Enriquez S, Vital-Gonzalez PA, Flores-Rodriguez FL, Macias-Silva M, Sosa-Garrocho M, et al. Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J. 2006;273(9):1975–88. Epub 2006/04/28. doi: EJB5214 [pii] 10.1111/j.1742-4658.2006.05214.x . [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Ruiz R, Uribe-Carvajal S, Devin A, Rigoulet M. Tumor cell energy metabolism and its common features with yeast metabolism. Biochim Biophys Acta. 2009;1796(2):252–65. Epub 2009/08/18. doi: S0304-419X(09)00051-1 [pii] 10.1016/j.bbcan.2009.07.003 . [DOI] [PubMed] [Google Scholar]
- 42.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–5. Epub 2002/04/12. 10.1074/jbc.M202487200 M202487200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 43.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22(16):4082–90. Epub 2003/08/13. 10.1093/emboj/cdg392 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.del Peso L, Castellanos MC, Temes E, Martin-Puig S, Cuevas Y, Olmos G, et al. The von Hippel Lindau/hypoxia-inducible factor (HIF) pathway regulates the transcription of the HIF-proline hydroxylase genes in response to low oxygen. J Biol Chem. 2003;278(49):48690–5. Epub 2003/09/25. 10.1074/jbc.M308862200 M308862200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 45.Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, et al. Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J Cell Sci. 2003;116(Pt 7):1319–26. Epub 2003/03/05. . [DOI] [PubMed] [Google Scholar]
- 46.Gossage L, Zaitoun A, Fareed KR, Turley H, Aloysius M, Lobo DN, et al. Expression of key hypoxia sensing prolyl-hydroxylases PHD1, -2 and -3 in pancreaticobiliary cancer. Histopathology. 2010;56(7):908–20. 10.1111/j.1365-2559.2010.03566.x . [DOI] [PubMed] [Google Scholar]
- 47.Su C, Huang K, Sun L, Yang D, Zheng H, Gao C, et al. Overexpression of the HIF hydroxylase PHD3 is a favorable prognosticator for gastric cancer. Med Oncol. 2012;29(4):2710–5. 10.1007/s12032-012-0171-6 . [DOI] [PubMed] [Google Scholar]
- 48.Chen S, Zhang J, Li X, Luo X, Fang J, Chen H. The expression of prolyl hydroxylase domain enzymes are up-regulated and negatively correlated with Bcl-2 in non-small cell lung cancer. Mol Cell Biochem. 2011;358(1–2):257–63. 10.1007/s11010-011-0976-1 . [DOI] [PubMed] [Google Scholar]
- 49.Fox SB, Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, et al. The prolyl hydroxylase enzymes are positively associated with hypoxia-inducible factor-1alpha and vascular endothelial growth factor in human breast cancer and alter in response to primary systemic treatment with epirubicin and tamoxifen. Breast Cancer Res. 2011;13(1):R16 10.1186/bcr2825 ; PubMed Central PMCID: PMCPMC3109585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka T, Kitamura H, Torigoe T, Hirohashi Y, Sato E, Masumori N, et al. Autoantibody against hypoxia-inducible factor prolyl hydroxylase-3 is a potential serological marker for renal cell carcinoma. J Cancer Res Clin Oncol. 2011;137(5):789–94. 10.1007/s00432-010-0940-6 . [DOI] [PubMed] [Google Scholar]
- 51.Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278(33):30772–80. Epub 2003/06/06. 10.1074/jbc.M304982200 M304982200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 52.Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279(11):9899–904. Epub 2004/01/01. 10.1074/jbc.M312254200M312254200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 53.Berchner-Pfannschmidt U, Tug S, Trinidad B, Oehme F, Yamac H, Wotzlaw C, et al. Nuclear oxygen sensing: induction of endogenous prolyl-hydroxylase 2 activity by hypoxia and nitric oxide. J Biol Chem. 2008;283(46):31745–53. Epub 2008/09/09. doi: M804390200 [pii] 10.1074/jbc.M804390200 . [DOI] [PubMed] [Google Scholar]
- 54.Nunez-O'Mara A, Gerpe-Pita A, Pozo S, Carlevaris O, Urzelai B, Lopitz-Otsoa F, et al. PHD3-SUMO conjugation represses HIF1 transcriptional activity independently of PHD3 catalytic activity. J Cell Sci. 2015;128(1):40–9. 10.1242/jcs.151514 . [DOI] [PubMed] [Google Scholar]
- 55.Lawrentschuk N, Poon AM, Foo SS, Putra LG, Murone C, Davis ID, et al. Assessing regional hypoxia in human renal tumours using 18F-fluoromisonidazole positron emission tomography. BJU Int. 2005;96(4):540–6. 10.1111/j.1464-410X.2005.05681.x . [DOI] [PubMed] [Google Scholar]
- 56.Parker C, Milosevic M, Toi A, Sweet J, Panzarella T, Bristow R, et al. Polarographic electrode study of tumor oxygenation in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58(3):750–7. 10.1016/S0360-3016(03)01621-3 . [DOI] [PubMed] [Google Scholar]
- 57.Ming L, Byrne NM, Camac SN, Mitchell CA, Ward C, Waugh DJ, et al. Androgen deprivation results in time-dependent hypoxia in LNCaP prostate tumours: informed scheduling of the bioreductive drug AQ4N improves treatment response. Int J Cancer. 2013;132(6):1323–32. 10.1002/ijc.27796 . [DOI] [PubMed] [Google Scholar]
- 58.Mabjeesh NJ, Willard MT, Frederickson CE, Zhong H, Simons JW. Androgens stimulate hypoxia-inducible factor 1 activation via autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3'-kinase/protein kinase B in prostate cancer cells. Clin Cancer Res. 2003;9(7):2416–25. . [PubMed] [Google Scholar]
- 59.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–3. Epub 2008/03/14. doi: nature06734 [pii] 10.1038/nature06734 . [DOI] [PubMed] [Google Scholar]
- 60.Dang CV. PKM2 tyrosine phosphorylation and glutamine metabolism signal a different view of the Warburg effect. Sci Signal. 2009;2(97):pe75. Epub 2009/11/19. doi: 2/97/pe75 [pii] 10.1126/scisignal.297pe75 . [DOI] [PubMed] [Google Scholar]
- 61.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. Epub 2009/05/23. doi: 324/5930/1029 [pii] 10.1126/science.1160809 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. 10.1016/j.cell.2012.01.021 ; PubMed Central PMCID: PMCPMC3437543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PC3 and LNCaP cells were transfected and cultured for 24 h in normoxia 20% O2 or hypoxia 0.1% O2. (A) HIF-1α and HIF-2α mRNA expression detection and quantification by qRT-PCR after treatment of cells with si-HIF-1α or si-HIF-2α (n = 4, Mean ± SEM, * P < 0.05, ** P < 0.01, *** P < 0.01 vs si-con Nox or Hox, ANOVA). (B) HRE reporter gene assay of transfected cells with si-PKM2 and si-HIF-1α (n = 6, Mean ± SEM, **** P < 0.0001, §§§§ P < 0.0001 vs si-con Hox).
(TIF)
PC3 and LNCaP cells were transfected and cultured for 24 hr in normoxia 20% O2 or hypoxia 0.1% O2. PKM2 mRNA expression detection and quantification by qRT-PCR after treatment of cells with si-PKM2 (n = 4, Mean ± SEM, *** P < 0.001, **** P < 0.0001 vs si-con Hox).
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.