Abstract
Background
Under-enrolling minority patients in clinical trials reduces generalizability. CLEAR III, a randomized controlled trial, presented an opportunity to assess African American (AA) participation.
Methods
AA enrollment was compared to U.S. population and NINDS trial data then stratified by region; census data for 42 recruitment cities were compared to screening and randomization percentages, using simple linear regression.
Results
AAs were 25% of screens and 45.1% of enrollments (n=370), more than twice the 19.8% participation rate reported by the 2011 NINDS Advisory Panel on Health Disparities Research and triple the projected 13.9% 2014 U.S. population. Conversion rates were (AA vs. non-AA): overall (8.7% vs. 3.4%, p<0.001); Northeast (7.7% vs. 2.9%, p<0.001); South (8.2% vs. 4.0%, p<0.001); Midwest (10.3% vs. 3.6%, p<0.01); and West (8.9% vs. 3.8%, p=0.02). AA enrollments ranged from 0% to 100% (mean: 40.4%). AA screening ranged from 0% to 63.7% (mean: 23.2%). AA city census ranged from 1.3% to 82.7% (mean: 28.0%); higher census was associated with higher screening (p<0.0001) and enrollment (p=0.004).
Conclusions
AAs were willing to enroll in an acute stroke trial. AA city census rates should be considered when selecting enrollment centers and setting recruitment goals. Factors leading to successful AA recruitment should be further investigated, as population-based participation is a goal in all trials.
Keywords: African American, Stroke, Health Disparities, Intracerebral Hemorrhage, Health Equity
INTRODUCTION
By 2050, racial and ethnic minorities will become the majority in the United States.1 While clinical trials are designed to inform the scientific workforce about the safety, efficacy, and effectiveness of medical strategies, treatments, or devices for evidence-based healthcare decision-making, the under-enrollment of minority patients reduces the generalizability of research findings.2 Enrolling an adequate proportion of minorities into clinical trials has proven difficult in the past; however, concerted efforts must be made to overcome barriers to enrollment.3–12 Proportional recruitment practices can provide data about health disparities and better serve the needs of minority populations.
Such is the case with hemorrhagic stroke. Hemorrhagic stroke is a devastating disease with a global mortality of 45%.13 Recent estimates indicate that 70,000 new hemorrhages occur in the United States each year.13 Minority patients are disproportionally affected in incidence and severity. African Americans, particularly, have a greater risk, incidence, prevalence, and mortality compared to white Americans.14–26 Not only does this evidence contribute to the overwhelming economic burden of sustained health disparities, but it also suggests a barrier to health equity and social justice. In 2009, the total direct and indirect cost of stroke in the United States was estimated at $68.9 billion.17 Minority populations contribute to a significant portion of stroke costs due to higher admission rates, greater severity and mortality, increased disability-adjusted life-years, and loss of productivity from stroke incidence at younger ages.13,16,27 Enrolling more minorities into stroke trials is an important part of any solution to alleviate the economic burden incurred through health disparities, improve the generalizability of trial results, and raise the standard of patient-centered stroke care.
Clot Lysis
Evaluating Accelerated Resolution of Intraventricular Hemorrhage III (CLEAR III), a 500-participant randomized controlled trial evaluation of alteplase in hemorrhagic stroke, presented an opportunity to assess African American (AA) trial enrollment in a hemorrhagic stroke population. We evaluated our screening and enrollment data to better understand if our recruitment efforts provided diversity and, more importantly, to improve our recruitment efforts in the future.
METHODS
Trial
This phase 3 randomized, double-blinded, placebo-controlled, multicenter trial was conducted at 73 sites in Brazil, Canada, Germany, Hungary, Israel, Spain, the United Kingdom, and the United States from 2009–2014.28 The investigators were either neurointensive care or neurosurgical service teams. This was a first-of-a-kind trial; it combined a catheter device with up to four days of ICU-based drug treatment. For the analysis of AA to non-AA participation, we limited the evaluation to U.S. sites. Over a 5-year period, investigators across 61 U.S. hospitals screened 8,587 patients (Figure 1), admitted to intensive care units in 42 U.S. cities, with stable, small non-traumatic intracerebral hemorrhage (ICH), intraventricular hemorrhage (IVH) with a clinical diagnosis of obstructive hydrocephalus, and an extraventricular drain (EVD) placed pre-trial. Participants were randomly assigned to receive alteplase (Genentech, Inc., San Francisco, CA, USA) or normal saline (placebo) via the EVD.
Figure 1.
CLEAR III trial screens from 2009 to 2014. AAs comprised 25.1% of the U.S. screens for which race was listed.
Subjects
Participants were aged 18–80 years with known symptom onset within 24 hours of the initial CT scan. CT scans were obtained every 24 hours throughout dosing. Initial eligibility criteria required supratentorial intracerebral hemorrhage volume 30 mL or less; additional criteria included a historical modified Rankin Scale (mRS) score of 1 or less (no disability prior to ICH), no limitations to hospital care, and no ongoing coagulopathy, suspicion of aneurysm, arteriovenous malformation, or other vascular anomaly.28
Consent
CLEAR III was a complex trial, with a long screening window of 72 hours. After the local principal investigator determined eligibility, the patient’s family was approached and informed of relevant risks, benefits, and alternative treatments. During the study, the investigators were provided guidelines, a checklist for consent, a smartphone application with procedural bedside guidance, and training consent videos modeling best and worst consent practices, both in the general case and specific to the CLEAR III intervention.29 The consent training program included an annual, mandatory refresher webinar on best practices, as well as training on how to engage colleagues to refer patients into the trial. After the families were given time to consider and comprehend the elements of participation, the families of fully-eligible patients were again approached, and informed consents were obtained or refused. We then compared AA and non-AA timelines for presentation, signed consent, and randomization.
Data
All data were captured electronically, and pertinent source documents were uploaded by local site personnel, using a web-based electronic data capture (EDC) system (VISION, Prelude Dynamics, LLC, Austin, TX, USA). All participants and trial personnel, except for the local and central pharmacists and the unblinded statistician, were masked to treatment assignments. Site personnel randomly assigned patients (1:1) within 72 hours of ictus. The EDC system transmitted a treatment allocation by email directly to the local, trained pharmacist.
Screening
The same EDC system was used to enter all participants screened. Study coordinators were trained to enter, in the electronic screening log, all admissions with a primary or secondary diagnosis of IVH. Protocol inclusion/exclusion criteria were collected in the EDC via pre-specified selections and then categorized as either medical reasons (e.g., biologically ineligible or pre-determined I/E ineligible) or non-medical reasons (e.g., access, personal choices, mistrust). EDC compliance was monitored, and sites were encouraged to make screening entries in real time. To limit coordinator burden, only a single exclusion factor was required for screen failures; sites were compensated for screening activities. Enrolling teams were trained to screen admissions, in person, every morning and afternoon or round with the ICU care teams. Remote screening, using electronic admission and medical records, was discouraged. Teams were trained to consider some I/E conditions as temporary and to conduct multiple screening attempts on such subjects during the 72-hour window.
Race/Ethnicity
Race was collected as part of screening data and entered locally into VISION. Investigators or study coordinators selected one or more of the following to report race: American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander, Black or African American, Asian, or White. From these categories, we grouped patients into two categories when race was listed, as either AA if Black or African American was selected (including those who chose other races in addition to AA) and non-AA if Black or African American was not selected (Figure 1).
Analysis
We analyzed AA participation using randomization (Stage 1) and screening (Stage 2) data. Our first inspection compared trial enrollment to an NIH aggregate report30 and to U.S. population data from 1990, 2000, and 2010, obtained via census.gov. To inform end-of-trial comparisons, forecast projections were calculated to determine the likely AA percentage for a 2014 U.S. population. With CLEAR III demonstrating such substantial AA participation and robust conversion rates, we stratified AA trial randomization rate by site geographic region. We then examined city census data at our CLEAR III locations, examining whether hospital location mattered. We retrieved census percentages31 and used simple linear regression modeling to assess the relationship between AA census in 42 cities and the AA percent screened, as well as the AA percent randomized in each city. Site and city data for CLEAR III sites that did not enroll any patients (regardless of race) were excluded from the analysis. We next stratified screening data by gender and age to test for significant demographic differences. Last, we interrogated the data for AA vs. non-AA distribution among medical, non-medical, or combination (both medical and non-medical) reasons for screen failure. Chi-square was used to compare the proportions between AA and non-AA for each screen failure reason.
RESULTS
Stage 1: African American vs Non-African American Enrollment
Overall
The U.S. respective trial enrollment rates were: African American, 45.1%; Asian, 3.5%; American Indian/Alaskan Native, 0.3%; Native Hawaiian or Other Pacific Islander, 0.8%; White, 48.6%; remaining mixed races, 0.3%; and Unknown, 1.4%. For our analyses, we grouped the race categories into AA and non-AA. When we compared CLEAR III recruitment to other National Institute of Neurological Disorders and Stroke (NINDS) participation data and to U.S. population data, during the same period as the trial, CLEAR III recruitment outperformed population expectations and that of other NINDS trials (Table 2). AAs comprised 45.1% of total U.S. enrollments (n=370), more than twice the 19.8% participation rate reported by NINDS in 201130 and triple the projected 13.9% U.S. population in 2014.
Table 2.
Conversion (Randomization) Rates: U.S. Overall and by Geographic Regions
| Regions (n=sites) | AA (%) | Non-AA (all other) (%) | P-value |
|---|---|---|---|
| U.S. overall (n=61) | 8.7 | 3.4 | <0.001 |
| Northeast (n=20) | 7.7 | 2.9 | <0.001 |
| South (n=16) | 8.2 | 4.0 | <0.001 |
| Midwest (n=16) | 10.3 | 3.6 | <0.01 |
| West (n=9) | 8.9 | 3.8 | 0.02 |
Conversion (Randomization) Rate by Geographic Region
Conversion rates for both AA and non-AA participants were calculated as total number of enrolled divided by total number screened (Table 2). Our planned conversion rate for trial enrollment was 5%. The randomized-to-screened ratio for AAs was 8.7% vs. 3.4% non-AA (p<0.001). Regional analysis showed similar differentials with AA conversion rates: Northeast (7.7% vs. 2.9%, p<0.001); South (8.2% vs. 4.0%, p<0.001); Midwest (10.3% vs. 3.6%, p<0.01); and West (8.9% vs. 3.8%, p=0.02).
Conversion (Randomization) Rate and City Census Comparisons
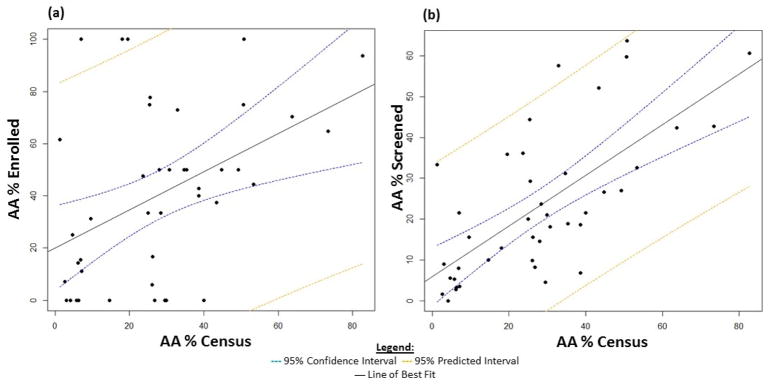
Trial sites were grouped by city, and their AA enrollment percentages were compared to corresponding city census data. The proportion of AAs enrolled per city ranged from 0% to 100%, with a mean of 40.4% (Figure 2a). The AA city census ranged from 1.3% to 82.7%, with a mean of 28.0%. The enrollment mean of 40.4% robustly exceeded the census mean (28.0%). Higher AA census was associated with higher AA enrollment percentage (R2 = 0.17, p-value = 0.004; β̂(95% CI) = 0.7 (0.25, 1.21)). The symbol, β̂, defines the slope of the regression line. The AA percent enrolled in a city increased, on average, 0.7% for each percent increase in AA census.
Figure 2.
a. AA enrollment by city (%) compared to AA census data (%) for each city. Cities with a higher percentage of AAs enrolled more AAs into the trial (p-value: 0.004, R2: 0.17). b. AA screening by city (%) compared to AA census data (%) for each city. Cities with a higher percentage of AAs screened more AAs into the trial (p-value: <0.001, R2: 0.46). 95% confidence interval indicates the likely location of the true population parameter, and 95% predicted interval forecasts where to expect the next data point sampled.
Comparing enrollment timelines, only randomization was statistically significant. African Americans randomized later than non-AAs, with an average difference of 5 hours. Time to informed consent approached significance, averaging approximately 2–3 hours longer.
Stage 2: African American vs non-African American Screening
We next looked at screening to understand conversion performance, assess who was excluded, and evaluate whether reasons for exclusion related to relevant demographic and biological variables.
Screening Rate and City Census Comparisons
The proportion of AAs screened per city ranged from 0% to 63.7%, with a mean of 23.2% (Figure 2b). Higher AA census was associated with higher AA screening percentage; the AA percent screened in a city increased, on average, 0.6 for each percent increase in AA census (R2 = 0.46, p-value < 0.001; β̂ (95% CI) = 0.62 (0.41, 0.83)). Comparing the census and screening means, CLEAR III investigators screened slightly less than the census mean (23.2% vs. 28%).
Screening by Gender and Age
We then assessed gender and age for overall U.S. screens and screen failures, where race was listed, to detect any significant demographic differences. Out of the 8,587 U.S. screens, race was reported for 7,663 participants (Figure 1). Of the 7,663 race-listed participants, 7,298 were screen failures and 365 were enrolled.
Of the 7,663 race-listed U.S. screens, gender was missing on four non-AA participants. Of the race-listed U.S. screens, AAs consisted of 918 (47.7%) females and 1,005 (52.3%) males. Equivalently, non-AAs consisted of 2,735 (47.7%) females and 3,001 (52.3%) males. As a subset of the 7,663 race-listed U.S. screens, the 7,298 screen failures contained the same four non-AA participants whose gender was missing. Of the screen failures, AAs consisted of 839 (47.8%) females and 917 (52.2%) males. Similarly, non-AAs consisted of 2,640 (47.6%) females and 2,898 (52.3%) males. There was no statistically significant difference in gender between race-listed U.S. screens and screen failures.
For race-listed U.S. screens, the average age of AA participants was 58 years old (standard deviation 13.7), compared to an average age of 66 years for non-AA participants (standard deviation 15.6) with a p-value <0.001. For the screen failure subset, similar results hold; the average age of AA participants was 58 years old (standard deviation 14.0), compared to an average age of 66 years for non-AA participants (standard deviation 15.7) with a p-value <0.001.
Medical vs. Non-Medical-Related Screen Failures
Upon review of screen failure reasons within the AA and non-AA race groups, African Americans were less frequently excluded due to biological/research strategy (I/E) reasons (Table 3).
Table 3.
Screen Failure Categories (N = 7,298)
| AA | Non-AA | P-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Medical | 1,292 | 73.6% | 4,581 | 82.7% | <0.001 |
|
| |||||
| Abnormal PTT, PLT < 100K, INR > 1.3 | 34 | 1.9% | 122 | 2.2% | 0.503 |
|
| |||||
| Age < 18 or > 80 years | 98 | 5.6% | 884 | 16.0% | <0.001 |
|
| |||||
| Aneurysm, mycotic aneurysm, moyamoya, etc. | 161 | 9.2% | 743 | 13.4% | <0.001 |
|
| |||||
| Craniectomy/other surgical procedures | 21 | 1.2% | 51 | 0.9% | 0.308 |
|
| |||||
| Etiology - tumor | 3 | 0.2% | 47 | 0.8% | 0.003 |
|
| |||||
| GCS < 3/herniation/brain dead/deceased | 25 | 1.4% | 43 | 0.8% | 0.014 |
|
| |||||
| Historic (pre-bleed) Rankin not 0 or 1 | 47 | 2.7% | 96 | 1.7% | 0.013 |
|
| |||||
| ICH > 30 cc on diagnostic CTC | 256 | 14.6% | 700 | 12.6% | 0.035 |
|
| |||||
| Infratentorial bleed | 150 | 8.5% | 451 | 8.1% | 0.591 |
|
| |||||
| No EVD placed | 220 | 12.5% | 733 | 13.2% | 0.449 |
|
| |||||
| No obstruction of 3rd and/or 4th | 261 | 14.9% | 636 | 11.5% | <0.001 |
|
| |||||
| Unstable bleeding | 16 | 0.9% | 75 | 1.4% | 0.146 |
|
| |||||
| Non-medical | 199 | 11.3% | 471 | 8.5% | <0.001 |
|
| |||||
| Improper screening | 9 | 0.5% | 21 | 0.4% | 0.446 |
|
| |||||
| Participation in another trial | 6 | 0.3% | 10 | 0.2% | 0.208 |
|
| |||||
| Patient eligible but refused consent | 54 | 3.1% | 66 | 1.2% | <0.001 |
|
| |||||
| Patient is DNR | 59 | 3.4% | 250 | 4.5% | 0.037 |
|
| |||||
| Study staff not notified within window | 11 | 0.6% | 21 | 0.4% | 0.171 |
|
| |||||
| Study staff unavailable | 2 | 0.1% | 8 | 0.1% | 0.764 |
|
| |||||
| Unable to dose within time window | 58 | 3.3% | 95 | 1.7% | <0.001 |
|
| |||||
| Combination medical and non-medical reasons | 265 | 15.1% | 490 | 8.8% | <0.001 |
|
| |||||
| MD/Surgeon chose not to enroll | 56 | 3.2% | 65 | 1.2% | <0.001 |
|
| |||||
| Not an IVH patient | 18 | 1.0% | 49 | 0.9% | 0.59 |
|
| |||||
| Other | 191 | 10.9% | 376 | 6.8% | <0.001 |
|
| |||||
| Total | 1,756 | 100.0% | 5,542 | 100.0% | |
For the medical screen failure category, AA had a lower percentage of patients excluded at the Upper Age Limit (AA: 5.6% vs. Non-AA: 16.0%), Aneurysm (AA: 9.2% vs Non-AA: 13.4%), and Etiology Tumor (AA: 0.2% vs Non-AA: 0.8%). However, AAs had a higher percentage of exclusions for GCS/Herniation/Brain Dead/Deceased (AA: 1.4% vs. Non-AA: 0.8%), Historic Rankin not 0 or 1 (AA: 2.7% vs. Non-AA: 1.7%), ICH > 30 cc (AA: 14.6% vs. Non-AA: 12.6%), and no obstruction of 3rd and/or 4th (AA: 14.9% vs. Non-AA: 11.5%). Other remaining screen failure reasons were statistically insignificant.
For non-medical reasons screen failure category, AAs had a lower percentage of patients who were DNR (AA: 3.4% vs. Non-AA: 4.5%) and a higher percentage of patients who were eligible but refused consent (AA: 3.1% vs. Non-AA: 1.2%). Remaining screen failure reasons for this category were statistically insignificant.
One category, “MD/Surgeon chose not to enroll,” had too broad a response, combining both medical and non-medical reasons. For screen failure category, AA had a higher percentage of screen failures for MD/Surgeon chose not to enroll (AA: 3.2% vs. Non-AA: 1.2%) and Other (AA: 10.9% vs. Non-AA: 6.8%). Other reasons were statistically insignificant. (See Table 3.)
DISCUSSION
AAs enrolled in CLEAR III at a rate greater than expected by available census data, regardless of city or geographic region. Although AAs refused consent at a greater rate, AAs enrolled 2.5 times more often than non-AAs. When we compared CLEAR III performance to other brain hemorrhage RCTs during the same period, CLEAR III enrolled AAs at 45.1% compared to 9% to 30% in the other trials, though AA screening and enrollment data are not available for some trials, limiting the comparison (Table 4). Further limiting comparison is that these trials were international and did not break out racial data by countries. 00251659264 When comparing reported enrollment windows and follow-up intervals, there is one notable difference—time from onset to randomization. CLEAR III participants had a much longer enrollment window, allowing more time to communicate with families. Moreover, the communication period occurred in the ICU rather than the ED. Prospective research on the relationship between enrollment windows, follow-up intervals, social support, recruitment monitoring, and minority enrollment/retention may provider stronger correlations. Gorelick et al. published the recruitment triangle in 1998,32 illustrating the social support triangle that reduces barriers and lessens disparities. The design of the 72-hour enrollment time window could be essential to enrollment and retention, particularly among AA participants, allowing communication time with the social support stakeholders and within the insulated ICU where trust reduces barriers, regardless of race or ethnicity. Initial and ongoing training of site teams emphasized that temporary I/E factors could resolve over a three-day period and the use of the entire time window.
Table 4.
Enrollment Window and Race Reporting in Major ICH Clinical Trials
| Trial | Inter-national | Medical or Surgical Trial | Enrollment Window (Hours) | F/U (Days) | Total Enrolled | % White Reported | % AA or Black Reported |
|---|---|---|---|---|---|---|---|
| CHANT | N | Medical | 6 | 90 | 607 | * | * |
| ICES | N | Surgical | 48 | 365 | 24 | 45.8 | 33.3 |
| FAST | Y | Medical | 4 | 90 | 841 | 9.0 | |
| ATACH-2 | Y | Medical | 4.5 | 90 | 1,000 | 13.1 | |
| PREDICT | Y | Medical | 6 | 90 | 268 | 86.0 | |
| Deferoxamine | N | Medical | 18 | 90 | 20 | 85.0 | |
| NovoSeven | Y | Medical | 3 | 90 | 399 | 81.0 | |
| MISTIE II | Y | Surgical | 48 | 365 | 96 | 56.0 | 30.0 |
| CLEAR III | Y | Medical | 72 | 365 | 500 | 61.0 | 34.0 |
| CLEAR III | U.S. only | Medical | 72 | 365 | 370 | 48.6 | 45.1 |
Race not reported
CLEAR III utilized intensive site management oversight with strong emphasis on best screening, consenting, and enrollment practices. We evaluated recruitment monthly and retrained annually on best consent practices, and we gave a presentation on common reasons for refusals both from families and investigators and on how to solve fixable refusal reasons. Furthermore, our training included the recruitment triangle social support principles:30 taking time and connecting with families; earning trust, not only of families but also of the ICU teams involved in the treatment and care of the patient; using best consent practices; providing family access to an interested and caring investigator; and respecting the cognitive and physical concerns of families in distress and sensory overload throughout the trial participation continuum.
LIMITATIONS
While biological/research strategy exclusions, city census, and being younger may contribute to CLEAR III’s high enrollment of AAs, any causal mechanisms behind these associations remain unclear. Several limitations impact the interpretation of our analysis.
Race categories were presented as checkboxes in the EDC and no specific definition for each category was provided, nor were directions for choosing race included in training. Thus, different interpretations of race categories may have occurred at the time of data entry. Furthermore, we recognize that there may have been inconsistencies across sites whether the race reported was determined by the patient, patient relative(s), medical record, site coordinator, or physician.
While race was more closely monitored for enrollment data, the same standards were not applied to screen failures. Of the 8,587 screens, 924 were missing race data (of which 5 were enrolled), introducing potential sampling error. Screen failure reasons such as “MD/Surgeon chose not to enroll,” “Patient eligible but refused consent,” and “Other” did not allow details, possibly obscuring causal factors related to race and recruitment. Another possible limitation is that the traditional categories “comorbidity,” “likely not able to complete the protocol,” and “…otherwise, in the investigator opinion, not eligible…” were grouped together and labeled as “Investigator Decision,” thus not identifying whether these screen failures were for medical or non-medical reasons or providing further details as to who made the decision.
Screening logs were not monitored prospectively. Tracking diversity in clinical trials is essential; and monitoring screening logs monthly for content (and not just submission) can determine how teams are doing, beyond overall screening and conversion rates, to recruit the underrepresented and underserved. Additionally, recognizing minority screen failures early allows the opportunity to redesign poorly constructed forms and retrain poorly performing teams. Last, including recruitment diversity and disparities metrics when publishing clinical trial results is imperative for comparative research where sub-populations are under active investigation.
Last, the analysis covered only city-level data; data is limited on the demographic characteristics of eligible patients at non-trial hospitals and patients coming to trial hospitals from other cities.
CONCLUSIONS
African Americans were willing to enroll in a novel, acute stroke trial, such as CLEAR III. Enrollment was systematically consistent in proportion to the subject’s demographic, taken from census data, suggesting higher enrollment was a function of the overall trial characteristics and national population characteristics. The enrollment of AAs was proportional to disease prevalence and allows for a robust estimate of minority population characteristics and responses. That CLEAR III AA enrollment exceeded census percentages is an important finding that requires further exploration. Cities densely populated by AAs should be considered when selecting recruitment sites. Census rates may be useful when setting recruitment goals, particularly for ICH trials.
Consent training in disparity recruitment methods appears to have been rewarded. Better screening instruments, screening standardization, and recruitment metrics will be important to the design of any trial. Prospective recruitment monitoring, and surveys and interviews following refusals, could improve understanding of screening-to-enrollment conversion rates among research participants.
Efforts are underway to understand and improve recruitment of African Americans and other underrepresented minorities into clinical trials. If we are to improve proportions of minorities enrolled into clinical trials, then we should apply the recruitment triangle to minority recruitment, interviewing, and data-entry training at investigator meetings and as part of best consent coaching.
This trial may provide some structure to those “trial-in-progress” practices. When designing clinical trials, determining underlying reasons for participation probably helps find solutions for eliminating disparities. Interestingly for CLEAR III, such an approach during the trial might have provided information about lower participation rates of non-AAs. When the incidence of stroke or other diseases is higher in minorities, we must develop minority-specific training programs to teach investigative teams about the importance of diversity.
Future trials should consider: incorporating minority recruitment goals in data collection design and consent training; incorporating targeted enrollment data into screening logs to manage enrollments during the trial to avoid falling short of minority representation; and bringing diversity awareness to the design of I/E criteria, data collection materials, and consent practices.
Table 1.
CLEAR III Enrollment Rates Compared to NINDS Rates and the U.S. Census Population During the Same Periods
| Period | AA Trial Representation (%) | U.S. Population (%, Year) | |
|---|---|---|---|
| Pre-NIH Revitalization Act | 1985–1995 | 11.6% | 12.1% (1990) |
| 56 NINDS trials | 1996–2008 | 19.8% | 12.9% (2000)* 13.0% (2010)** |
| CLEAR III U.S. trial subjects (AAs) | 2009–2014 | 45.1% | 14.1% (2014)*** |
Includes persons identifying as African American and one or more additional races
An additional 1% of the U.S. population identified as African American in addition to one or more other races
Projected U.S. population
Acknowledgments
The authors thank the CLEAR III patients and their families for participating in the trial and contributing to this very important research to find a treatment for a devastating and otherwise untreatable form of stroke. We especially acknowledge and thank our African American patients, who historically share the greater burden of severity, mortality, and disability, for improving the generalizability of the CLEAR III trial results and raising the standard of patient-centered stroke care. We also thank Megan Clark for her editorial assistance.
CLEAR III is supported by the grant 5U01 NS062851-05, awarded to Daniel Hanley from the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS). This work is also supported through a NINDS for Conference Funding Support of the Health Equity Symposium (Grant R13NS101924, 6th World Intracranial Hemorrhage Conference Grant), awarded to Karen Lane. We also thank Genentech, Inc. for its donation of alteplase for use in the CLEAR III trial.
Footnotes
ClinicalTrials.gov, NCT00784134: CLEAR III: Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage III
References
- 1.Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. Current Population Reports P25-1143. 2015:9. census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf.
- 2.Paskett ED, Reeves KW, McLaughlin JM, et al. Recruitment of minority and underserved populations in the United States: the Centers for Population Health and Health Disparities experience. Contemporary Clinical Trials. 2008;29(6):847–61. doi: 10.1016/j.cct.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner GJ, Miles TP. Participation of African Americans in clinical research. Neuroepidemiology. 1997;16(6):281–4. doi: 10.1159/000109698. [DOI] [PubMed] [Google Scholar]
- 4.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. The Gerontologist. 2003;43(1):18–26. doi: 10.1093/geront/43.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Harris Y, Gorelick PB, Samuels P, Bempong I. Why African Americans may not be participating in clinical trials. Journal of the National Medical Association. 1996;88(10):630. [PMC free article] [PubMed] [Google Scholar]
- 6.Dancy BL, Wilbur J, Talashek M, Bonner G, Barnes-Boyd C. Community-based research: barriers to recruitment of African Americans. Nursing Outlook. 2004;52(5):234–40. doi: 10.1016/j.outlook.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. Journal of Clinical Epidemiology. 1999;52(12):1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 8.Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. Journal of General Internal Medicine. 1999;14(9):537–46. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branson RD, Davis K, Butler KL. African Americans’ participation in clinical research: importance, barriers, and solutions. American Journal of Surgery. 2007;193(1):32–9. doi: 10.1016/j.amjsurg.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Chandra A, Paul DP., III African American participation in clinical trials: recruitment difficulties and potential remedies. Hospital Topics. 2003;81(2):33–8. doi: 10.1080/00185860309598020. [DOI] [PubMed] [Google Scholar]
- 11.Huang H-h, Coker AD. Examining issues affecting African American participation in research studies. Journal of Black Studies. 2010;40(4):619–36. [Google Scholar]
- 12.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology. 2002;12(4):248–56. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Global Heart. 2014;9(1):101–6. doi: 10.1016/j.gheart.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Howard G, Cushman M, Howard VJ, et al. Risk Factors for Intracerebral Hemorrhage. Stroke. 2013;44(5):1282–7. doi: 10.1161/STROKEAHA.111.000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi AI, Giles WH, Croft JB. Racial differences in the incidence of intracerebral hemorrhage Effects of blood pressure and education. Neurology. 1999;52(8):1617–21. doi: 10.1212/wnl.52.8.1617. [DOI] [PubMed] [Google Scholar]
- 16.Rincon F, Mayer SA. The epidemiology of intracerebral hemorrhage in the United States from 1979 to 2008. Neurocritical Care. 2013;19(1):95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- 17.Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8(3):319. doi: 10.1007/s13311-011-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaherty ML, Woo D, Haverbusch M, et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36(5):934–7. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 19.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. New England Journal of Medicine. 1992;326(11):733–6. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- 20.Broderick J, Brott T, Kothari R, et al. The greater Cincinnati/northern Kentucky stroke study. Stroke. 1998;29(2):415–21. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 21.Collaborators NMSS, Sacco RL, Boden-Albala B, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. American Journal of Epidemiology. 1998;147(3):259–68. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 22.Sturgeon JD, Folsom AR, Longstreth W, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38(10):2718–25. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 23.Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurologic Clinics. 2008;26(4):871–95. doi: 10.1016/j.ncl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Labovitz DL, Halim A, Boden-Albala B, Hauser W, Sacco R. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65(4):518–22. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 25.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50(5):1413–8. doi: 10.1212/wnl.50.5.1413. [DOI] [PubMed] [Google Scholar]
- 26.Howard G, Anderson R, Sorlie P, Andrews V, Backlund E, Burke GL. Ethnic differences in stroke mortality between non-Hispanic whites, Hispanic whites, and blacks. The National Longitudinal Mortality Study. Stroke. 1994;25(11):2120–5. doi: 10.1161/01.str.25.11.2120. [DOI] [PubMed] [Google Scholar]
- 27.Boan AD, Feng WW, Ovbiagele B, et al. Persistent racial disparity in stroke hospitalization and economic impact in young adults in the buckle of stroke belt. Stroke. 2014;45(7):1932–8. doi: 10.1161/STROKEAHA.114.004853. [DOI] [PubMed] [Google Scholar]
- 28.Hanley DF, Lane K, McBee N, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11. doi: 10.1016/S0140-6736(16)32410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman W, Macek M, Lane K, et al. Simulation of Best-Consent and Failed-Consent Elements from CLEAR III: A Randomized, Placebo Controlled Trial. Poster 170. Paper presented at the 12th Annual Neurocritical Care Society Meeting; Seattle, WA. 2014. [Google Scholar]
- 30.Burke J, Brown D, Lisabeth L, Sanchez B, Morgenstern L. Enrollment of women and minorities in NINDS trials. Neurology. 2011;76(4):354–60. doi: 10.1212/WNL.0b013e3182088260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Census Bureau. [Accessed February 1, 2017];American FactFinder. factfinder.census.gov/
- 32.Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS) Journal of the National Medical Association. 1998;90(3):141. [PMC free article] [PubMed] [Google Scholar]