Abstract
Habitat-forming species sustain biodiversity and ecosystem functioning in harsh environments through the amelioration of physical stress. Nonetheless, their role in shaping patterns of species distribution under future climate scenarios is generally overlooked. Focusing on coastal systems, we assess how habitat-forming species can influence the ability of stress-sensitive species to exhibit plastic responses, adapt to novel environmental conditions, or track suitable climates. Here, we argue that habitat-former populations could be managed as a nature-based solution against climate-driven loss of biodiversity. Drawing from different ecological and biological disciplines, we identify a series of actions to sustain the resilience of marine habitat-forming species to climate change, as well as their effectiveness and reliability in rescuing stress-sensitive species from increasingly adverse environmental conditions.
Positive species interactions under climate change
Anthropogenic climate change is causing unprecedented alterations to Earth’s ecosystems [1,2]. Modifications in species distribution and abundance as a consequence of altered environmental conditions can be the direct result of physiological and/or phenological responses [3]. More often, climate-induced modifications in individual physiology, phenology, and behavior scale up to the community level through the filter of species interactions [4]. Nonetheless, species interactions are still seldom incorporated into models aiming to forecast species distribution under future climate scenarios [5,6].
Although terrestrial and marine studies have started addressing the effects of climate change on the balance between negative and positive species interactions (Box 1) [7–11], the role of habitat-formers (Box 1) in shaping future patterns of species distribution is yet to be fully explored. This is at odds with compelling evidence showing that habitat-formers frequently facilitate other species in otherwise hostile environments [5,8,12–15] and can enhance conservation and restoration success [16–18]. Habitat-formers have allowed species to persist under dramatic changes in climate in the past and acted as important evolutionary forces. For instance, environmental stress amelioration by canopy-forming Quaternary plants has allowed Tertiary plant lineages adapted to moist conditions to persist despite the onset of an unfavorable climate [19]. Indeed, biogenic modification of abiotic conditions (Box 1) underpins pivotal chapters in the evolution of life on Earth; in the Cambrian Period, the development of biomineralised skeletons (e.g., trilobites and other arthropods), a response to the advent of predation, caused reworking and oxygenation of ocean sediments (i.e., the burrowing revolution), giving rise to the ancestors of many modern groups of animals [20]. Milder conditions due to warming may reduce the reliance of extant species on habitat-formers in some extreme environments, such as alpine and arctic tundra [7]. There is, however, indisputable evidence that increasingly harsher physical conditions are a major driver of the current biodiversity crisis across ecosystems on Earth [1,2], suggesting that the importance of physical stress amelioration by habitat-formers is set to increase under future climate scenarios.
Box 1. Glossary
Positive species interactions: Interactions among species, also referred to as facilitative interactions or facilitation, in which at least one of the participants benefits from the presence of the other, while neither is disadvantaged. These include interactions between coevolved, mutually obligate organisms as well as looser, facultative interactions between species that did not coevolve.
Habitat-former: A species able to support the persistence of other species by providing suitable environmental conditions, enhancing the availability of or access to limiting resources, or reducing the effects of negative species interactions, such as competition, predation, and diseases. Habitat-formers include ecosystem engineers, which are defined as organisms that affect other species through the creation, modification, and maintenance of habitat. Biotic and abiotic conditions are not necessarily optimal (relative to other habitats) for all the species found in the presence of a habitat-former.
Biogenic modification of environmental conditions: Modification of environmental conditions operated by a living organism (i.e., a habitat-former). Similarly, biogenic amelioration or buffering of environmental stress refers to the case in which the presence of a living organism reduces the intensity of stressful environmental conditions for other species.
Biogenic refugia: Habitats formed by living organisms and of limited spatial extent that allow other species to escape adverse environmental or biological conditions and from which they can subsequently expand when suitability of external conditions is restored.
Benefactor and beneficiary species: The benefactor is a species able to deliver benefits to other species, defined as beneficiary species. A species may behave as a benefactor under some environmental conditions or resource availability levels but not under others. For example, an intertidal canopy-forming macroalga (i.e., the benefactor) can benefit understory species (i.e., the beneficiaries), reducing heat and desiccation at high-shore levels. By contrast, it can negatively influence understory species lower on the shore, where heat and desiccation stress are less severe.
Epigenetic mechanisms: Mechanisms that form the basis of the dynamic regulation of gene expression through chromatin remodeling, DNA methylation, noncoding RNA-associated genes, and histone modification. Epigenetic changes can be inherited but do not involve changes in the underlying DNA sequence.
Assortative mating: Nonrandom mating model in which the frequency of mating between individuals with a similar genotype and/or phenotype is higher than that expected by chance.
Climate rescuer: A habitat-former resistant/resilient to climate change providing suitable environmental conditions to species that would otherwise be unable to maintain viable populations under future climate scenarios.
Habitat-formers are key in shaping community structure and ecosystem functioning in marine environments through both local and long-distance positive interactions that extend across coastal landscapes [12,13,21]. In transitional and shallow-water environments, the habitat-former concept has traditionally been applied to sessile species, such as mangroves, salt-marsh plants, seagrasses, macroalgae, bivalves, and corals [22] (Fig 1A–1E). However, mobile species that modify the characteristics of sediments through their burrowing or feeding activity (i.e., bioturbators, Fig 1F), such as holothurians, crustaceans, and polychaetes, could play a similar role from tidal flats to abyssal plains [23]. Here, we assess the circumstances under which biogenic amelioration of environmental stress may sustain coastal biodiversity and ecosystem functioning in the face of climate change and, hence, be used as a nature-based solution for coastal conservation and restoration.
Fig 1. Habitat-formers in intertidal and subtidal environments.
(A) Clumps of the mussel Mytilus edulis on a tidal flat in the Wadden Sea, the Netherlands (Photo credit: B.K.E. Eriksson); (B) mangrove trees of the species Avicennia marina along the central coasts of the Red Sea (Photo credit: T. Dailianis); (C) fronds of the brown seaweed Fucus vesiculosus at low tide on a rocky shore of the Iberian Peninsula (Photo credit: E. Serrão); (D) the seagrass Posidonia oceanica in shallow waters of Crete in the Aegean Sea (Photo credit: T. Dailianis); (E) multi-specific canopy stands formed by the brown seaweeds Cystoseira barbata, C. compressa, and C. crinita on shallow rocky reefs of Croatia in the northeast Adriatic Sea (Photo credit: L. Iveša); (F) burrowing by the sea cucumber Holothuria scabra exposes anoxic sediments on a reef flat in Fiji (Photo credit: S. Lee).
Biogenic refugia against climate change
Biogenic buffering of environmental stress has been documented in harsh, transitional habitats, such as intertidal rocky and sandy shores, mudflats, and salt marshes [12]. For example, intertidal macroalgal canopies or mussels beds reduce heat and desiccation stress during emersion, sustaining diversity and productivity of benthic communities [24,25]. However, while the role of geomorphological refugia for species persistence in the face of past and current changes in climate is recognized [26], that of biogenic refugia (Box 1) remains unexplored. Benefactors (Box 1) may provide climatically suitable habitat for stress-susceptible species, increasing their survival during acute climate-driven disturbance events, such as heatwaves or sea storms. For example, intertidal mussel clumps enhance cordgrass survival during severe drought events and function as nuclei for vegetative recovery in the aftermath [11]. In subtidal environments, macrophyte photosynthetic activity buffers calcifying organisms from ocean acidification by increasing pH [27,28]. Daily uptake of carbon dioxide (CO2) by plants increases pH within the surrounding diffusive boundary layer, and these effects can scale up to adjacent habitats, such as stony corals or mussel beds [28,29]. Subtidal canopies also attenuate wave action and, at shallow depths, light stress [30,31]. Below the sediment surface, biogenic activity can reduce the impacts of seasonal hypoxia driven by heatwaves. Seawater flushing and particle mixing by large burrowing marine invertebrates (i.e., bioturbation and bioirrigation) facilitate oxygenation of sedimentary pore water spaces and the burial of organic matter, ameliorating biogeochemical conditions within sediments [32,33]. Indeed, reduction of physical stress by bioturbators (e.g., temperature-driven hypoxia) may explain why the proportion of benthic species on soft sediments shifting their trailing edge at the pace predicted by seawater warming rates is lower than expected [34].
Facilitation can expand the distribution of beneficiary species beyond the range predicted from their physiological tolerance matrices [35–37]. The magnitude of the biogenic reduction of thermal stress may exceed—by far—the increment expected under warming climates. For example, intertidal canopies of the seaweed Ascophyllum nodosum reduced summer maximum rock temperatures in New England by as much as approximately 8 °C [24], and mussels and algal turfs ameliorated lethal and sublethal thermal stress over 14° of latitude [35].
Reliance of beneficiaries on biogenic amelioration of environmental conditions may increase under future climates, at least until beneficiary species possibly adapt to the new conditions. Thus, a large proportion of species in a community might become obligate associates with habitat-formers. The survival of beneficiary species would depend, first, upon the spatial and temporal extent of the biogenic refugia and, second, their fitness therein. Refugia might be too small to allow beneficiaries to maintain viable populations. In addition, life in biogenic habitats can entail costs due to competition either with the benefactor itself or other associated species [37].
Adapt, move, or perish: The role of biogenic habitat
A species that is currently neither resistant (unaffected) nor resilient (able to recover) to climate change must either adapt or move to persist. Can habitat-formers influence the mechanisms underpinning species potential to i) exhibit plastic responses, ii) genetically adapt to novel environmental conditions, or iii) track suitable climates?
-
i)
Pre-existing phenotypic plasticity, allowing individuals to acclimate, may sustain short-term population persistence before evolutionary adaptation can take place [3]. Rapid adaptation to novel environmental conditions through the activation of alternative metabolic pathways or the modification of gene expression levels by epigenetic mechanisms (Box 1) has been demonstrated in marine organisms [38,39]. Acclimation can also influence subsequent generations, and biogenic habitats may facilitate species acclimation via developmental or transgenerational plasticity exposing individuals to sublethal temperatures during extreme events, such as heatwaves [40, 41].
-
ii)
Adaptation to changing climate by selection of individual traits across generations can require time, especially for long-lived organisms. Body mass, reproduction type (e.g., sexual versus vegetative), and generation time influence local adaptation rates [42]. By virtue of their smaller body mass and shorter generation times, adaptation can be expected to be generally more rapid in beneficiary species than in habitat-formers. However, given that current climate-driven changes may modify marine habitats at rates fast exceeding the potential for adaptive, genetic change within populations, habitat-formers may buy population persistence time for stress-sensitive species. The evolutionary potential of positive interactions remains unquantified [43], but small-scale variation in the intensity of negative biotic interactions (e.g., predation) has been shown to promote rapid adaptive differentiation [44].
Several lines of argument do suggest that biogenic habitats may influence fine-scale genetic structures of associated species. First, at the seascape scale, patches of habitat-formers alternating with open surfaces—a common configuration of transitional coastal environments—increase spatial heterogeneity in selective pressures, thus sustaining genetic polymorphism. This may explain the inverted dominance of two alleles found homozygous in barnacles living in exposed sites versus underneath a canopy-forming macroalga [45]. Second, enhanced aggregation of individuals seeking shelter in biogenic habitats, in association with limited dispersal and occurrence of within-habitat environmental gradients [10], can influence genetic structuring through isolation by distance [46]. Third, habitat-formers can elicit phenotypic variations in beneficiary species that, when involving reproductive traits, may enhance fine-scale genetic structuring through assortative mating (Box 1) [46,47]. Biogenic enhancement of genetic variation would be particularly important in populations at range edges since they may have lower genetic variability compared to central populations [48].
-
iii)
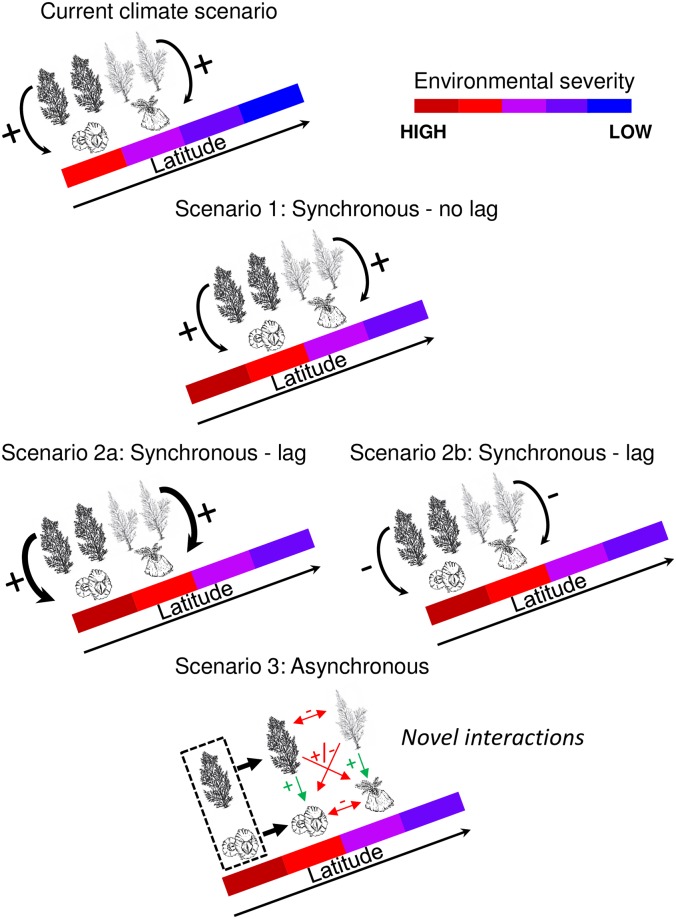
Under lethal climate-driven stress, the synchrony of migration capacities determines species interaction outcomes at the leading edge of range shifts. Three different scenarios describe how climate change can alter species interactions [49]. In the first, all the species within a community migrate synchronously to track climate change without noteworthy modification of the interaction environment. Thus, facilitative effects of habitat-formers could be maintained in newly colonized areas (Fig 2, scenario 1).
Fig 2. Alternative scenarios of interaction between benefactor and beneficiary species after climate change.
Under the current climate, southern and northern canopy-forming macroalgae facilitate different species of barnacles (in the northern hemisphere, in this example). Under scenario 1, species migrate synchronously to track a suitable climate, resulting in no significant modifications of the interacting environment and no generation of novel interactions; extant positive interactions are maintained. Under scenario 2, all species exhibit the same time lag in migration and interact in harsher environmental conditions, resulting in either (a) a strengthening of positive interactions or (b), in the case in which levels of stress become excessive, in the collapse of facilitation. Under scenario 3, species migration is asynchronous, generating novel interactions. In this example, southern species migrate poleward and start interacting with extant, nonmigrating species. Positive interactions between each original pair of canopy-forming macroalgae and barnacles are likely to be maintained (green arrows). Novel interactions (red arrows) between canopy-formers and barnacles can be either positive or negative, while novel interactions between canopy-formers and between barnacles are likely to be negative.
In the second scenario, all species exhibit the same migration lag, thus interacting under changing environmental conditions. Enhanced levels of environmental stress may increase the frequency and/or intensity of positive interactions [15] (Fig 2, scenario 2a). For example, along the east coast of the United States, intertidal macroalgal canopies fostered cirriped survival at thermally stressful southern sites [24]. By contrast, at northern cooler sites, benefits were overridden by increased whelk predation. Progressive warming may strengthen stress mitigation benefits, shifting the net effect of canopies from negative to positive also at northern sites. Alternatively, facilitation may collapse if environmental stress becomes extreme and impairs the ability of the benefactor to deliver benefits [50] (Fig 2, scenario 2b).
In the third scenario, some species migrate toward cooler climates and start interacting with resident, nonmigrating species (Fig 2, scenario 3). Such novel interactions can be either positive or negative. Recruitment through seeds, spores, or larvae represents a critical stage of range shifts. Juvenile stages are often less tolerant to stressful conditions than adults, and biogenic stress amelioration might be crucial to enable their recruitment outside their current distributional range. For instance, on the east coast of the United States, salt marsh vegetation facilitates recruitment of the black mangrove Avicennia germinans at its northward distributional limit [51]. Positive effects do not necessarily stem from environmental stress reduction but might be generated by alleviation of resource limitation, competition, or predation pressure. For example, reefs formed by the Pacific oyster north of its former range provide native mussels with shelter from crab predation [52].
What makes a habitat-former a climate rescuer species?
Ecosystem-wide effects of environmental stress buffering
The first requisite of a climate rescuer (Box 1) is the ability to sustain biodiversity and ecosystem functioning through stress alleviation (Fig 3). This effect is not limited to temperature or desiccation but extends to other climate-related stressors, such as ocean acidification, hypoxia, increased UV radiation, and changing hydrodynamic regimes. Ideally, positive effects should not be limited to single habitats but should propagate to other ecosystems. Primary habitat-formers can provide substrates for other habitat-formers (facilitation cascades: [53]) or promote other species across the landscape (habitat cascades: [54]) through long-distance interactions [55]. Within this context, stress-tolerant species that facilitate other species both within and across habitats should be considered standout climate rescuers.
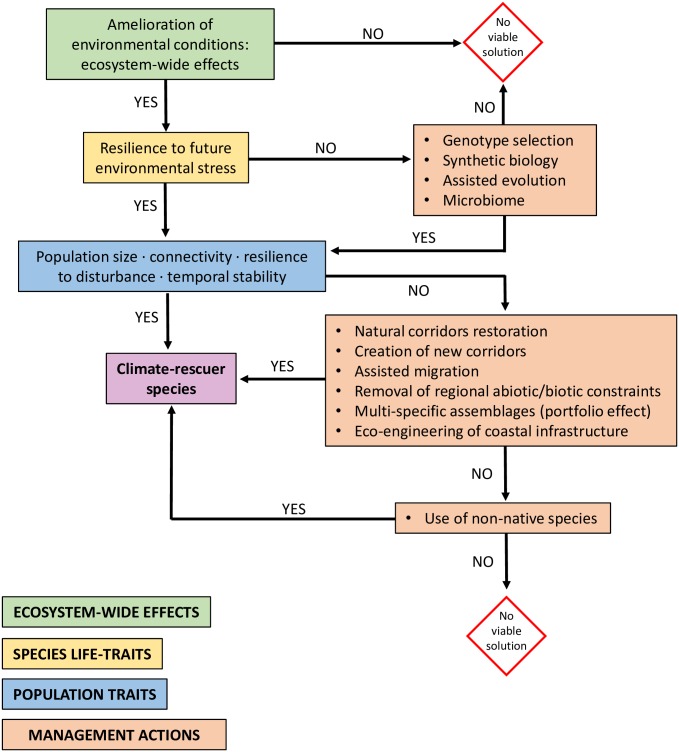
Fig 3. Climate rescuer identification and management.
The diagram describes sequential steps toward the identification of a climate rescuer species and possible management actions aimed to sustain i) life traits underpinning its resistance to future environmental stress and ii) population traits that determine the strength and reliability of its positive effects on stress-sensitive species.
Resilient morphology and phenology under changing climates
Climate rescuer species should be able to persist in increasingly stressful environments without facing morphological or phenological modifications that undermine their facilitative functionality (Fig 3). Climate rescuing would be supported if the benefactor can withstand a greater magnitude of change in a given climate-driven stressor than its beneficiaries whilst still sustaining function. Thus, the success of the benefactor–beneficiary relationship hinges on the relationship between the (climate) response traits of the benefactor relative to its ability to express the (ameliorating) functional effect trait supporting the beneficiary under a changing environment.
Functional effect traits of a habitat-former are often related to morphology and can be altered by climate change. Known changes in species morphology associated with climate changes include reduced average body size in ectotherms [56]. Likewise, calcifying organisms, including important habitat-formers such as bivalves, may reduce their growth to compensate for increased metabolic costs incurred in acidified seawater [57]. Reduced size may confer weaker ability to deliver benefits to other species. Calcifiers may also experience changes in the chemical makeup of their shells under ocean acidification and warming. This may render them less structurally robust to physical forcing, reducing their ability to serve as anchoring structures for marine diversity [58].
Modifications in phenology may also reduce stress-buffering capacity. For example, on the coasts of British Columbia, experimental warming delayed the development of annual intertidal algae [59], potentially exposing associated species to desiccation and heat stress during spring low tides.
Viable populations under changing climates
The ability of a habitat-former to maintain viable populations at the edges of its distribution or within warming hotspots determines its potential to act as a critical refugium (Fig 3). In some cases, habitat-former populations have collapsed at the warmer limit of their distribution [60,61]. In other cases, poleward shifts have occurred without changes at the equatorial range edge. For example, reduced risk of winter freezing has promoted poleward migration of some mangroves at the expense of salt marshes but with no significant equatorial edge contraction [62]. In the southern hemisphere, tropical corals and seagrasses have expanded toward higher latitudes without modifying their northernmost boundaries [63,64].
In addition, climate rescuer populations should not undergo thinning during hot seasons or extreme atmospheric events (i.e., exhibit large temporal fluctuations) because they might become too sparse to buffer environmental stress. Since habitat modification is often density dependent [65], assessing whether there is a minimal (threshold) population density or size that is needed for benefits to accrue seems crucial.
Active management of habitat-formers to mitigate biodiversity loss
By virtue of their potential to ameliorate environmental stress, habitat-former populations could be managed as a tool against climate-driven loss of biodiversity (Fig 3). Major threats to marine habitat-formers and approaches to their conservation have been thoroughly reviewed elsewhere [66] and will not be reiterated here. Instead, we outline a number of actions to sustain habitat-formers facing novel climatic conditions as well as population traits enhancing their effectiveness and reliability in rescuing stress-sensitive species.
Enhancing habitat-former tolerance to novel climatic conditions
Genotype selection
Persistence of target habitat-former populations can be enhanced by selecting stress-tolerant genotypes. Genetic variation in traits relevant under global change seems high in coastal biota [67], and novel quantitative genetic analyses can provide accurate estimates of persistence probability of wild populations [68]. High genetic variation occurs among populations with reduced gene flow but also within the same population. For example, resilience to heatwaves differs between shallow and deep genotypes of the same populations of the seagrass Posidonia oceanica [39]. If this is caused by inherited genetic adaptation rather than acclimation to different developmental depths, then assisted relocation of such stress-tolerant genotypes—reared either in the lab or in the field—could rescue declining populations and enhance subpopulation connectivity.
Synthetic biology
Although its application in the field of conservation is still in its infancy, synthetic biology is moving fast and may represent a strategic tool under future climates if accompanied by thorough risk-assessment and complying with environmental ethics [69]. Organisms have been genetically modified to enhance their resistance to biotic (e.g., disease) and abiotic (e.g., drought, salinity, heat) stressors, both in terrestrial and marine environments [70,71]. Gene editing of a single habitat-forming species may indirectly enhance the persistence of an entire suite of stress-susceptible species under adverse climates. The molecular basis for tolerance to environmental stress has been identified in key habitat-forming species, such as oysters and corals [72,73]. New genome editing techniques, such as CRISPR/Cas9, may rapidly advance this field.
Assisted evolution
Tolerance to stress can be enhanced through human-assisted acceleration of natural processes [74]. Short-term variance in biotic or abiotic pressures is critical to build stress tolerance [75]. For example, rapid fluctuations between benign and severe conditions accelerated adaptation to warming in the marine diatom Thalassiosira pseudonana, since population size expansion during favorable periods increased the probability of fixing beneficial mutations [76]. Thus, controlled alternation of high- and low-stress phases in mesocosms—climate incubators—may act as an accelerator for adaptation to climate change, as high-stress phases cause selective mortality of sensitive genotypes, while stress relaxation phases allow surviving genotypes to recover and, possibly, reproduce [77].
The microbiome
Microbial symbionts influence host physiology, behavior, and resistance to disease [78]. High genetic diversity and fast generational turnover of symbionts can allow rapid adaptation to novel climatic conditions, potentially raising host fitness [79]. Laboratory thermal selection could expand the temperature tolerance range of the coral-dinoflagellate Symbiodinium after approximately 80 asexual generations, corresponding to just two and a half years [80]. Although the mechanisms regulating property transfer from the microbiome to the host (i.e., emergence of stress tolerance at the holobiont level) are yet to be fully understood, assisted microbiome evolution might be a formidable tool for raising habitat-former tolerance to novel climatic conditions.
Enhancing habitat-former population traits under novel climatic conditions
Conservation biology
By drawing on conservation and restoration knowledge, population viability of potential climate rescuers can be actively sustained (Fig 3). Habitat-former population size and resilience can be enhanced by supporting connectivity through protection of source populations, restoration of natural migration corridors, or the creation of new ones [81]. In some cases, managed relocation (or assisted migration) of habitat-formers at strategic sites might enhance connectivity among their populations as well as among populations of beneficiary species. Likewise, herbivore release from predation can result in the overgrazing of habitat-forming macrophytes, and trophic cascade restoration could be necessary to foster their persistence [82].
Mitigation of other anthropogenic stressors
Control of local/regional anthropogenic perturbations potentially exacerbating the impact of climate stressors will likely enhance habitat-former population resilience to climate and nonclimate stressors [66,83]. For example, removal of excess nutrients enhances the tolerance of canopy-forming macroalgae to increased temperature [83].
Biodiversity
A large body of literature suggests a positive relationship between biodiversity and both resilience and temporal stability [84]. Thus, promoting multispecies assemblages of habitat-formers that are, to some degree, functionally interchangeable, may increase the reliability of their positive effects on other species under changing environmental conditions. In addition, greater microhabitat availability in multispecies assemblages of habitat-formers may enhance the coexistence among beneficiary species and, hence, broaden the number of species sheltered from adverse climatic conditions [84]. When desirable, the formation and maintenance of multispecies assemblages could be pursued through active control of competitively dominant species that would otherwise form monospecific stands or through the seeding of subordinate species. Similar actions could be implemented to enhance genotype diversity, although they would require better understanding of competitive hierarchies between clonal genotypes.
Ecoengineering
Maritime infrastructures, off-shore installations, and hard coastal defences (breakwaters, seawalls) significantly change species distribution and ecological connectivity [85]. Ecoengineering designs of artificial habitats including conservation or restoration objectives have the potential to turn these changes into an opportunity to sustain climate rescuer populations by supplying suitable habitats or providing new dispersal routes facilitating their migrations and that of beneficiary species. As previously demonstrated in the fields of restoration and conservation [16,17], engineering man-made structures for sustaining target habitat-forming species would be sufficient for attracting a suite of facultative and obligate associated species and represents, therefore, a cost-effective approach.
Non-native species
Where native habitat-formers are lacking, non-native species might be considered as alternative climate rescuers, as they may revitalize functionalities that would be otherwise lost, including the support of diverse communities and the provision of climate refuges. The use of non-native species in conservation is still highly debated, but in extreme cases, they may be the only chance of avoiding massive species loss when key habitat-formers decline due to global and regional human-driven changes (Box 2).
Box 2. The role of non-native species as climate rescuers
The view that all non-native species represent a threat to native biodiversity has been challenged on the grounds that some of them cause no harm and can contribute to achieving conservation and restoration goals [86,87].
Climate change is predicted to foster invasions via enhanced propagule dispersal and decreased biotic resistance of native communities [86,88]. In addition, poleward shifts of coastal species have been documented throughout the globe [88]. By virtue of their better adaptation to novel climate conditions, non-native species may be the primary cause of native species decline or local extinction. On the other hand, non-natives may replace natives when they decline as a consequence of other anthropogenic stressors. Although the effects of non-native habitat-formers on marine biodiversity are often complex and variable [89,90], there are examples of non-native species compensating, to some extent, for native habitat-former loss. For example, in areas of Chesapeake Bay where native eelgrass beds have retreated, the macroalga Gracilaria vermiculophylla provides suitable habitat for the native blue crab Callinectes sapidus, a highly valued recreational and commercial species [91]. Positive effects of non-native habitat-formers can scale up to whole communities and influence ecosystem functioning. For example, long-term bioirrigation by the non-native polychaetes Marenzelleria spp. alleviates soft-sediment hypoxia in the Baltic Sea [92]. Likewise, the non-native seaweed Sargassum muticum confers benthic assemblages greater resistance to warming and acidification than native macroalgal canopies [93].
Of course, the benefits and risks of using non-native species as climate rescuers do not differ from those already described for restoration or conservation practice [94]. Many aspects of biological invasions, including their perception and management, are still highly controversial [95,96]. By no means do we negate the capacity of non-native species to alter native biodiversity and to impair ecosystem functioning; rather, we suggest that their potential to rescue native species from changing climates should not be discarded a priori but benefits and risks fully evaluated on a case-by-case basis.
Concluding remarks
Amelioration of physical stress by habitat-formers sustains species persistence in harsh environments [14,15]. This service might become increasingly important under future climates. The potential of habitat-formers to act as climate rescuers relies on their ability to maintain key individual and population traits in the face of climate changes. Likewise, the strength of rescuing effects depends upon source-sink dynamics and the interplay of stabilizing and destabilizing forces regulating the coexistence between the benefactor and the beneficiaries as well as among beneficiaries. Thus, current ability to ameliorate environmental conditions is not sufficient in itself to make a habitat-former a climate rescuer species. Nonetheless, some habitat-forming species display the right individual and population traits (Box 3). Drawing from different ecological and biological disciplines, a series of management actions can sustain the strength and reliability of their climate-rescuing effects. Within a multidisciplinary framework (Fig 3), understanding how biogenic habitats influence evolutionary adaptation of beneficiary species to changing conditions and their ability to track suitable climates should be considered a priority. Developing the concept of sustaining habitat-former populations as a nature-based solution to climate change will likely depend on our ability and willingness to address ethical issues in modern conservation, such as those related to the use of synthetic biology, non-native species, assisted species evolution, and species relocation. Finally, the general features of one or a few species that reduce climate-driven abiotic stress for other species that we describe in coastal systems are likely to be found also in other types of ecosystems. For example, heat tolerance of freshwater gastropods is lowered in hypoxic conditions [97] and may be sustained by macrophyte oxygen production. In high-alpine systems, some cushion plants mitigate the effects of warming on native grasses [9]. Likewise, during drought events, canopy-forming mosses enhance the survival of smaller mosses and hepatics in their understory [98]. Thus, the broad conclusions we derive for coastal ecosystems under climate change may also apply to other ecosystems.
Box 3. Examples of potential climate rescuers
Climate rescuer on the sand
Sea cucumbers play an important role in coastal environments since they bioturbate sediments and recycle nutrients, sustaining the diversity and functioning of benthic communities [99]. The sea cucumber Holothuria scabra (the “sandfish,” Fig 1F) is distributed throughout the Indo-Pacific region, between 30° N and 30° S of latitude. It is an active burrower and enhances sediment oxygenation, buffering negative effects of hypoxia caused by eutrophication and warming [33]. In addition, it can foster seagrass growth and productivity via remineralization of nutrients and/or their release from sediment pore water [99], potentially triggering a facilitation cascade. This species is cultured, and it seems able to rapidly adapt to variable environmental conditions (e.g., salinity, temperature) through behavioral and molecular mechanisms [100,101]. For instance, in aquaculture facilities, extreme water temperatures exceeding 31 °C caused no mortality of juveniles and, indeed, fostered their growth [102]. Finally, the entire mitochondrial genome of this species has been sequenced [103]. For the reasons above, this species may offer a nature-based solution for alleviating the impact of temperature-driven hypoxia.
Climate rescuer on the rocks
The brown macroalga Fucus vesiculosus (Fig 1C) occupies wide ecological and geographical ranges. Presently, it spans latitudes from above 70° N (Norway) to near 30° N (Morocco), withstanding, at low tide, extreme freezing (e.g., Labrador Sea), extreme heat (e.g., above 40 °C in Iberia), and variable salinities (estuaries, the Baltic Sea). It can function as climate rescuer for taxa beyond the southern limits of most intertidal fucoid seaweeds of the NE Atlantic, which can be vertically compressed and geographically restricted beyond the northwest Iberian climate refugium [104]. In contrast, F. vesiculosus extends further south, persisting in more extreme conditions. Although it suffered the loss of many populations of a southern genetic lineage [105], reciprocal transplants showed that populations that persisted from this southern lineage have better adaptive traits for their habitat [106]. In this species, the costs of thermal stress to cellular metabolism (recorded as molecular heat shock response) can be escaped when high temperatures co-occur with rapid extreme desiccation [36]. Producing large quantities of recruits of F. vesiculosus is a standard procedure because this species has been widely used for decades as a model in developmental biology, reproductive ecology, and ecophysiology, including in experimental field outplants [107]. Because the species is easily propagated and the southern populations have the capacity to withstand heat stress and maintain large canopies in areas where few other large intertidal canopies exist, it may offer a nature-based solution for alleviating the impact of multiple stressors on intertidal community diversity and abundance along its warm range limits.
Acknowledgments
We sincerely thank three anonymous reviewers for providing comments and constructive criticism on an earlier draft. F.B. wishes to thank the University of Pisa for providing access to facilities during the workshop POSTCLIMA.
Funding Statement
EuroMarine - European Marine Research Network http://euromarinenetwork.eu/. Funds were granted to FB for the organization of the Foresight Workshop POSTCLIMA. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Provenance: Not commissioned; externally peer reviewed.
References
- 1.Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC, Chen I-C, et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science. 2017; 355(6332): eaai9214. 10.1126/science.aai9214 [DOI] [PubMed] [Google Scholar]
- 2.Scheffers BR, De Meester L, Bridge TCL, Hoffmann AA, Pandolfi JM, Corlett RT, et al. The broad footprint of climate change from genes to biomes to people. Science. 2016; 354(6313): aaf7671. 10.1126/science.aaf7671 [DOI] [PubMed] [Google Scholar]
- 3.Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol. 2010; 213(6): 912–20. 10.1242/jeb.037473 [DOI] [PubMed] [Google Scholar]
- 4.Ockendon N, Baker DJ, Carr JA, White EC, Almond REA, Amano T, et al. Mechanisms underpinning climatic impacts on natural populations: altered species interactions are more important than direct effects. Global Change Biol. 2014; 20(7): 2221–9. 10.1111/gcb.12559 [DOI] [PubMed] [Google Scholar]
- 5.Cavieres LA, Brooker RW, Butterfield BJ, Cook BJ, Kikvidze Z, Lortie CJ, et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol Lett. 2014; 17(2): 193–202. 10.1111/ele.12217 [DOI] [PubMed] [Google Scholar]
- 6.Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev. 2013; 88(1): 15–30. 10.1111/j.1469-185X.2012.00235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthelme F, Cavieres LA, Dangles O. Facilitation among plants in alpine environments in the face of climate change. Front Plant Sci. 2014; 5(387). 10.3389/fpls.2014.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, et al. Facilitation in plant communities: the past, the present, and the future. J Ecol. 2008; 96(1): 18–34. 10.1111/j.1365-2745.2007.01295.x [DOI] [Google Scholar]
- 9.Cavieres LA, Sierra-Almeida A. Facilitative interactions do not wane with warming at high elevations in the Andes. Oecologia. 2012; 170(2): 575–84. 10.1007/s00442-012-2316-x [DOI] [PubMed] [Google Scholar]
- 10.Jurgens LJ, Gaylord B. Edge effects reverse facilitation by a widespread foundation species. Sci Rep. 2016; 6: 37573 10.1038/srep37573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelini C, Griffin JN, van de Koppel J, Lamers LPM, Smolders AJP, Derksen-Hooijberg M, et al. A keystone mutualism underpins resilience of a coastal ecosystem to drought. Nat Commun. 2016; 7: 12473 10.1038/ncomms12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulleri F. Facilitation research in marine systems: state of the art, emerging patterns and insights for future developments. J Ecol. 2009; 97(6): 1121–30. 10.1111/j.1365-2745.2009.01567.x [DOI] [Google Scholar]
- 13.Donadi S, van der Heide T, van der Zee EM, Eklöf JS, de Koppel Jv, Weerman EJ, et al. Cross-habitat interactions among bivalve species control community structure on intertidal flats. Ecology. 2013; 94(2): 489–98. 10.1890/12-0048.1 [DOI] [PubMed] [Google Scholar]
- 14.He Q, Bertness MD, Altieri AH. Global shifts towards positive species interactions with increasing environmental stress. Ecol Lett. 2013; 16(5): 695–706. 10.1111/ele.12080 [DOI] [PubMed] [Google Scholar]
- 15.Bertness MD, Callaway R. Positive interactions in communities. Trends Ecol Evol. 1994; 9(5): 191–3. 10.1016/0169-5347(94)90088-4 [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Aparicio L, Zamora R, Gómez JM., Hódar JA., Castro J, Baraza E. Applying plant facilitation to forest restoration: a meta-analysis of the use of shrubs as nurse plants. Ecol Appl. 2004; 14(4): 1128–38. 10.1890/03-5084 [DOI] [Google Scholar]
- 17.Halpern BS, Silliman BR, Olden JD, Bruno JP, Bertness MD. Incorporating positive interactions in aquatic restoration and conservation. Front Ecol Environ. 2007; 5(3): 153–60. 10.1890/1540-9295(2007)5[153:ipiiar]2.0.co;2 [DOI] [Google Scholar]
- 18.Byers JE, Cuddington K, Jones CG, Talley TS, Hastings A, Lambrinos JG, et al. Using ecosystem engineers to restore ecological systems. Trends Ecol Evol. 2006; 21(9): 493–500. 10.1016/j.tree.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 19.Valiente-Banuet A, Rumebe AV, Verdú M, Callaway RM. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proc Natl Acad Sci U S A. 2006; 103(45): 16812–17. 10.1073/pnas.0604933103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meysman FJR, Middelburg JJ, Heip CHR. Bioturbation: a fresh look at Darwin's last idea. Trends Ecol Evol. 2006; 21(12): 688–95. 10.1016/j.tree.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 21.Jvd Koppel, Tvd Heide, Altieri AH, Eriksson BK, Bouma TJ, Olff H, et al. Long-distance interactions regulate the structure and resilience of coastal ecosystems. Annu Rev Mar Sci. 2015; 7(1): 139–58. 10.1146/annurev-marine-010814-015805 [DOI] [PubMed] [Google Scholar]
- 22.Altieri AH, van de Koppel J, Foundation species in marine environments In: Bertness MD, Bruno JF, Silliman BR, Stachowicz JJ editors. Marine Community Ecology and Conservation. Sinauer Associates, Inc., Sunderland; 2014. pp. 37–56. [Google Scholar]
- 23.Solan M, Cardinale BJ, Downing AL, Engelhardt KAM, Ruesink JL, Srivastava DS. Extinction and ecosystem function in the marine benthos. Science. 2004; 306(5699): 1177–80. 10.1126/science.1103960 [DOI] [PubMed] [Google Scholar]
- 24.Leonard GH. Latitudinal variation in species interactions: a test in the New England rocky intertidal zone. Ecology. 2000; 81(4): 1015–30. 10.1890/0012-9658(2000)081[1015:lvisia]2.0.co;2 [DOI] [Google Scholar]
- 25.Silliman BR, Bertness MD, Altieri AH, Griffin JN, Bazterrica MC, Hidalgo FJ, et al. Whole-community facilitation regulates biodiversity on Patagonian rocky shores. PLoS ONE. 2011; 6(10): e24502 10.1371/journal.pone.0024502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keppel G, Van Niel KP, Wardell-Johnson GW, Yates CJ, Byrne M, Mucina L, et al. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecol Biogeogr. 2012; 21(4): 393–404. 10.1111/j.1466-8238.2011.00686.x [DOI] [Google Scholar]
- 27.Hendriks IE, Olsen YS, Ramajo L, Basso L, Steckbauer A, Moore TS, et al. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences. 2014; 11(2): 333–46. 10.5194/bg-11-333-2014 [DOI] [Google Scholar]
- 28.Wahl M, Schneider Covachã S, Saderne V, Hiebenthal C, Müller JD, Pansch C, et al. Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol. Oceaogr. 10.1002/lno.10608 [DOI] [Google Scholar]
- 29.Unsworth RKF, Collier CJ, Henderson GM, McKenzie LJ. Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environ Res Lett. 2012; 7(2): 024026 10.1088/1748-9326/7/2/024026 [DOI] [Google Scholar]
- 30.Pinsky ML, Guannel G, Arkema KK. Quantifying wave attenuation to inform coastal habitat conservation. Ecosphere. 2013; 4(8): 1–16. 10.1890/es13-00080.1 [DOI] [Google Scholar]
- 31.Bennett S, Wernberg T, de Bettignies T, Kendrick GA, Anderson RJ, Bolton JJ, et al. Canopy interactions and physical stress gradients in subtidal communities. Ecol Lett. 2015; 18(7): 677–86. 10.1111/ele.12446 [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez JL, Jones CG, Byers JE, Arkema KK, Berkenbusch K, Commito JA, et al. Physical ecosystem engineers and the functioning of estuaries and coasts In: Wolanski E, McLusky D editors. Treatise on Estuarine and Coastal Science. Elsevier, Amsterdam, The Netherlands; 2011. pp. 53–81. [Google Scholar]
- 33.Lee S, Ford AK, Mangubhai S, Wild C, Ferse SCA. Effects of sandfish (Holothuria scabra) removal on shallow-water sediments in Fiji. PeerJ. 2018; 6: e4773 10.7717/peerj.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiddink JG, Burrows MT, Molinos JG. Temperature tracking by North Sea benthic invertebrates in response to climate change. Global Change Biol. 2015; 21(1): 117–29. 10.1111/gcb.12726 [DOI] [PubMed] [Google Scholar]
- 35.Jurgens LJ, Gaylord B. Physical effects of habitat-forming species override latitudinal trends in temperature. Ecol Lett. 21(2): 190–6. 10.1111/ele.12881 [DOI] [PubMed] [Google Scholar]
- 36.Mota CF, Engelen AH, Serrao EA, Pearson GA. Some don't like it hot: microhabitat-dependent thermal and water stresses in a trailing edge population. Funct Ecol. 2015; 29(5): 640–9. 10.1111/1365-2435.12373 [DOI] [Google Scholar]
- 37.Bulleri F, Bruno JF, Silliman BR, Stachowicz JJ. Facilitation and the niche: implications for coexistence, range shifts and ecosystem functioning. Funct Ecol. 2016; 30(1): 70–8. 10.1111/1365-2435.12528 [DOI] [Google Scholar]
- 38.Torda G, Donelson JM, Aranda M, Barshis DJ, Bay L, Berumen ML, et al. Rapid adaptive responses to climate change in corals. Nat Clim Change. 2017; 7(9): 627–36. 10.1038/nclimate3374 [DOI] [Google Scholar]
- 39.Marín-Guirao L, Entrambasaguas L, Dattolo E, Ruiz JM, Procaccini G. Molecular mechanisms behind the physiological resistance to intense transient warming in an iconic marine plant. Front Plant Sci. 2017; 8(1142). 10.3389/fpls.2017.01142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donelson JM, Munday PL, McCormick MI, Nilsson GE. Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Global Change Biol. 2011; 17(4): 1712–9. 10.1111/j.1365-2486.2010.02339.x [DOI] [Google Scholar]
- 41.Putnam HM, Gates RD. Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J Exp Biol. 2015; 218(15): 2365–72. 10.1242/jeb.123018 [DOI] [PubMed] [Google Scholar]
- 42.Stillwell RC, Fox CW. Geographic variation in body size, sexual size dimorphism and fitness components of a seed beetle: local adaptation versus phenotypic plasticity. Oikos. 2009; 118(5): 703–12. 10.1111/j.1600-0706.2008.17327.x [DOI] [Google Scholar]
- 43.Soliveres S, Smit C, Maestre FT. Moving forward on facilitation research: response to changing environments and effects on the diversity, functioning and evolution of plant communities. Biol Rev. 2015; 90(1): 297–313. 10.1111/brv.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanford E, Kelly MW. Local adaptation in marine invertebrates. Annu Rev Mar Sci. 2011; 3: 509–35. 10.1146/annurev-marine-120709-142756 [DOI] [PubMed] [Google Scholar]
- 45.Schmidt PS, Rand DM. Adaptive maintenance of genetic polymorphism in an intertidal barnacle: Habitat- and life-stage-specific survivorship of Mpi genotypes. Evolution. 2001; 55(7): 1336–44. [DOI] [PubMed] [Google Scholar]
- 46.Castellanos MC, Donat-Caerols S, Gonzalez-Martinez SC, Verdu M. Can facilitation influence the spatial genetics of the beneficiary plant population? J Ecol. 2014; 102(5): 1214–21. 10.1111/1365-2745.12278 [DOI] [Google Scholar]
- 47.Liancourt P, Choler P, Gross N, Thibert-Plante X, Tielbörger K. How facilitation may interfere with ecological speciation. Internat. J Ecol. 2012; 725487. 10.1155/2012/725487 [DOI] [Google Scholar]
- 48.Diekmann OE, Serrao EA. Range-edge genetic diversity: locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Mol Ecol. 2012; 21(7): 1647–57. 10.1111/j.1365-294X.2012.05500.x [DOI] [PubMed] [Google Scholar]
- 49.Alexander JM, Diez JM, Hart SP, Levine JM. When climate reshuffles competitors: A call for experimental macroecology. Trends Ecol Evol. 2016; 31(11): 831–41. 10.1016/j.tree.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michalet R, Brooker RW, Cavieres LA, Kikvidze Z, Lortie CJ, Pugnaire FI, et al. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol Lett. 2006; 9(7): 767–73. 10.1111/j.1461-0248.2006.00935.x [DOI] [PubMed] [Google Scholar]
- 51.Guo HY, Zhang YH, Lan ZJ, Pennings SC. Biotic interactions mediate the expansion of black mangrove (Avicennia germinans) into salt marshes under climate change. Global Change Biol. 2013; 19(9): 2765–74. 10.1111/gcb.12221 [DOI] [PubMed] [Google Scholar]
- 52.Reise K, Buschbaum C, Büttger H, Wegner KM. Invading oysters and native mussels: from hostile takeover to compatible bedfellows. Ecosphere. 2017; 8(9). 10.1002/ecs2.1949 [DOI] [Google Scholar]
- 53.Altieri AH, Silliman BR, Bertness MD. Hierarchical organization via a facilitation cascade in intertidal cordgrass bed communities. Am Nat. 2007; 169(2): 195–206. 10.1086/510603 [DOI] [PubMed] [Google Scholar]
- 54.Thomsen MS, Wernberg T, Altieri AH, Tuya F, Gulbransen D, McGlathery KJ, et al. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr Comp Biol. 2010; 50(2): 158–75. 10.1093/icb/icq042 [DOI] [PubMed] [Google Scholar]
- 55.van de Koppel J, van der Heide T, Altieri AH, Eriksson BK, Bouma TJ, Olff H, et al. Long-distance interactions regulate the structure and resilience of coastal ecosystems. Annu Rev Mar Sci. 2015; 7: 139–58. 10.1146/annurev-marine-010814-015805 [DOI] [PubMed] [Google Scholar]
- 56.Daufresne M, Lengfellner K, Sommer U. Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci U S A. 2009; 106(31): 12788–93. 10.1073/pnas.0902080106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garilli V, Rodolfo-Metalpa R, Scuderi D, Brusca L, Parrinello D, Rastrick SPS, et al. Physiological advantages of dwarfing in surviving extinctions in high-CO2 oceans. Nat Clim Change. 2015; 5: 678 10.1038/nclimate2616 [DOI] [Google Scholar]
- 58.Gaylord B, Hill TM, Sanford E, Lenz EA, Jacobs LA, Sato KN, et al. Functional impacts of ocean acidification in an ecologically critical foundation species. J Exp Biol. 2011; 214(15): 2586–94. 10.1242/jeb.055939 [DOI] [PubMed] [Google Scholar]
- 59.Kordas RL, Dudgeon S, Storey S, Harley CDG. Intertidal community responses to field-based experimental warming. Oikos. 2015; 124(7): 888–98. 10.1111/oik.00806 [DOI] [Google Scholar]
- 60.Thomson JA, Burkholder DA, Heithaus MR, Fourqurean JW, Fraser MW, Statton J, et al. Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Global Change Biol. 2015; 21(4): 1463–74. 10.1111/gcb.12694 [DOI] [PubMed] [Google Scholar]
- 61.Rilov G. Multi-species collapses at the warm edge of a warming sea. Sci Rep. 2016; 6: 36897 10.1038/srep36897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osland MJ, Day RH, Hall CT, Brumfield MD, Dugas JL, Jones WR. Mangrove expansion and contraction at a poleward range limit: climate extremes and land-ocean temperature gradients. Ecology. 2017; 98(1): 125–37. 10.1002/ecy.1625 [DOI] [PubMed] [Google Scholar]
- 63.Wernberg T, Bennett S, Babcock RC, de Bettignies T, Cure K, Depczynski M, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016; 353(6295): 169–72. 10.1126/science.aad8745 [DOI] [PubMed] [Google Scholar]
- 64.Gorman D, Turra A, Bergstrom ER, Horta PA. Population expansion of a tropical seagrass (Halophila decipiens) in the southwest Atlantic (Brazil). Aquat Bot. 2016; 132: 30–6. 10.1016/j.aquabot.2016.04.002 [DOI] [Google Scholar]
- 65.Bouma TJ, Friedrichs M, Van Wesenbeeck BK, Temmerman S, Graf G, Herman PMJ. Density-dependent linkage of scale-dependent feedbacks: a flume study on the intertidal macrophyte Spartina anglica. Oikos. 2009; 118(2): 260–8. 10.1111/j.1600-0706.2008.16892.x [DOI] [Google Scholar]
- 66.O'Leary JK, Micheli F, Airoldi L, Boch C, De Leo G, Elahi R, et al. The resilience of marine ecosystems to climatic disturbances. Bioscience. 2017; 67(3): 208–20. 10.1093/biosci/biw161 [DOI] [Google Scholar]
- 67.Reusch TBH. Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evol Appl. 2014; 7(1): 104–22. 10.1111/eva.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gienapp P, Fior S, Guillaume F, Lasky JR, Sork VL, Csilléry K. Genomic quantitative genetics to study evolution in the wild. Trends Ecol Evol. 32(12): 897–908. 10.1016/j.tree.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 69.Piaggio AJ, Segelbacher G, Seddon PJ, Alphey L, Bennett EL, Carlson RH, et al. Is it time for synthetic biodiversity conservation? Trends Ecol Evol. 32(2): 97–107. 10.1016/j.tree.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 70.Harfouche A, Meilan R, Altman A. Tree genetic engineering and applications to sustainable forestry and biomass production. Trends Biotechnol. 2011; 29(1): 9–17. 10.1016/j.tibtech.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 71.Levin RA, Voolstra CR, Agrawal S, Steinberg PD, Suggett DJ, van Oppen MJH. Engineering strategies to decode and enhance the genomes of coral symbionts. Front Microbiol. 2017; 8: 1220 10.3389/fmicb.2017.01220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR. Genomic basis for coral resilience to climate change. Proc Natl Acad Sci U S A. 2013; 110(4): 1387–92. 10.1073/pnas.1210224110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang GF, Li L, Meng J, Qi HG, Qu T, Xu F, et al. Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu Rev Anim Biosci. 2016; 4: 357–81. 10.1146/annurev-animal-022114-110903 [DOI] [PubMed] [Google Scholar]
- 74.van Oppen MJH, Oliver JK, Putnam HM, Gates RD. Building coral reef resilience through assisted evolution. Proc Natl Acad Sci U S A. 2015; 112(8): 2307–13. 10.1073/pnas.1422301112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carpenter SR, Brock WA, Folke C, van Nes EH, Scheffer M. Allowing variance may enlarge the safe operating space for exploited ecosystems. Proc Natl Acad Sci U S A. 2015; 112(46): 14384–9. 10.1073/pnas.1511804112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaum CE, Buckling A, Smirnoff N, Studholme DJ, Yvon-Durocher G. Environmental fluctuations accelerate molecular evolution of thermal tolerance in a marine diatom. Nat Comm 2018; 9(1): 1719 10.1038/s41467-018-03906-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wahl M, Buchholz B, Winde V, Golomb D, Guy-Haim T, Muller J, et al. A mesocosm concept for the simulation of near-natural shallow underwater climates: The Kiel Outdoor Benthocosms (KOB). Limnol Oceanogr-Meth. 2015; 13(11): 651–63. 10.1002/lom3.10055 [DOI] [Google Scholar]
- 78.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012; 336(6086): 1255–62. 10.1126/science.1224203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008; 32(5): 723–35. 10.1111/j.1574-6976.2008.00123.x [DOI] [PubMed] [Google Scholar]
- 80.Chakravarti LJ, Beltran VH, van Oppen MJH. Rapid thermal adaptation in photosymbionts of reef-building corals. Global Change Biol. 2017; 23(11): 4675–88. 10.1111/gcb.13702 [DOI] [PubMed] [Google Scholar]
- 81.Rudnick DA, Ryan SJ, Beier P, Cushman SA, Dieffenbach F, Epps CW, et al. The role of landscape connectivity in planning and implementing conservation and restoration priorities. Issues Ecol 2012; 16:1–23. [Google Scholar]
- 82.Östman Ö, Eklöf J, Eriksson BK, Olsson J, Moksnes P-O, Bergström U. Top-down control as important as nutrient enrichment for eutrophication effects in North Atlantic coastal ecosystems. J Appl Ecol. 2016; 53(4): 1138–47. 10.1111/1365-2664.12654 [DOI] [Google Scholar]
- 83.Strain EMA, van Belzen J, van Dalen J, Bouma TJ, Airoldi L. Management of local stressors can improve the resilience of marine canopy algae to global stressors. PLoS ONE. 2015; 10(3): e0120837 10.1371/journal.pone.0120837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, et al. Biodiversity loss and its impact on humanity. Nature. 2012; 486: 59–67. 10.1038/nature11148 [DOI] [PubMed] [Google Scholar]
- 85.Bishop MJ, Mayer-Pinto M, Airoldi L, Firth LB, Morris RL, Loke LHL, et al. Effects of ocean sprawl on ecological connectivity: impacts and solutions. J Exp Mar Biol Ecol. 2017; 492: 7–30. 10.1016/j.jembe.2017.01.021 [DOI] [Google Scholar]
- 86.Walther GR, Roques A, Hulme PE, Sykes MT, Pysek P, Kuhn I, et al. Alien species in a warmer world: risks and opportunities. Trends Ecol Evol. 2009; 24(12): 686–93. 10.1016/j.tree.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 87.Schlaepfer MA. Do non-native species contribute to biodiversity? PLoS Biol. 2018; 16(4): e2005568 10.1371/journal.pbio.2005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sorte CJB, Williams SL, Carlton JT. Marine range shifts and species introductions: comparative spread rates and community impacts. Global Ecol Biogeogr. 2010; 19(3): 303–16. [Google Scholar]
- 89.Maggi E, Benedetti-Cecchi L, Castelli A, Chatzinikolaou E, Crowe TP, Ghedini G, et al. Ecological impacts of invading seaweeds: a meta-analysis of their effects at different trophic levels. Divers Distrib. 2015; 21(1): 1–12. 10.1111/ddi.12264 [DOI] [Google Scholar]
- 90.Guy-Haim T, Lyons DA, Kotta J, Ojaveer H, Queiros AM, Chatzinikolaou E, et al. Diverse effects of invasive ecosystem engineers on marine biodiversity and ecosystem functions: A global review and meta-analysis. Global Change Biol. 2018; 24(3): 906–24. 10.1111/gcb.14007 [DOI] [PubMed] [Google Scholar]
- 91.Johnston CA, Lipcius RN. Exotic macroalga Gracilaria vermiculophylla provides superior nursery habitat for native blue crab in Chesapeake Bay. Mar Ecol Prog Ser. 2012; 467: 137–46. 10.3354/meps09935 [DOI] [Google Scholar]
- 92.Norkko J, Reed DC, Timmermann K, Norkko A, Gustafsson BG, Bonsdorff E, et al. A welcome can of worms? Hypoxia mitigation by an invasive species. Global Change Biol. 2012; 18(2): 422–34. 10.1111/j.1365-2486.2011.02513.x [DOI] [Google Scholar]
- 93.Olabarria C, Arenas F, Viejo RM, Gestoso I, Vaz-Pinto F, Incera M, et al. Response of macroalgal assemblages from rockpools to climate change: effects of persistent increase in temperature and CO2. Oikos. 2013; 122(7): 1065–79. 10.1111/j.1600-0706.2012.20825.x [DOI] [Google Scholar]
- 94.Schlaepfer MA, Sax DF, Olden JD. The potential conservation value of non-native species. Conserv Biol. 2011; 25(3): 428–37. 10.1111/j.1523-1739.2010.01646.x [DOI] [PubMed] [Google Scholar]
- 95.Davis MA, Chew MK, Hobbs RJ, Lugo AE, Ewel JJ, Vermeij GJ, et al. Don’t judge species on their origins. Nature. 2011; 474: 153 10.1038/474153a [DOI] [PubMed] [Google Scholar]
- 96.Simberloff D. Non-natives: 141 scientists object. Nature. 2011; 475: 36 10.1038/475036a [DOI] [PubMed] [Google Scholar]
- 97.Koopman KR, Collas FPL, van der Velde G, Verberk W. Oxygen can limit heat tolerance in freshwater gastropods: differences between gill and lung breathers. Hydrobiologia. 2016; 763(1): 301–12. 10.1007/s10750-015-2386-y [DOI] [Google Scholar]
- 98.Mulder CPH, Uliassi DD, Doak DF. Physical stress and diversity-productivity relationships: The role of positive interactions. Proc Natl Acad Sci U S A. 2001; 98(12): 6704–8. 10.1073/pnas.111055298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Purcell SW, Conand C, Uthicke S, Byrne M. Ecological roles of exploited sea cucumbers. Oceanogr Mar Biol Annu Rev. 2016; 54: 367–86. [Google Scholar]
- 100.Kamyab E, Kuhnhold H, Novais SC, Alves LMF, Indriana L, Kunzmann A, et al. Effects of thermal stress on the immune and oxidative stress responses of juvenile sea cucumber Holothuria scabra. J Comp Physiol B. 2017; 187(1): 51–61. 10.1007/s00360-016-1015-z [DOI] [PubMed] [Google Scholar]
- 101.Mercier A, Battaglene SC, Hamel JF. Daily burrowing cycle and feeding activity of juvenile sea cucumbers Holothuria scabra in response to environmental factors. J Exp Mar Biol Ecol. 1999; 239(1): 125–56. 10.1016/s0022-0981(99)00034-9 [DOI] [Google Scholar]
- 102.Lavitra T, Fohy N, Gestin PG, Rasolofonirina R, Eeckhaut I. Effect of water temperature on the survival and growth of endobenthic Holothuria scabra (Echinodermata: Holothuroidea) juveniles reared in outdoor ponds. SPC Beche-de-Mer Inform Bull. 2010; 30: 25–8. [Google Scholar]
- 103.Xia JJ, Ren CH, Yu ZH, Wu XY, Qian J, Hu CQ. Complete mitochondrial genome of the sandfish Holothuria scabra (Holothuroidea, Holothuriidae). Mitochondrial DNA Part A. 2016; 27(6): 4174–5. 10.3109/19401736.2014.1003899 [DOI] [PubMed] [Google Scholar]
- 104.Neiva JSE, Assis J, Pearson GA, Coyer JA, Olsen JL, et al. Climate oscillations, range shifts and phylogeographic patterns of North Atlantic Fucaceae In: Hu Z-M, Fraser C, editors. Seaweed Phylogeography. Adaptation and Evolution of Seaweeds under Environmental Change. Springer, Netherlands; 2016. pp. 279–308. [Google Scholar]
- 105.Nicastro KR, Zardi GI, Teixeira S, Neiva J, Serrao EA, Pearson GA. Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biol. 2013; 11: 6 10.1186/1741-7007-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saada G, Nicastro KR, Jacinto R, McQuaid CD, Serrao EA, Pearson GA, et al. Taking the heat: distinct vulnerability to thermal stress of central and threatened peripheral lineages of a marine macroalga. Divers Distrib. 2016; 22(10): 1060–8. 10.1111/ddi.12474 [DOI] [Google Scholar]
- 107.Ladah L, Bermudez R, Pearson G, Serrao E. Fertilization success and recruitment of dioecious and hermaphroditic fucoid seaweeds with contrasting distributions near their southern limit. Mar Ecol Prog Ser 2003; 262: 173–83. 10.3354/meps262173 [DOI] [Google Scholar]