Abstract
Salmonella Typhi and Salmonella Paratyphi A are the leading causative agents of enteric fever which cause morbidity and mortality worldwide. Currently, there is no combination vaccine which could protect infection from both the strains. In this paper, we are focusing on the development of a novel bivalent typhoidal Outer Membrane Vesicles (OMVs) based immunogen against enteric fever. We have isolated Salmonella Typhi and Paratyphi A OMVs and also characterized OMVs associated antigens. Then we immunized adult mice with three doses of our newly formulated bivalent immunogen orally (25 μg/200 μl). After three doses of oral immunization, we found our immunogen could significantly induce humoral response. We have also found serum IgG against LPS, Vi-polysaccharide etc. OMV immunization induces CD4, CD8 and CD19 population in immunized mice spleen. It also induces Th1 and Th17-cell mediated immunity. We also found bivalent OMVs immunization can prevent more than lethal dose of heterologous Salmonella strains mediated systemic infection in adult mice model. We determined that, the protective immune responses depend on the humoral and cell-mediated immune response. Furthermore, we have evaluated the mode of protective immune response carried out by anti-OMVs antibody by significantly inhibiting bacterial motility and mucin penetration ability. Taken together, these findings suggest that our bivalent immunogen could be used as a novel candidate vaccine against enteric fever.
Introduction
Enteric fever, a serious invasive febrile illness of the human, caused by Salmonella enterica serovers Typhi and Paratyphi A (S. Typhi and S. Paratyphi A, respectively) is considered to be a major global burden in developed and developing countries [1]. Globally S.Typhi affects 21.7 million people and 200,000 deaths per year. Although S. Typhi is more prevalent until recently; S.Paratyphi (A, B and C) too can cause significant morbidity and mortality especially in Asia and in travelers returning from these endemic areas. Recent trends of enteric fever suggest a change in disease pattern, typically a rise of paratyphoid fever in the Asian and other developing countries [2, 3].
In this changing disease pattern, it has been hypothesized that, soon S. Paratyphi A infection would become the prime strain with significant human morbidity and mortality [2, 3]. S. Typhi and Paratyphi A are transmitted by ingestion of contaminated food and water. After ingestion, salmonellae adhere to the mucosa and invade intestinal epithelial cells especiallythrough Microfold (M) cells. After invasion, organisms translocate into intestinal lymphoid organs and some passes on to the liver and spleen [4]. During this stage of infection Salmonella activates different mucosal and systemic immune response which is very crucial for strategic suitable vaccine development.
Currently, there are three globally licensed vaccines available against S.Typhi, a live-attenuated oral vaccine Ty21a (galE mutant of the wild type Ty2 strain) and parenteral polysaccharide Vi-vaccine (Vi-vaccine) [5]. The third one is a recent tetanus toxoid-conjugated vaccine which is also against S.Typhi. Although the oral vaccine somewhat cross-protect paratyphoid infection, but because Vi polysaccharide is generally absent in Paratyphi, the Vi vaccine is ineffective against Paratyphi infection. Therefore, a better understanding of the immunology towards protection is required to allow more rational approach to vaccine development. In this situation, need for a new vaccine seems obvious which will protect against both S. Typhi and S. Paratyphi A infection.
Outer Membrane Vesicles (OMVs) are secreted naturally from several Gram-negative bacteria including typhoidal salmonellae. These native OMVs are being used as a next generation acellular vaccine, which induces long-term protective immune response without any additionally added adjuvant. OMVs have LPS and membrane proteins as a natural adjuvant [6, 7, 8, 9, 10, 11]. Biochemical and proteomics studies have revealed that OMVs consists of different Pathogen-Associated Molecular Patterns (PAMPs) such as LPSs, outer membrane proteins, outer membrane lipids, periplasmic proteins, DNA, RNA etc. which can activate innate as well as adaptive immune response, which is a crucial characteristic for an ideal vaccine.
The only licensed OMV-vaccine is of Neisseria meningitides serogroup-B vaccine, MeNZB, which is safe, immunogenic and protective in nature [12, 13]. It has showed nearly 80% protective efficacies during an epidemic meningococcal serotype B outbreak in Norway and New Zealand in both adults and children. In the past, in our laboratory, we have developed different multivalent OMV based vaccine against different enteric organisms like V.cholerae, Shigella sp. etc. [14, 15, 16, 17].
In this study, we are focusing onto the development of an oral bivalent OMV based typhoidal vaccine which can induce long-term broad spectrum protective efficacy against both S. Typhi and Paratyphi A. Another aim of our study was to prevent the entry of Salmonella through intestine as well as reduction in the bacterial load after systemic infection by activating mucosal as well as systemic immune response.
Methods
Bacterial strains and culture conditions
OMV antigens were prepared from S.Typhi C-6953 and S. Paratyphi A C-6915, and for challenge study, S. Typhi C-6.946 and S. Paratyphi A BCR 148 were collected from National Institute of Cholera and Enteric Diseases (NICED) culture bank. All strains were kept in 20% glycerol in brain heart infusion broth (Difco, USA) at −80° C. Prior to experimentation, each strain was grown in Tryptic Soy Broth (TSB; Difco, USA) at 37° C under shaking conditions (100 rpm) or on plates in Tryptic Soy Agar (TSA; Difco, NJ, USA).
Characterization of the strains used
Strains were characterized by both PCR-based method and serotyping. For PCR, the following genes were selected and their respective primer sequences [18]have been shown in bracket:prtA-parat-s (5’-CTT GCT ATG GAA GAC ATA ACG AAC C-3’); parat-as (5’-CGT CTC CAT CAA AAG CTC CAT AGA-3’); fliCd-as (5’-GCA TAG CCA CCA TCA ATA ACC-3’);fliCa-as (5’-TAG TGC TTA ATG TAG CCG AAG G-3’); fliCcom-s (5’-AAT CAA CAACAA CCT GCA GCG-3’). Afterwards, anti O- and H-antibodies were used for serotypic characterization of the strains used in the study. White-Kauffmann-Le Minor scheme for Antigenic Formulae was followed during the experiment [19, 20] (S1 File).
Antibiotic-resistance pattern of the strains was also checked by Bauer method [21]. Selection of antibiotics was dependent on both WHO, CLSI guidelines [22, 23] and the availability of antibiotics in our facility. Antibiotic disks (AMP 10 μg, CTX 30μg, CFZ 30 μg, CFX 5μg, CRO 30 μg, CHL 30 μg, I 10 μg, NAL 30 μg, CO 25 μg, TET 30 μg, TMP 30 μg, SXT 23.75 μg, LO 30 μg, AZ 15 μg, CIP 5 μg, STR 10 μg, KAN 30 μg) were purchased from BD Bioscience, USA (S2 File).
Preparation of OMVs
OMVs were prepared from two Salmonella enterica strains as shown in Table 1 following the method adapted by Sinha et al. with slight modifications [15]. Briefly, cells were grown till exponential phase at 37°C under shaking condition followed by centrifugation at 8000 rpm for a total of 40 minutes at 4°C. Following filtration by 0.22 μm bacterial filters (Millipore, USA), OMVs were subsequently purified by ultra-centrifugation (4 h, 140,000 x g, 4°C) using a Sorvall T-865 rotor, and re-suspended in Phosphate-Buffered Saline (PBS, pH 7.4). Protein concentration was determined by the modified Lowry protein assay kit (Pierce, USA). Reducing sugar in LPS O-Ag was determined by a method used by Dubois et al [24].
Table 1. Concentration of protein and O-antigen in OMV samples.
| Name of OMVs | Mean concentration of protein (μg/ml) | Mean concentration of O-antigen (μg/ml) | Source or reference |
|---|---|---|---|
| S. Typhi C-6953 | 414 | 250 | This study |
| S.Paratyphi A C-6915 | 430 | 235 | This study |
The amount of protein and O-antigen of LPS associated with isolated OMVs were measured by Lowry and Dubois methods, respectively. The amount of protein varied between 400–440 μg/ml in every batch. The exact amount was calculated every time before the introduction of the immunogen in animals or in in vitro study.
Negative staining of OMVs and OMV-secreting bacteria
A 5 μl aliquot of secreted OMVs were placed on a carbon coated grid and left for 1 minute for proper absorption. The grid was then washed with two drops of Tris-HCl buffer. After blotting excess fluid, the sample was stained with 2% aqueous solution of uranyl acetate. In case of negative staining of bacteria, the same procedure was followed with log-phase live bacterial cells. Both the negatively stained OMVs and bacteria-secreting OMVs were observed under Tecnai 12 (Bio Twin Transmission Electron Microscope, FEI, the Netherlands) operating at 80 kV (Hayat & Miller, 1990).
MALDI-TOF/TOF
Standard procedure was followed for the preparation of samples for MALDI-TOF/TOF [25]. Coomassie stained SDS-PAGE gel pieces were excised. Gel pieces were processed using in-gel tryptic digestion kit (Pierce, Thermo scientific). In brief, they were reduced with Tris (2-carboxyethyl) phosphine in ammonium bicarbonate buffer (25 mM), incubated with iodo-acetamide in dark for 1 h and subsequently digested overnight with trypsin (100 ng/ sample) in ammonium bicarbonate buffer.
Gel pieces were washed with acetonitrile, dried and further rehydrated with trypsin containing digestion buffer. Peptides were extracted from gel, dried, and dissolved in acetonitrile (50%) in trifluoroacetic acid (0.1%). Subsequently, they were spotted on a target MALDI plate using Cyano Hydroxy Cinnamic Acid (CHCA) as a matrix and analyzed using MALDI-TOF mass-spectrometer (Applied Biosystem, Foster City, CA). Spectra were calibrated using the matrix and tryptic auto-digestion ion peaks of Calmix, a standard mixture of six peptides.
Spectral data were analyzed from PMF in combination with MS/MS spectra by searching against the database using the MASCOT (Matrix Science Ltd., London, UK) version 2.2 and basic local alignment search tool (BLAST) of ABI GPS Explorer software, version 3.6 (Applied Biosystems). For database searching the following parameters were used. Peak list-generating software: 4000 series explorer software version 3.5; taxonomy: all entries; database: MSDB version 2.1.0 dated 27.02.2005; No of entries: Database-MSDB20050227 (1942918 sequences; 629040812 residues); cleavage enzyme: trypsin; variable modifications: oxidation on methionine; fixed modification: carbamidomethylation; missed cleavages permitted: one missed cleavages; minimum signal to noise ratio (S/N): 10; peptide charge: +1; precursor mass tolerance: +/- 100 ppm; mass tolerance for the MS/MS search: +/- 0.2 Da. Significance of data was selected according to their p value (p < 0.05) where p is the probability that the observed match in a random event. Therefore, Mascot search engine is setting the threshold ions score [-10*Log(p)] on its own based on the type of analysis, number of spectra to be analyzed etc.
Animals
Seven weeks old female BALB/c mice of either sex were taken from the animal resource division of NICED, Kolkata. Female mice were caged separately as groups of 10 and maintained at a temperature of 25°C with humidity at 75%. Mice were fed sterile food and water. All the animal experiments were conducted following the standard operating procedure as outlined by Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA), Ministry of environment and forest, Government of India. The animal experimental protocol was approved by the Institutional Animal Ethical Committee of NICED with the project approval no. PRO/117/June, 2015–June, 2018.
Oral immunization
Seven weeks old female BALB/c mice were kept empty stomach for (3–4 h) hours before the immunization date, water ad libitum. Mice were immunized orally on day 0th, 14th and 28th with 25 μg of purified S. Typhi, or S. Paratyphi A OMV and a combination of both the OMVs in a 1:1 formulation in 200 μL of PBS following the protocol as explained previously as shown (S3 File).
Collection of serum and stool
Blood was collected from the lateral tail vein at different time intervals on the 0th, 14th, 21st, 28th, 35th, 78th and 90thday of first oral immunizationas shown in S3 File. The collected blood was taken in BD Microtainer (BD, NJ, USA) followed by centrifugation (1000 rpm, 10 min and 4°C).
Stools were collected aseptically from immunized and non-immunized mice at the same time intervals as blood. Stools were then homogenized in protease inhibitor (MP Biomedicals, USA)-containing PBSand centrifuged at 10000 x g for 10 min to remove the debris. The supernatant was collected and stored at -20°C for further use.
Isolation of outer membrane proteins (OMPs) of S. Typhi and Paratyphi A
Isolation of OMP was carried out as stated elsewhere with modifications [26]. For isolation of outer membrane proteins of two Salmonella strains, 50 ml of the respective strains were grown till late exponential phase. Cells were harvested (4,500 x g, 15 min, 4°C), washed once and then re-suspended in 10 mM HEPES, pH 7.5, with protease inhibitor (MP Biomedicals) and then sonicated in a Hielscher (UP100H) ultrasonic processor. Unbroken cells were removed by centrifugation (13,000 x g, 1 min). The supernatant containing the OM proteins was transferred into a new tube and centrifuged again (13,000 x g, 30 min, 4°C). The pellet was re-suspended in 0.8 ml of 10mM HEPES, pH 7.5, plus 1% sarcosyl (Sigma) and incubated for 30 min. After centrifugation (13,000 x g, 30 min, 4°C), the pellet was washed once with 1 ml of 10 mM HEPES, pH 7.5, and re-suspended in 50 μl of 10 mM HEPES, pH 7.5. The protein concentration was determined as described above and adjusted to 2.5 μg/μl by using 10 mM HEPES, pH 7.5. Purified OM proteins were stored at -80°C for further use.
Isolation of LPS
LPS was isolated by the protocol followed previously [27]. Briefly, overnight cultures of two strains were centrifuged followed by washing with PBS. Phenol-saturated 3-[N-morpholino] propen sulfonic acid (MOPS) was added and centrifuged at 15000 x g for 20 minutes. Four volume of chilled ethanol was then added and kept at -20°C overnight. Next day, the LPS was isolated and dissolved in autoclaved, distilled water and kept at -20°C for further use.
TCA precipitation
Culture supernatant was taken out and TCA was used to precipitate the proteins dissolved in it as described elsewhere [28].
Isolation of whole cell lysate (WCL)
Bacteria were grown overnight and centrifuged at 8000 rpm for 10 minutes, washed with PBS (Sigma, USA, pH 7.4) and sonicated in a Heilcher UP100H sonicator at 100% amplitude/cycle for at least 12 cycles of 30 seconds each. Cells were then checked for membrane lysis and centrifuged at 10,000 rpm for 10 minutes. The supernatant was collected and stored at -80°C for future use.
SDS-PAGE and immunoblot
Total protein content of the OMVs along with OMV-associated LPS profile in the isolated OMVs, mature LPS and OMPs from Salmonella strains were determined by SDS-PAGE. OMVs were incubated with Proteinase-K and SDS at 60°C for 2 hours to isolate the OMV-associated LPS.80 μg of OMVs, OMPs, were boiled in SDS-PAGE buffer and all four LPS samples were boiled in LPS sample buffer. The samples were then loaded onto a 12% SDS-PAGE gel separately depending on their staining reagent. 100 V was then applied for running the gel in an AE-6530 SDS-PAGE apparatus from ATTO Corporation (Japan). The gel was then stained by either coomassie or silver stain.
Afterwards, we did immunoblot assay to determine immunogeniccomponents present in the same samples along with WCL. The samples were electrophoresed together, transferred ontonitrocellulose membrane (Bio Rad, USA) by using ATTO Corporation AE 6687 (Japan) semi-dry blot apparatus for Western blot analysis. Anti-OMV serum from immunized mice was used. Presence of immunogenic components in OMVs was further determined by using convalescent serum from mice. Here, only OMVs were electrophoresed and blotted using convalescent serum. Blots were either observed by using alkaline phosphatase substrate BCIP/NBT (MP, USA, Cat# 980621) or Amersham™ ECL™ Prime Western Blotting Detection Reagent (GE Healthcare, UK, Lot 15839044). In all the cases, same condition and equipment were being followed and used, respectively.
Dot blot assay
Dot blot analysis was done as described previously [27]. Briefly, LPS from two strains were taken and blotted directly onto a nitrocellulose membrane in different concentrations. The membrane was then washed with Tris-Buffered Saline (TBS) containing 0.1% Tween-20. The membrane was then incubated with anti-OMV anti-sera from immunized mice and anti-mouse IgG-HRP secondary antibody (Sigma, USA) successively and the blot was then finally developed by chemiluminescence.
ELISA
Different immunoglobulins; e.g. IgG and its sub-types (IgG1, IgG2a, IgG3), and IgA, sIgA and IgM were measured by ELISA as stated by Keren [29]. Briefly, disposable polystyrene micro-titer wells (Nunc, Denmark) were separately coated with two different typhoidal OMVs (5 μg/well) as shown in Table 1 and incubated for 18 h at 4°C. Wells were washed and blocked with Bovine Serum Albumin (BSA; Sigma-Aldrich, USA). After washing the wells with PBS-T (PBS with 0.5% Tween-20, Sigma-Aldrich, USA) and incubated with serially diluted serum samples, 100μL HRP-conjugated goat anti-mouse immunoglobulin was added and incubated. After washing with PBS, the substrate o-phenyl-Di-amine (OPD) was added to each well followed by stopping the reaction after 10 min by adding 100 μL of 2 N sulphuric acid. OD492 was taken afterwards. The experiments were repeated three times for each immunoglobulin, with the immunized and non-immunized serum, collected from individual mice, before, during and after immunization. The same procedure was carried out when ELISA were done against Vi-polysaccharide (Batch number: T030516, Bio-Med, India) of S. Typhi.
Measurements of CD4+, CD8+ and CD19 expression in splenic cells by flow cytometry analysis
Spleens were collected from both naïve and immunized BALB/c mice and stained for FACS analysis. Expression of CD4, CD8a and CD19 were checked by using PE-tagged antibodies from Miltenyi Biotech, Germany (CD4-PE: 130-116-509; CD8a-PE: 130-109-247; CD19-PE: 130-12-035, REA control: 130-104-613) [30]. Expression was checked using a FACS Aria II flow cytometry machine. Data was being calculated using a FACSDiva version 6.1.3 software. Percent up-regulation was measured using the following formula:
Where delta mean fluorescence intensity was the difference between delta mean fluorescence intensity of immunized–delta mean fluorescence intensity of control.
Cytokine assays
i. Splenocyte re-stimulation assay
After 1 week from the end of last immunization, splenic cells from immunized BALB/c mice were cultured for 2 hours in complete RPMI 1640 (MP Biomedicals, USA) containing 10% FBS (Gibco, Life Technologies, USA). Cells were then treated with 10 μg/ml of bivalent OMV and incubated in 37°C for 24 hours in presence of 5% CO2 [15]. Different cytokines, namely IFN-γ, IL-6 and IL-17 were then measured by cytokine ELISA kit (Invitrogen, USA).
ii. Isolation of bone marrow-derived dendritic cells (BMDCs)
Immature bone marrow was isolated from naïve BALB/c mice and kept in RPMI 1640 medium supplemented with 10% FBS, Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) until their maturation. Matured BMDCs were stimulated with 10 μg/ml of bivalent OMV for 24 hours in presence of 5% CO2 [15]. Th17-polarizing cytokines were then measured using cytokine ELISA kit (Invitrogen, USA).
iii. BMDC-splenic CD4 T cell co-culture
BMDCs were isolated from naïve BALB/c mice and cultured like mentioned above. Splenic cells from both naïve and immunized BALB/c mice were collected, CD4 T cells were isolated (Dynal® Mouse CD4 Negative Isolation Kit, Cat. No. 114.15D, Invitrogen Dynal AS, Oslo, Norway) and cultured with BMDCs at a 4:1 ratio for 8 hours in an U-bottom microtiter plate under the same condition with a stimulation of 10 μg/ml bivalent OMV [31]. Th1 and Th17-polarizing cytokines were measured using cytokine ELISA kits from Invitrogen, USA according to the manufacturer’s instructions.
Intra-peritoneal mice model for typhoidal and paratyphoidal salmonellae and determination of LD50
Mice were challenged intra-peritoneally with 1 x 103 to1 x 109 doses of challenge bacteria for the determination of LD50 value. A total of 24 mice were divided into four groups. 6 mice per group were challenged intra-peritoneallywith one of the following four bacterial concentrations: 1 x 103 CFU/ml, 1 x 105 CFU/ml, 1 x 107 CFU/ml and 1 x 109 CFU/ml. The mice were then kept for 7 days to record mortality based on humane endpoint analysis. In all cases, including survival analyses, the following points were considered while determining the humane endpoint: reduction in body weight (more than equal to 20% than that of the healthy animals), with severe lethargy, reduction in rectal temperature (more than equal to 3 degree C than that of the healthy animal). Mice were then kept for 4 more hours to observe whether their health situation can revert back to normal. After the elapse period, they were euthanized using Ketamin hydrochloride via parenteral route. This preliminary study on the mortality of mice indicated the LD50 value must lie somewhere between 1 x 107 CFU/ml and 1 x 109 CFU/ml. So, we then challenged a group of 12 mice (which were divided into 2 separate groups; i.e. 6 in each group) fresh mice with the following concentrations of bacteria: 2 x 107 CFU/ml and 2 x 108 CFU/ml. After finding the LD50 value to be 2 x 107 CFU/ml for both the challenge strains, mice were infected intra-peritoneally [32] with LD50 dose to check the systemic spread of the bacteria. They were kept in normal condition in the animal cages. The infected mice were also observed for physiological changes in their body such as hunched back, lethargy, piloerection etc. Internal organs like spleen and liver were isolated on 1 to 5 dayspost infection (1–5 dpi) for the detection of bacteria in the organs. Colonization in small, large intestine and stool were also evaluated. After the proper repetition and development of this mice model, we moved forward towards functional protective efficacy studies. In all the cases, remaining mice were euthanized at the end of the experiment. Mice were checked frequently at a 1-hour interval.
Protective efficacy studies
i. Challenge study
In challenge study, we have taken three groups of mice, 10 animals per group. The animals of each non-immunized, immunized and PBS group were challenged intra-peritoneally with (>LD50) 1 x 108 CFU/ml of heterologous virulent bacteria after one week of last immunization. After challenge, the infected mice were kept for 9 days for monitoring and survival and colonization studies. There was one more group, namely the PBS-challenged group which was inoculated with 100 μl PBS and kept for 9 days. The same system was followed for mice when they were challenged with sub-lethal dose (3 x 105 CFU/ml) heterologous typhoidal strains. Spleen and liver were isolated, homogenized, serially diluted and plated to see the bacterial content of the infected animals 3 Days Post Infection (DPI). Bacterial load in small intestine was evaluated on 3–5 DPI from 3 mice per group.
ii. Adoptive transfer
Serum and spleen from immunized and non-immunized mice were obtained 1 week after the last immunization. For serum transfer, each naïve mouse was injected via tail vein with 100 μl from either OMV-immunized or non-immunized mice. For splenocyte transfer, spleens were disassociated with a cell strainer and a sterile plunger from a 10-ml syringe. After the R.B.C.s were lysed by R.B.C. lysis solution (Sigma-Aldrich, USA), splenocytes were re-suspended in PBS and 100 μl of this cell suspension (containing 1 x 106 splenocytes) was injected in naïve mice via tail vein. Mice were then challenged intra-peritoneally with heterologous strains of the same species at a concentration of 1 x 106 CFU/ml to observe the passive protection, if any. One group of mice was challenged on the same day of the adoptive transfer and the other group was challenged after seven days. Spleen and liver were isolated on 3rd day post-infection, homogenized, serially diluted and plated as indicated before.
Serum agglutination
Challenge strains were transformed with GFP plasmid. 1 x 108 CFU/ml log-phase GFP-tagged challenge cells were incubated with heat-inactivated non-immunized and immunized sera at 37°C for 1 hour for classical serum agglutination to occur. Visible clumps were then observed under fluorescence microscope.
Motility assay
Motility assay was done as previously described, with modifications [33]. Briefly, heat-inactivated immunized and non-immunized serum samples were mixed with PBS at a concentration of 1:400 and poured on soft agar (0.3%) plates. The plates were kept for an hour to get the serum mixed PBS to soak in the plate. After the plates became dry, log-phase bacteria (OD600 = 0.8) were pricked in the middle of the plate. The plates were then incubated at 37°C for 24 hours.
Mucin penetration assay
The assay was performed according to previously described protocol [34]. In brief, 1% mucin columns were prepared in1 ml syringes. Bacteria were incubated with heat-inactivated non-immunized and immunized sera for 1 hour at 37°C. Cultures were then taken and 100 μl of culture containing equal number bacteria (1 x 107 CFU/ml) were added from the top of 1% mucin columns. Columns were then kept at 37° C under static conditions. After 30 min of incubation 500 μl fractions were collected from the bottom of the columns, serially diluted and plated onto TSA to measure the bacterial count.
Opsonization assay
Opsonization by peritoneal macrophage was done as described previously [35]. Briefly, mouse peritoneal macrophages were harvested by flushing the peritoneal cavity of BALB/c mice with ice-cold PBS. Collected cells were then centrifuged and re-suspended in pre-warmed RPMI (Gibco, USA) media supplemented with 10% FBS. Approximately, 5 x 105cells were seeded to each well in 12-well plates and cultivated in CO2-incubator (Thermo Fisher Scientific, USA) for 2 hours at 37°C. Cells were washed and then log-phase bacteria (either immune serum-treated or non-immune serum-treated) were added at a M.O.I. of 1:100 and were kept for 30 minutes. Cells were washed with PBS and incubated with pre-warmed RPMI media for another 30 minutes. Then the cells were washed and incubated in pre-warmed RPMI media for 0 and 60 minutes. Finally, the cells were lysed with 0.1% Triton X-100 and serially diluted and plated.
Statistical analysis
In the majority of cases, the data presented are not normally distributed due to biological variation. Therefore, non-parametric tests were used for all data analysis. Comparison between two categorical variables was made using the two-tailed student’s t test. Comparison between multiple categorical variables was made using the one-tailed student’s t test. Each experiment was repeated at least three times. A P value of either <0.05 or <0.01 were considered significant. GraphPad Prism 5 and Microsoft Excel, both were used in Windows OS for all the statistical analyses.
Results
Basic characterization of salmonella strains used in the study
As our strains were clinical isolates, we wanted to be assured about their genotypic and phenotypic properties. Presence of genus-specific prtA in all four strains and presence of fliC-j in S. Typhi strains and fliC-a in S. Paratyphi A strains were observed. Presence of FliC-j was determined by PCR-based method, whereas only FliC-d was observed via serological studies. This has been reported previously and perfectly coincides with a previous data and supports the genotypic evidence that our strains were indeed typhoidal salmonellae as shown in S1 File [18, 36, 37]. Very small change in gene level may sometimes be unrecognizable in serological motility assays, which increases the chance of a false result (S1 File) [35, 38]. But since our objective was just to prove that the strains are indeed either S. Typhi or S. Paratyphi A, we have not done further classifications.
Isolation and characterization of S. Typhi and S. Paratyphi A OMVs
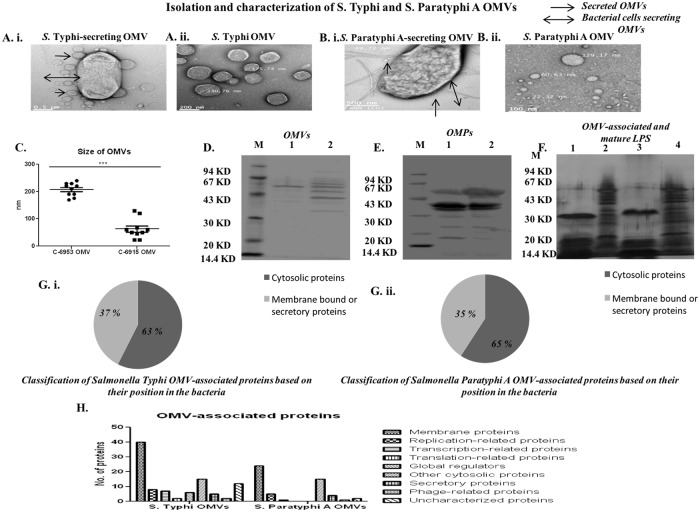
Both S. Typhi and Paratyphi A secrete OMVs during log phase of growth (Fig 1Ai and 1Bi). In electron microscopic analysis, we found different size of isolated OMVs. The sizes of these OMV’s were distributed between 130–300 nm (Fig 1Aii and 1Bii) and the S. Typhi OMVs were relatively larger in size in comparison to the S. Paratyphi A (Fig 1C). Then, we quantified the protein and O antigen of both OMVs and we found both OMVs contained nearly 400 μg/ml protein and 250 μg/ml O-Ag as shown in Table 1 above. Furthermore, SDS-PAGE analysis showed protein bands of 40KD to 62KD in case of S. Typhi OMVs, whereas, bands of 33KD to 73KD were shown in case of S. Paratyphi A OMVs. Other than that, faint bands were observed in both cases (Fig 1D). We further evaluated the native OMPs of these two typhoidal salmonellae to compare the protein profile with isolated OMVs. A vast difference in banding patterns (Fig 1E) indicated that our OMVs contain constituents other than the OMPs.
Fig 1. Isolation and characterization of OMVs.
A. i. OMVs attached to S. Typhi bacteria and A. ii. Isolated OMVs from S. Typhi. B. i. OMVs attached to S. Paratyphi A and B. ii. Isolated OMVs from S. Paratyphi A. C. Sizes of isolated OMVs.10 different OMVs were chosen from 10 different fields and their sizes were noted down. Each dot represents an individual OMVs size. D.SDS-PAGE profile of OMVs extracted from two strains of typhoidal salmonellae. Lane M: Low molecular weight marker (Bangalore GeNei), Lane 1: S. Typhi, Lane 2: S. Paratyphi A OMVs. E.SDS-PAGE profile of OMPs extracted from two strains of typhoidal salmonellae. Lane M: Low molecular weight marker (Bangalore GeNei), Lane 1: S. Typhi, Lane 2: S. Paratyphi A OMPs. F. Different natures of OMV-associated and mature LPS. Lanes 1 & 3: Proteinase K-treated OMV of S. Typhi and S. Paratyphi A, respectively. Lanes 2 & 4: Extracted mature LPS of S. Typhi and S. Paratyphi A, respectively. G. i. Pie-chart showing the presence of hypothetical cytosolic and membrane-bound proteins in S. Typhi OMVs. G. ii. Pie-chart showing the presence of hypothetical cytosolic and membrane-bound proteins in S. Paratyphi A OMVs. Both cases indicate the abundance of cytosolic proteins instead of membrane-bound proteins for both OMVs. H. Classification of OMV-associated hypothetical proteins according to their functions. The OMV-associated proteins unique to S. Typhi and Paratyphi A were grouped based on their biological processes and molecular functions, and their proportions are plotted.
We further evaluated the OMV-associated LPS after chemically neutralizing the proteins with Proteinase-K. Proteinase-K treated OMVs were found to be associated with LPS of low molecular weight which might be an effect of the change in the structure of OMVs after the treatment (Fig 1F). Lanes 1 and 3 in Fig 1F are OMV-associated LPS isolated from the OMVs. On the other hand, lanes 2 and 4 in the same figure are native, mature LPS isolated from the bacteria itself. The bands located between the core and lipid A represent LPS synthesis intermediates consisting of the core and different amounts of oligosaccharide subunits in case of native LPS preparation [39]. Literature suggests, the native LPS isolated from the bacteria itself contains O-Ag, core polysaccharide and lipid A. On the other hand, the OMV-associated LPS contain mature LPS structure and free lipid A. In case of S. Typhimurium, OMVs contain deacylated LPS, but the bacteria have hexa-acylated LPS [40]. This might be another reason of the observed change in banding pattern. This also indicates the vesicles not remaining intact once the treatment was over. This might also be the reason of the structural dissimilarity observed. As the banding pattern was much different in TCA precipitation, our immunogen must contain OMVs as a major constituent (S12 File).
Determination of the OMV proteome
The MALDI-TOF/TOF spectrums were shown in S4 and S5 Files. A total of 100 hypothetical proteins in case of S. Typhi OMVs as shown S6–S9 Files and 42 hypothetical proteins in case of S. Paratyphi A OMVs as shown in S10 and S11 Files were found. The comparison of the sub-cellular locations of the OMV protein components with that of Salmonella genome-inferred proteins revealed that OMVs derived in the exponential phase of growth cycle contains both cytosolic as well as membrane proteins (Fig 1Gi and 1Gii). The proteomic profiling of these OMVs (Fig 1H) revealed hypothetical protein compositions in the isolated OMVs, which indicates that the OMVs cargo depends on the growth conditions and provides a deeper insight into how Salmonella utilizes OMVs to adapt to environmental changes. Although the proteomic analysis does not suggest that these proteins are the sole proteins that are present in OMVs, but these are the proteins which occur most abundantly in OMVs. There might be a number of other proteins which we were unable to isolate using SDS-PAGE.
Immunogenicity of bivalent OMVs
i. Activation of humoral immune response by bivalent OMVs
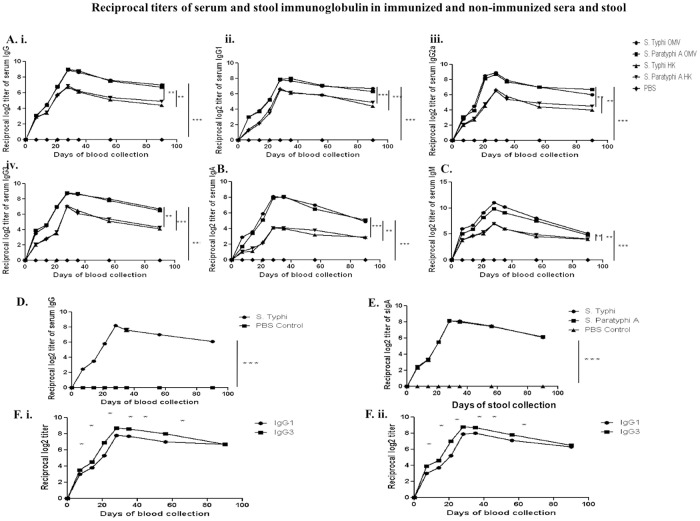
In the next experiment, we measured the generation of systemic as well as mucosal humoral immune response after oral immunization of female mice with four doses (25 μg/dose) of bivalent immunogen. Our data showed significantly higher levels of serum IgG, IgG1, IgG2a, IgG3, IgM, IgA antibodies against each OMV up to 90-days post 1st immunization. The responses differed from each other significantly (P value < 0.005). Significant higher level of IgG1, IgG2a, IgA were seen after the second dose of immunization. Although the titers were found to be above the detection level even after 90-days from day 0, the highest peaks were found to be between day 28 and day 35. Simultaneously, we compared immune responses between OMVs and heat-killed immunogen and we found bivalent OMVs are more immunogenic than heat-killed immunogen. (Fig 2A, 2B and 2C).
Fig 2. Serum immunoglobulin titers in immunized sera were separately measured against each component OMVs of bivalent OMV and heat-killed (HK) formulations.
ELISA plates were coated with either each component of OMVs or heat-killed immunization and either bivalent immunized mice serum or heat-killed immunized mice serum was used, at appropriate times. While doing ELISA against Vi-polysaccharide, ELISA plates were coated with market-available Vi-polysaccharide. A. Serum IgG (i), IgG1 (ii), IgG2a (iii), IgG3 (iv); B. Serum IgA; C. Serum IgM; D. Anti-Vi serum IgG; E. sIgA; F. i. ii. Serum IgG1 and IgG3 response against each of the two OMVs, Salmonella Typhi and Paratyphi A, respectively at pre-immunization, immunization and post-immunization periods. High serum IgG3 titer against IgG1 indicates higher Th1 cell-mediated immune response in adult mice sera after three doses of immunization. The horizontal axis indicates the days of blood collection. Data represented here are the mean values +/- Standard Deviation (SD) of three independent experiments. No statistically significant difference in overall antibody response was observed against each of the two OMVs (P values >0.05). The differences in post-immunization day wise response of each of the studied antibodies against each of the two OMVs were highly significant (P value< 0.005).
S. Typhi has a capsular Vi-polysaccharide on the outer membrane [41]. In our study, we have found that adequate amount of Vi-polysaccharide specific anti-Vi-polysaccharide serum IgG was generated after oral immunization (Fig 2D). We also observed mucosal immune response from stool diluents and found the same trend as noted before in case of serum antibodies (Fig 2E).
Moreover, our bivalent typhoidal salmonellae OMV formulation induces a higher IgG3 response than IgG1, against each component of OMVs (Fig 2Fi and 2Fii). This result indicates that, bivalent OMVs immunization elicited Th1-cell mediated immunity, manifested by higher IgG3 response than IgG1 against each component of the OMV. The overall antibody titer was increased during the immunization process and it reached a peak near 28-day post-immunization. The immunoglobulins were detectable until day 90. From, the above result we can conclude that our bivalent immunogen is immunogenic and more immunogenic than heat-killed immunogen.
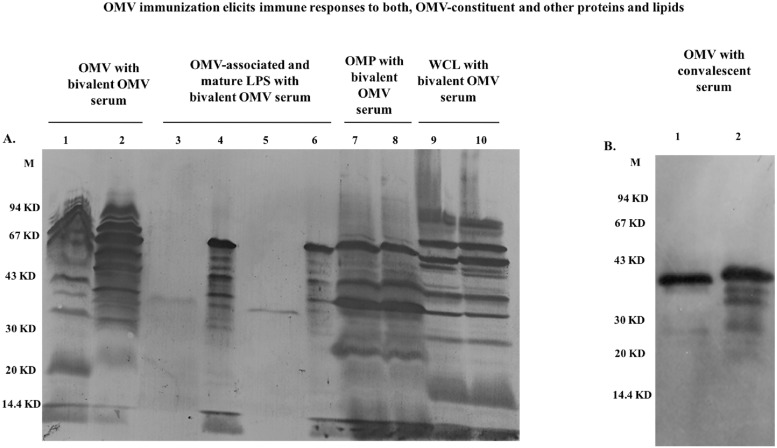
ii. Specificity of the antibody response via immunoblot
In immunoblot analysis, immune-dominant bands were seen using anti-OMVs serum (Fig 3A). Lane 1 and 2 shows immune-dominant bands from OMVs separated by SDS-PAGE. A range of proteins, starting from 20 and ending near 90 KD regions, were found to be immunogenic. Immune-dominant bands against OMV-associated and native, mature LPS was seen in lane 3, 4, 5 and 6. Bivalent OMV-immunized mice serum was also used to detect immunogenicity of mature LPS from these two strains by dot blot analysis (S12 File). Although the OMV-associated LPS and native LPS are structurally different, but they both were recognized by the polyclonal anti-OMV serum raised in mice. In case of purified outer membrane proteins of S.Typhi and Paratyphi A against OMV-immunized sera, several outer membrane proteins of S.Typhi and Paratyphi A were found to be immunogenic in nature (lane 7, 8). The last two lanes (lanes 9 and 10), are showing the immunogenic nature of our immunogen against whole cell lysate isolated from these two strains. We found a number of proteins to be immunogenic in nature. To detect immunogenic proteins of OMVs, we also generated convalescent sera in mice by infecting them separately with wild type strains of S. Typhi and Paratyphi A intra-peritoneally. We did immunoblot using these immunized sera against two OMVs and we found immune-dominant bands in the region of nearly 20–40 KD (Fig 3B). From this study, we can conclude that both OMVs contained different immunogenic proteins and also different immunogenic outer membrane proteins.
Fig 3. Representative immunoblot analysis against OMVs, proteinase K-treated OMVs, isolated OMPs, whole cell lysate (WCL) using the same anti-OMV mouse serum and immunoblot of OMVs using convalescent sera of two typhoidal strains.
A. Immunoblot against each component of the OMVs, LPS, OMPs and WCL of the bivalent formulation probed with 28th day’s anti-bivalent OMVs serum from a single mouse. Lane M: Pre-stained molecular weight marker (Bangalore Genei), Lane 1: S. Typhi, Lane 2: S. Paratyphi A OMV. Lane: 3, 5: OMV-associated LPS of S.Typhi and S. Paratyphi A, respectively. Lane 4, 6: Isolated native LPS from S.Typhi and S. Paratyphi A, respectively. Lane: 7, 8: S.Typhi and S.Paratyphi A OMPs. Lane: 9, 10: S. Typhi and S.Paratyphi A WCL. B. Immunoblot analysis against each component of the OMVs of the bivalent formulation using convalescent sera of mice. Lane: 1 and 2: S.Typhi and S.Paratyphi A OMVs.
Induction of cell-mediated immune response—Cytokines from BMDCs, splenic cells and BMDC-splenic CD4 T cell co-culture and FACS analysis
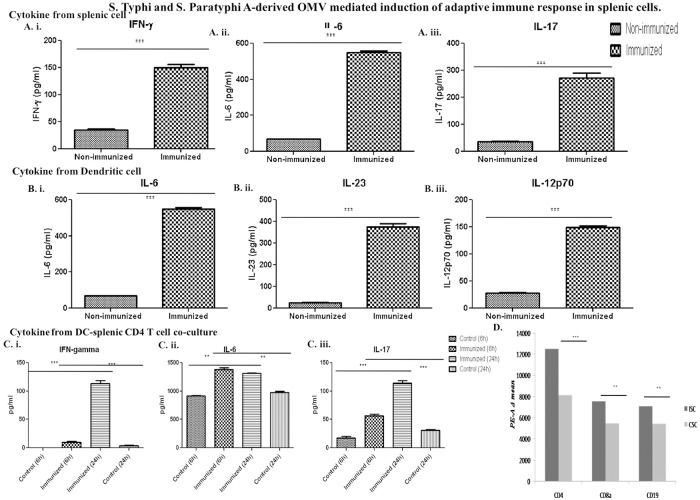
i. Production of Th1-Th17 immune response provoking cytokines were evident ex vivo
After three successive oral immunizations, on the 35th day, splenic cells were isolated from both immunized and non-immunized mice and were treated with bivalent OMVs for 24 hours in vitro at 37°C, in 5% CO2. Protein-level up-regulation of IFN- γ, IL-6 and IL-17 were found (Fig 4Ai, 4Aii and 4Aiii).
Fig 4. Bivalent OMVs induces the production of Th1 and Th17 polarizing cytokines.
A. Splenic cells after immunization and B. In naïve matured ex vivo stimulated BMDCs. Splenic cells collected from immunized mice and BMDCs were collected from non-immunized and kept in GM-CSF supplemented media until their maturation and then were treated with 10 μg/ml bivalent OMV antigens. After 24 hours, levels of IFN-γ, IL-6 and IL-17 from splenic cells and IL-6, IL-23, IL-12p70 were measured by ELISA (n = 3, each group). C.BMDCs from naïve mice were collected and incubated until maturation and splenic CD4 T cells from immunized and naïve mice collected and co-culture with the matured BMDCs. Stimulation of these co-cultured cells with the same OMVs produced significant amounts of IFN-γ, IL-6 and IL-17 which were measured by ELISA (n = 3, each group)(*** P < 0.005). D. Up-regulation of CD4, CD8a and CD19 was shown in terms of PE-A values. (n = 3, each group) (*** P < 0.005).
Matured dendritic cells were also found to be secreting significant amounts of IL-6, IL-23 and IL-12p70 (Fig 4Bi, 4Bii and 4Biii). Co-cultured matured BMDCs and splenic CD4 T cells were found to be secreting significant amounts of IFN-γ, IL-6 and IL-17 (Fig 4Ci, 4Cii and 4Ciii). All these results indicate a strong Th1-Th17-biased immune response in immunized mice following OMV immunization.
ii. FACS–up-regulation of B- and T-cell specific surface markers indicates the activation of an adaptive immune response following OMV immunization in mice
We tested the changes in expression of CD4, CD8a and CD19 on splenic cells. The expression of CD4, CD8a and CD19 was greatly increased (S13 File, Fig 4D; Table 2). This result indicates up-regulation of both humoral and cellular arms of the immune response.
Table 2. Up-regulation of different surface markers by FACS.
| Markers | CSC | Mean | Δ mean from 3 animals | STDEV of mean | ISC | Mean | Δ mean from 3 animals | STDEV of mean | Difference in Δ mean | % up-regulation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st experiment | CD4 | 8022 | 8144 | 8654 | 8273.33 | 8151.77 | 105.44 | 12908 | 12304 | 12475 | 12562.33 | 12535.11 | 62.14 | 4383.33 | 34.96 | |
| CD8a | 5754 | 5123 | 5671 | 5516 | 5503.44 | 18.93 | 7668 | 7126 | 7892 | 7562 | 7551.33 | 32.02 | 2047.88 | 27.11 | ||
| CD19 | 5475 | 5124 | 5612 | 5403.66 | 5456.55 | 45.84 | 7099 | 6830 | 7109 | 7012.66 | 7089.55 | 83.27 | 1633 | 23.03 | ||
| 1st repeat | CD4 | 7900 | 8044 | 8311 | 8085 | 13123 | 12401 | 12213 | 12579 | |||||||
| CD8a | 5500 | 5256 | 5689 | 5481.60 | 7789 | 7098 | 7843 | 7576.66 | ||||||||
| CD19 | 5322 | 5221 | 5900 | 5481 | 7121 | 6900 | 7213 | 7078 | ||||||||
| 2nd repeat | CD4 | 8245 | 7810 | 8236 | 8097 | 12823 | 12132 | 12437 | 12464 | |||||||
| CD8a | 5409 | 5651 | 5478 | 5512.60 | 7956 | 7245 | 7345 | 7515.33 | ||||||||
| CD19 | 5345 | 5145 | 5965 | 5485 | 7231 | 6846 | 7457 | 7178 | ||||||||
Values are means ± SD of triplicate biological and technical repeats.
Protective efficacy study in intra-peritoneal challenge adult mice model
We have standardized the intra-peritoneal mice model of typhoidal Salmonella. We saw a clear dose-dependent response in terms of survival of the infected mice, with the LD50 value to be 2 x 107 CFU/ml for both the challenge strains of S. Typhi and S.Paratyphi A (S14 File). Along with that, we have found strong association of dose-dependent response with colonization rate as well. In our time-dependent approach, we have found both typhoidal salmonellae strains to be present in the spleen and liver after 2–3 days.
After developing the mice model, we wanted to check how the immunogen was working. Before assessing the effectiveness of bivalent immunogen, we wanted to check the nature of protection in monovalent OMV-immunized mice. We found that, monovalent OMV-immunized mice were protected against homologous strains but not against heterologous strains (S15 File). Then we moved onto deciphering the effect of bivalent OMV.
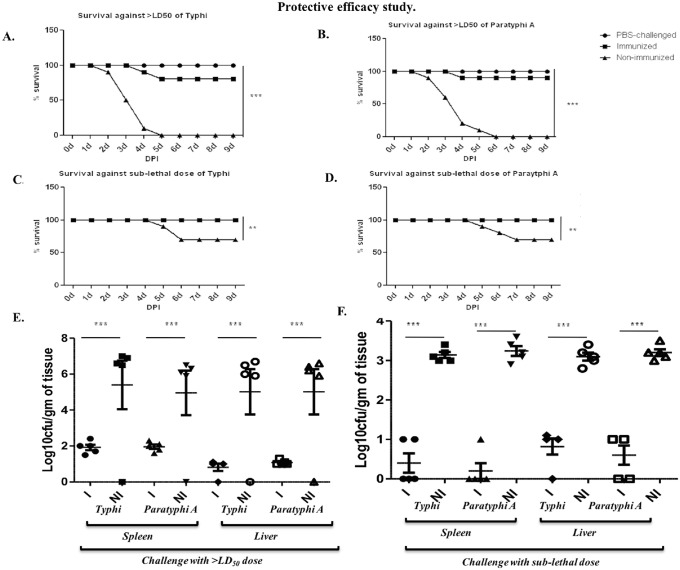
In protective efficacy study, we used the same adult mice model. Immunized and non-immunized mice were intra-peritonealchallenged with the doses described in the methods. We kept a negative control which we termed PBS-challenged to eliminate any bias. In case of non-immunized mice, all the mice died within 3–5 days (Fig 5A and 5B). But, 80% and 90% immunized mice were survived after 9 days. The sub-lethal dose, on the other hand, was only able to kill 30% mice in non-immunized challenged groups (Fig 5C and 5D). All the immunized mice survived this infection. Negligible effect was observed in terms of change in body weight of the immunized mice. Colonization data suggests the immunogen’s potent nature against systemic spread and infection of typhoidal salmonellae in mice and it indeed was protecting the mice infected with both the doses of infection (Fig 5E and 5F).
Fig 5. Immunization with the bivalent OMVs provides protection in adult mice model.
Mice were immunized with 1:1 mixture of typhoidal OMVs using 25 μg total OMVs per dose and a three-dose immunization. Mice were then challenged with 1 x 108 CFU/ml and 3 x 105 CFU/ml of each challenge strains and observed for the period of survival for 9 days. A., B. Percent survival against S. Typhi and S. Paratyphi A lethal challenge and C., D. Percent survival against S. Typhi and S. Paratyphi A sub-lethal challenge. E. An infection with lethal dose of typhoidal strains caused a huge colonization of systemic organs after both S. Typhi and S. Paratyphi A infection. F. A lower level of colonization of systemic organs was seen in mice challenged with sub-lethal dose of infection.
Reduction in body weight was observed in non-immunized mice after both lethal and sub-lethal challenges (S16 File). Although much less reduction in body weight was observed after sub-lethal challenge, a higher thanLD50 dose caused significant change in body weight. Bivalent OMV-immunized mice were shielded from the infection that occurred in the non-immunized mice (Fig 5 and S16 File). At 3 DPI, we observed 105 –108CFU/gm of infection in spleen, liver in case of non-immunized mice, whereas, 101−102 CFU/gm of infection in spleen, liver was the highest colonization found in immunized mice after higher than LD50 challenge (Fig 5E). After sub-lethal challenge lesser level of colonization was found as expected (Fig 5F). Colonization in small intestine (S17 File).
Adoptive transfer of splenocytes and sera from immunized mice protects naïve mice from typhoidal salmonellae-induced lethal infection
To find out whether humoral or T-cell induced cellular immunity is important for the protection against Salmonella, we transferred serum and splenocytes via tail vein in non-immunized mice (S18 and S19 Files). We found both immunized serumand splenocytes had protected the naïve mice almost equally. We did survival assay of these mice against challenge bacteria and found it to be 70–100% protective in both cases after challenging them on day 0. We have also measured the systemic spread of the challenge strains in adoptive transferred naïve mice. Colonization in spleen and liver were found to be in the range of 1 x 106–1 x 107 CFU/gm of the respective organs in case of control or non-immunized mice (S18 File). Whereas, immunized sera and splenocytes recipient mice showed significantly lower colonization. We challenged the other adoptively transferred mice on day 7 and a slight increase in the colonization was seen (S19 File). This result suggested that protection against Salmonella depends on both humoral and cell mediated immunity.
Mode of protection
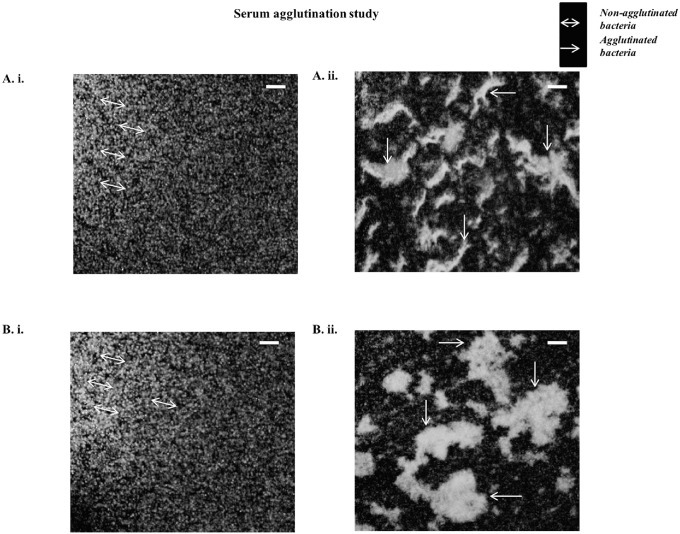
Bivalent OMV-immunized mice sera agglutinate wild-type bacteria in vitro
To check if the LPS and Vi-polysaccharide specific antibody generated by OMVs immunization can detect the bacterial outer surface of both S. Typhi and Paratyphi A we did agglutination test in presence of OMVs immunized sera and non-immunized sera (heat-inactivated). We found non-immnunized sera treated wild-type bacteria did not show any agglutination (Fig 6Ai and 6Bi). On the other hand, visible clump or bacterial agglutination in immunized sera treated group under fluorescence microscope (Fig 6Aii and 6Bii). Specific arrows were being used to indicate both non-agglutinated and agglutinated bacteria. This result indicates that; our bivalent OMV-immunized sera can activate complement mediated killing or innate immune response.
Fig 6. Heat-inactivated serum from bivalent immunized mice forms agglutination when comes in contact with the GFP-tagged clinical isolates of S. Typhi and Paratyphi A.
A. i.S. Typhi treated with heat-inactivated non-immunized serum, A. ii. S.Typhi treated with heat-inactivated immunized serum. B. i. S. Paratyphi A treated with heat-inactivated non-immunized serum, B. ii. S. Paratyphi A treated with heat-inactivated immunized serum. Bar represents 10 μm.
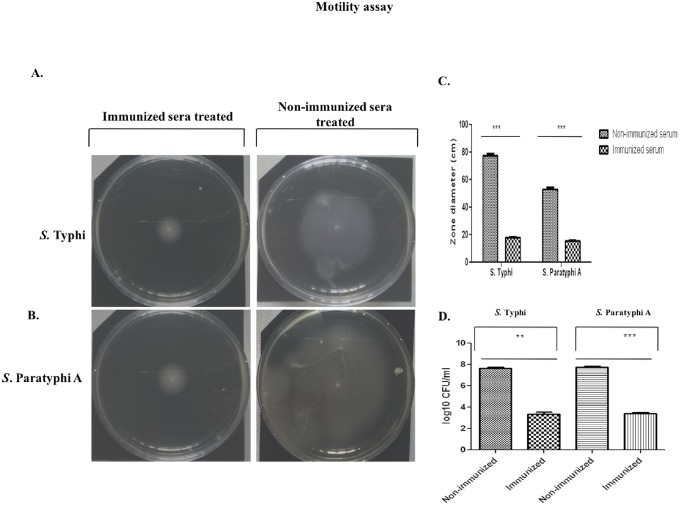
Inhibition of S. Typhi and Paratyphi A motility and mucin penetration ability in vitro by antibodies from OMV- immunized mice
In our next experiment, we did heat–inactivation of immunized sera and non-immunized sera to deactivate the complement and incubated heat-inactivated immunized and non-immunized serum with in vitro grown Salmonella strains. We found significant inhibition of the Salmonella motility. Immunized serum, compared to non-immunized serum showed significant difference in the zone of spread of bacteria (Fig 7A, 7B and 7C). This result suggests that our bivalent formulation is indeed resulted in agglutination of bacteria which could be a way towards protective nature of this immunogen.
Fig 7. Serum from bivalent immunized mice inhibits S. Typhi and Paratyphi A motility.
Serum from non-immunized or immunized mice was mixed 1:400 with PBS and was spreaded over the soft agar (0.3% agar) plates. Plates were dried and the plates were punctured at the center either with one colony of S. Typhi or with one colony of S. Paratyphi A and kept at 37°C for 24 hours. A. The spread of S. Typhi and B. S. Paratyphi A was C. Significantly reduced in case of serum collected from immunized mice D. Bacteria treated with non-immunized or immunized serum were loaded on top of the 1 ml mucin column and allowed to penetrate. Immunized serum agglutinated bacteria showed reduced ability to penetrate mucin.
Next, we investigated mucin penetration ability of both typhoidal Salmonella strains in presence of heat-inactivated immunized and non-immunized sera. We found immunized serum-treated bacteria showed lesser mucin penetration ability in the range of 2 x 103 CFU/ml to 3 x 103 CFU/ml. Whereas, the ability of penetration by non-agglutinated bacteria were much higher, in the range of 3 x 107 CFU/ml to 4 x 107 CFU/ml (Fig 7D). This data indicate that OMVs specific sera significantly inhibit motility which can inhibit mucin penetration that can help to inhibit colonization or invasion into the epithelial cells.
OMVs specific serum induces opsonization in peritoneal macrophage
The bacteria-killing ability of the isolated immunized sera was evaluated in peritoneal macrophages. Bacteria incubated with mouse anti-bivalent OMV sera were killed more efficiently by the macrophages than that of the bacteria incubated with non-immunized sera. Bivalent sera had efficiently increased phagocytosis ability, thus increasing the bacteria killing property (Table 3).
Table 3. Opsonization activity of immunized and non-immunized sera.
| Bacteria | Treated with | Mean CFU x 105 / well | Source or reference | |
|---|---|---|---|---|
| Incubation condition | ||||
| 0 minute | 60 minutes | |||
| S. Typhi C-6.946 | Immunized serum | 2.3±0.3 | 9.3±0.7** | This study |
| Non-immunized serum | 2.1±0.5 | 50.5±0.1 | ||
| S.Paratyphi A BCR 148 | Immunized serum | 2.5±0.2 | 10.4±0.8** | This study |
| Non-immunized serum | 2.6±0.4 | 53.6±0.7 | ||
Values are means ± SD of triplicate samples.
**p < 0.005 (highly significant) when compared with the respective result of non-immune sera.
All the above results suggest that both humoral and cellular immunity confer the protective effect of S. Typhi and S. Paratyphi A bivalent OMVs-immunization.
Discussion
There is an urgent need for a suitable broad spectrum typhoidal Salmonella vaccine [1]. Still no such vaccines have the ability to provide a complete protection against S. Typhi and Paratyphi A together. Moreover, no licensed vaccine is available for paratyphoid fever. One of the major bottlenecks for the development of such vaccine is lack in the identification of protective antigens. Recent study showed that secreted or surface-associated antigens of Salmonella can induce strong protective humoral and cell mediated immune response [42]. Surface associated antigens are more protective than internal antigen because of physical association with PAMPs such as lipopolysaccharide that can activate both innate and adaptive immunity.
Recent studies demonstrated that OMVs of gram-negative bacteria are one of the promising candidate vaccines [43, 44, 45, 46, 47, 48]. The composition of OMVs reflects outer membrane structure of the bacteria. So, we have isolated the OMVs from S. Typhi and Paratyphi A and characterized them. We found that both the OMVs contained LPS, different outer membrane, inner membrane, cytosolic and virulence proteins. Our study indicates that, OMV-constituents bridge their potent immunogenic nature with protective immuneresponse against enteric fever. To develop a complete broad range of protective immune response against both S. Typhi and Paratyphi A, we have designed a bivalent OMV-based vaccine and measured protective immune response in mice model. After oral immunization of three doses of bivalent Salmonella OMVs immunogen, we found antigen specific antibody responses, namely, anti-LPS, anti-Vi polysaccharide, anti-OMPs, anti-WCL. This result indicated that our formulation was immunogenic in nature. Furthermore, our data also indicates that OMVs are more immunogenic than heat-killed immunogen generating higher response for the production of antigen specific antibodies. Our results have also suggested that OMVs have an ability to induce humoral as well as cell mediated immune response. We found that after oral immunization, the populations of CD4 T and CD8 T cells in spleen were significantly increased. That data indicates that our immunogen activates T cell-mediated immune response. From this study, we can conclude that our immunogen significantly induce adaptive immune response; both humoral and cell mediated immune responses which are very crucial for generating long term protective immune response. The notion that, generation of both humoral and cellular arms of the immune system is necessary for the generation of long-term immune response was supported by previous studies as well [49, 50, 51]. LPS and Vi-polysaccharide are crucial antigenic components of typhoidal vaccine [52, 53]. Both LPS and Vi-polysaccharides are T–independent antigens [54]. Vi-polysaccharide typhoidal vaccine generates Vi-polysaccharide specific IgG by T-independent manner. For this reason, Vi-polysaccharide based vaccine was unable to generate significant long term protection (only 55% of the population was protected) [55]. Further studies showed that conjugated Vi-polysaccharide vaccine is more effective to generate long term protection than the native one [56, 57]. Vi-polysaccharide was conjugated with tetanus toxoid which induces significant T-cell depended immune response [56, 57]. Recent study showed that different outer membrane proteins, such as, OmpF, OmpC, OmpS1 and OmpS2 of S.Typhi are immunogenic and acts like adjuvant, they also induce T-dependent immune response [58]. Our proteomics data and immunoblot result showed that OMVs contained several outer membrane proteins and they are immunogenic. This data indicates that proteins of OMVs act as an adjuvant which can significantly induce long term Vi-polysaccharide and LPS specific antibodies in a T-depended manner.
On the other perspective, cell-mediated immune responses are necessary to prevent intracellular Salmonella infection and spreading [59]. In our study, we found our bivalent immunogen to be significantly producing Th1 and Th17 polarizing cytokine which indicate activation of innate and cell mediated immune response [60, 61, 62]. Our result showed that our immunogen significantly induces Th1 and Th17 mediated immune response; generating significant amount of IFN-γand IL-17. BMDCs-splenic CD4 T cell co-culture could actually mimic the condition of antigen presentation and up-regulation of different arms of the immune system. Elevation of IFN-γ and IL-12 indicates that the immune response may activate both CTL and Th1-response. IL-6 has a reputation of bridging the gaps between innate and adaptive immune response as well [63]. Up-regulation of CD4, CD8a and CD19+ cells indicate the activation of both B- and T-cells of the immune system [64]. Th1 cell mediated immune response is very crucial for Salmonella specific protective immune response [65]. A study showed that less IFN-γ producing mice are more susceptible to Salmonella Typhimurium infections compared with wild-type mice [66]. IFN-γ has a distinct role to activate macrophage for the clearance of invasive bacteria [67, 68]. Furthermore, IL-17-producing Th17 cells are necessary for vaccine-induced protection against bacterial infection by enhancing neutrophil recruitment to infection sites and direct bacterial killing property [69]. Both IL-17 and IL-22 are produced in the intestinal mucosa early after oral Salmonella infection [70]. After Salmonella infection, IL-17 deficient mice demonstrate a modest increase in bacterial dissemination, suggesting that IL-17 contributes to the maintenance of the mucosal barrier [71]. Indeed, depletion of intestinal CD4+ T cells that accompanies simian immunodeficiency virus infection selectively blunted the intestinal IL-17 response in rhesus macaques, allowing increased translocation of Salmonella to the mesenteric lymph nodes and spleen [72]. Thus, although Th1 cells are critical for bacterial clearance in systemic tissues, Th17 cells most likely play an important additional protective role in preventing bacterial dissemination from the intestine.
In the protective study, we have challenged the immunized and non-immunized mice intraperitoneally with lethal dose of lethal wild type Salmonella Typhi and Paratyphi A strains. This mice model is wildly used and most acceptable mice model from others like iron overload model due to the reproducibility and easy for the vaccine study for Typhi infection (32). We found almost 80–90% survival rate in immunized group and the bacterial burden in different organs of immunized group were significantly less in compare in immunized group in compare with non-immunized group after challenge. We can conclude that our formulated immunogen can provide broader range of protection against typhoidal fever.
Furthermore, we have checked the effect of both humoral and cell mediated immune response on protective efficacy by adoptive transfer of serum and spenocytes [73, 74]. This finding suggested that both humoral and cell mediated immune responses are crucial to induce protection against typhoidal Salmonella infection. Different serum transfer studies suggested that Salmonella specific antibodies are essential to complement fixation or opsonization of free bacteria [75]. Recent study showed that B cell-deficient mice did not produce Th1 and protective immune response after oral challenge with wild type S. Typhimurium strains and protective immune response cannot be restored by adoptive transfer of Salmonella specific antibodies [76]. Previous study also showed that B cells function as antigen presenting cells and an important source of inflammatory cytokines during Salmonella infection [77, 78]. So, both B and T cells are essential for Salmonella-specific immunity. Furthermore, much less colonization in the liver of immunized animals could be a result of neutrophils playing their part in providing protection [79]. In future, we will focus on which immune responses (B or T cells) is essential for clearance of diseases burden using different gene knock-out mice for direct evidence.
Here, we have further checked the mode of action of OMV specific antibody on S. Typhi and Paratyphi A. We found that OMV specific sera can agglutinate both S. Typhi and Paratyphi A which indicates the activation of complement mediated killing [80]. Agglutination was found to be occurring even after heat inactivation of complement in the sera which then helped in the inhibition of motility. Our result showed that OMV specific antibody significantly inhibits motility of S. Typhi and Paratyphi A. Bacterial motility is very crucial for penetrating the mucin layer, attachment and invading the intestinal epithelial cells [81]. Recent studies showed that V. cholerae OMV specific antibody significantly reduce adherence by inhibiting V. cholerae motility [82]. So, our data indicate that our immunogen also induced protective immune response by blocking initial bacterial entry via intestinal epithelial cells. Moreover, we found that typhoidal Salmonella OMV specific antibody enhance the macrophage mediated opsonization for killing the invasive strain. Overall our data suggest that our immunogen significantly induce first line defense of immune system to block and for clearance of typhoidal Salmonella infection.
Conclusions
S. Typhi and S.Paratyphi A naturally secrets OMVs during their growth cycle. These OMVs are rich in outer-, inner-membrane associated and cytosolic proteins as well as LPS. 1:1 mixture of these typhoidal Salmonella OMVs is immunogenic in nature. Up-regulation of different cytokines and surface marker expression proves the elevation of both humoral and cellular arms of the immune response in mice model. These two arms of the immune response play together in providing protection in adult intra-peritoneal mice model. This bivalent formulation can be used in future in phase-I trial.
Supporting information
All strains were characterized by both serotyping and PCR-based methods. Specific primers and anti O- and H-antigens were used for this purpose.
(TIF)
Antibiotic susceptibility pattern was tested by Kirby-Bauer disc diffusion assay.
(TIF)
Mice were immunized by oral gavage on day 0 and then two subsequent booster doses follow as stated. Mice were then challenged on day 35 via intra-peritoneal infection model. Blood were drawn as shown by the dotted arrows.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
A. Dot blot analysis against extracted LPS from two typhoidal strains. Lane 1: S. Typhi native LPS, Lane 2: S. Paratyphi A native LPS. Here, 1, 2, and 3 denotes three different concentrations of LPSs against which the dot blot analysis was performed. B.TCA precipitation of culture supernatant. Lane 1: S. Typhi culture supernatant, Lane 2: S. Paratyphi A culture supernatant.
(TIF)
50,000 events in total population were taken in each and every case and the mean value of area under PE in P2 population was taken into account whilst calculating the result. i. Unstained cells, ii., iii., iv. Up-regulation of CD4, CD8a and CD19, respectively in immunized and control mice’s spleen. v. REA-cloned Iso-Type Control (ITC) for all three markers.
(TIF)
6 mice were per group were challenged with different concentrations of bacteria. Mice were observed for 7 days. The effective dose for LD50 or 50% mortality rate was found to be 2 x 107 CFU/ml for both the challenge strains.
(TIF)
A., B. Colonization in spleen and liver in S. Typhi OMV-immunized mice were less when they were challenged with S. Typhi. C. D.Colonization in spleen and liver in S. Paratyphi A OMV-immunized mice were less when they were challenged with S. Paratyphi A. In both cases,monovalent OMV immunization could not be able to hinder the infection from a heterologous strain.
(TIF)
A., B. A drastic change in body weight was seen in non-immunized mice challenged with >LD50 dose of infection. C. D. A lower level ofchange in body weight was seen in mice challenged with sub-lethal dose of infection.
(TIF)
Three mice per group were challenged either sub-lethal dose or with greater than LD50 dose. Bacterial colonization in the small intestine was estimated since 3DPI until 5DPI. Higher colonization was observed in mice group challenged with greater than LD50 dose.
(TIF)
Both B- and T-cell mediated immune response induced by OMVs is necessary for the protective immunity to bacterial infection. Splenocytes (1 x 106) and serum (100 μl) were injected via the tail vain. One group of mice was challenged with 2 x 107 CFU/ml of heterologous strains of bacteria after two hours of the adoptive transfer and kept for three days. A., B. Systemic infection of S. Typhi in spleen and liver was observed in the early group 3 days post infection. Similarly, C. D. Systemic infection of S. Paratyphi A in spleen and liver was observed 3 days post infection.
(TIF)
This group was challenged with the same dose as mentioned above after seven days of the adoptive transfer. A., B. Systemic infection of S. Typhi in spleen and liver wasobserved 3 days post infection. Similarly, C., D. Systemic infection of S. Paratyphi A in spleen and liver was observed 3 days post infection.
(TIF)
Acknowledgments
We are grateful to Ms. Rhishita Chourashi, Mr. Sandip Chakraborty, Mr. Subrata Singha for their technical and meaningful help.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was done with very limited funding from Indian Council of Medical Research (ICMR), India and the first author only receives his fellowship from Council of Scientific and Industrial Research (CSIR), India and a marginal 20,000 INR as a contingency grant per year. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010. January 15;50(2):241–6. 10.1086/649541 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John J, Van Aart CJ, Grassly NC. The Burden of Typhoid and Paratyphoid in India: Systematic Review and Meta-analysis. PLoSNegl Trop Dis. 2016. April 15;10(4):e0004616. 10.1371/journal.pntd.0004616 eCollection 2016 Apr. Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teh CS, Chua KH, Thong KL. Paratyphoid fever: splicing the global analyses. Int J Med Sci. 2014. May 14;11(7):732–41. 10.7150/ijms.7768 eCollection 2014. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur J, Jain SK. Role of antigens and virulence factors of Salmonella entericaserovarTyphi in its pathogenesis. Microbiol Res. 2012. April 20;167(4):199–210. 10.1016/j.micres.2011.08.001 Epub 2011 Sep 25. Review. . [DOI] [PubMed] [Google Scholar]
- 5.Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res. 2012;135:161–9. Review. [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee D, Chaudhuri K. Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 protein-dependent manner and induce dendritic cell-mediated Th2/Th17 cell responses. J Biol Chem. 2013. February 8;288(6):4299–309. 10.1074/jbc.M112.408302 Epub 2012 Dec 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schild S, Nelson EJ, Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun. 2008. October;76(10):4554–63. 10.1128/IAI.00532-08 Epub 2008 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009. January;77(1):472–84. 10.1128/IAI.01139-08 Epub 2008 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop AL, Tarique AA, Patimalla B, Calderwood SB, Qadri F, Camilli A. Immunization of mice with vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission. J Infect Dis. 2012. February 1;205(3):412–21. 10.1093/infdis/jir756 Epub 2011 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop AL, Schild S, Patimalla B, Klein B, Camilli A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect Immun. 2010. October;78(10):4402–20. 10.1128/IAI.00398-10 Epub 2010 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roier S, Fenninger JC, Leitner DR, Rechberger GN, Reidl J, Schild S. Immunogenicity of Pasteurellamultocida and Mannheimiahaemolytica outer membrane vesicles. Int J Med Microbiol. 2013. July;303(5):247–56. 10.1016/j.ijmm.2013.05.001 Epub 2013 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oster P, Lennon D, O’Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005. March 18;23(17–18):2191–6. 10.1016/j.vaccine.2005.01.063 . [DOI] [PubMed] [Google Scholar]
- 13.Holst J, Oster P, Arnold R, Tatley MV, Næss LM, Aaberge IS, Galloway Y, McNicholas A, O’Hallahan J, Rosenqvist E, Black S. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum VaccinImmunother. 2013. June;9(6):1241–53. 10.4161/hv.24129 Epub 2013 Mar 7. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy N, Barman S, Ghosh A, Pal A, Chakraborty K, Das SS, Saha DR, Yamasaki S, Koley H. Immunogenicity and protective efficacy of Vibrio cholerae outer membrane vesicles in rabbit model. FEMS Immunol Med Microbiol. 2010. October;60(1):18–27. 10.1111/j.1574-695X.2010.00692.x . [DOI] [PubMed] [Google Scholar]
- 15.Sinha R, Koley H, Nag D, Mitra S, Mukhopadhyay AK, Chattopadhyay B. Pentavalent outer membrane vesicles of Vibrio cholerae induce adaptive immune response and protective efficacy in both adult and passive suckling mice models. Microbes Infect. 2015. March;17(3):215–27. 10.1016/j.micinf.2014.10.011 Epub 2014 Nov 15. . [DOI] [PubMed] [Google Scholar]
- 16.Mitra S, Barman S, Nag D, Sinha R, Saha DR, Koley H. Outer membrane vesicles of Shigellaboydii type 4 induce passive immunity in neonatal mice. FEMS Immunol Med Microbiol. 2012. November;66(2):240–50. 10.1111/j.1574-695X.2012.01004.x Epub 2012 Aug 1. . [DOI] [PubMed] [Google Scholar]
- 17.Mitra S, Chakrabarti MK, Koley H. Multi-serotype outer membrane vesicles of Shigellae confer passive protection to the neonatal mice against shigellosis. Vaccine. 2013. June 28;31(31):3163–73. 10.1016/j.vaccine.2013.05.001 Epub 2013 May 15. . [DOI] [PubMed] [Google Scholar]
- 18.Hirose K, Itoh K, Nakajima H, Kurazono T, Yamaguchi M, Moriya K, Ezaki T, Kawamura Y, Tamura K, Watanabe H. Selective amplification of tyv (rfbE), prt(rfbS), viaB, and fliC genes by multiplex PCR for identification of Salmonella entericaserovarsTyphi and Paratyphi A. J ClinMicrobiol. 2002. February;40(2):633–6. 10.1128/JCM.40.2.633-636.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patrick A.D. Grimont& François-Xavier Weill. Antigenic formulae of the Salmonellaserovars. WHO Collaborating Centre for Reference and Research on Salmonella. 2007 9th Edition.
- 20.Kauffmann F. Salmonella In: Kauffmann F, editor. The bacteriology of Enterobacteriaceae. Collected studies of the author and his co-workers. Copenhagen: Munksgard; 1966. p. 53−304. [Google Scholar]
- 21.Srinivasan VB, Singh BB, Priyadarshi N, Chauhan NK, Rajamohan G. Role of novel multidrug efflux pump involved in drug resistance in Klebsiella pneumoniae. PLoS One. 2014. May 13;9(5):e96288 10.1371/journal.pone.0096288 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.External Quality Control System (EQAS). Global Foodborne Infections Network (GFN). World Health Organization (WHO), 2017. [Google Scholar]
- 23.Jaiswal A, Sarkar S, Das P, Nandy S, Koley H, Sarkar B. Trends in the genomic epidemiology of Vibrio cholerae O1 isolated worldwide since 1961. Int J Antimicrob Agents. 2015. October;46(4):460–4. 10.1016/j.ijantimicag.2015.06.012 Epub 2015 Jul 22. . [DOI] [PubMed] [Google Scholar]
- 24.DuBois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956. 28 (3), 350–356. [Google Scholar]
- 25.Khatua B, Van Vleet J, Choudhury BP, Chaudhry R, Mandal C. Sialylation of outer membrane porin protein D: a mechanistic basis of antibiotic uptake in Pseudomonas aeruginosa. Mol Cell Proteomics. 2014. June;13(6):1412–28. 10.1074/mcp.M113.030999 Epub 2014 Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobb RI, Fields JA, Burns CM, Thompson SA. Evaluation of procedures for outer membrane isolation from Campylobacter jejuni. Microbiology. 2009. March;155(Pt3):979–88. 10.1099/mic.0.024539-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee P, Raychaudhuri S, Nag D, Sinha R, Howlader DR, Mukhopadhyay AK, Koley H. Evaluation of immunogenicity and protective efficacy of combination heat-killed immunogens from three entero-invasive bacteria in rabbit model. Immunobiology. 2016. August;221(8):918–26. 10.1016/j.imbio.2016.03.002 Epub2016 Mar 18. . [DOI] [PubMed] [Google Scholar]
- 28.Hwang BJ, Chu G. Trichloroacetic acid precipitation by ultracentrifugation to concentrate dilute protein in viscous solution. Biotechniques. 1996. June;20(6):982–4. . [DOI] [PubMed] [Google Scholar]
- 29.Keren DF. Enzyme-linked immunosorbent assay for immunoglobulin G and immunoglobulin A antibodies to Shigellaflexneri antigens. Infect Immun. 1979. May;24(2):441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez T, Pérez O, Ménager N, Ugrinovic S, Bracho G, Mastroeni P. Interactions of proteoliposomes from serogroup B Neisseria meningitides with bone marrow-derived dendritic cells and macrophages: adjuvant effects and antigen delivery. Vaccine. 2005. January 26;23(10):1312–21. 10.1016/j.vaccine.2004.07.049 . [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol. 2011. February;89(2):235–49. 10.1189/jlb.0310154 Epub 2010 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paliwal PK, Bansal A, Sagi SS, Sairam M. Intraperitoneal immunization of recombinant HSP70 (DnaK) of Salmonella Typhi induces a predominant Th2 response and protective immunity in mice against lethal Salmonella infection. Vaccine. 2011. September 2;29(38):6532–9. 10.1016/j.vaccine.2011.07.005 Epub 2011 Jul 19. . [DOI] [PubMed] [Google Scholar]
- 33.Turnbull L, Whitchurch CB. Motility assay: twitching motility. Methods Mol Biol. 2014;1149:73–86. 10.1007/978-1-4939-0473-0_9 . [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Wang Y, Liu S, Sheng Y, Rueggeberg KG, Wang H, Li J, Gu FX, Zhong Z,Kan B, Zhu J. Vibrio cholerae represses polysaccharide synthesis to promotemotility in mucosa. Infect Immun. 2015. March;83(3):1114–21. 10.1128/IAI.02841-14 Epub 2015 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Kalev OK, Balabanova MS, Mitov IG. Protective efficacy of IgA monoclonal antibodies to O and H antigens in a mouse model of intranasal challenge with Salmonella enterica serotype Enteritidis. Microbes Infect. 2004. August;6(10):901–10. 10.1016/j.micinf.2004.05.007 . [DOI] [PubMed] [Google Scholar]
- 36.Frankel G, Newton SM, Schoolnik GK, Stocker BA. Intragenic recombination in a flagellin gene: characterization of the H1-j gene of Salmonella typhi. EMBO J.1989. October;8(10):3149–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joys TM. The covalent structure of the phase-1 flagellar filament protein of Salmonella typhimurium and its comparison with other flagellins. J Biol Chem. 1985. December 15;260(29):15758–61. . [PubMed] [Google Scholar]
- 38.Kuwajima G. Flagellin domain that affects H antigenicity of Escherichia coli K-12. J Bacteriol. 1988. January;170(1):485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidhu VK, Vorhölter FJ, Niehaus K, Watt SA. Analysis of outer membrane vesicle associated proteins isolated from the plant pathogenic bacterium Xanthomonascampestrispv. campestris. BMC Microbiol. 2008. June 2;8:87 10.1186/1471-2180-8-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elhenawy W, Bording-Jorgensen M, Valguarnera E, Haurat MF, Wine E, Feldman MF. LPS Remodeling Triggers Formation of Outer Membrane Vesicles in Salmonella. MBio. 2016. July 12;7(4). 10.1128/mBio.00940-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmadi H, Tabaraie B, Maleknia S, Shapouri R, Nejati M, Pour Mirza Gholi F, Hedayati M, Sadati M, Zahednia S, SharifatSalmani A. Immunological evaluation of Vi capsular polysaccharide of Salmonella enterica subsp. Typhi vaccine by serum bactericidal assay. J Med Microbiol. 2013. February;62(Pt 2):283–6. 10.1099/jmm.0.047159-0 Epub 2012 Oct 25. . [DOI] [PubMed] [Google Scholar]
- 42.Barat S, Willer Y, Rizos K, Claudi B, Mazé A, Schemmer AK, Kirchhoff D, Schmidt A, Burton N, Bumann D. Immunity to intracellular Salmonella depends on surface-associated antigens. PLoSPathog. 2012;8(10):e1002966 10.1371/journal.ppat.1002966 Epub 2012 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015. September;10(11):1689–706. 10.1002/biot.201400395 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins BS. Gram-negative outer membrane vesicles in vaccine development. Discov Med. 2011. July;12(62):7–15. Review. . [PubMed] [Google Scholar]
- 45.Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, Putnam D. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl AcadSci U S A. 2010. February 16;107(7):3099–104. 10.1073/pnas.0805532107 Epub 2010 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen I, Amano A. Outer membrane vesicles—offensive weapons or good Samaritans? J Oral Microbiol. 2015. April 1;7:27468 10.3402/jom.v7.27468 eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SB, Jang HB, Nho SW, Cha IS, Hikima J, Ohtani M, Aoki T, Jung TS. Outer membrane vesicles as a candidate vaccine against edwardsiellosis. PLoS One. 2011. March 8;6(3):e17629 10.1371/journal.pone.0017629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv. 2017. September;35(5):565–574. 10.1016/j.biotechadv.2017.05.003 Epub 2017 May 15. Review. . [DOI] [PubMed] [Google Scholar]
- 49.Amanna IJ, Slifka MK. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011. March 15;411(2):206–15. 10.1016/j.virol.2010.12.016 Epub 2011 Jan 8. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaplin DD. Overview of the immune response. J Allergy ClinImmunol. 2010. February;125(2 Suppl 2):S3–23. 10.1016/j.jaci.2009.12.980 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, Lee CY. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis. 2008. May 15;197(10):1419–26. 10.1086/587695 . [DOI] [PubMed] [Google Scholar]
- 52.Mekara Y, Maneekarn N, Vithayasai V, Makonkawkeyoon S. Determination of antibody from typhoid patients against lipopolysaccharide and protein antigens of Salmonella typhi. Asian Pac J Allergy Immunol. 1990. December;8(2):95–101. . [PubMed] [Google Scholar]
- 53.Ochiai RL, Khan MI, Soofi SB, Sur D, Kanungo S, You YA, Habib MA, Sahito SM, Manna B, Dutta S, Acosta CJ, Ali M, Bhattacharya SK, Bhutta ZA, Clemens JD. Immune responses to Vi capsular polysaccharide typhoid vaccine in children 2 to 16 years old in Karachi, Pakistan, and Kolkata, India. Clin Vaccine Immunol. 2014. May;21(5):661–6. 10.1128/CVI.00791-13 Epub 2014 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall JL, Flores-Langarica A, Kingsley RA, Hitchcock JR, Ross EA, López-Macías C, Lakey J, Martin LB, Toellner KM, MacLennan CA, MacLennan IC, Henderson IR, Dougan G, Cunningham AF. The capsular polysaccharide Vi from Salmonella typhi is a B1b antigen. J Immunol. 2012. December 15;189(12):5527–32. 10.4049/jimmunol.1103166 Epub 2012 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine. 1996. April;14(5):435–8. . [DOI] [PubMed] [Google Scholar]
- 56.Mai NL, Phan VB, Vo AH, Tran CT, Lin FY, Bryla DA, Chu C, Schiloach J, Robbins JB, Schneerson R, Szu SC. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Engl J Med. 2003. October 2;349(14):1390–1. 10.1056/NEJM200310023491423 . [DOI] [PubMed] [Google Scholar]
- 57.Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, Khiem HB, Trach DD, Karpas A, Hunt S, Bryla DA, Schneerson R, Robbins JB, Szu SC. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun. 1999. November;67(11):5806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreno-Eutimio MA, Tenorio-Calvo A, Pastelin-Palacios R, Perez-Shibayama C, Gil-Cruz C, López-Santiago R, Baeza I, Fernández-Mora M, Bonifaz L, Isibasi A, Calva E, López-Macías C. Salmonella Typhi OmpS1 and OmpS2 porins are potent protective immunogens with adjuvant properties. Immunology. 2013. August;139(4):459–71. 10.1111/imm.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J Immunol. 1999. May 1;162(9):5398–406. . [PubMed] [Google Scholar]
- 60.Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naive to memory and everything in between. AdvPhysiol Educ. 2013. December;37(4):273–83. 10.1152/advan.00066.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010. January;1183:211–21. 10.1111/j.1749-6632.2009.05133.x Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holley MM, Kielian T. Th1 and Th17 cells regulate innate immune responses and bacterial clearance during central nervous system infection. J Immunol. 2012. February 1;188(3):1360–70. 10.4049/jimmunol.1101660 Epub 2011 Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naive to memory and everything in between. AdvPhysiol Educ. 2013. December;37(4):273–83. 10.1152/advan.00066.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014. May 9;114(10):1640–60. 10.1161/CIRCRESAHA.114.302761 Review. . [DOI] [PubMed] [Google Scholar]
- 65.Mizuno Y, Takada H, Nomura A, Jin CH, Hattori H, Ihara K, Aoki T, Eguchi K, Hara T. Th1 and Th1-inducing cytokines in Salmonella infection. ClinExpImmunol. 2003. January;131(1):111–7. 10.1046/j.1365-2249.2003.02060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bao S, Beagley KW, France MP, Shen J, Husband AJ. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology. 2000. March;99(3):464–72. 10.1046/j.1365-2567.2000.00955.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon MA, Jack DL, Dockrell DH, Lee ME, Read RC. Gamma interferon enhances internalization and early nonoxidative killing of Salmonella entericaserovar Typhimurium by human macrophages and modifies cytokine responses. Infect Immun. 2005. June;73(6):3445–52. 10.1128/IAI.73.6.3445-3452.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS One. 2011. May 2;6(5):e19105 10.1371/journal.pone.0019105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khader SA, Gopal R. IL-17 in protective immunity to intracellular pathogens. Virulence. 2010. Sep-Oct;1(5):423–7. 10.4161/viru.1.5.12862 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siegemund S, Schütze N, Schulz S, Wolk K, Nasilowska K, Straubinger RK, Sabat R, Alber G. Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella entericaserovarEnteritidis. IntImmunol. 2009. May;21(5):555–69. 10.1093/intimm/dxp025 Epub 2009 Mar 18. . [DOI] [PubMed] [Google Scholar]
- 71.Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J ClinImmunol. 2010. March;30(2):196–203. 10.1007/s10875-010-9368-7 Epub 2010 Feb 2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Bäumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008. April;14(4):421–8. 10.1038/nm1743 Epub 2008 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Genovesi EV, Pettey CL, Collins JJ. Use of adoptive transfer and Winn assay procedures in the further analysis of antiviral acquired immunity in mice protected against Friend leukemia virus-induced disease by passive serum therapy. Cancer Res. 1984. April;44(4):1489–98. . [PubMed] [Google Scholar]
- 74.Kraaijeveld CA, Benaissa-Trouw BJ, Harmsen M, Snippe H. Adoptive transfer of immunity against virulent Semliki Forest virus with immune spleen cells from mice infected with avirulent Semliki Forest virus. Arch Virol. 1986;91(1–2):83–92. . [DOI] [PubMed] [Google Scholar]
- 75.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl AcadSci U S A. 2010. February 16;107(7):3070–5. 10.1073/pnas.0910497107 Epub 2010 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol. 2012. December 15;189(12):5503–7. 10.4049/jimmunol.1201413 Epub 2012 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen P, Fillatreau S. Suppressive functions of B cells in infectious diseases. IntImmunol. 2015. October;27(10):513–9. 10.1093/intimm/dxv037 Epub 2015 Jun 10. Review. . [DOI] [PubMed] [Google Scholar]
- 78.Barr TA, Brown S, Mastroeni P, Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol. 2010. September 1;185(5):2783–9. 10.4049/jimmunol.1001431 Epub 2010 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conlan JW. Neutrophils prevent extracellular colonization of the livermicrovasculature by Salmonella typhimurium. Infect Immun. 1996. March;64(3):1043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McQuillen DP, Gulati S, Rice PA. Complement-mediated bacterial killing assays. Methods Enzymol. 1994;236:137–47. . [DOI] [PubMed] [Google Scholar]
- 81.Liu Z, Miyashiro T, Tsou A, Hsiao A, Goulian M, Zhu J. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc Natl AcadSci U S A. 2008. July 15;105(28):9769–74. 10.1073/pnas.0802241105 Epub 2008 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chourashi R, Mondal M, Sinha R, Debnath A, Das S, Koley H, Chatterjee NS. Role of a sensor histidine kinase ChiS of Vibrio cholerae in pathogenesis. Int J Med Microbiol. 2016. December;306(8):657–665. 10.1016/j.ijmm.2016.09.003 Epub 2016 Sep 19. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All strains were characterized by both serotyping and PCR-based methods. Specific primers and anti O- and H-antigens were used for this purpose.
(TIF)
Antibiotic susceptibility pattern was tested by Kirby-Bauer disc diffusion assay.
(TIF)
Mice were immunized by oral gavage on day 0 and then two subsequent booster doses follow as stated. Mice were then challenged on day 35 via intra-peritoneal infection model. Blood were drawn as shown by the dotted arrows.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
A. Dot blot analysis against extracted LPS from two typhoidal strains. Lane 1: S. Typhi native LPS, Lane 2: S. Paratyphi A native LPS. Here, 1, 2, and 3 denotes three different concentrations of LPSs against which the dot blot analysis was performed. B.TCA precipitation of culture supernatant. Lane 1: S. Typhi culture supernatant, Lane 2: S. Paratyphi A culture supernatant.
(TIF)
50,000 events in total population were taken in each and every case and the mean value of area under PE in P2 population was taken into account whilst calculating the result. i. Unstained cells, ii., iii., iv. Up-regulation of CD4, CD8a and CD19, respectively in immunized and control mice’s spleen. v. REA-cloned Iso-Type Control (ITC) for all three markers.
(TIF)
6 mice were per group were challenged with different concentrations of bacteria. Mice were observed for 7 days. The effective dose for LD50 or 50% mortality rate was found to be 2 x 107 CFU/ml for both the challenge strains.
(TIF)
A., B. Colonization in spleen and liver in S. Typhi OMV-immunized mice were less when they were challenged with S. Typhi. C. D.Colonization in spleen and liver in S. Paratyphi A OMV-immunized mice were less when they were challenged with S. Paratyphi A. In both cases,monovalent OMV immunization could not be able to hinder the infection from a heterologous strain.
(TIF)
A., B. A drastic change in body weight was seen in non-immunized mice challenged with >LD50 dose of infection. C. D. A lower level ofchange in body weight was seen in mice challenged with sub-lethal dose of infection.
(TIF)
Three mice per group were challenged either sub-lethal dose or with greater than LD50 dose. Bacterial colonization in the small intestine was estimated since 3DPI until 5DPI. Higher colonization was observed in mice group challenged with greater than LD50 dose.
(TIF)
Both B- and T-cell mediated immune response induced by OMVs is necessary for the protective immunity to bacterial infection. Splenocytes (1 x 106) and serum (100 μl) were injected via the tail vain. One group of mice was challenged with 2 x 107 CFU/ml of heterologous strains of bacteria after two hours of the adoptive transfer and kept for three days. A., B. Systemic infection of S. Typhi in spleen and liver was observed in the early group 3 days post infection. Similarly, C. D. Systemic infection of S. Paratyphi A in spleen and liver was observed 3 days post infection.
(TIF)
This group was challenged with the same dose as mentioned above after seven days of the adoptive transfer. A., B. Systemic infection of S. Typhi in spleen and liver wasobserved 3 days post infection. Similarly, C., D. Systemic infection of S. Paratyphi A in spleen and liver was observed 3 days post infection.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.