Abstract
The health of an organism is intricately linked to its gut microbiome. However, the mechanisms by which the microbiome affect the host gene regulation are still not well established. A new study by Tuorto et al (2018) shows that queuine, a nitrogenous base obtained from the gut microbiota, is used to modify tRNAs and affects cellular behavior. Dietary queuine is required for proper protein synthesis, and its depletion activates cellular stress responses in vitro and in vivo.
Subject Categories: Metabolism, Protein Biosynthesis & Quality Control, RNA Biology
In recent years, a growing body of evidence suggests that post‐transcriptional modifications of tRNAs, the molecules that physically connect the mRNA with its corresponding amino acids at the ribosome, and the enzymes catalyzing such modifications play a role in gene regulation and are involved in human pathologies. In eukaryotes, tRNAs are decorated with a variety of more than 100 chemically different modifications, the majority of which are conserved among species (Boccaletto et al, 2018). In fact, with nearly 12% of its total residues modified, tRNA is the most modified known RNA species (Kirchner & Ignatova, 2015). While the identification of chemical modifications dates back to the early 60s, the function of several tRNA modifications has only recently been elucidated. Nonetheless, the role of the majority of tRNA modifications has remained unexplored.
In 1966, Francis Crick hypothesized that anticodons participate in non‐canonical Watson–Crick base pairing, termed wobble (Crick, 1966). The wobble hypothesis explains how 64 combinations of codons only encode 20 amino acids. Interestingly, position 34 of the tRNA anticodon, the so‐called wobble position, is highly modified. Modifications at this site are associated with increased diversity of codon recognition by allowing codon–anticodon wobbling (Torres et al, 2014) and have been previously shown to play a role in fine‐tuning regulation of translation in yeast and C. elegans (Nedialkova & Leidel, 2015; Chou et al, 2017).
One of the wobble modifications is a guanosine analog called queuosine (Q). This modification is very intriguing because in animals Q and its precursor queuine can only be obtained as a micronutrient from dietary sources and from the gut microbiota (Fergus et al, 2015). The function of Q in mammals remains poorly understood, mainly because of difficulties in achieving its depletion. Tuorto and colleagues employed ribosome profiling and proteomics in human cell lines and in germ‐free mice fed on a Q‐deficient diet to answer the long‐standing question of the role of Q in protein translation.
The authors first examined human cell lines grown in synthetic serum with or without the addition of chemically synthesized Q. Using a special Northern blot analysis in which Q‐tRNA migrates slower than normal tRNA, they confirmed the reduction in Q‐tRNA over time in Q‐deficient medium. The presence of Q has been previously reported to affect DNMT2‐dependent tRNAAsp methylation at position 38 in fission yeast (Müller et al, 2015). Indeed, bisulfite sequencing revealed a significant reduction in C38 methylation when cells were grown in Q‐depleted medium, supporting the enhancement role of Q on DNMT2 activity in mammalian cells.
Next, the team set out to determine how the presence of Q on tRNAs affects translation speed using ribosome profiling. In this method, ribosome‐protected mRNA fragments are deep sequenced to map the ribosomal location across transcripts at a single‐nucleotide resolution. This analysis revealed higher density of ribosomes at Q‐decoded codons, suggesting slower translation of these codons in the absence of Q. Stable isotope protein labeling and mass spectrometry analysis (SILAC) further supported the ribosome profiling results—demonstrating reduced expression of proteins enriched for slow‐translated codons following Q deprivation.
In animal cells, alterations in the abundance of Q have been shown to correlate with a variety of processes including stress tolerance, cell proliferation, and tumor growth (Fergus et al, 2015). Tuorto et al (2018) examined the mRNAs that were differentially translated and observed enrichment of genes involved in stress signaling. Q deprivation resulted in decreased cell growth and proliferation. To address whether the growth defects and the activation of stress signaling were caused by misfolded proteins, the authors used a reporter assay and electron microscopy in human cell lines. Indeed, they observed the formation of protein aggregates in Q‐depleted cells accompanied by irregular morphology of the endoplasmic reticulum (ER). These findings, supported by the identification of additional ER stress markers, suggest that Q deficiency leads to the accumulation of misfolded proteins that induce a cellular stress response. Does Q depletion affect mice the same way it affects human cell lines? It seems the answer is yes. The authors fed germ‐free mice with Q‐free synthetic diet and analyzed their tissues. Indeed, reduced levels of Q‐tRNA were detected, and these were rescued by addition of synthetic Q to the diet. Interestingly, Q‐starved mice displayed reduced body weight, increased expression of ER stress markers, and reduced mRNA translation, recapitulating the cell‐based observations.
Collectively, the study by Tuorto et al (2018) convincingly shows that the absence of Q affects protein synthesis and that this effect is associated with ER stress caused by a misfolded protein response (Fig 1). Nevertheless, several questions remain unsolved. First, what is the exact mechanism by which depletion of Q‐tRNA affects translation? Can it be explained by codon–anticodon affinity at the ribosome, or perhaps the misincorporation of wrong amino acids to the growing polypeptide chain? Second, what is the mechanism that leads to ER stress? The authors mention a slower rate of translation as the main cause, but the possibility that translation fidelity is affected needs to be addressed too. Lastly, recent studies demonstrated that tRNA‐modifying enzymes can be implicated in disease. For example, a recent study provided evidence that certain wobble‐modifying enzymes are involved in resistance to cancer targeted therapy (Rapino et al, 2018). Since queuine is obtained from the diet, it will be interesting to determine whether Q deficiency or excess can result in disease. Additionally, it will be interesting to determine whether QTRT1, the enzyme that catalyzes the base exchange of a guanine residue with queuine at position 34, is implicated in human diseases. Altogether, this study nicely demonstrates the impact that nutrition and microbiome have on cell‐intrinsic mechanisms such as protein synthesis, paving the way for potential therapeutic manipulation of these environmental factors.
Figure 1. Dietary queuine and gut microbiota‐derived queuine regulate protein synthesis via tRNA queuosinylation.
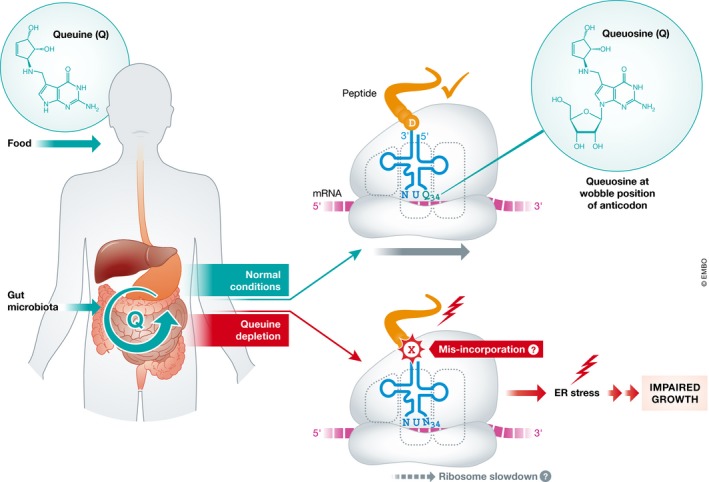
Queuine is a micronutrient obtained from food and the gut microbiota. Under normal conditions, queuine is used to modify the wobble position of certain tRNAs and this modification is required for proper translation. Queuine depletion results in deregulation of translation (possibly due to amino acid misincorporation or ribosome slowdown) that leads to ER stress and impaired growth in a mouse model system.
The EMBO Journal (2018) 37: e100405
See also: https://doi.org/10.15252/embj.201899777 (September 2018)
References
- Boccaletto P, MacHnicka MA, Purta E, Pitkowski P, Baginski B, Wirecki TK, De Crécy‐Lagard V, Ross R, Limbach PA, Kotter A, Helm M, Bujnicki JM (2018) MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 46: D303–D307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HJ, Donnard E, Gustafsson HT, Garber M, Rando OJ (2017) Transcriptome‐wide analysis of roles for tRNA modifications in translational regulation. Mol Cell 68: 978–992.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FHC (1966) Codon—anticodon pairing: the wobble hypothesis. J Mol Biol 19: 548–555 [DOI] [PubMed] [Google Scholar]
- Fergus C, Barnes D, Alqasem MA, Kelly VP (2015) The queuine micronutrient: charting a course from microbe to man. Nutrients 7: 2897–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner S, Ignatova Z (2015) Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet 16: 98–112 [DOI] [PubMed] [Google Scholar]
- Müller M, Hartmann M, Schuster I, Bender S, Thüring KL, Helm M, Katze JR, Nellen W, Lyko F, Ehrenhofer‐Murray AE (2015) Dynamic modulation of Dnmt2‐dependent tRNA methylation by the micronutrient queuine. Nucleic Acids Res 43: 10952–10962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedialkova DD, Leidel SA (2015) Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell 161: 1606–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapino F, Delaunay S, Rambow F, Zhou Z, Tharun L, De Tullio P, Sin O, Shostak K, Schmitz S, Piepers J, Ghesquière B, Karim L, Charloteaux B, Jamart D, Florin A, Lambert C, Rorive A, Jerusalem G, Leucci E, Dewaele M et al (2018) Codon‐specific translation reprogramming promotes resistance to targeted therapy. Nature 558: 605–609 [DOI] [PubMed] [Google Scholar]
- Torres AG, Batlle E, Ribas de Pouplana L (2014) Role of tRNA modifications in human diseases. Trends Mol Med 20: 306–314 [DOI] [PubMed] [Google Scholar]
- Tuorto F, Legrand C, Cirzi C, Federico G, Liebers R, Müller M, Ehrenhofer‐Murray AE, Dittmar G, Gröne HJ, Lyko F (2018) Queuosine‐modified tRNAs confer nutritional control of protein translation. EMBO J 37: e99777 [DOI] [PMC free article] [PubMed] [Google Scholar]


