Abstract
Background
By transdifferentiation, proximal tubular cells (PTC) have been considered as a source of interstitial myofibroblasts. We examined the combined effect of transforming growth factor-beta-1 (TGF-β1) stimulation and contact with type I collagen on PTC transdifferentiation.
Methods
Human kidney-2 cells were grown on type I substratum with the concurrent stimulation of TGF-β1.
Results
Following addition of TGF-β1, cells acquired an elongated fibroblastic appearance and an increase in α-smooth muscle actin (α-SMA) expression, a myofibroblastic marker. Upon addition of TGF-β1, E-cadherin expression, an epithelial marker, was reduced, while cytokeratin expression, another epithelial marker, remained unaltered. Following removal of TGF-β1, PTC regained an epithelial appearance and E-cadherin expression reverted to the unstimulated level, suggesting incomplete and reversible transdifferentiation. Addition of TGF-β1 to cells grown on type I collagen demonstrated a cooperatively increased α-SMA expression and decreased E-cadherin and cytokeratin expressions, suggesting more complete transdifferentiation. Co-stimulation of TGF-β1 and contact with type I collagen led to a stable cell phenotype and persistently decreased E-cadherin, which was not reversed upon removal of TGF-β1, indicating irreversible transdifferentiation. Addition of TGF-β1 or type I collagen caused a 4-fold increase in migratory cell number as compared to the control, whereas addition of both TGF-β1 and type I collagen led to an 11-fold increase.
Conclusions
TGF-β1 alone results in a reversible and incomplete transdifferentiation. The combination of TGF-β1 and exposure to type I collagen leads to an irreversible and complete PTC transdifferentiation.
Keywords: α-Smooth muscle actin, E-cadherin, Transdifferentiation, Transforming growth factor-beta-1, Type I collagen
At a glance commentary
Scientific background of the subject
Proximal tubular cells (PTC) have been considered as a source of interstitial myofibroblasts and transforming growth factor-beta-1 (TGF-β1) is crucial to induce phenotypic alterations of PTC to form fibroblastoid morphology. But previous studies imply that there are other factors required to cooperate with TGF-β1 to complete terminal transdifferentiation.
What this study adds to the field
This study demonstrates that TGF-β1 alone causes incomplete and reversible transdifferentiation. In contrast, combination of TGF-β1 stimulation and contact with type I collagen substratum cooperatively causes complete and irreversible PTC transdifferentiation.
It is now clear that progression of renal insufficiency is closely correlated to the degree of renal interstitial fibrosis [1], [2]. Despite conflicting evidence arguing the origin of myofibroblasts [3], [4], it has been demonstrated that renal proximal tubular cells (PTC) can contribute to progression of renal fibrosis through a process called epithelial-mesenchymal transition (EMT) or transdifferentiation, in which PTC exert a phenotypic conversion to acquire characteristics of mesenchymal cells [5], [6], [7]. Early work of Strutz et al. suggested that PTC may express fibroblast-specific markers in vitro and in vivo in a murine model of anti-tubular basement membrane and anti-glomerular basement membrane model of nephritis [8]. Ng et al. have demonstrated de novo expression of α-smooth muscle actin (α-SMA), a marker of myofibroblast phenotype, by PTC, associated with disruption of the tubular basement membrane in 5/6 nephrectomized rats [9]. Li et al. have also demonstrated that advanced glycation end products and induced epithelial-myofibroblast transdifferentiation in PTC [10].
Transforming growth factor-beta-1 (TGF-β1), a prototypic member of the TGF-β superfamily, exerts a broad range of biological activities. It plays pivotal roles during embryonic development where it is involved in induction of cell differentiation and organogenesis. Furthermore, TGF-β1 has been implicated in the pathogenesis of renal fibrosis in both experimental and human disease [11], [12], [13], [14]. In addition, TGF-β1 has been shown to be involved in oncogenesis as it can induce EMT of mammary epithelial cells, thought to be important during transformation of squamous carcinoma to invasive spindle cell carcinoma [15], [16]. In vitro studies also suggest that TGF-β1-induced phenotypic alterations of PTC to form fibroblastoid morphology and α-SMA expression and loss of epithelial markers, suggesting induction of EMT [17], [18], [19].
Early studies have demonstrated that tubular basement membrane is an essential structure for maintenance of epithelial phenotype as EMT of PTC is frequently associated with damage of tubular basement membrane [9], [20], [21], [22]. Zeisberg et al. have clearly shown that disruption of the structure of type IV collagen, a major component of basement membrane, is essential for PTC transdifferentiation. These studies would therefore suggest that although TGF-β1 is a critical cytokine in the process of transdifferentiation, other factors are likely to act in a cooperative way to complete the process.
Since type I collagen is the most abundant extracellular matrix protein in the renal interstitium and the most possible candidate that transdifferentiated PTC may frequently contact with following migration through disrupted basement membrane into the interstitium, the aim of this study was to examine the combined effect of TGF-β1 stimulation and contact with type I collagen on PTC phenotype and function. The data demonstrate that contact with type I collagen have synergistic effects with TGF-β1, promoting irreversible PTC transdifferentiation.
Materials and methods
Cell culture
Human kidney-2 (HK2) cells (human renal PTC immortalized by transduction with human papilloma virus 16 E6/E7 genes [22]) were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM/Ham's F12 medium (Invitrogen Ltd.) supplemented with 10% fetal calf serum (Biological Industries Ltd.), 2 μM glutamine (Life Technologies Ltd.), 20 mM HEPES buffer (Gibco BRL), 5 μg/mL insulin, 5 μg/mL transferrin and 5 ng/mL sodium selenite (Sigma). Cells were grown at 37 °C in 5% CO2 and 95% air. Cells were grown to confluence and then serum deprived for 48 h prior to experimental manipulation. All experiments were performed under serum free conditions. For cell culture plate coated with type I collagen, 2 mg/mL of sterilized type I collagen (Sigma) was added to tissue culture plate overnight to form a monolayer of gel and cells were then seeded upon the gel on the next day.
Immunocytochemistry
Immunocytochemistry was performed on cells grown in 8-well chamber slides (Nunc, Gibco/BRL Life Technologies Ltd.) [23]. Cells were grown to confluence and stimulated under serum free conditions. Culture medium was subsequently removed and the cell monolayer washed with sterile phosphate buffered saline (PBS). For immunostaining of cytokeratin, cells were fixed in acetone-methanol (1:1 vol/vol) for 20 min at −20 °C. For the staining of α-SMA and E-cadherin, cells were fixed in 3.5% paraformaldehyde for 15 min at room temperature, and permeabilized with 0.1% triton in PBS for 5 min at room temperature. Following fixation, slides were blocked with 5% Bovine serum albumin (BSA) for 1 h at room temperature prior to a further washing step with PBS. Subsequently slides were incubated with the primary antibody diluted in 1% BSA/PBS for 1 h at room temperature. Primary antibodies included: Murine monoclonal anti-α-SMA clone 1A4 (Sigma), murine monoclonal anti-cytokeratin (DAKO), and murine monoclonal anti-E-cadherin (Transduction Laboratories). Following a further washing step slides were incubated with the appropriate fluorescein isothiocyanate-conjugated secondary antibody. After washing with PBS, cells were mounted with FluorSave™ reagent (Calbiochem) and analyzed by confocal microscope (Leica TCS 4D).
Western blot analysis
Total cellular protein extraction was performed as described previously [24]. Protein samples mixed with reducing sodium dodecyl sulfate (SDS) sample buffer were resolved on a 10% SDS-polyacrylamide gel electrophoresis and then electroblotted. Nonspecific binding was blocked with a 5% nonfat milk solution. The membrane was then incubated with primary antibody overnight at 4 °C followed by incubation with a horseradish peroxidase-conjugated secondary antibody. Proteins were visualized using enhanced chemiluminescence (Amersham Bio-sciences, Amersham, UK).
Cell motility assay
Cell motility was assessed by migration assay using tissue culture Transwell filters (Boyden chamber) [25]. Cells were seeded onto the top of the Boyden chamber (8 μm pore) and when confluent, cells were serum deprived prior to adding stimuli to the lower chamber under serum free conditions. At the end of the experimental period, filters of the Boyden chamber were fixed with 3% paraformaldehyde with 1% crystal violet in PBS. The upper surface of the filter was carefully wiped with a cotton-tipped applicator. Cells that passed through the pores were counted in four random nonoverlapping fields (×20) and photographed with a Nikon microscope.
Zymography
In order to determine the effect of TGF-β1 on gelatinolytic activity, confluent monolayers of cells were stimulated under serum free conditions with recombinant TGF-β (10 ng/mL) for up to 4 days. The conditioned medium was then collected and subjected to zymography. Samples were run at 4 °C on 7.5% nonreducing SDS-polyacrylamide gels containing gelatin at a final concentration of 1 mg/mL at 4 °C. The gels were then washed in 2.5% Triton at room temperature for 1 h prior to incubation overnight at 37 °C in 50 mM tris HCl pH 7.6 containing 10 mM CaCl2 and 0.05% Brij. The presence of gelatinase activity was demonstrated by zones of lysis in the Coomassie Blue stained gel [26].
Statistical analysis
All the data are presented as means ± standard error of the mean. Statistical analysis was performed using the unpaired Student's t-test. A value of p < 0.05 was considered to represent a significant difference.
Results
Cooperative effects of TGF-β1 and contact with type I collagen on alteration of cell phenotype.
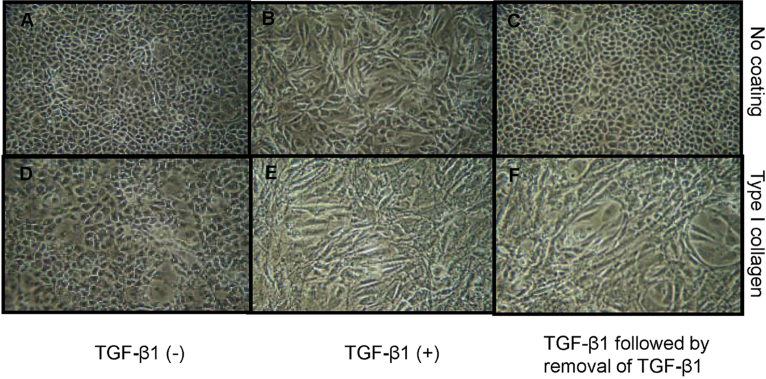
Addition of recombinant TGF-β1 to growth arrested monolayers of HK-2 cells led to a marked alteration in cell morphology [Fig. 1B]. Three days after its addition cells lost cell–cell contact and become elongated, acquiring a fibroblastic phenotype. Terminal transdifferentiation implies acquisition of a stable phenotype, removal of TGF-β1 and addition of culture medium containing 10% fluorescence correlation spectroscopy (FCS) for further 3 days resulted in reversal of the TGF-β1 mediated alteration of cell phenotype [Fig. 1C], such that the cells were indistinguishable from the unstimulated cells [Fig. 1A].
Fig. 1.
Cooperative effect of transforming growth factor-beta-1 and type I collagen substratum on alteration in cell phenotype. Human kidney-2 cells were grown on a plastic surface (A–C) or type I collagen substratum (D–F) to confluence followed by serum deprivation for 2 days. Cells were then incubated in the absence (A and D) or presence of transforming growth factor-beta-1 (10 ng/mL) (B, C, E and F) for 3 days. Subsequently, stability of cell phenotype was determined by removal of transforming growth factor-beta-1 and addition of medium containing 10% fluorescence correlation spectroscopy for further 3 days (C and F). Cell morphology was monitored and photographed by phase contrast microscopy, ×100.
To examine the effect of contact of cells with extracellular matrix components, tissue culture flasks, or six well plates were coated with type I collagen. Cells were seeded onto the coated plastic in the presence of 10% FCS. After 24 h, cells were serum deprived for a further 48 h and cell phenotype monitored by light microscopy either in the absence or presence of recombinant TGF-β1 for 3 days. Cells grown on type I collagen showed no gross change in cell phenotype [Fig. 1D] when compared to that grown on the plastic [Fig. 1A]. Addition of TGF-β1 to cells grown on type I collagen-coated dishes resulted in a fibroblastoid morphologic change [Fig. 1E]. However, unlike cells grown on the plastic, which returned to the epithelial morphology following removal of TGF-β1, cells grown on type I collagen substratum did not revert to the epithelial phenotype upon withdrawal of TGF-β1 and addition of normal medium [Fig. 1F].
Cooperative effects of TGF-β1 and contact with type I collagen on alteration of phenotypic marker expression.
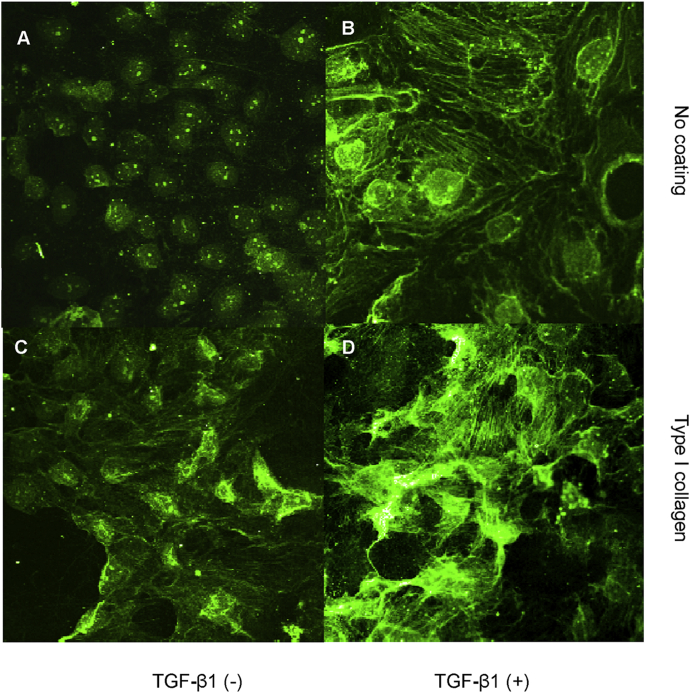
Acquisition of myofibroblastic markers was assessed using immunocytochemical localization of α-SMA. Laser scanning confocal microscopy, demonstrated very little staining in control cells [Fig. 2A]. Up-regulation of α-SMA in fine reticular pattern throughout the cell cytoplasm, was seen following addition of recombinant TGF-β1 [Fig. 2B]. When cells were grown on culture dishes coated with type I collagen, some cells at the edge of cell monolayer displayed α-SMA expression [Fig. 2C]. Addition of TGF-β1 to cells seeded onto type I collagen-coated dishes led to a marked increase in the expression of this myofibroblastic marker [Fig. 2D].
Fig. 2.
Effect of transforming growth factor-beta-1 and contact with type I collagen on a myofibroblastic marker, α-smooth muscle actin expression. Cells were grown on a plastic surface (A and B) or type I collagen substratum (C and D) in the absence (A and C) or presence (B and D) of transforming growth factor-beta-1 (10 ng/mL) under serum free condition for 3 days. Subsequently, cells were fixed with 3.5% paraformaldehyde and α-smooth muscle actin expression was assessed by immunocytochemistry and confocal microscopy as described in materials and methods, ×400.
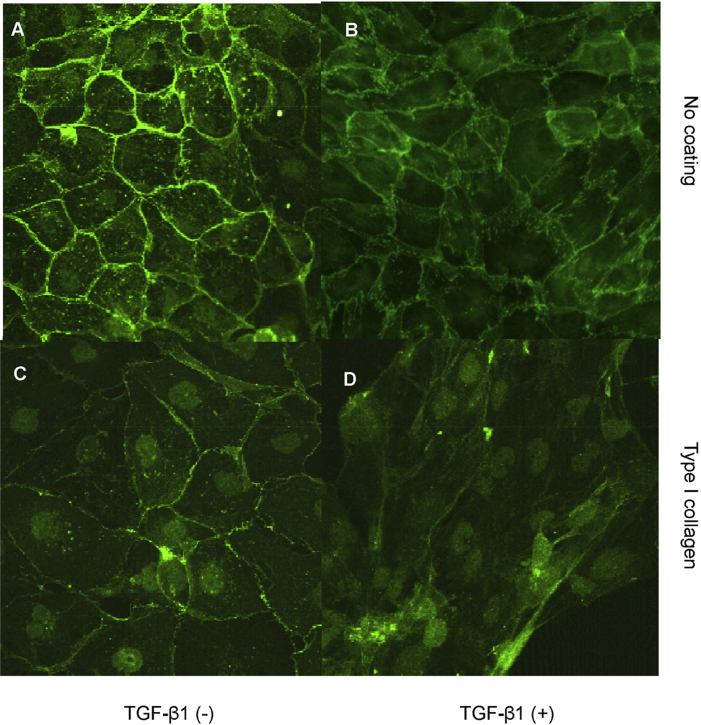
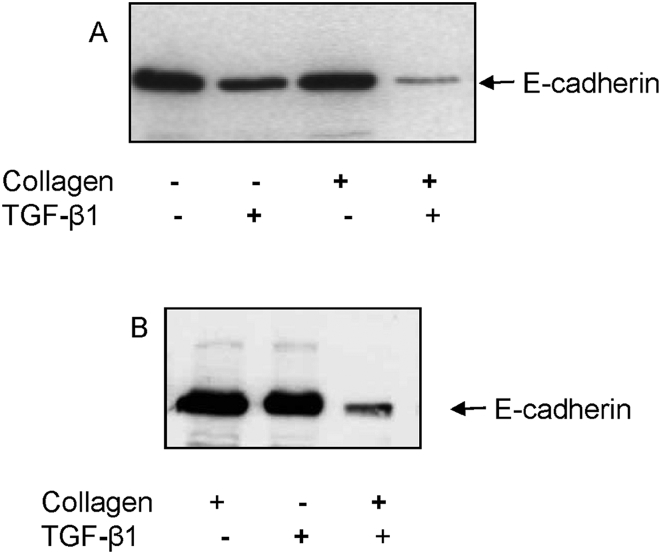
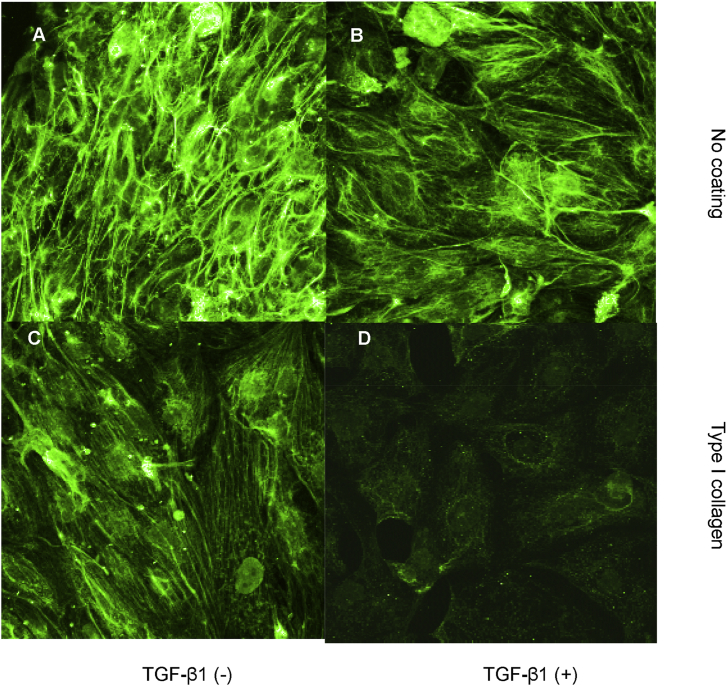
Both E-cadherin and cytokeratin were used as markers of epithelial phenotype. In unstimulated growth arrested HK-2 cells, examination of fluorescence immunostaining for E-cadherin under a confocal laser scanning microscope clearly outlined cell contours [Fig. 3A]. The contour immunofluorescence of E-cadherin decreased and became discontinuous either incubation with TGF-β1 [Fig. 3B]. Cells grown on type I collagen substratum displayed some disruption of E-cadherin expression although most outlined E-cadherin remained intact [Fig. 3C]. Contrarily, simulation of cells seeded on type I collagen coupled with TGF-β1 resulted in a remarkable loss of E-cadherin immunostaining [Fig. 3D]. E-cadherin expression was also examined by western blot analysis. Despite some disruption of E-cadherin expression assessed by immunocytochemistry in cells grown on type I collagen substratum, total E-cadherin expression determined by western blot analysis was not altered [Fig. 4A]. In agreement with results of immunocytochemistry, stimulation with TGF-β1 and growth on a collagen substratum led to a dramatic decrease of E-cadherin expression [Fig. 4A]. As with alteration in cell morphology, alteration in E-cadherin expression following addition of TGF-β1 alone was reversible upon withdrawal of TGF-β1 and re-introduction of normal 10% FCS medium for 3 days [Fig. 4B, the fourth lane vs. the second lane]. In contrast, the combination of TGF-β1 together with growth on type I collagen led to down-regulation of E-cadherin expression that was no affected by withdrawal of TGF-β1 and re-introduction of normal 10% FCS medium [Fig. 4B, the fifth lane vs. the third lane].
Fig. 3.
Effect of transforming growth factor-beta-1 and contact with type I collagen on an epithelial marker, E-cadherin expression. Cells were grown on a plastic surface (A and B) or type I collagen substratum (C and D) in the absence (A and C) or presence (B and D) of transforming growth factor-beta-1 (10 ng/mL) under serum free condition for 3 days. Subsequently, cells were fixed with 3.5% paraformaldehyde and E-cadherin expression was assessed by immunocytochemistry and confocal microscopy as described in materials and methods, ×400.
Fig. 4.
Regulation of E-cadherin expression by transforming growth factor-beta-1 and contact with type I collagen. (A) Cells seeded on a plastic surface or type I collagen substratum were serum deprived for 2 days and then incubated in the absence or presence of transforming growth factor-beta-1 (10 ng/mL) under serum free condition for further 3 days. (B) To examine the reversibility of E-cadherin expression, culture medium containing transforming growth factor-beta was removed and replaced by normal culture medium containing 10% fluorescence correlation spectroscopy for 3 days. At the end of each experiment, cells were lysed and subjected to western blot analysis for E-cadherin expression described in methodologies.
Synergistic effect of collagen and TGF-β1 were also seen on cytokeratin expression [Fig. 5A]. Addition of TGF-β1 alone did not lead to a decrease in cytokeratin expression [Fig. 5B], suggesting that TGF-β1 alone is insufficient to cause complete loss of epithelial markers. Contact with type I collagen resulted in a relative decrease in the expression of cytokeratin [Fig. 5C], while obvious loss of cytokeratin expression was only seen when both TGF-β1 and the type I collagen substratum were applied [Fig. 5D]. This result suggests that TGF-β1 and contact with type I collagen can induce more complete transdifferentiation.
Fig. 5.
Effect of transforming growth factor-beta and contact with type I collagen on another epithelial marker, cytokeratin expression. Cells were grown on a plastic surface (A and B) or type I collagen substratum (C and D) in the absence (A and C) or presence (B and D) of transforming growth factor-beta-1 (10 ng/mL) under serum free condition for 3 days. Subsequently, cells were fixed with acetone-methanol (1:1) and cytokeratin expression was assessed by immunocytochemistry and confocal microscopy as described in Materials and methods, ×400.
Cooperative effect of TGF-β1 and contact with type I collagen on alteration of extracellular matrix protein production.
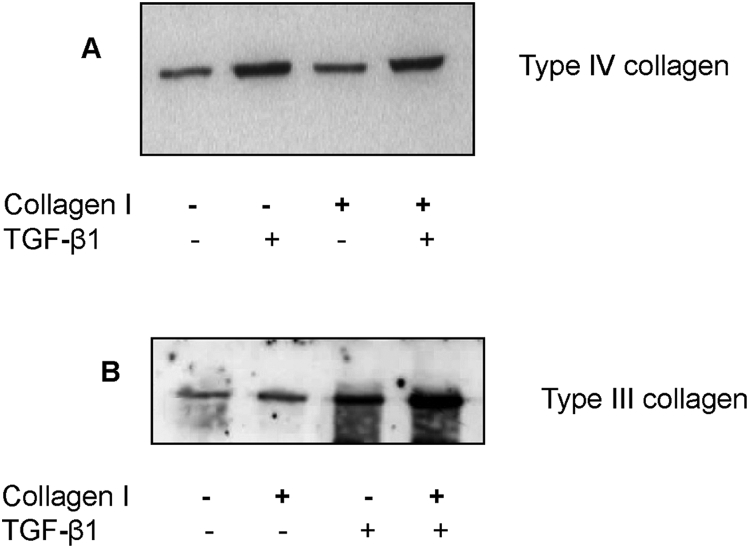
Type III and IV collagen expression in cell culture supernatant was examined to determine if alteration in cell phenotype was associated with alteration in the generation of extracellular matrix. Stimulation with TGF-β1, led to a marked increase in the generation of type IV collagen [Fig. 6A]. Contact with type I collagen had little effect of type IV collagen generation, while addition of TGF-β1 and concurrent contact with type I collagen had no greater effect on type IV collagen generation than addition of TGF-β1 alone. In contrast, stimulation with either TGF-β1, or contact with type I collagen led to stimulation of type III collagen [Fig. 6B]. The combination of TGF-β1 and a type I substratum led to a synergistic increase in type III collagen in the cell culture supernatant. There was no cross-reactivity of the antibody used for detection of type III collagen, with the type I collagen used in these experiments. These results therefore suggest that transformation of epithelial cells is associated with an alteration in the ratio of type III to type IV collagen synthesis.
Fig. 6.
Type IV or III collagen protein expression in the cell supernatant following stimulation. Confluent monolayers of human kidney-2 cells were stimulated with recombinant transforming growth factor-beta-1 (10 ng/mL) under serum free conditions either in cells grown on tissue culture plastic, or cells grown on a type I collagen substratum for 3 days. Subsequently, the culture supernatant was subjected to western blot analysis to measure type IV collagen (A) or type III collagen (B) production.
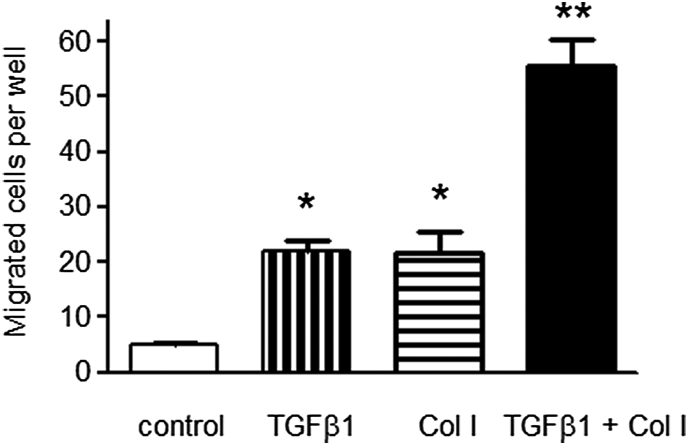
Cooperative effect of TGF-β1 and contact with type I collagen on enhancement of cell migration. In order to understand how TGF-β1 influences PTC migration and hence their potential entry into the interstitial compartment, we utilized a Transwell migration assay by counting the number of cells migrating through the Boyden chamber. In control experiments, in which cells were bathed in serum free culture medium in both upper and lower chambers, very few cells migrated to the lower aspect of the culture chamber. As shown in Fig. 7, addition of TGF-β1 to the lower chamber led marked increase in cell migration manifested as a 4-fold increase in the number of cells migrating through the Boyden chamber (5.1 ± 0.3 vs. 21.9 ± 2.1, n = 8, p < 0.001). Addition of type I collagen to the lower chamber also increased cell number migrating through the chamber (5.1 ± 0.3 vs. 21.8 ± 3.8, n = 8, p < 0.001). Addition of both TGF-β1 and type I collagen to the lower chamber led to a synergistic enhancement in cell migration with an 11-fold increase in cell number as compared to the control (5.1 ± 0.3 vs. 55.5 ± 4.9, n = 8, p < 0.001).
Fig. 7.
Migration of proximal tubular epithelial cells by different stimulations. Human kidney-2 cells were seeded on the filters of the Boyden chamber (pore size 8 μm). Confluent serum deprived cells were stimulated with transforming growth factor-beta-1 (10 ng/mL) (transforming growth factor), type I collagen (500 μg/mL) (Col I) or the combination of both stimuli (Col I + transforming growth factor) added to the lower chamber for further 2 days. In control experiments serum free medium only was added to the lower chamber (control). In all experiment serum free medium alone was added to the upper chamber. At the end of the experiment, filters were fixed with 3% paraformaldehyde with 1% crystal violet in phosphate buffered saline. The upper surface of the filter was carefully wiped with a cotton-tipped applicator and the number of cells migrating through the pore onto the bottom surface of the filter was calculated. Data represents mean ± standard error, n = 8 *p < 0.001 compared to both control value or in the case of combination, compared to either stimulus alone. **p < 0.01 compared to control.
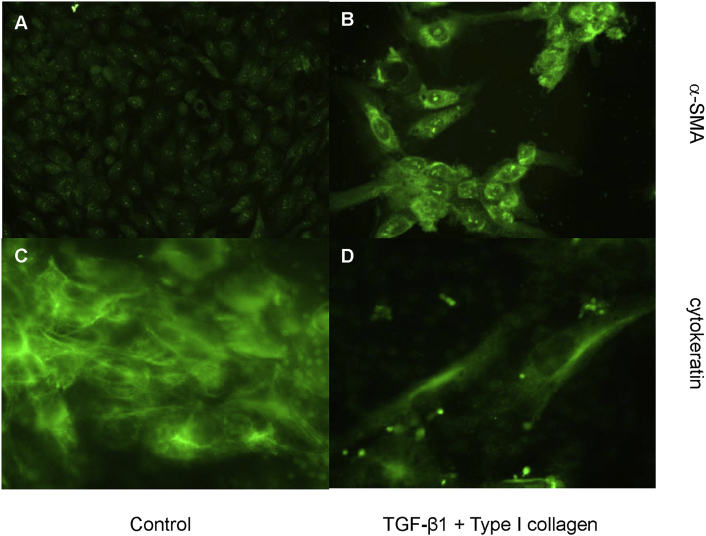
To assess if the migrated cells expressed myofibroblastic characteristics, cells were grown on the filter of the Boyden chamber and stimulated with the combination of TGF-β1 and type I collagen added to the lower chamber for 2 days. Immunofluorescent staining demonstrated that the cells migrating through the Boyden chamber displayed a strong α-SMA expression [Fig. 8B] and a decrease of cytokeratin expression [Fig. 8D] when compared to unstimulated cells on the upper chamber [Fig. 8A and C]. This finding clearly indicated that the cells with increased migration capacity became myofibroblastoid and lost their epithelial characteristics.
Fig. 8.
The expression of α-smooth muscle actin and cytokeratin in migrated cells. Cells were seeded on the transwell filters (Boyden chamber) and serum deprived for 2 days. Subsequently, cells were stimulated with the combination of transforming growth factor-beta-1 (10 ng/mL) and type I collagen (500 μg/mL) added to the lower chamber for further 2 days. The cells were then fixed with 3% paraformaldehyde and the upper surface of the filter was carefully wiped with a cotton-tipped applicator. The cells migrating through the chamber were immunostained with α-smooth muscle actin (B) or cytokeratin (D). In the control, cells were grown only in the serum free medium for 2 days. The cells on the lower surface of the filter were removed and those remained on the upper surface were immunostained with α-smooth muscle actin (A) or cytokeratin (D), (A and B: ×200, B and D, ×400.
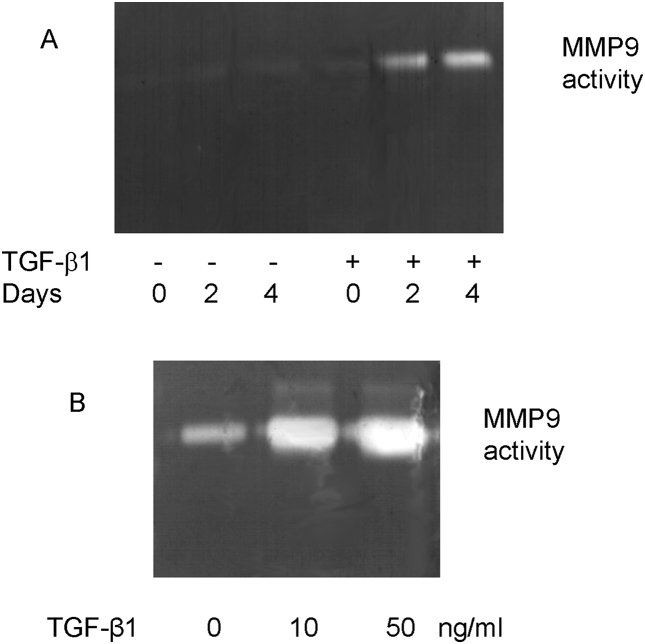
One important aspect of this work is how PTC could come into contact with interstitial collagen. Since matrix metalloproteinases (MMPs) are major enzymes for extracellular matrix degradation to allow PTC to contact the interstitium, we examined the effect of TGF-β1 on matrix degradation by examining MMP9 activity by substrate zymography. Induction of MMP9 activity following addition of TGF-β1 was apparent at day 2 [Fig. 9A]. There was also a dose-dependent relationship with the concentration of TGF-β1 added [Fig. 9B].
Fig. 9.
Stimulation of matrix metalloproteinases-9 activity following addition of transforming growth factor-beta-1. Confluent monolayers of cells were stimulated with recombinant transforming growth factor-beta (10 ng/mL) for up to 4 days (A) or increasing doses (0, 10, 50 ng/mL) for 4 days (B). Supernatant samples were collected and gelatinase activity assessed by substrate zymography as described in Materials and methods.
Discussion
With increasing awareness of the mechanisms of renal fibrosis, PTC, which make up the bulk of the tubulointerstitium, have been demonstrated to play a pro-fibrotic role in the development of tubulointerstitial fibrosis in numerous ways. PTC react to proteinuria by generation of chemokines which initiate interstitial inflammatory infiltrates [27], [28], [29]. In addition they may alter extracellular matrix turnover [26], [30], [31] and generate pro-fibrotic cytokines such as TGF-β1 [12], [32], [33], [34]. In recent years, increasing evidence demonstrates that PTC can contribute to tubulointerstitial fibrosis through EMT induced by different stimuli such as advanced glycated end products and TGF-β1 to transdifferentiate into myofibroblasts [10], [17], [35].
TGF-β1 is one of the most important cytokines involved in EMT in many different tissues [36], [37]. The process of EMT involves loss of epithelial phenotype, de novo expression of α-smooth muscle actin and actin reorganization, disruption of the tubular basement membrane and enhanced migration and invasive capacity of the transformed cells. Although addition of TGF-β1 is a known modulator or PTC phenotype and function [35], numerous studies have demonstrated that TGF-β1 together with other cytokines such as epidermal growth factor (EGF) [38], or fibroblast growth factor [39] are required to optimally affect EMT at least in vitro.
Studies by Zeisberg et al. using a murine cell line have demonstrated that integrity of basement membrane collagen has a significant effect on EMT in vitro. Their studies demonstrate that type IV collagen, a major component of the tubular basement membrane, contributes to the maintenance of the epithelial phenotype of PTC, whereas inhibition of type IV collagen assembly stimulates the production of TGF-β1 thus facilitating phenotypic change [40]. Ng et al. have shown in tubulointerstitial fibrosis of the nephrectomized rats that PTC expressing α-SMA are associated with disruption of the tubular basement membrane Furthermore, Humphreys et al. have also demonstrated a failure of tubular epithelial cells to undergo EMT following kidney injury if PTC are confined with the tubular basement membrane [3]. These results suggest the importance of the contact with the interstitial components in the induction of EMT. Since type I collagen is the most abundant interstitial matrix in the tubulointerstitium of renal fibrosis and provided by evidence that TGF-β1 is a major culprit of renal fibrosis, we hypothesized that the concurrent stimulation with TGF-β1 and contact with type I collagen may facilitate transdifferentiation of PTC into myofibroblasts. In line with our previous work [31], [35], [41], the results of this study demonstrated that stimulation with TGF-β1 caused a phenotypic change from epithelial morphology to a fibroblastic morphology and also resulted in a decrease in the expression of E-cadherin, an epithelial marker, and an increase in the expression of α-SMA, a myofibroblast marker. Interestingly, stimulation of TGF-β1 alone did not reduce the expression of cytokeratin, another epithelial marker, suggesting incomplete transdifferentiation of PTC under stimulation of TGF-β1 alone. In contrast, while contact with type I collagen alone did not cause a significantly morphological change or reduce the expression of E-cadherin and cytokeratin or acquest the expression of α-SMA, concurrent stimulation of TGF-β1 and contact with type I collagen led to a more significant decrease in the E-cadherin expression and a more prominent increase in the α-SMA expression, and the most importantly, a decrease in the cytokeratin expression, suggesting more complete transdifferentiation. Interestingly, Okada et al. have demonstrated that growth of murine PTC on a type I collagen substratum also led to transdifferentiation as assessed by the expression of FSP1, a fibroblast-specific protein associated with mesenchymal cell morphology [38]. Our work using PTC would confirm the hypothesis that contact with type I collagen would facilitate TGF-β1-induced transdifferentiation.
Transdifferentiation of epithelial cells to fibroblastic cells is generally accompanied by a change in the extracellular matrix synthetic profile from basement membrane to interstitial components reflected by alterations in type III to IV collagen synthesis ratios [38]. In this study, we also examined collagen generation as a further measure of change in functionality. As with all other parameters used to examine epithelial-mesenchymal transdifferentiation, although TGF-β1 alone increased type III collagen synthesis, the effect on type III collagen synthesis was far greater when both stimuli were applied together, suggesting more complete transdifferentiation in cell function.
Once PTC transdifferentiate into myofibroblasts and move into the interstitium, in theory, they cannot rapidly undergo reverse phenotypic change from myofibroblasts back to PTC. In this study, following removal of TGF-β1 for 72 h, TGF-β1-stimulated transformed PTC promptly regained their epithelial morphology. In contrast, transdifferentiated PTC induced by concurrent stimulation of TGF-β1 and contact with type I collagen remained their fibroblastic morphology even if TGF-β1 was removed. We speculate that transdifferentiated PTC once move into the interstitium and are then stuck in microenvironment full of type I collagen, causing an irreversible phenotypic alteration.
In addition to phenotypic and functional alterations, transdifferentiated PTC need to move into the interstitial compartment if they are to function as myofibroblasts. Our results using a Transwell system demonstrate that PTC respond to chemotactic stimulation by TGF-β1. Furthermore, the chemotactic effect of TGF-β1 was augmented by the presence of type I collagen in the lower chamber. This would suggest that exposure of PTC on their basolateral aspect to type I collagen facilitates migration, and is in agreement with our previous studies demonstrating that TGF-β1 mediated loss of cell–cell contact and disassembly of adherens junctions were seen following addition of TGF-β1 to the basolateral aspect of the cells [41].
As Zeisberg et al. and Ng et al. have emphasized the importance of an intact tubular basement membrane in preservation of an epithelial phenotype during the process of transdifferentiation [9], [40], how might alterations in organization and composition of the basement membrane be mediated? MMPs have been demonstrated to be implicated in renal fibrosis [30], [42], [43]. Addition of TGF-β1 in our experimental system led to both a time and dose-dependent induction of MMP-9 in PTC. This suggests that TGF-β1 may be involved in basement membrane disruption to allow PTC to contact with the interstitial components. Further support for the importance of the interaction between TGF-β1 and MMP comes from a study demonstrating that MMP9 can trigger EMT in murine PTC and TGF-β1-induced EMT is blocked by MMP2/9 inhibitors [44]. In addition, Cheng et al. also show that gelatinase A (MMP2) were co-localized to sites of active epithelial-mesenchymal transformation and basal lamina disruption, suggesting a possibility that MMP causes complex rearrangement of microenvironment in EMT [30]. We have previously demonstrated that MMP2/9 inhibitors abrogated EGF and TGF-β1-co-stimulated EMT and cell migration, further verifying the importance of MMPs in EMT [31]. Nevertheless, whether other factors that can disrupt the integrity of basement membrane structure also facilitate EMT formation requires further investigation.
The mechanism beyond the synergistic effect of contact with type I collagen and TGF-β1 stimulation remains unclear. We have previously demonstrated that TGF-β1 and EGF can synergistically induce PTC transdifferentiation through sustained activation of extracellular signal-regulated kinase (ERK) signals [31]. As contact with type I collagen also induces activation of ERK signals [45], [46], [47], it is tempting to speculate that combination of TGF-β1 stimulation and contact with type I collagen cooperatively causes sustained activation of the ERK cascade to maintain complete transdifferentiation. In vitro studies have reported in some cell types that grown on type I collagen gel leads to an invasive morphological change accompanied by increased expression of some mesenchymal markers and loss of epithelial markers. In this study, grown on type I collagen substratum alone also caused an increase in α-SMA expression and migratory cell number. However, unlike TGF-β1, it did not enhance type IV collagen synthesis or reduce E-cadherin expression determined western blot analysis despite some disruption of E-cadherin assessed by immunostaining. We speculate that activation of some specific pathways in contact with type I collagen or TGF-β1 stimulation may be attributed to the discrepancy in these two different reactions. For example, the classical Smad pathway specifically induced by TGF-β1 but not by contact with type I collagen (our observation) may bring about different responses.
In summary, the current study has demonstrated induction of a stable transformed phenotype of proximal tubular epithelial cells in which all of parameters indicative of a fibroblastic transformation may be regulated in a cooperative manner by the pro-fibrotic cytokine TGF-β1 and exposure of PTC to an interstitial extracellular matrix component, type I collagen.
Source of support
Nil.
Conflicts of interest
None declared.
Acknowledgments
This study was supported by grants from the National Science Council of Taiwan to Dr. Y-C Tian and Dr. Y-J Li (NSC 102-2628-B-182A-004-MY3, 102-2314-B-182A-103-MY3) and grants from the Chung Gang Medical Research Project to Dr. YC Tian and Dr. Y-J Li (CMRPG3B1341-3, CMRPG390921).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Bohle A., Mackensen-Haen S., von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol. 1987;7:421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- 2.Mackensen-Haen S., Bader R., Grund K.E., Bohle A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol. 1981;15:167–171. [PubMed] [Google Scholar]
- 3.Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S.L., Kisseleva T., Brenner D.A., Duffield J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan H.Y. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 7.Zeisberg M., Strutz F., Müller G.A. Renal fibrosis: an update. Curr Opin Nephrol Hypertens. 2001;10:315–320. doi: 10.1097/00041552-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Strutz F. Novel aspects of renal fibrogenesis. Nephrol Dial Transpl. 1995;10:1526–1532. [PubMed] [Google Scholar]
- 9.Ng Y.Y., Huang T.P., Yang W.C., Chen Z.P., Yang A.H., Mu W. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998;54:864–876. doi: 10.1046/j.1523-1755.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- 10.Li J.H., Wang W., Huang X.R., Oldfield M., Schmidt A.M., Cooper M.E. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Border W.A., Noble N.A., Yamamoto T., Tomooka S., Kagami S. Antagonists of transforming growth factor-beta: a novel approach to treatment of glomerulonephritis and prevention of glomerulosclerosis. Kidney Int. 1992;41:566–570. doi: 10.1038/ki.1992.83. [DOI] [PubMed] [Google Scholar]
- 12.Tian Y.C., Chen Y.C., Hung C.C., Chang C.T., Wu M.S., Phillips A.O. Leptospiral outer membrane protein induces extracellular matrix accumulation through a TGF-beta1/Smad-dependent pathway. J Am Soc Nephrol. 2006;17:2792–2798. doi: 10.1681/ASN.2006020159. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T., Nakamura T., Noble N.A., Ruoslahti E., Border W.A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T., Noble N.A., Miller D.E., Border W.A. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int. 1994;45:916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- 15.Piek E., Moustakas A., Kurisaki A., Heldin C.H., ten Dijke P. TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112(Pt 24):4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- 16.Yamamura Y., Hua X., Bergelson S., Lodish H.F. Critical role of Smads and AP-1 complex in transforming growth factor-beta -dependent apoptosis. J Biol Chem. 2000;275:36295–36302. doi: 10.1074/jbc.M006023200. [DOI] [PubMed] [Google Scholar]
- 17.Fan J.M., Ng Y.Y., Hill P.A., Nikolic-Paterson D.J., Mu W., Atkins R.C. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 18.Bhowmick N.A., Ghiassi M., Bakin A., Aakre M., Lundquist C.A., Engel M.E. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellenrieder V., Hendler S.F., Boeck W., Seufferlein T., Menke A., Ruhland C. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61:4222–4228. [PubMed] [Google Scholar]
- 20.Jinde K., Nikolic-Paterson D.J., Huang X.R., Sakai H., Kurokawa K., Atkins R.C. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis. 2001;38:761–769. doi: 10.1053/ajkd.2001.27693. [DOI] [PubMed] [Google Scholar]
- 21.Ng Y.Y., Fan J.M., Mu W., Nikolic-Paterson D.J., Yang W.C., Huang T.P. Glomerular epithelial-myofibroblast transdifferentiation in the evolution of glomerular crescent formation. Nephrol Dial Transpl. 1999;14:2860–2872. doi: 10.1093/ndt/14.12.2860. [DOI] [PubMed] [Google Scholar]
- 22.Ryan M.J., Johnson G., Kirk J., Fuerstenberg S.M., Zager R.A., Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 23.Tian Y.C., Phillips A.O. TGF-beta1-mediated inhibition of HK-2 cell migration. J Am Soc Nephrol. 2003;14:631–640. doi: 10.1097/01.asn.0000053418.56286.5e. [DOI] [PubMed] [Google Scholar]
- 24.Li Y.J., Wu H.H., Weng C.H., Chen Y.C., Hung C.C., Yang C.W. Cyclophilin A and nuclear factor of activated T cells are essential in cyclosporine-mediated suppression of polyomavirus BK replication. Am J Transpl. 2012;12:2348–2362. doi: 10.1111/j.1600-6143.2012.04116.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang J., Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips A.O., Morrisey K., Steadman R., Williams J.D. Decreased degradation of collagen and fibronectin following exposure of proximal cells to glucose. Exp Nephrol. 1999;7:449–462. doi: 10.1159/000020624. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Chen J., Chen L., Tay Y.C., Rangan G.K., Harris D.C. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol. 1997;8:1537–1545. doi: 10.1681/ASN.V8101537. [DOI] [PubMed] [Google Scholar]
- 28.Wada T., Furuichi K., Sakai N., Iwata Y., Yoshimoto K., Shimizu M. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 29.Tesch G.H., Schwarting A., Kinoshita K., Lan H.Y., Rollins B.J., Kelley V.R. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng S., Lovett D.H. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol. 2003;162:1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian Y.C., Chen Y.C., Chang C.T., Hung C.C., Wu M.S., Phillips A. Epidermal growth factor and transforming growth factor-beta1 enhance HK-2 cell migration through a synergistic increase of matrix metalloproteinase and sustained activation of ERK signaling pathway. Exp Cell Res. 2007;313:2367–2377. doi: 10.1016/j.yexcr.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Han D.C., Isono M., Hoffman B.B., Ziyadeh F.N. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-beta. J Am Soc Nephrol. 1999;10:1891–1899. doi: 10.1681/ASN.V1091891. [DOI] [PubMed] [Google Scholar]
- 33.Phillips A.O., Steadman R., Donovan K.D., Williams J.D. A new antibody capture enzyme linked immunoassay specific for transforming growth factor beta 1. Int J Biochem Cell Biol. 1995;27:207–213. doi: 10.1016/1357-2725(94)00077-o. [DOI] [PubMed] [Google Scholar]
- 34.Phillips A.O., Topley N., Steadman R., Morrisey K., Williams J.D. Induction of TGF-beta 1 synthesis in D-glucose primed human proximal tubular cells by IL-1 beta and TNF alpha. Kidney Int. 1996;50:1546–1554. doi: 10.1038/ki.1996.470. [DOI] [PubMed] [Google Scholar]
- 35.Tian Y.C., Fraser D., Attisano L., Phillips A.O. TGF-beta1-mediated alterations of renal proximal tubular epithelial cell phenotype. Am J Physiol. 2003;285:F130–F142. doi: 10.1152/ajprenal.00408.2002. [DOI] [PubMed] [Google Scholar]
- 36.Lee E.H., Joo C.K. Role of transforming growth factor-beta in transdifferentiation and fibrosis of lens epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:2025–2032. [PubMed] [Google Scholar]
- 37.Miettinen P.J., Ebner R., Lopez A.R., Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127(6 Pt 2):2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada H., Danoff T.M., Kalluri R., Neilson E.G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273(4 Pt 2):F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 39.Strutz F., Zeisberg M., Ziyadeh F.N., Yang C.Q., Kalluri R., Müller G.A. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 40.Zeisberg M., Bonner G., Maeshima Y., Colorado P., Müller G.A., Strutz F. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Y.C., Phillips A.O. Interaction between the transforming growth factor-beta type II receptor/Smad pathway and beta-catenin during transforming growth factor-beta1-mediated adherens junction disassembly. Am J Pathol. 2002;160:1619–1628. doi: 10.1016/s0002-9440(10)61109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Zhou Y., Tan R., Xiong M., He W., Fang L. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2010;299:F973–F982. doi: 10.1152/ajprenal.00216.2010. [DOI] [PubMed] [Google Scholar]
- 43.Nair S., Phillips A.O., Norton N., Spurlock G., Williams H.J., Craig K.J. Further evidence for the association of MMP9 with nephropathy in type 2 diabetes and application of DNA pooling technology to candidate gene screening. J Nephrol. 2008;21:400–405. [PubMed] [Google Scholar]
- 44.Tan T.K., Zheng G., Hsu T.T., Wang Y., Lee V.W., Tian X. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am J Pathol. 2010;176:1256–1270. doi: 10.2353/ajpath.2010.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montesano R., Soriano J.V., Hosseini G., Pepper M.S., Schramek H. Constitutively active mitogen-activated protein kinase kinase MEK1 disrupts morphogenesis and induces an invasive phenotype in Madin-Darby canine kidney epithelial cells. Cell Growth Differ. 1999;10:317–332. [PubMed] [Google Scholar]
- 46.Fassett J.T., Tobolt D., Nelsen C.J., Albrecht J.H., Hansen L.K. The role of collagen structure in mitogen stimulation of ERK, cyclin D1 expression, and G1-S progression in rat hepatocytes. J Biol Chem. 2003;278:31691–31700. doi: 10.1074/jbc.M300899200. [DOI] [PubMed] [Google Scholar]
- 47.Chu C.L., Reenstra W.R., Orlow D.L., Svoboda K.K. Erk and PI-3 kinase are necessary for collagen binding and actin reorganization in corneal epithelia. Invest Ophthalmol Vis Sci. 2000;41:3374–3382. [PMC free article] [PubMed] [Google Scholar]