Abstract
Background
Breast cancer risk factors have been examined extensively in Western setting and more developed Asian cities/countries. However, there are limited data on developing Asian countries. The purpose of this study was to examine breast cancer risk factors and the change of selected risk factors across birth cohorts in Malaysian women.
Methods
An unmatched hospital based case-control study was conducted from October 2002 to December 2016 in Selangor, Malaysia. A total of 3,683 cases and 3,980 controls were included in this study. Unconditional logistic regressions, adjusted for potential confounding factors, were conducted. The breast cancer risk factors were compared across four birth cohorts by ethnicity.
Results
Ever breastfed, longer breastfeeding duration, a higher soymilk and soy product intake, and a higher level of physical activity were associated with lower risk of breast cancer. Chinese had the lowest breastfeeding rate, shortest breastfeeding duration, lowest parity and highest age of first full term pregnancy.
Conclusions
Our study shows that breastfeeding, soy intake and physical activity are modifiable risk factors for breast cancer. With the increasing incidence of breast cancer there is an urgent need to educate the women about lifestyle intervention they can take to reduce their breast cancer risk.
Background
Breast cancer risk factors have been examined extensively and the common ones include early age of menarche, late age of menopause, short breastfeeding duration, late age of first full term pregnancy, nulliparity and low parity [1–5]. However, most of these studies were conducted predominantly in developed countries in a Western setting. Although a limited number of studies examining women living in Asian countries also supported the association of these common risk factors with breast cancer [6–10], they were conducted in the more developed Asian cities/countries, or have been limited to sample sizes of several hundred women and mostly limited to one ethnicity. Therefore, there is a need to conduct a more extensive study with a larger sample size to determine whether these risk factors also play a similar role among Asian populations in developing countries, as this evidence should contribute importantly to the development of appropriate strategies for breast cancer prevention and control in Asia.
Malaysia offers a unique opportunity to examine breast cancer risk factors in Asian populations because of its multi-cultural and multi-religious setting, both of which might influence lifestyle and reproductive characteristics, and hence, breast cancer risk. Notably, the three main ethnicities in Malaysia, namely, Malay, Chinese and Indian, represent the three largest ethnic groups in Asia. Breast cancer is the most common cancer among Malaysian women and accounted for 31% of total female cancers [11]. The age-adjusted breast cancer incidence in Malaysia is 47.4/100,000, about half of that in North America [12]. Chinese have the highest incidence (59.9/100,000) followed by Indians (54.2/100,000) and Malays (34.9/100,000) [11]. Like many developing Asian countries, Malaysia is undergoing a transition toward a Westernized diet that is high in fat and sugar, an increasingly sedentary lifestyle [13] and also experiencing changes in reproductive characteristics [14]. Thus, there is an urgent need to examine the impact of these changes on breast cancer risk.
In this paper, we report the association between clinical, exogenous hormonal, menstrual, reproductive, anthropometric and lifestyle factors with breast cancer from a hospital-based case-control study of 7,663 women in Malaysia. We also present the change of selected breast cancer-related factors across birth cohorts and their implication for breast cancer in Malaysia and potentially other developing Southeast Asian countries.
Materials and methods
The study was approved by the Independent Ethics Committee, Ramsay Sime Darby Health Care (reference nos: 201109.4 and 201208.1), and the Medical Ethics Committee, University Malaya Medical Centre (reference no: 842.9). All participants provided written informed consent. The study was performed in accordance with the Declaration of Helsinki.
The Malaysian Breast Cancer Genetic Study (MyBrCa), initiated in 2002, is a hospital-based case-control study of breast cancer risk factors. The study participants are recruited from two participating hospitals in Selangor, Malaysia: University Malaya Medical Centre (UMMC), a public hospital, and Subang Jaya Medical Centre (SJMC), a private hospital. All patients diagnosed clinically with breast carcinoma are eligible for inclusion as cases. Cases from UMMC were recruited since October 2002, and from SJMC, since September 2012. Controls are healthy women between ages 40 and 74 with no personal history of breast cancer and recruited in the Malaysian Mammography Study (MyMammo) at UMMC and SJMC. At SJMC, MyMammo is a subsidized opportunistic mammogram screening programme that was initiated in 2011; while at UMMC, MyMammo started recruitment in 2014 from patients attending routine opportunistic screening in UMMC.
All participants were interviewed by trained interviewers at the hospitals. The participants completed questionnaire that included items related to demographics, personal and family history of cancers, history of breast surgery, menstrual and reproductive history, use of oral contraceptive and hormone replacement therapy (HRT), breast cancer diagnosis (cases only) and history of and motivation of attending mammography screening (controls) only. The participants provided a blood sample that was processed and stored.
Statistical analysis
To date, a total of 4,056 cases and 4,145 controls were recruited and interviewed. Only participants recruited before 1 January 2017 were included in this study. After removing duplicates, males and non-breast cancer cases, the remaining cohort consists of 3,683 cases and 3,980 controls.
Ever had breast surgery was defined as whether the participant had surgery for a benign lump or cyst in the breast. Women who had sisters/mothers/daughters with breast cancer were categorized as having a first-degree family history of breast cancer. Ever used oral contraceptives and HRT was defined as at least one month of usage. Post-menopausal status was defined as no menses for the past one year. The participants were categorized as parous if they had at least one full term pregnancy (live or still birth). BMI was calculated as dividing weight (kg) by the square of height (m). Soy products intake included the consumption of tofu, fermented soybeans, tofu pudding, and soy products other than soymilk. The participants reported their average duration of strenuous, moderate and gentle physical activity of three periods: childhood (before 18 years old), young adulthood (18–30 years), and the recent years. Weekly metabolic equivalent (MET)-hours were obtained by multiplying the corresponding MET value of each intensity of physical activity (7, 4, 3 for strenuous, moderate and gentle activities, respectively) with the average time spent on physical activity [15].
Cases and controls were compared using chi-square tests for categorical variables and t-tests for continuous variables. Unconditional logistic regressions were conducted to assess the association between risk factors and breast cancer, adjusting for potential confounders and other risk factors. The first models were adjusted for age, ethnicity, and hospital; for history of breast surgery, and anthropometric and lifestyle variables, the models were adjusted for age, ethnicity and education, and only participants from private hospital were included. In the second models, other breast cancer risk factors such as age of menarche, age of menopause, ever had full term pregnancy, first degree family history of breast cancer, and age of first full term pregnancy were added when appropriate. Conditional logistic regression using hospital-, ethnicity- and age- (±5 years) matched samples and unconditional logistic regressions stratified by pre- and post-menopausal status were also conducted. However, the results were similar to the unconditional and unstratified analysis thus they are not reported here.
The participants were categorized based on their year of birth into four birth cohorts: those born before 1949, between 1950–59, between 1960–69, and after 1969, and their breast cancer risk factors were compared across the birth cohorts. To compare across ethnicity, analysis of variances (ANOVAs) were conducted for continuous variables while chi-square tests were conducted for categorical variables. To determine whether there was a change of trend in the selected variables across birth cohorts, trend analyses were conducted by entering the birth cohort variable as continuous parameter in the regression models.
All analyses were conducted using R [16].
Results
Table 1 is the demographic comparisons of cases and controls. Controls were significantly older than cases, with mean ages of 54.0 years and 50.8 years, respectively (p<0.001) and significantly more controls had received secondary education. There were significantly more Chinese among the cases.
Table 1. Socio-demographic characteristics of 3,683 breast cancer cases and 3,980 controls in a hospital-based case-control study of breast cancer.
| Variables | Cases (N = 3,683) | Controls (N = 3,980) | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | <0.001 | ||||
| <45 | 1051 | 29.35 | 595 | 15.02 | |
| 45–54 | 1268 | 35.41 | 1556 | 39.27 | |
| 55–64 | 851 | 23.76 | 1297 | 32.74 | |
| >65 | 411 | 11.48 | 514 | 12.97 | |
| Ethnicity | <0.001 | ||||
| Chinese | 2481 | 68.74 | 2106 | 53.11 | |
| Indian | 436 | 12.08 | 860 | 21.69 | |
| Malay | 610 | 16.9 | 846 | 21.34 | |
| Other | 82 | 2.27 | 153 | 3.86 | |
| Education | <0.001 | ||||
| Primary or less | 214 | 17.16 | 308 | 8.31 | |
| Secondary | 556 | 44.59 | 2010 | 54.25 | |
| Tertiary | 477 | 38.25 | 1387 | 37.44 | |
| Monthly household income (RM) | 0.672 | ||||
| <5,000 | 703 | 57.67 | 2267 | 58.81 | |
| 5–10,000 | 319 | 26.17 | 1003 | 26.02 | |
| >10,000 | 197 | 16.16 | 585 | 15.18 | |
We conducted unconditional logistic regression to examine the association of clinical, exogenous hormonal, menstrual and reproductive factors with breast cancer (Table 2). Compared with those who had never had breast surgery, participants who had breast surgery to remove cysts and lumps were 2.3 times (95% CI = 1.82–2.83) more likely to develop breast cancer after adjusting for demographics and other risk factors. First-degree family history of breast cancer was associated with 19% increased risk of breast cancer after adjusting for demographics and other risk factors. Post-menopausal women had a 52% increased risk of breast cancer after adjusting for demographics and other risk factors. The use of oral contraceptives and HRT were not significantly associated with breast cancer risk after adjustment of other breast cancer risk factors.
Table 2. Clinical, exogenous hormonal, and menstrual and reproductive factors and their association with breast cancer.
| Variables | Cases/Controls | OR(95%CI)3 | OR(95%CI)4 |
|---|---|---|---|
| Ever had breast surgery | |||
| No | 972/1,807 | ||
| Yes | 249/214 | 2.42(1.96–3.00)*** | 2.27(1.82–2.83)*** |
| First degree family history of breast cancer | |||
| No | 3,170/3,478 | ||
| Yes | 513/502 | 1.10(0.96–1.27) | 1.19(1.02–1.38)* |
| Ever use oral contraceptives | |||
| Never | 2,452/2,806 | ||
| Ever | 935/1,145 | 1.02(0.91–1.14) | 0.99(0.88–1.11) |
| Ever use hormonal replacement therapy1 | |||
| Never | 419/1,452 | ||
| Ever | 51/259 | 0.52(0.44–0.61)*** | 0.48(0.4–0.58)*** |
| Age of menarche | |||
| ≤12 | 1,178/1,611 | ||
| >12 | 1,714/2,329 | 1.04(0.94–1.15) | 1.04(0.94–1.16) |
| Menopausal status | |||
| Pre/Peri-menopausal | 1,149/1,550 | ||
| Post-menopausal | 1771/2,408 | 1.53(1.33–1.76)*** | 1.52(1.32–1.75)*** |
| Age of menopause (years)1 | |||
| ≤50 | 217/862 | ||
| >50 | 246/822 | 0.86(0.71–1.03) | 0.89(0.73–1.08) |
| Ever had full term pregnancy | |||
| Nulliparous | 473/552 | ||
| Parous | 2,778/3,366 | 1.05(0.91–1.20) | 1.13(0.97–1.31) |
| Parity2 | |||
| 1 | 319/337 | ||
| 2 | 828/974 | 0.97(0.81–1.18) | 1.09(0.82–1.46) |
| 3 | 800/1,020 | 0.97(0.8–1.17) | 0.98(0.73–1.32) |
| 4 | 460/586 | 1.12(0.91–1.38) | 0.99(0.72–1.38) |
| ≥5 | 370/449 | 1.49(1.19–1.86)*** | 1.20(0.85–1.69) |
| Age of first full term pregnancy2 | |||
| <25 | 925/940 | ||
| 25–29 | 1,039/1,506 | 1.09(0.87–1.37) | 1.0(0.79–1.28) |
| ≥30 | 744/845 | 1.38(1.08–1.77)* | 1.29(0.99–1.67) |
| Ever breastfed2 | |||
| Never | 532/684 | ||
| Ever | 942/2,514 | 0.56(0.48–0.65)*** | 0.65(0.55–0.78)*** |
| Breastfeeding duration in months2 | |||
| None | 532/684 | ||
| ≤12 | 766/1,678 | 0.60(0.51–0.7)*** | 0.73(0.61–0.88)*** |
| >12 | 173/836 | 0.40(0.32–0.5)*** | 0.30(0.22–0.4)*** |
*p<0.05;
***p<0.001
1Natural menopause;
2Parous women only
3 Adjusted for age, ethnicity, and hospital, except history of breast surgery, which is adjusted for age, ethnicity, and education and only participants from private hospital are included
4Adjusted for age, ethnicity, hospital and breast cancer risk factors (age of menarche, menopausal status, ever had full term pregnancy, first degree family history of breast cancer, parity, age of first full term pregnancy, ever breastfed, breastfeeding duration when appropriate), except history of breast surgery, which is adjusted for age, ethnicity, education and relevant breast cancer risk factors, and only participants from private hospital are included
Of the menstrual and reproductive factors examined, breastfeeding had the strongest protective effect against breast cancer (Table 2). Among parous women, those who ever breastfed had 35% lower risk in the fully adjusted models; compared with those who did not breastfeed, the reduction of risk for those who breastfed between 1–12 months and those who breastfed more than 12 months was 30% and 70% respectively.
We also examined the association between anthropometric and lifestyle factors and breast cancer (Table 3). A higher BMI was associated with a lower risk of breast cancer; those who are overweight (BMI = 23.0–27.4kg/m2) had 33% reduced risk and those who are obese (BMI ≥ 27.5kg/m2) had 53% reduced risk, after controlling for other risk factors (Table 3). Those who consumed one cup or more soymilk per week and soy products once or more per week had 75% and 60% reduction in breast cancer risk, respectively. We did not find any significant association between smoking status and breast cancer. Women who drink less than 1 glass of alcohol per week and 1 glass per week or more had 55% and 48% reduced risk of breast cancer. It is noteworthy that the prevalence of those who reported alcohol intake in our cohort is low at 14%. A higher level of physical activity during childhood, young adulthood and recent period were also significantly associated with reduced risk of breast cancer before and after adjusting for other risk factors.
Table 3. Anthropometric and lifestyle factors and their association with breast cancer.
| Variables | Cases/Controls | OR(95%CI)1 | OR(95%CI)2 |
|---|---|---|---|
| Height | |||
| <1.53 | 224/399 | ||
| 1.53–1.57 | 401/666 | 1.10(0.88–1.37) | 1.07(0.84–1.35) |
| >1.57 | 589/950 | 1.08(0.88–1.34) | 1.06(0.84–1.33) |
| BMI | |||
| <23.0 | 654/757 | ||
| 23.0–27.4 | 388/758 | 0.68(0.57–0.81)*** | 0.67(0.56–0.81)*** |
| ≥27.5 | 165/493 | 0.53(0.43–0.67)*** | 0.47(0.37–0.61)*** |
| Ever smoked | |||
| Never | 1202/3,745 | ||
| Ever | 93/214 | 0.97(0.74–1.28) | 0.75(0.56–1.01) |
| Alcohol intake | |||
| Non-drinker | 1073/3,394 | ||
| Less than 1 glass per week | 98/279 | 0.71(0.55–0.91)** | 0.45(0.34–0.59)*** |
| 1 glass per week or more | 71/169 | 0.75(0.55–1.02) | 0.52(0.37–0.71)*** |
| Soy milk intake | |||
| None | 795/2362 | ||
| 1 cup per week or less | 382/1036 | 1.17(1–1.37)* | 1.24(1.03–1.49)* |
| 1 cup or more per week | 72/521 | 0.36(0.27–0.48)*** | 0.25(0.18–0.33)*** |
| Soy products intake | |||
| Once a week/less | 403/401 | ||
| Once a week or more | 614/1,476 | 0.39(0.33–0.47)*** | 0.40(0.33–0.48)*** |
| Physical activity, childhood (METS-hours/week) | |||
| <10 | 368/404 | ||
| 10–20 | 282/576 | 0.58(0.47–0.72)*** | 0.55(0.44–0.7)*** |
| >20 | 425/942 | 0.59(0.48–0.72)*** | 0.58(0.47–0.72)*** |
| Physical activity, mid-adulthood (METS-hours/week) | |||
| <10 | 524/518 | ||
| 10–20 | 307/639 | 0.52(0.43–0.63)*** | 0.50(0.41–0.61)*** |
| >20 | 243/785 | 0.35(0.29–0.43)*** | 0.35(0.29–0.44)*** |
| Physical activity, recent (METS-hours/week) | |||
| <10 | 518/706 | ||
| 10–20 | 407/759 | 0.73(0.61–0.87)*** | 0.72(0.59–0.87)*** |
| >20 | 168/490 | 0.45(0.36–0.56)*** | 0.42(0.33–0.53)*** |
*p<0.05;
**p<0.01;
***p<0.001
1Adjusted for age, ethnicity, and education. Only participants from private hospital were included
2Adjusted for age, ethnicity, education, and breast cancer risk factors (age of menarche, menopausal status, ever had full term pregnancy, first degree family history of breast cancer, parity, age of first full term pregnancy, ever breastfed, breastfeeding duration, ever had breast surgery when appropriate). Only participants from private hospital were included
Change of risk factors by birth cohorts
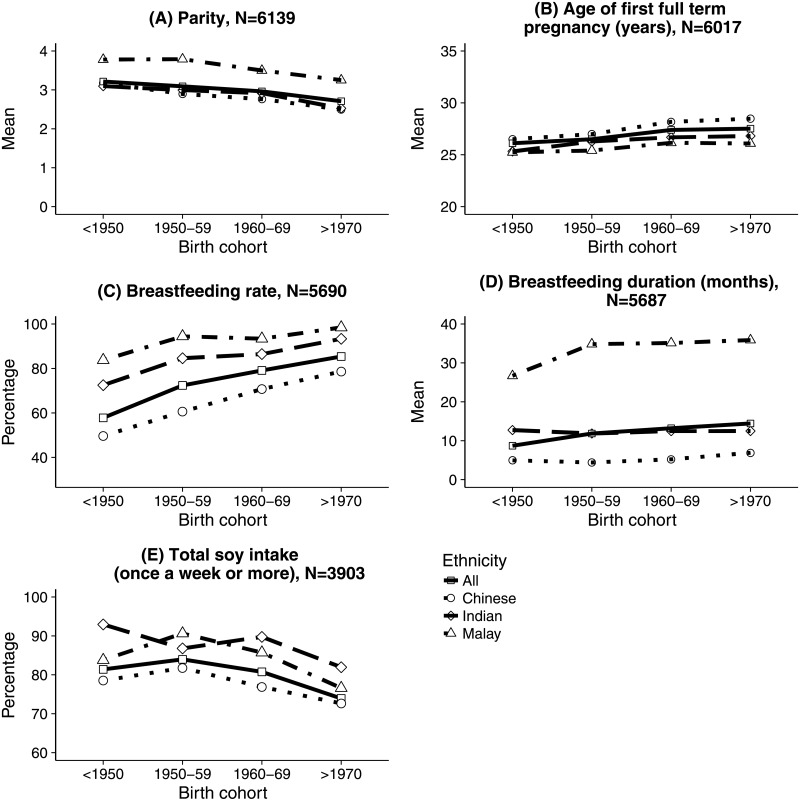
We examined the change of all risk factors across birth cohorts of both controls and cases in the three major ethnic groups in Malaysia and here we report the variables that had significantly changed across birth cohorts. Fig 1 showed the change of parity, age of first full term pregnancy, breastfeeding rate, breastfeeding duration and total soy intake. Compared with Indians and Malays, Chinese have the lowest parity, oldest age of first full term pregnancy, lowest breast feeding rate and shortest breastfeeding duration (p<0.001). All ethnic groups were experiencing significant reduction in parity (p<0.001 for all races) and significant increase of age of first full term pregnancy (p<0.001 for Chinese and p<0.001 for Malays and Indians) across birth cohorts.
Fig 1. Change of breast cancer risk factors across birth cohorts.
All ethnic groups had significant increase of breastfeeding rate across birth cohorts (p<0.001). The increase was more noticeable among Chinese; there was an increase from 50% among the oldest cohort to 79% among the youngest cohort. Only Chinese had a significant increase of breastfeeding duration across birth cohorts (p<0.001); however, breastfeeding duration among Chinese remained low compared with Malays and Indians.
There was a significant decrease of total soy intake among Chinese (p<0.001) and Malay (p<0.05) across birth cohorts. Compared with Malays and Indians, Chinese consumed significantly less soy products (p<0.001). However, the number of Malays and Indians who reported their intake of soy products were small compared with that of Chinese.
Discussion
In this hospital-based case-control study of 7,663 Malaysian women, we showed that a higher breastfeeding rate and duration, soy intake and level of physical activity were associated with a reduced risk of breast cancer among Southeast Asian women. Although Southeast Asian countries are experiencing a substantial increase in the burden of breast cancer, there have been limited studies in risk factors for breast cancer in these populations. Before the Malaysian Breast Cancer Genetic study, the previous largest study on breast cancer risk factors in Southeast Asia was from Indonesia and included 526 cases and 1,052 controls [17, 18]. A large scale prospective cohort study that followed 35,303 women in which 629 developed breast cancer has been conducted in Singapore, however, it focused mainly on soy intake and breast cancer risk and was limited to Chinese only [19]. Our current study included a large sample size and examined a wide range of breast cancer risk factors.
The strongest predictor of breast cancer in our study was breastfeeding, and the inverse association between breastfeeding and breast cancer risk is well documented [6, 7, 20–22]. Our study also showed an increasing trend of breastfeeding across birth cohorts in all ethnicity; however, among Chinese the breastfeeding rate and duration were still relatively low. The low breastfeeding rate and short breastfeeding duration may contribute to the highest breast cancer incidence (59.9/100,000) among Chinese in Malaysia compared with Indians (54.2/100,000) and Malays (34.9/100,000) [11]. Thus, the results of our study could be helpful in public health strategies to reduce risk of breast cancer through modifiable lifestyle choices including breastfeeding.
Our study also found that higher intake of soymilk and soy products is associated with lower risk of breast cancer. Soy is a major food in many parts of Asia and retrospective cross-sectional cohort studies in China and Japan show that increased soy protein intake is associated with reduced breast cancer risk in pre- and post-menopausal women [23, 24]. A study in Singapore showed that increased soy intake was significantly associated with reduced breast cancer risk among pre-menopausal women but not post-menopausal women [9] while another study in China found no significant association between soy protein intake and breast cancer risk. While there is some heterogeneity across these Asian studies, meta-analyses of observational studies in both Caucasian and Asian countries have consistently shown that high soy intake is associated to a lower risk of breast cancer, particularly among Asian women [25–30]. Given that our results shows declining soy intake across birth cohorts, future studies are required to confirm the benefit of soy in reducing population risk of breast cancer, as well as to also identify effective strategies to increase soy intake among Asian women, for whom a soy intervention may be an affordable and acceptable strategy for breast cancer prevention.
Another lifestyle factor that is shown to be associated with decreased breast cancer risk in our study is physical activity. This is consistent with the latest World Cancer Research Fund report which showed strong probable evidence that regular physical activity of various intensity decreases the risk of breast cancer among post-menopausal women while among pre-menopausal women, regular vigorous physical activity is associated with reduced risk [31]. A recent systematic review evaluated 80 studies and found that moderate-vigorous physical activity is associated with lower breast cancer risk among pre-menopausal (RR = 0.80, 95% CI = 0.74–0.87) and post-menopausal cohort studies (RR = 0.79, 95% CI = 0.76–0.84) [32]. Another systematic review that examined the dose-response between physical activity and major non-communicable diseases, which included breast cancer, found that compared with insufficiently active women, the reduction of risk of breast cancer among the low active, moderately active and highly active was 3%, 6% and 14% respectively [33]. Compared with other populations, Malaysian women have a higher prevalence of physical inactivity [34] and in our study there was no significant change of physical activity across birth cohorts. Thus, there is a need to construct innovative strategy to increase the level of physical activity in order to reduce future breast cancer risk.
In our study, two risk factors were associated with breast cancer risk in the contradictory direction. First, the consumption of alcohol was associated with a decreased risk of breast cancer in our study. The association of alcohol consumption with increased breast cancer risk has long been established [35]. However, in our study, only 6% reported an intake of more than 1 glass of alcohol per week, which is low compared with other populations. The second risk factor that had a contradictory association with breast cancer in our study was a higher BMI, which was associated with a lower risk of breast cancer after adjustment for major breast cancer risk factors. Past studies have shown that a higher BMI is associated with increased risk among post-menopausal women and reduced risk among pre-menopausal women [36]. However, when stratified by menopausal status, our analysis showed that a higher BMI was still significantly associated with lower breast cancer risk in both pre- and post-menopausal women. More studies need to be conducted among the Malaysian women to further explore the link between BMI and breast cancer risk.
In addition, our study did not find a significant association between parity, age of first full term pregnancy, age of menarche and menopause and breast cancer, which is inconsistent with other studies [2–5, 8, 10, 37–41]. Our study also found only a slight association between first-degree family history of breast cancer and increased risk of breast cancer risk, while other studies show that family history is strongly associated with increased breast cancer risk [20, 21, 39, 41–44].
Since this is a hospital-based case-control study rather than population-based, it might be subject to selection bias. The two hospitals where our participants were recruited were located in urban areas and rural Malaysian women were not included. However, it is noteworthy that these hospitals treat more than 10% of the breast cancer cases in Malaysia. The controls of our study were enriched for women who had a family history of breast cancer because they were participants of an opportunistic mammography screening programme.
In conclusion, our study shows that breastfeeding, soy intake and physical activity are modifiable risk factors for breast cancer; and with the increasing incidence of breast cancer there is an urgent need to educate the women about lifestyle intervention they can take to reduce their risk of breast cancer.
Supporting information
This file contains the questionnaire items used in this study.
(DOCX)
Acknowledgments
We want to thank Pui-Yoke Kwan, Norhashimah Hassan, Peter Choon-Eng Kang, In-Nee Kang, Kah-Nyin Lai, Hanis Hasmad, Jin-Tong Ng, Dr. Gaik-Theng Toh, Nancy Geen-See Tan, Dr. Suhaida Selamat, Dr. Rohaya Mohd Kasim, Dr. Malkit Kaur Dhillon, Dr. Thin-Chai Liu, Ernie Azwa, Hanani Che Halim, Leelavathy Krishnan, Don-Na Tan, Sweet-Lin Goh, Nur Naquiah Kamaruddin, Faridah Bakri, the participants of this study, and all staff at Cancer Research Malaysia, University Malaya, and Sime Darby Medical Centre who assisted in recruitment and interviews.
Data Availability
The data collected in this study are compliant with the Data Protection Act in Malaysia and can only be shared with research groups that contact Cancer Research Malaysia directly. All requests for data should be sent to Joanna Lim at the Data Access Committee of Cancer Research Malaysia using the following email address: genetics@cancerresearch.my.
Funding Statement
This study was supported by grants from Newton-Ungku Omar Fund [grant no: MR/P012930/1] and Wellcome Trust [grant no: v203477/Z/16/Z]. The Malaysian Breast Cancer Genetic Study was established using funds from the Malaysian Ministry of Science, and the Malaysian Ministry of Higher Education High Impact Research Grant [grant no: UM.C/HIR/MOHE/06]. The Malaysian Mammographic Density Study was established using funds raised through the Sime Darby LPGA tournament and the High Impact Research Grant. Additional funding was received from Yayasan Sime Darby, PETRONAS and other donors of Cancer Research Malaysia. The Newton-Ungku Omar Fund (grant no: MR/P012930/a), https://www.britishcouncil.my/programmes/newton-ungku-omar-fund was used to establish the cohort; Wellcome Trust (grant no: v203477/Z/16/Z), https://wellcome.ac.uk/funding, was used to establish the cohort; and Malaysian Ministry of Higher Education High Impact Research Grant (grant no: UM.C/HIR/MOHE/06, https://www.mohe.gov.my/en/initiatives-2/187-program-utama/penyelidikan/548-research-grants-information, was used to establish the cohort.
References
- 1.McPherson K, Steel C, Dixon J. ABC of breast diseases: breast cancer—epidemiology, risk factors, and genetics. Br Med J. 2000;321(7261):624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambe M, Hsieh C-C, Chan H-W, Ekbom A, Trichopoulos D, Adami H-O. Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Res Treat. 1996;38(3):305–11. [DOI] [PubMed] [Google Scholar]
- 3.Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23(38):6379–91. 10.1038/sj.onc.1207899 [DOI] [PubMed] [Google Scholar]
- 4.Titus-Ernstoff L, Longnecker MP, Newcomb PA, Dain B, Greenberg ER, Mittendorf R, et al. Menstrual factors in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prevent. 1998;7(9):783–9. [PubMed] [Google Scholar]
- 5.Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre-and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86(5):723–7. 10.1038/sj.bjc.6600124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao SC, Yu MC, Ross RK, Xiu KW. Risk factors for breast cancer in Chinese women of Beijing. Int J Cancer. 1988;42(4):495–8. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Ross R, Yu M, Ning J, Henderson B, Kimm H. A case-control study of breast cancer in Tianjin, China. Cancer Epidemiol Biomarkers Prevent. 1992;1(6):435–9. [PubMed] [Google Scholar]
- 8.Hu YH, Nagata C, Shimizu H, Kaneda N, Kashiki Y. Association of body mass index, physical activity, and reproductive histories with breast cancer: a case-control study in Gifu, Japan. Breast Cancer Res Treat. 1997;43(1):65–72. [DOI] [PubMed] [Google Scholar]
- 9.Lee HP, Gourley L, Duffy SW, Estève J, Lee J, Day NE. Risk factors for breast cancer by age and menopausal status: a case-control study in Singapore. CCC. 1992;3(4):313–22. [DOI] [PubMed] [Google Scholar]
- 10.Ng EH, Gao F, Ji CY, Ho GH, Soo KC. Risk factors for breast carcinoma in Singaporean Chinese women. Cancer. 1997;80(4):725–31. [DOI] [PubMed] [Google Scholar]
- 11.Lim GCC, Rampal S, Yahaya H. Cancer incidence in Peninsular Malaysia, 2003–2005: The Third Report of the National Cancer Registry, Malaysia: National Cancer Registry; 2008.
- 12.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 13.Popkin BM. Urbanization, lifestyle changes and the nutrition transition. World Dev. 1999;27(11):1905–16. [Google Scholar]
- 14.Yip CH, Taib N, Mohamed I. Epidemiology of breast cancer in Malaysia. Asia Pac Popul J. 2006;7(3):369–74. [PubMed] [Google Scholar]
- 15.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr., Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Medicine and science in sports and exercise. 2011;43(8):1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria; 2016. [Google Scholar]
- 17.Cornain S, Ohno Y, Prihartono J, Sakamoto G, Tjahjadi G, Tjindarbumi D, et al. Similar and dissimilar findings in Japan-Indonesia case-control study on breast cancer: two phases study. J Epidemiol. 1996;6(4sup):175–80. [Google Scholar]
- 18.Budiningsih S, Ohno Y, Prihartono J, Ramli M, Wakai K, Cornain S, et al. Epidemiological analysis of risk factors for breast cancer in Indonesian females. Med J Indonesia. 1995;4(3):163–8. [Google Scholar]
- 19.Wu A, Koh W, Wang R, Lee H, Yu M. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer. 2008;99(1):196–200. 10.1038/sj.bjc.6604448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matalqah L, Radaideh K, Yusoff ZM, Awaisu A. Predictors of breast cancer among women in a northern state of Malaysia: a matched case-control study. Asian Pac J Cancer Prev. 2011;12(6):1549–53. [PubMed] [Google Scholar]
- 21.Hejar A, Chong F, Rosnan H, Zailina H. Breast cancer and lifestyle risks among Chinese women in the Klang Valley in 2001. The Med Malaysia. 2004;59(2):226–32. [PubMed] [Google Scholar]
- 22.Kamarudin R, Shah SA, Hidayah N. Lifestyle factors and breast cancer: a case-control study in Kuala Lumpur, Malaysia. Asian Pac J Cancer Prev. 2006;7(1):51–4. [PubMed] [Google Scholar]
- 23.Dai Q, Shu X, Jin F, Potter J, Kushi L, Teas J, et al. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br J Cancer. 2001;85(3):372–8. 10.1054/bjoc.2001.1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95(12):906–13. [DOI] [PubMed] [Google Scholar]
- 25.Wu A, Yu M, Tseng C, Pike M. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98(1):9–14. 10.1038/sj.bjc.6604145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, et al. Association between soy isoflavone intake and breast cancer risk for pre-and post-menopausal women: a meta-analysis of epidemiological studies. PloS one. 2014;9(2):e89288 10.1371/journal.pone.0089288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong J-Y, Qin L-Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125(2):315–23. 10.1007/s10549-010-1270-8 [DOI] [PubMed] [Google Scholar]
- 28.Nagata C, Mizoue T, Tanaka K, Tsuji I, Tamakoshi A, Matsuo K, et al. Soy intake and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2014;44(3):282–95. 10.1093/jjco/hyt203 [DOI] [PubMed] [Google Scholar]
- 29.Mourouti N, Kontogianni MD, Papavagelis C, Panagiotakos DB. Diet and breast cancer: a systematic review. Int J Food Sci Nutr. 2015;66(1):1–42. 10.3109/09637486.2014.950207 [DOI] [PubMed] [Google Scholar]
- 30.Fritz H, Seely D, Flower G, Skidmore B, Fernandes R, Vadeboncoeur S, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PloS One. 2013;8(11):e81968 10.1371/journal.pone.0081968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Cancer Research Fund International. Diet, Nutrition, Physical Activity and Breast Cancer. London: American Institute for Cancer Research; 2017. [Google Scholar]
- 32.Neilson HK, Farris MS, Stone CR, Vaska MM, Brenner DR, Friedenreich CM. Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: a systematic review and meta-analysis. Menopause. 2017;24(3):322–44. 10.1097/GME.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 33.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857 10.1136/bmj.i3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–57. 10.1016/S0140-6736(12)60646-1 [DOI] [PubMed] [Google Scholar]
- 35.Key J, Hodgson S, Omar RZ, Jensen TK, Thompson SG, Boobis AR, et al. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes & Control. 2006;17(6):759–70. [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. J Amer Med Assoc. 1997;278(17):1407–11. [PubMed] [Google Scholar]
- 37.Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, Wen W, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer. 2000;87(2):295–300. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer. 1990;46(5):796–800. [DOI] [PubMed] [Google Scholar]
- 39.Razif SM, Sulaiman S, Hanie SS, Aina EN, Rohaizak M, Fuad I, et al. The contribution of reproductive factors and family history towards premenopausal breast cancer risk in Kuala Lumpur, Malaysia. Med J Malaysia. 2011;66(3):220–6. [PubMed] [Google Scholar]
- 40.Rejali L, Jaafar MH, Ismail NH. Serum selenium level and other risk factors for breast cancer among patients in a Malaysian hospital. Environ Health Prev Med. 2007;12(3):105–10. 10.1007/BF02898024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norsa adah B, Rusli B, Imran A, Naing I, Winn T. Risk factors of breast cancer in women in Kelantan, Malaysia. Singapore Med J. 2005;46(12):698–705. [PubMed] [Google Scholar]
- 42.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997;71(5):800–9. [DOI] [PubMed] [Google Scholar]
- 43.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet. 2001;358(9291):1389–99. 10.1016/S0140-6736(01)06524-2 [DOI] [PubMed] [Google Scholar]
- 44.Colditz GA, Willett WC, Hunter DJ, Stampfer MJ, Manson JE, Hennekens CH, et al. Family history, age, and risk of breast cancer: prospective data from the Nurses’ Health Study. J Amer Med Assoc. 1993;270(3):338–43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains the questionnaire items used in this study.
(DOCX)
Data Availability Statement
The data collected in this study are compliant with the Data Protection Act in Malaysia and can only be shared with research groups that contact Cancer Research Malaysia directly. All requests for data should be sent to Joanna Lim at the Data Access Committee of Cancer Research Malaysia using the following email address: genetics@cancerresearch.my.