Abstract
Aims
Although therapeutic drug monitoring of plasma mycophenolic acid (MPA) concentrations has been recommended to individualize dosage in transplant recipients, little is known regarding lymphocyte concentrations of MPA, where MPA inhibits inosine monophosphate dehydrogenase (IMPDH). This study investigated the utility of measuring predose MPA concentrations in peripheral blood mononuclear cells (C0C) and predose IMPDH activity, as predictors of graft rejection in renal transplant recipients.
Methods
Forty‐eight patients commencing mycophenolate mofetil (1 g twice daily) in combination with tacrolimus and prednisolone were recruited. Blood was collected for determination of trough total (C0P) and unbound (C0u) plasma MPA concentrations. Peripheral blood mononuclear cells were isolated for determination of C0C and IMPDH activity. The incidence of rejection within 2 days of sample collection was determined histologically and classified according to the Banff 2007 criteria.
Results
There was no association between MPA C0C and C0P (rs = 0.28, P = 0.06), however, MPA C0C were weakly correlated with MPA C0u (rs = 0.42, P = 0.013). Multivariate analysis indicated that MPA C0C was the only covariate independently associated with rejection (FDR‐adjusted P = 0.033). The receiver operating characteristic area under the curve (AUC) for the prediction of severe rejection using MPA C0C was 0.75 (P = 0.013), with 73% sensitivity and specificity at a C0C threshold of 0.5 ng 10–7 cells. However, predose IMPDH activity was not a predictor of rejection (P > 0.15).
Conclusions
MPA C0C measurement within the early post‐transplant period may be useful to facilitate early titration of MPA dosing to significantly reduce rejection.
Keywords: immunosuppression, pharmacokinetics, renal transplantation, therapeutic drug monitoring
What is Already Known About this Subject
Mycophenolic acid (MPA) is an immunosuppressant commonly used to prevent rejection following renal transplantation.
MPA prevents lymphocyte proliferation by inhibiting inosine monophosphate dehydrogenase activity, the rate limiting enzyme for de novo purine synthesis.
Several clinical trials have demonstrated that therapeutic drug monitoring of plasma MPA concentrations reduces the risk of rejection.
What this Study Adds
Peripheral blood mononuclear cell (PBMC) concentrations of MPA were better predictors of rejection risk, compared to measuring trough plasma MPA concentrations or PBMC inosine monophosphate dehydrogenase activity.
Trough plasma MPA concentrations were relatively poor predictors of PBMC concentrations.
Predose PBMC MPA concentrations <0.5 ng/107 cells predicted severe rejection with 73% sensitivity and specificity.
Introduction
Mycophenolic acid (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6832), one of the primary immunosuppressants administered to prevent rejection following renal transplantation 1, exerts antiproliferative effects on lymphocytes as its major mode of action 2. It is a potent, selective, reversible and noncompetitive inhibitor of inosine monophosphate dehydrogenase (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2625) type II, a rate‐limiting enzyme involved in de novo purine synthesis that is selectively required for lymphocyte proliferation 2. Inhibition of this pathway prevents the proliferation of lymphocytes and the activation of T‐cells, which consequently contributes to the prevention of graft rejection.
The clinical use of MPA is compromised by MPA having a narrow therapeutic index and large inter‐patient variability in pharmacokinetics 3. Numerous studies have investigated the plasma MPA concentration‐effect relationship, and recent consensus reports indicate significant benefits of monitoring area under the plasma concentration–time curve (AUCP) with respect to rejection 3, 4. In general, although there is also a relationship between MPA trough concentrations (C0P) and the risk of graft rejection, the data are more equivocal due to the variable enterohepatic recirculation of MPA and its inhibition by cyclosporin (CsA; but not tacrolimus, TAC), potentially resulting in different relationships between C0P and AUCP when coadministered with CsA compared to TAC 3, 4.
Despite application of therapeutic drug monitoring (TDM) strategies that target patients to narrow plasma MPA concentrations (C0P: 1.9–3.5 mg l–1) or AUCP ranges (0–12 h: 30–60 mg h l–1) when coadministered with TAC 3, 5, rejection rates in Australian renal transplant recipients remain relatively high (13–24% in the first 6 months post‐transplantation) 1. This suggests that monitoring of plasma MPA concentrations alone may be inadequate to predict graft rejection and may not be the most appropriate predictor of target lymphocyte (site of action) concentrations, and hence, clinical outcomes. Direct measurement of lymphocyte MPA concentrations may provide a better understanding of its immunosuppressive efficacy and distribution during graft rejection. The importance of such an approach has already been demonstrated for CsA 6, 7, 8, 9 and TAC 10, 11 by direct quantification of peripheral blood mononuclear cells (PBMCs), representing the target site of action. It has been suggested that lower PBMC CsA 9 and TAC 10 concentrations are associated with significantly higher incidences of graft rejection, which were not reflected by whole blood concentrations.
Little is known regarding the relationship between PBMC and plasma MPA concentrations. In 40 kidney transplant recipients, no association between MPA C0P and predose PBMC (C0C) MPA concentrations was found 12. However, a later study conducted by the same group reported significant correlations between plasma and PBMC MPA concentrations at 1.5 and 3.5 h postdose, but not between MPA C0P and C0C concentrations on days 2, 4 and 10 post‐transplantation 13. No other studies have investigated the factors that determine PBMC MPA concentrations. Two important modulators of these concentrations may include the binding of MPA to plasma albumin, and the possible role of PBMC uptake and efflux transporters in modulating intracellular concentrations. MPA is a substrate for the efflux transporter multidrug resistance‐associated protein 2 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=780) 14, which is also expressed in lymphocytes 15. Genetic variability in the ABCC2 gene that encodes MRP2 may, therefore, influence PBMC MPA concentrations and modulate the efficacy and/or safety of MPA therapy.
Pharmacodynamic monitoring of MPA by the measurement of IMPDH activity has also been investigated in PBMCs 13, 16, 17, 18, 19, 20, 21. In general, there is an inverse relationship between plasma MPA concentrations and total PBMC IMPDH activity (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2624 and II). Within a dosing interval, maximum inhibition of activity coincides with the maximum plasma MPA concentration, and activity returns to predose levels by between 3.5 and 11 h postdose 13, 16, 19, 21. Population pharmacokinetic–pharmacodynamic (Emax) modelling has been used to calculate EC50 values for the inhibition of IMPDH activity by MPA, which range from 0.97 to 5.4 mg l–1, in both adult and paediatric renal transplant recipients 16, 19, 21. However, the relationship between trough plasma MPA concentrations and IMPDH activity is less clear 13, 18, 20. Similarly, the relationship between IMPDH activity and clinical outcomes is not well defined. An increased risk of acute rejection in patients with high pretransplant IMPDH activities has been reported 17, but there appears to be no relationship post‐transplant between predose IMPDH activity and acute rejection 20.
This study aimed to investigate PBMC MPA concentrations and intracellular IMPDH activity as predictors of early graft rejection in kidney transplant recipients. We hypothesized that PBMC MPA concentrations within the early post‐transplant period may have greater relevance for predicting IMPDH activity and the incidence of graft rejection, compared to plasma MPA concentrations.
Methods
Study population, pharmacokinetic and clinical data
Forty‐eight kidney transplant recipients gave written informed consent to participate in this prospective clinical study, which was approved by the Royal Adelaide Hospital Research Ethics Committee (approval number 130109). The study was conducted in accordance with the Declaration of Helsinki and the Australian NHMRC Statement on Ethical Conduct in Human Research. Recipients were transplanted between June 2013 and November 2014 with kidneys from living and deceased donors. They received the MPA prodrug, mycophenolate mofetil (MMF, 1 g twice daily) for maintenance immunosuppression in combination with TAC and prednisolone. For each recipient, a fine‐needle graft biopsy was taken between 5 and 22 days post‐transplantation. On the same day, blood was drawn to assess trough plasma MPA concentrations. Total trough plasma MPA concentrations (C0P) were determined using a validated high‐performance liquid chromatography (HPLC) method 22, and matching unbound (C0u) plasma MPA concentrations were determined following temperature controlled (37°C) ultrafiltration of plasma samples. MPA‐d3 internal standard (Toronto Research Chemicals, Toronto, Canada) in methanol was added to ultrafiltrate, vortexed and centrifuged prior to analysis by liquid chromatography using an Acquity UPLC HSS T3 C18 analytical column (1.8 μm, 2.1 × 100 mm) and Acquity BEH C18 precolumn (1.7 μm, VanGuard 2.1 × 5 mm) maintained at 40°C, with gradient elution using 2 mmol l–1 ammonium acetate and 0.1% formic acid in methanol (mobile phase B) or water (mobile phase A). Multiple reaction monitoring was carried out using positive electrospray ionization and detection of MPA 321.2 > 207.2 and MPA‐d3 324.3 > 310.2 transitions. The assay was linear between 5–1500 μg l–1 (10 μl injection) with intra‐assay imprecision <4% and interassay imprecision <9% and inaccuracy <5%. Postpreparation sample stability was at least 24 h at 25 °C, carry‐over was <0.1% at the highest calibrator, analyte recovery was 100–101%, and matrix effects were <10%. Demographic, pharmacokinetic and clinical data were obtained from original patient case notes and the data collected were: recipient and donor ages; sex; ethnicity (self‐report); donor type (living or deceased); human leucocyte antigen (HLA) mismatches; cold ischaemia time (CIT); panel‐reactive antibodies (PRA); plasma creatinine; albumin and bilirubin concentrations; pretransplant angiotensin II type‐1 receptor (AT1R) antibody levels; donor‐specific HLA antibodies; trough whole blood TAC concentrations; and the incidences of rejection and delayed graft function (DGF). Rejection status within the period of ±2 days from sample collection was determined based on histological evidence of rejection in protocol and for‐cause biopsies (5–22 days post‐transplant), and classified for severity according to Banff 2007 criteria 23 as: no rejection; subclinical or borderline; or clinically evident cellular/vascular rejection (Type 1A/2 or 2A/B). The incidence of DGF was identified by the lack of spontaneous decline in serum creatinine or requirement for haemodialysis within 7 days post‐transplantation.
ABCC2 genotyping and haplotype predictions
Blood samples from recipients were also used for determination of the common ABCC2 allele variants (–24 C > T, 1249 G > A and 3972 C > T) 24, and ABCC2 haplotypes were inferred by the use of PHASE software version 2.1.1 25. The recipients were divided into high, wild‐type (WT) or low MRP2 expressor groups according to ABCC2 haplotypes reported previously by Laechelt et al. 26.
Isolation of PBMCs from whole blood
Additional duplicate blood samples (2 × 9 ml) were collected in EDTA tubes at the same time as the C0P sample [median (range): 13 (5–22) days post‐transplant] and were processed individually within 4 h after collection to ensure maximal PBMC yield. PBMC were isolated with Lymphoprep (Axis‐Shield, Oslo, Norway) according to the manufacturer's protocol with several modifications. In brief, 9 ml of patient's blood were diluted with an equal volume of 0.9% ice‐cold NaCl, underlayered with 9 ml Lymphoprep and centrifuged without brakes at 1200 g at 4°C for 20 min. After centrifugation, PBMCs were harvested from the plasma/Lymphoprep interface and washed three times with 30 ml of 0.9% NaCl (centrifugation at 1200 g at 4°C for 10 min). The washed PBMC pellets were resuspended in 5 ml of 0.9% NaCl and 500 μl used for cell counting (in duplicate) on a haemocytometer. Each duplicate sample was centrifuged (1200 g, 4°C, 10 min), and each corresponding PBMC pellet was stored at –80°C for later determination of MPA C0C concentrations and IMPDH activity, respectively. Fresh blood (150 ml) obtained from healthy volunteers was used to isolate PBMCs (treated as 9 ml aliquots as described above) for the preparation of calibrator and quality control (QC) samples.
Measurement of predose PBMC MPA (C0C) concentrations
Measurement of C0C concentrations from patient samples was based on our previously published liquid chromatography–tandem mass spectrometry (LC–MS/MS) method for the quantification of MPA in human kidney transplant biopsies with slight modifications 27. On the day of the assay, frozen patient and blank PBMC pellets were thawed at 4°C and placed on ice. Subsequently, 200 μl of ice‐cold phosphate buffered saline (PBS) pH 7.4 was added to each thawed patient cell pellet and was mixed thoroughly. The calibrators and QC samples were prepared from blank PBMC pellets (containing 107 cells), to which 100 μl of PBS solution was added followed by 100 μl of MPA working solutions (prepared in 50% MeOH), to attain final concentrations of 0.1, 0.2, 0.5, 1.0, 3.0 and 5.0 ng ml–1 for calibrators and 0.3, 1.5 and 2.0 ng ml–1 for QC samples. To each calibrator, QC and patient sample, 60 μl of 0.4 mol l–1 HCl, 10 μl of 0.2 μg ml–1 MPA‐d3 internal standard and 1 ml of tertiary‐butyl methyl ether were added. MPA extraction and cell lysis were performed by gently mixing on a roller mixer for 10 min followed by centrifugation at 1900 g at 4°C for 10 min. The organic layer was removed, transferred to a 5 ml disposable glass tube and evaporated to dryness using an evacuated centrifuge at 45°C for 20 min. The dried residues were reconstituted with 50 μl of 50% methanol, vortexed for 2 min and 10 μl of the reconstituted solutions injected onto the LC–MS/MS for analysis. Analytical and LC–MS/MS conditions are described elsewhere 27.
The assay was fully validated according to the US Food and Drug Administration guidelines for bioanalytical methods 28 with assessment for linearity, accuracy, precision, extraction efficiency, matrix effects and stability. The calibration curves were linear, with coefficients of determination (R 2) greater than 0.99 (n = 5), and intra‐ and interassay inaccuracy and imprecision were <15% (n = 5). MPA extraction efficiency displayed good reproducibility, with coefficient of variations (CVs) ranging from 0.1 to 5.2% and matrix effects were minimal (< 10%); both were assessed at three MPA concentrations (1.0, 5.0 and 20.0 ng ml–1) in duplicate. MPA was stable when spiked into blank PBMCs, with no significant degradation after 12 h at room temperature or 6 months at –80°C, nor in postprocessing samples left in the autosampler (4°C) for 24 h. Other immunosuppressants likely to be administered with MPA (i.e. TAC, CsA, prednisolone, sirolimus and everolimus) had no significant effects on either MPA or MPA‐d3 peak areas. The measured MPA C0C concentrations were adjusted according to the number of PBMC extracted and expressed as ng 10–7 cells.
Measurement of PBMC IMPDH enzyme activity
Measurement of IMPDH activity from lysed PBMCs was based on previously published HPLC methods for the quantification of IMPDH activity in PBMCs 16, 29. In brief, after thawing at 4°C, the PBMC pellets were resuspended in 900 μl ice‐cold Millipore water and insoluble fragments of disrupted cells were removed by centrifugation at 15 800 g at room temperature (RT) for 2 min. The PBMC lysate was used for protein content (20 μl) and IMPDH enzymatic activity (50 μl) determinations. The measurement of lysate protein concentration was performed with Bio‐Rad Protein Assay Reagent (Bio‐Rad Laboratories, California, USA) using bovine serum albumin as standard according to the manufacturer's protocol. IMPDH activity in PBMCs was determined from the conversion of inosine 5′‐monophosphate (IMP) to xanthosine 5′‐monophosphate (XMP) based on methods described previously 16, 29. Briefly, the IMPDH incubation mixture (pH 7.4) consisted of 1 mmol l–1 IMP, 0.5 mmol l–1 NAD+, 40 mmol l–1 NaH2PO4 and 100 mmol l–1 KCl. The enzymatic reaction was initiated by the addition of 50 μl of the PBMC lysate to 120 μl of reaction mixture and incubated at 37°C for 2.5 h. After incubation, the reaction was terminated by adding 20 μl of 4 mol l–1 ice‐cold HCIO4, vortexing for 10 s, and the deproteinised solution was centrifuged at 15 800 g at RT for 2 min. Subsequently, 170 μl of supernatant was neutralised by adding 17 μl of 5 mol l–1 ice‐cold K2CO3, vortexing for 10 s, and storing the samples for 30 min at –80°C. After thawing and centrifugation at 15 800 g at RT for 2 min, 25 μl of the supernatant was immediately injected onto the HPLC column for analysis.
Chromatographic detection of XMP production was achieved using a Synergi HydroRP 80A column (4 μmol l–1, 250 × 3 mm; Phenomenex, Lane Cove, NSW, Australia) maintained at 45°C on an Agilent HPLC system, with two mobile phases: A) 50 mmol l–1 potassium phosphate (KH2PO4) and 7 mmol l–1 TBA buffer (pH 5.5); and B) 100% MeOH. The mobile phases were pumped at a flow rate of 0.7 ml min–1 using a semigradient programme of: 94% A and 6% B for 0–13.0 min; 80% A and 20% B for 13.1–23.0 min; and 95% A and 5% B for 23.1–40.0 min. Injection volume was 25 μl with ultraviolet detection at a wavelength of 254 nm.
Specificity was tested in control incubations containing IMP in the absence of cosubstrate NAD+, or containing NAD+ in the absence of IMP. No endogenous XMP was detected in samples incubated without IMP or NAD+, and no interfering peaks were observed at the retention time of XMP. Linearity of XMP formation with protein content was confirmed for protein concentrations up to 2.7 mg ml–1 and time of incubation up to 200 min. IMPDH activity was expressed as XMP produced (nmol) per incubation time (h) per mg protein (nmol h–1 mg protein–1).
Data analyses
Normality of data distribution was assessed by the D'Agostino and Pearson omnibus normality test. Correlations between MPA C0C, C0P and C0u concentrations and IMPDH activity were assessed using a Spearman's rank correlation (rs). Differences in MPA C0C, C0P and C0u concentrations, and IMPDH activity, between patients with and without graft rejection were evaluated using Mann–Whitney rank sum test. Receiver operating characteristic (ROC) curve analysis was performed to assess the ability of either MPA C0C concentrations or IMPDH activity to predict graft rejection. Associations between MPA C0C, C0P and C0u concentrations, and IMPDH activity, with the severity of rejection (no evidence of rejection, subclinical or borderline rejection, and severe cellular or vascular rejection) were assessed using Kruskal–Wallis tests (with Dunn's posthoc for multiple comparisons). The Mann–Whitney rank sum test (CIT, PRA, creatinine, bilirubin, AT1R and TAC C0 concentration), unpaired t‐test (recipient and donor ages, HLA and albumin) and Fisher's Exact test (sex, ethnicity, donor‐specific HLA antibodies post‐transplant, type of donor graft and DGF) were used to investigate the differences in demographic and clinical covariates between patients with or without rejection episodes. All analyses were performed using Prism version 6.0 (GraphPad Software, Inc, La Jolla, CA, USA). Differences between MPA C0C, C0P and C0u concentrations, and IMPDH activity, with the severity of rejection were also analysed using Jonckheere–Terpstra tests (SPSS, version 19, IBM, Armonk, NY, USA). All data are presented as either mean ± standard deviation for continuous parametric data, median (range) for continuous nonparametric data or frequencies (absolute numbers) for categorical data. Statistical significance was considered for P‐values <0.05.
Multivariate analyses were performed using R 30, with false discovery rate (FDR) adjusted P‐values <0.05 considered significant. A step‐up logistic regression model with step‐wise addition of factors was used to compare current TDM practice using blood TAC and MPA C0P with MPA C0C, as predictors of rejection. In addition, a step‐up linear model of multivariate regression with step‐wise addition of factors was used to investigate associations between recipient MRP2 phenotype, plasma albumin concentration, MPA C0P and MPA C0C.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 31 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 32, 33.
Results
Patient characteristics
Patient demographic and biological characteristics are shown in Table 1. Thirty‐nine patients (81%) were Caucasians and nine were Indigenous Australians. Sixteen patients (33%) experienced graft rejection during this study at an average ± standard deviation (range) of 12 ± 5 days (5–22 days) post‐transplantation. There were no significant differences (P ≥ 0.15) between the rejection and no rejection groups with respect to recipient and donor ages, sex, ethnicity, HLA mismatches, CIT, PRA, AT1R antibody levels, serum concentrations of creatinine, albumin or bilirubin, or trough whole blood (C0B) TAC concentrations (Table 1). TAC doses had been adjusted according to TDM practice and, at the time of study, only four patients had concentrations below the recommended therapeutic range. No MMF dosage adjustment had been performed and all subjects received 2 g day–1.
Table 1.
Demographics and biological characteristics of 48 renal transplant recipients
| All (n = 48) | Rejectiona group (n = 15) | No rejection group (n = 33) | P‐value | |
|---|---|---|---|---|
| Pretransplant | ||||
| Sex (male/female) | 25/23 | 8/7 | 17/16 | 0.99 |
| Ethnicity (Caucasian/Indigenous Australian) | 39/9 | 12/3 | 27/6 | 0.99 |
| Recipient age (years) | 50.5 (20–69) | 42 (28–65) | 52 (20–69) | 0.15 |
| Donor age (years) | 48 (17–74) | 41 (18–71) | 50 (17–74) | 0.24 |
| HLA mismatches | 4 (0–6) | 4 (2–6) | 4 (0–6) | 0.45 |
| PRA | 0 (0–90) | 0 (0–3) | 0 (0–90) | 0.18 |
| CIT (h) | 11 (3–34) | 11 (4–26) | 11 (3–34) | 0.70 |
| AT1R antibody level (U l –1 ) | 9 (0–40) | 11 (0–28) | 9 (0–40) | 0.61 |
| Donor graft (living/deceased) | 12/36 | 5/10 | 7/26 | 0.48 |
| Post‐transplant | ||||
| DSA (present/absent) | 13/35 | 4/11 | 9/24 | 0.99 |
| DGF (yes/no) | 19/29 | 5/10 | 14/19 | 0.75 |
| Serum creatinine (μmol l –1 ) b | 150 (56–965) | 130 (58–913) | 159 (56–965) | 0.75 |
| Albumin (g l –1 ) b | 32 (27–43) | 32 (27–43) | 32 (28–43) | 0.55 |
| Bilirubin (μmol l –1 ) b | 8 (3–25) | 7 (3–25) | 8 (4–23) | 0.50 |
| TAC C 0B (μg l –1 ) b | 7.5 (2.5–23.6) | 6.7 (2.5–14.2) | 7.7 (4.3–23.6) | 0.23 |
Rejection status within ±2 days from sample collection
At same time as MPA sample collection
Data are given as median (range) or frequencies (absolute numbers) depending on data type
AT1R, angiotensin II type‐1 receptor; CIT, cold ischaemia time; DGF, delayed graft function; DSA, donor‐specific HLA antibodies; HLA, human leucocyte antigen; PRA, panel reactive antibodies; TAC C0B: trough whole blood tacrolimus concentrations
ABCC2 polymorphisms and inferred MRP2 phenotype
Allele and genotype frequencies for the study cohort are shown in Supporting Table S1. Genotype frequencies did not deviate from Hardy–Weinberg equilibrium (P ≥ 0.1). Eight ABCC2 haplotypes were identified (Table S2), with the four most common being H1 CGC (44.3%, WT), H12 TGT (21.5%, low protein expression), H2 CAC (14.7% high protein expression) and H9 CGT (12.4%, low protein expression). The frequencies of patients with inferred high, moderate and low expressor phenotypes was 12.5, 48 and 39.5%, respectively.
Correlations between MPA C0C, C0P and C0u concentrations, and IMPDH activity
MPA C0C concentrations ranged from 0.1 to 3.9 ng 10–7 cells (median = 0.68 ng 10–7 cells). The corresponding MPA C0P concentrations ranged from 0.45 to 6.5 mg l–1 (median = 2.1 mg l–1), with 19 (40%) below, 17 (35%) within and 12 (25%) above the notional therapeutic range. Pre‐dose IMPDH activity ranged from 0.9 to 33.9 nmol h–1 mg–1 protein (median = 11.9 nmol–1 h–1 mg protein), similar to previous studies 16, 17. There was no correlation between MPA C0C and C0P concentrations (P = 0.055, Table 2), and no correlations between predose IMPDH activity and either C0C (P = 0.066) or C0P concentrations (P = 0.64, Table 2). There was no effect of inferred MRP2 phenotype on either C0C or the ratio of C0C/C0P (P > 0.43, data not shown).
Table 2.
Spearman rank correlation coefficients (rs) and P‐values for mycophenolic acid (MPA) pharmacokinetic variables and predose inosine monophosphate dehydrogenase (IMPDH) activity
| C0P | C0u | IMPDH | |
|---|---|---|---|
| C 0C | rs = 0.279 P = 0.055 | rs = 0.418 P = 0.013 | rs = –0.267 P = 0.066 |
| C 0P | rs = 0.638 P < 0.0001 | rs = –0.070 P = 0.636 | |
| C 0u | rs = –0.027 P = 0.878 |
C0C, trough PBMC MPA concentration; C0P, trough plasma MPA concentration; C0u, trough unbound plasma MPA concentration; IMPDH, predose PBMC IMPDH activity
MPA C0u plasma concentrations (n = 34) ranged from 1.0 to 166.4 μg l–1 (median = 19.2 μg l–1) and were significantly correlated with MPA C0C and MPA C0P (P = 0.013 and < 0.0001, Table 2, Figure 1) concentrations, but not with predose IMPDH activity (P = 0.878, Table 2).
Figure 1.
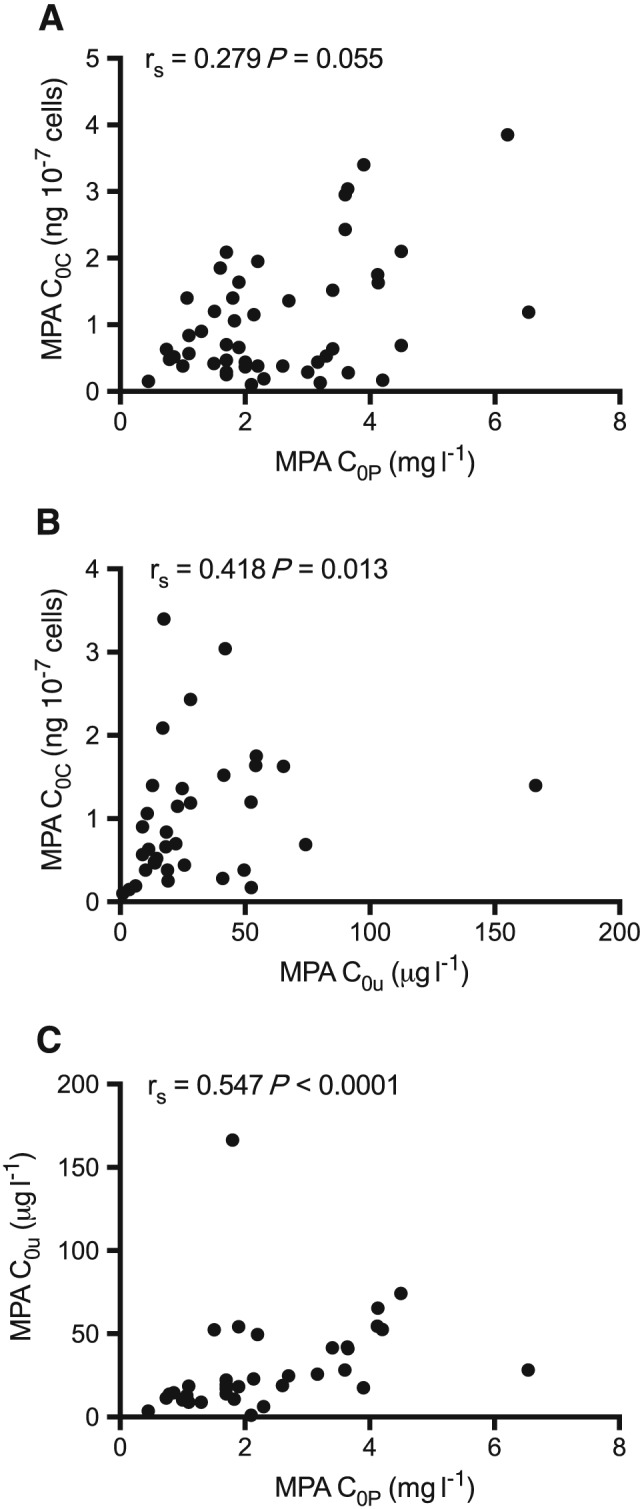
Spearman rank correlations between: (A) trough plasma mycophenolic acid (MPA) concentrations (C0P) and predose MPA PBMC concentrations (C0C); (B) trough plasma unbound MPA concentrations (C0u) and C0C; and (C) C0P and C0u in 48 renal transplant recipients
Relationships between rejection and MPA C0C, C0P and C0u concentrations, and IMPDH activity
In the rejection group (n = 15), 11 (73%) patients developed severe cellular or vascular rejection, and four (27%) patients were classified as subclinical or borderline at the time of protocol or for‐cause biopsies. There was no difference in MPA C0P, C0u or predose IMPDH activity between recipients with or without rejection (P > 0.197, Table 3). However, median MPA C0C were 59% lower in recipients with rejection compared to those without rejection (P = 0.029), and there was a statistically significant concentration‐effect relationship between MPA C0C and the severity of rejection (Jonckheere–Terpstra trend test, P = 0.015, Table 3, Figure 2).
Table 3.
Median (range) mycophenolic acid (MPA) pharmacokinetic parameters and predose inosine monophosphate dehydrogenase (IMPDH) activity in renal transplant recipients with no, all, borderline and severe rejection
| No rejection | All rejection | Borderline rejection | Severe rejection | P‐value No vs. all rejection (Mann–Whitney) | P‐value No, borderline, severe (Jonckheere–Terpstra) | |
|---|---|---|---|---|---|---|
| C 0C (ng 10 –7 cells) | 1.06 (0.10–3.85) (n = 33) | 0.44 (0.13–1.64) (n = 15) | 0.84 (0.42–1.64) (n = 4) | 0.38 (0.13–1.36) (n = 11) | 0.029 | 0.015 |
| C 0P (mg l –1 ) | 2.20 (0.45–6.54) (n = 33) | 2.00 (0.79–3.65) (n = 15) | 2.02 (1.5–3.3) (n = 4) | 2.00 (0.79–3.65) (n = 11) | 0.197 | 0.177 |
| C 0u (μg l –1 ) | 19.2 (1.0–166.4) (n = 25) | 20.7 (6.2–54.3) (n = 10) | 38.6 (22.9–54.3) (n = 2) | 16.1 (6.2–49.6) (n = 8) | 0.583 | 0.324 |
| IMPDH (nmol –1 h –1 mg –1 ) | 10.6 (0.9–33.8) (n = 33) | 13.7 (3.4–33.9) (n = 15) | 10.6 (3.4–22.3) (n = 4) | 13.9 (5.6–33.9) (n = 11) | 0.197 | 0.151 |
C0C, trough PBMC MPA concentration; C0P, trough plasma MPA concentration; C0u, trough unbound plasma MPA concentration; IMPDH, predose PBMC IMPDH activity
Figure 2.
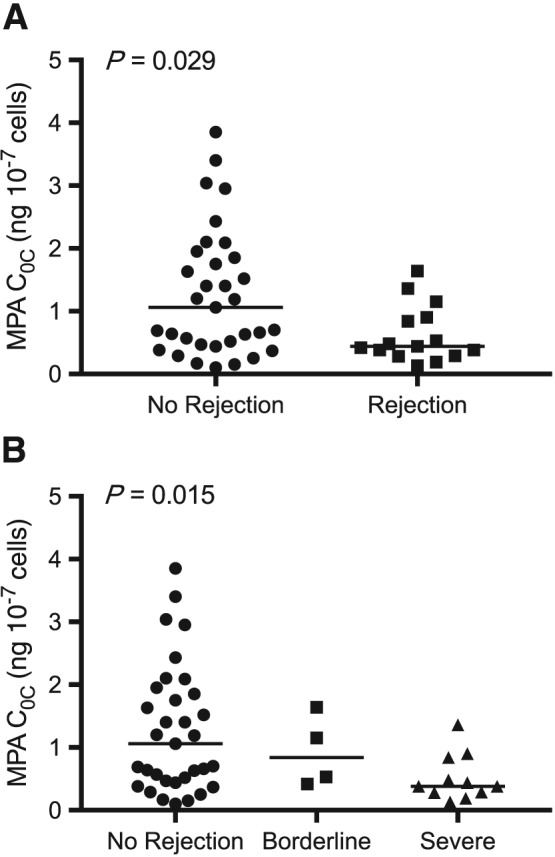
Comparison of mycophenolic acid C0C (ng 10–7 cells) with (A) all rejection and (B) severity of graft rejection. Lines indicate median values. P‐values are shown for Mann–Whitney (A) and Jonckheere–Terpstra test for trend (B)
ROC curve analyses were performed to provide threshold data for predicting the risk of rejection using MPA C0C. The ROC area under the curve (AUC) for the prediction of all rejection using MPA C0C concentrations was 0.70 (P = 0.03), with a threshold of 0.55 ng 10–7 cells providing 70% sensitivity, 67% specificity and a likelihood ratio of 2.09 (Figure 3A). The ROC AUC for the prediction of severe (cellular/vascular) rejection using MPA C0C concentrations was 0.75 (P = 0.013; Figure 3B), with a C0C threshold of 0.5 ng 10–7 cells providing 73% sensitivity and specificity, and a likelihood ratio of 2.68.
Figure 3.
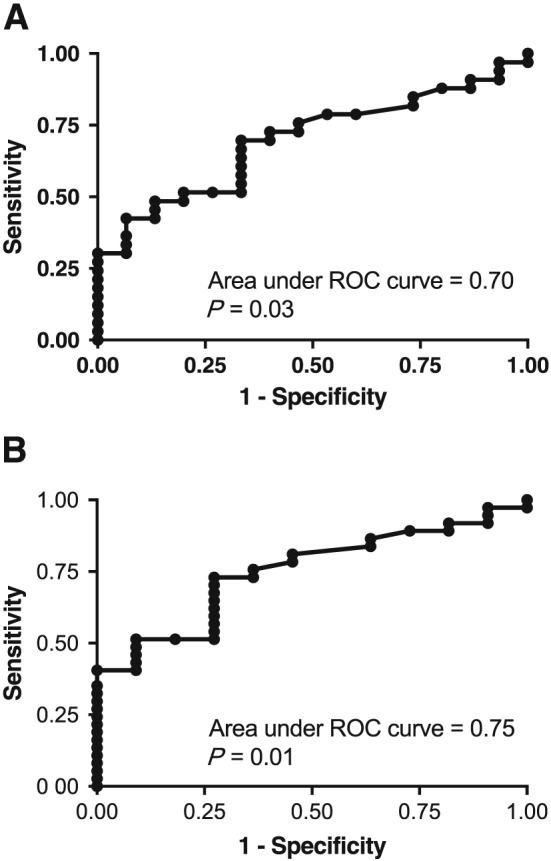
Receiver operating characteristic (ROC) curves using MPA C0C concentrations for the prediction of (A) all rejection and (B) severe (cellular/vascular) rejection
Multivariate analyses of pharmacokinetic variables associated with rejection, and PBMC MPA concentrations
Multivariate logistic regression analysis demonstrated that MPA C0C (P = 0.011, FDR‐adjusted P = 0.033) was the only significant independent predictor of rejection with lower MPA C0C predicting rejection with a ROC AUC of 0.72. In contrast, there was no association between rejection and TAC C0B (FDR‐adjusted P = 0.395) or MPA C0P (FDR‐adjusted P = 0.129), used for current TDM. With regard to prediction of MPA C0C, the final multivariate regression model incorporating only MPA C0P, predicted 19% of variability in MPA C0C (FDR‐adjusted P = 0.003). There was no significant effect of recipient MRP2 phenotype (FDR‐adjusted P = 0.691) or plasma albumin concentrations (FDR‐adjusted P = 0.482) on MPA C0C. Since there was a strong association between C0P and C0u, and there were several missing C0u values, C0u was not tested as a predictor of C0C in the multivariate model.
Discussion
There has been considerable effort to understand the relationship between MPA pharmacokinetics and pharmacodynamics, in an attempt to reduce the risk of rejection after renal transplantation. As for other immunosuppressants (TAC or CsA), MPA concentrations in PBMCs may better predict efficacy compared to blood/plasma concentrations 9, 10, 11, as they may better reflect intralymphocyte immunosuppressant concentrations. This study investigated differences in the associations between MPA C0C or IMPDH activity and graft rejection, versus MPA C0P and graft rejection.
The primary outcome of this study supports our hypothesis that obtaining PBMC MPA concentrations may provide greater prediction of graft rejection compared to measuring trough MPA concentrations alone, as there was a concentration–effect relationship between MPA C0C and severity of rejection, but no relationship between MPA C0P and rejection. In addition, multivariate analysis confirmed that MPA C0C was the dominant pharmacokinetic factor predicting rejection in our study population, who were already receiving TDM for trough blood TAC concentrations.
In the multivariate analysis, MPA C0P was the only significant pharmacokinetic variable associated with MPA C0C. However, it only explained 19% of the variability in MPA C0C; consistent with no correlation observed between MPA C0C and C0P. The lack of prediction of graft rejection by MPA C0P concentrations is consistent with previous findings in renal transplant recipients also receiving TAC 34, 35, and suggests that MPA C0P concentrations may not be the best predictor of rejection. The current MPA therapeutic range is derived primarily from plasma total AUC data 3, 4, which is a better predictor of rejection than trough plasma concentrations. However, full AUC monitoring in clinical practice is impractical (requiring intense sampling during a 12 h dosing interval), labour‐intensive and costly. Alternatively, a Bayesian population forecasting model 36 may have been more suitable to predict individual MPA exposures and should be compared to C0C in future studies.
Although univariate regression analysis showed a weak correlation between MPA C0C and C0u concentrations, C0u was not a significant predictor of rejection in univariate analyses. This was surprising as it is unbound MPA which exerts pharmacological effects 37 to inhibit IMPDH activity. Nonetheless, this observation is in agreement with previous findings 38, 39. It is possible that the smaller number of recipients for whom C0u (73% of cohort) were available may have resulted in a type II statistical error. Alternatively, it is possible that carrier‐mediated MPA uptake into or efflux out of PBMCs complicates the relationship between C0u and unbound C0C (not measured in this study). MPA is a substrate of the efflux pump, MRP2 14, so that ABCC2 polymorphisms affecting the expression and/or function of MRP2, may be a source of the variability observed in MPA C0C. For example, the ABCC2 CAC (‐24C/1249A/3972C) haplotype is associated with significantly higher MRP2 expression and activity 26, and may therefore modulate MPA immunosuppressive efficacy. However, in this study, recipient ABCC2 haplotypes were not significantly associated with MPA C0C, which may therefore reflect a weak expression of MRP2 in PBMCs 15, or a subtle effect of ABCC2 genetics that is not detected by our small sample size. MPA is also a substrate for P‐glycoprotein 40 that is similarly expressed in PBMCs 41. Determination of differences in the expression and/or function of this transporter may also help explain some of this MPA pharmacokinetic variability.
This study also investigated the potential for IMPDH activity in PBMCs as a useful biomarker, as it may correlate more closely to the biological response of MPA than plasma concentrations. Although predose IMPDH activity was not a significant predictor of rejection, our relatively small sample size could have resulted in a type II statistical error. However, in a study of 101 renal transplant recipients, Sombogaard et al. similarly found no association between post‐transplant predose IMPDH activity and acute rejection 20. It may be that the time at which IMPDH activity is assessed is important. Glander et al. reported that patients with high pretransplant IMPDH activity (hence requiring greater inhibition post‐transplant) had a 3.6–fold higher incidence of graft rejection compared to patients with low pretransplant IMPDH activity 17. In addition, many studies have demonstrated that minimum IMPDH activity occurs shortly after MMF administration, coinciding with peak plasma MPA concentrations, followed by recovery of IMPDH activity by 3.5–11 h, to predose levels 13, 16, 19, 21. Thus, our study may have been limited using only a single predose measurement taken 5–22 days post‐transplantation. Consequently, the measurement of IMPDH activities at multiple time points, which we were not able to perform in this study, may be needed to further elucidate the predictive nature of this factor.
Measurement of IMPDH messenger RNA (mRNA) has also been investigated as a possible predictor of rejection, particularly to differentiate between the two types of IMPDH isoforms: type I, which is expressed in all cell types; and type II, which is expressed only in activated lymphocytes 2. In isolated human T lymphocytes, both IMPDH type I and type II mRNA is increased following activation, correlating with increased total IMPDH activity 42. However, like the measurement of IMPDH activity, the relationship between IMPDH mRNA expression and rejection is not clear. One study in renal transplant recipients found that low post‐transplant expression of both type I and II mRNA was associated with acute rejection, although there was no correlation between total IMPDH activity and type I or II mRNA expression 20, whilst a later study reported an association between rejection and high pretransplant expression of both type I and II mRNA 18.
In conclusion, the results from our study suggest that MPA C0C may represent an additional tool for individualization of MMF dose following renal transplantation. Its clinical application may be most practical as a single test during the early post‐transplantation period, when the risk of developing graft rejection is highest, and when protocol or for‐cause biopsies are also usually performed.
Competing Interests
There are no competing interests to declare.
The authors would like to acknowledge the work of the following people: Prof G. Russ, Ms T. East, Ms D. Spellacy, Drs C. Hope and A. Fuss, transplant surgeons and clinical staff at the Central and Northern Adelaide Renal and Transplantation Service, Adelaide SA, for their assistance with blood and tissue sample collection; and Prof A. Vinks and Ms S. Cox at the Cincinnati Children's Hospital Medical Center, Cincinnati OH, for their help in setting up the IMPDH assay. Z.I.M.D. was the recipient of an Endeavour Postgraduate Scholarship funded by the Australian Government.
Contributors
Z.I.M.D., patient recruitment, acquisition of data, analyses and interpretation; contribution to study design, drafting of manuscript. J.K.C., patient recruitment, data analysis and interpretation, drafting and critical review of manuscript. R.P.C., data analysis and interpretation, critical review of manuscript. J.T., data analysis and interpretation, critical review of manuscript. B.C.M., acquisition of data, analysis and critical review of manuscript. A.A.S., data interpretation, drafting and critical review of manuscript. B.C.S., study conception, design and coordination, data interpretation, drafting and critical review of manuscript.
Supporting information
Table S1 Recipient allele and genotype frequencies (%) for the C‐24 T, G1249A and C3972T SNPs of ABCC2
Table S2 ABCC2 haplotype frequencies (%) in 48 kidney transplant recipients
Md Dom, Z. I. , Coller, J. K. , Carroll, R. P. , Tuke, J. , McWhinney, B. C. , Somogyi, A. A. , and Sallustio, B. C. (2018) Mycophenolic acid concentrations in peripheral blood mononuclear cells are associated with the incidence of rejection in renal transplant recipients. Br J Clin Pharmacol, 84: 2433–2442. 10.1111/bcp.13704.
References
- 1. ANZDATA Registry . 39th Report, Chapter 8: Transplantation. Australia and New Zealand Dialysis and Tansplant Registry, Adelaide, Australia. 2016. Available at http://www.anzdata.org.au (last accessed 14 December 2017).
- 2. Carr SF, Papp E, Wu JC, Natsumeda Y. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem 1993; 268: 27286–27290. [PubMed] [Google Scholar]
- 3. Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, et al Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol 2010; 5: 341–358. [DOI] [PubMed] [Google Scholar]
- 4. van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, et al Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit 2006; 28: 145–154. [DOI] [PubMed] [Google Scholar]
- 5. Shaw LM, Holt DW, Oellerich M, Meiser B, van Gelder T. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit 2001; 23: 305–315. [DOI] [PubMed] [Google Scholar]
- 6. Barbari A, Masri MA, Stephan A, Mokhbat J, Kilani H, Rizk S, et al Cyclosporine lymphocyte versus whole blood pharmacokinetic monitoring: correlation with histological findings. Transplant Proc 2001; 33: 2782–2785. [DOI] [PubMed] [Google Scholar]
- 7. Barbari AG, Masri MA, Stephan AG, El Ghoul B, Rizk S, Mourad N, et al Cyclosporine lymphocyte maximum level monitoring in de novo kidney transplant patients: a prospective study. Exp Clin Transplant 2006; 4: 400–405. [PubMed] [Google Scholar]
- 8. Falck P, Guldseth H, Asberg A, Midtvedt K, Reubsaet JL. Determination of ciclosporin A and its six main metabolites in isolated T‐lymphocytes and whole blood using liquid chromatography‐tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 852: 345–352. [DOI] [PubMed] [Google Scholar]
- 9. Falck P, Asberg A, Guldseth H, Bremer S, Akhlaghi F, Reubsaet JL, et al Declining intracellular T‐lymphocyte concentration of cyclosporine a precedes acute rejection in kidney transplant recipients. Transplantation 2008; 85: 179–184. [DOI] [PubMed] [Google Scholar]
- 10. Capron A, Lerut J, Latinne D, Rahier J, Haufroid V, Wallemacq P. Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: preliminary results of a prospective study. Transplant Int 2012; 25: 41–47. [DOI] [PubMed] [Google Scholar]
- 11. Capron A, Musuamba F, Latinne D, Mourad M, Lerut J, Haufroid V, et al Validation of a liquid chromatography‐mass spectrometric assay for tacrolimus in peripheral blood mononuclear cells. Ther Drug Monit 2009; 31: 178–186. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen Thi MT, Capron A, Mourad M, Wallemacq P. Mycophenolic acid quantification in human peripheral blood mononuclear cells using liquid chromatography‐tandem mass spectrometry. Clin Biochem 2013; 46: 1909–1911. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen Thi MT, Mourad M, Capron A, Tshinanu FM, Vincent MF, Wallemacq P. Plasma and intracellular pharmacokinetic‐pharmacodynamic analysis of mycophenolic acid in de novo kidney transplant patients. Clin Biochem 2014; 48: 401–405. [DOI] [PubMed] [Google Scholar]
- 14. El‐Sheikh AA, Koenderink JB, Wouterse AC, van den Broek PH, Verweij VG, Masereeuw R, et al Renal glucuronidation and multidrug resistance protein 2–/multidrug resistance protein 4‐mediated efflux of mycophenolic acid: interaction with cyclosporine and tacrolimus. Transl Res 2014; 164: 46–56. [DOI] [PubMed] [Google Scholar]
- 15. Laupeze B, Amiot L, Payen L, Drenou B, Grosset JM, Lehne G, et al Multidrug resistance protein (MRP) activity in normal mature leukocytes and CD34‐positive hematopoietic cells from peripheral blood. Life Sci 2001; 68: 1323–1331. [DOI] [PubMed] [Google Scholar]
- 16. Fukuda T, Goebel J, Thøgersen H, Maseck D, Cox S, Logan B, et al Inosine monophosphate dehydrogenase (IMPDH) activity as a pharmacodynamic biomarker of mycophenolic acid effects in pediatric kidney transplant recipients. J Clin Pharmacol 2011; 51: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glander P, Hambach P, Braun KP, Fritsche L, Giessing M, Mai I, et al Pre‐transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant 2004; 4: 2045–2051. [DOI] [PubMed] [Google Scholar]
- 18. Molinaro M, Chiarelli LR, Biancone L, Castagneto M, Boschiero L, Pisani F, et al Monitoring of inosine monophosphate dehydrogenase activity and expression during the early period of mycophenolate mofetil therapy in de novo renal transplant patients. Drug Metab Pharmacokinet 2013; 28: 109–117. [DOI] [PubMed] [Google Scholar]
- 19. Rother A, Glander P, Vitt E, Czock D, von Ahsen N, Armstrong VW, et al Inosine monophosphate dehydrogenase activity in paediatrics: age‐related regulation and response to mycophenolic acid. Eur J Clin Pharmacol 2012; 68: 913–922. [DOI] [PubMed] [Google Scholar]
- 20. Sombogaard F, Peeters AM, Baan CC, Mathot RA, Quaedackers ME, Vulto AG, et al Inosine monophosphate dehydrogenase messenger RNA expression is correlated to clinical outcomes in mycophenolate mofetil‐treated kidney transplant patients, whereas inosine monophosphate dehydrogenase activity is not. Ther Drug Monit 2009; 31: 549–556. [DOI] [PubMed] [Google Scholar]
- 21. Tang JT, de Winter BC, Hesselink DA, Sombogaard F, Wang LL, van Gelder T. The pharmacokinetics and pharmacodynamics of mycophenolate mofetil in younger and elderly renal transplant recipients. Br J Clin Pharmacol 2017; 83: 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Westley IS, Sallustio BC, Morris RG. Validation of a high‐performance liquid chromatography method for the measurement of mycophenolic acid and its glucuronide metabolites in plasma. Clin Biochem 2005; 38: 824–829. [DOI] [PubMed] [Google Scholar]
- 23. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8: 753–760. [DOI] [PubMed] [Google Scholar]
- 24. Naesens M, Kuypers DR, Verbeke K, Vanrenterghem Y. Multidrug resistance protein 2 genetic polymorphisms influence mycophenolic acid exposure in renal allograft recipients. Transplantation 2006; 82: 1074–1084. [DOI] [PubMed] [Google Scholar]
- 25. Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 2001; 68: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laechelt S, Turrini E, Ruehmkorf A, Siegmund W, Cascorbi I, Haenisch S. Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. Pharmacogenomics J 2011; 11: 25–34. [DOI] [PubMed] [Google Scholar]
- 27. Md Dom ZI, Noll BD, Coller JK, Somogyi AA, Russ GR, Hesselink DA, et al Validation of an LC–MS/MS method for the quantification of mycophenolic acid in human kidney transplant biopsies. J Chromatogr B Analyt Technol Biomed Life Sci 2014; 945–946: 171–177. [DOI] [PubMed] [Google Scholar]
- 28. Guidance for Industry: Bioanalytical Method Validation. US Food and Drug Administration, Center for Drug Evaluation and Research. 2001. Available at http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm070107.pdf (last accessed 14 December 2017).
- 29. Glander P, Sombogaard F, Budde K, van Gelder T, Hambach P, Liefeldt L, et al Improved assay for the nonradioactive determination of inosine 5′‐monophosphate dehydrogenase activity in peripheral blood mononuclear cells. Ther Drug Monit 2009; 31: 351–339. [DOI] [PubMed] [Google Scholar]
- 30. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing. In, Vienna, Austria URL. Available at http://www.r-project.org/, 2017 (last accessed 14 December 2017).
- 31. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to Pharmacology in 2018: updates and expansion to encompass the new guide to Immunopharmacology. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to Pharmacology 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to Pharmacology 2017/18: Transporters. Br J Pharmacol 2017; 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long‐term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther 2004; 75: 434–447. [DOI] [PubMed] [Google Scholar]
- 35. Mourad M, Malaise J, Chaib Eddour D, De Meyer M, Konig J, Schepers R, et al Pharmacokinetic basis for the efficient and safe use of low‐dose mycophenolate mofetil in combination with tacrolimus in kidney transplantation. Clin Chem 2001; 47: 1241–1248. [PubMed] [Google Scholar]
- 36. Premaud A, Le Meur Y, Debord J, Szelag JC, Rousseau A, Hoizey G, et al Maximum a posteriori bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther Drug Monit 2005; 27: 354–361. [DOI] [PubMed] [Google Scholar]
- 37. Nowak I, Shaw LM. Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem 1995; 41: 1011–1017. [PubMed] [Google Scholar]
- 38. Atcheson BA, Taylor PJ, Mudge DW, Johnson DW, Hawley CM, Campbell SB, et al Mycophenolic acid pharmacokinetics and related outcomes early after renal transplant. Br J Clin Pharmacol 2004; 59: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber LT, Shipkova M, Armstrong VW, Wagner N, Schutz E, Mehls O, et al The pharmacokinetic‐pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: a report of the German study group on mycophenolate mofetil therapy. J Am Soc Nephrol 2002; 13: 759–768. [DOI] [PubMed] [Google Scholar]
- 40. Sawamoto T, Van Gelder T, Christians U, Okamura N, Jacobsen W, Benet L. Membrane transport of mycophenolate mofetil and its active metabolite, mycophenolic acid in MDCK and MDR1‐MDCK cell monolayers. J Heart Lung Transplant 2001; 20: 234–235. [DOI] [PubMed] [Google Scholar]
- 41. Klimecki WT, Futscher BW, Grogan TM, Dalton WS. P‐glycoprotein expression and function in circulating blood cells from normal volunteers. Blood 1994; 83: 2451–2458. [PubMed] [Google Scholar]
- 42. Dayton JS, Lindsten T, Thompson CB, Mitchell BS. Effects of human T lymphocyte activation on inosine monophosphate dehydrogenase expression. J Immunol 1994; 152: 984–991. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Recipient allele and genotype frequencies (%) for the C‐24 T, G1249A and C3972T SNPs of ABCC2
Table S2 ABCC2 haplotype frequencies (%) in 48 kidney transplant recipients


