Abstract
Aims
Recent data suggest that antidepressants are associated with incident diabetes but the possible pharmacological mechanism is still questioned. The aim of the present study was to evaluate antidepressant's risk for reporting diabetes using disproportionality analysis of the FDA adverse events spontaneous reporting system (FAERS) database and to investigate possible receptor/transporter mechanisms involved.
Methods
Data from 2004 to 2017 were analysed using OpenVigil2 and adjusted reporting odds ratio (aROR) for reporting diabetes was calculated for 22 antidepressants. Events included in the narrow scope of the SMQ ‘hyperglycaemia/new‐onset diabetes mellitus’ were defined as cases and all the other events as non‐cases. The pharmacodynamic profile was extracted using the PDSP and IUPHAR/BPS databases and the occupancy on receptors (serotonin, alpha adrenoreceptors, dopamine, muscarinic, histamine) and transporters (SERT, NET, DAT) was estimated. The relationship between aROR for diabetes and receptor occupancy was investigated with Pearson's correlation coefficient (r) and univariate linear regression.
Results
Six antidepressants were associated with diabetes: nortriptyline with aROR [95% CI] of 2.01 [1.41–2.87], doxepin 1.97 [1.31–2.97], imipramine 1.82 [1.09–3.06], sertraline 1.47 [1.29–1.68], mirtazapine 1.33 [1.04–1.69] and amitriptyline 1.31 [1.09–1.59]. Strong positive correlation coefficients between occupancy and aROR for diabetes were identified for the receptors M1, M3, M4, M5 and H1.
Conclusion
Most of the tricyclic antidepressants, mirtazapine and sertraline seem to be associated with reporting diabetes in FAERS. Higher degrees of occupancy on muscarinic receptors and H1 may be a plausible pharmacological mechanism. Further clinical assessment and pharmacovigilance data is needed to validate this potential safety signal.
Keywords: drug safety, antidepressants, pharmacodynamics, endocrinology, pharmacovigilance, diabetes
What is Already Known About this Subject
Pharmacovigilance data suggest a link between antidepressants and type 2 diabetes.
The exact mechanism of antidepressants' diabetogenic properties is questioned.
The risk for diabetes could be elucidated by considering the diverse pharmacodynamic profile of antidepressants.
What this Study Adds
The disproportionality analysis of the FDA adverse events spontaneous reporting system detected a differential risk for reporting diabetes among antidepressants.
Most of the tricyclic antidepressants, mirtazapine and sertraline seem to carry a higher risk for reporting diabetes.
A plausible pharmacological mechanism is the antagonism of muscarinic and H1 receptors, but other mechanisms should also be expected.
Introduction
Drug‐induced metabolic side effects are a major concern in psychopharmacology. Antipsychotics are strongly associated with the core components of metabolic syndrome (weight gain, disrupted glucose handling and dyslipidaemia), whereas antidepressants were thought initially to have quite neutral effects on glucose metabolism. Conversely, a recent meta‐analysis of 20 prospective studies suggested that antidepressants are associated with incident diabetes, with a relative risk of 1.27 (95% CI: 1.19–1.35) 1, while a systematic review proposed a possible causal relationship between antidepressant and new‐onset diabetes 2.
The exact mechanism of antidepressants' diabetogenic properties is still questioned and most studies suggest that the risk for diabetes could be further elucidated by considering the diverse pharmacodynamic profile of the drugs 1, 2. The better‐studied antipsychotics could provide insights on the possible mechanisms of antidepressant‐induced diabetes. Several receptor mechanisms of antipsychotic‐induced metabolic side effects have been suggested, such as antagonism of dopamine, histamine, muscarinic and serotonin receptors 3. A recent pharmacoepidemiological‐pharmacodynamic study in VigiBase suggested that antipsychotics with http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=262 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=8 antagonistic properties were associated with diabetes 4. Similar receptor mechanisms could mediate antidepressant‐induced metabolic side effects; in support, observational studies suggested that http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=926 inhibition, as well as antagonism on 5‐HT2C, H1 5, 6 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=15 receptors 7, could explain the risk for diabetes.
In the present study, a combined pharmacovigilance‐pharmacodynamic approach was used 4, 8, 9: (i) to detect a potential safety signal of antidepressants‐induced type 2 diabetes (T2D) using disproportionality analysis in the FDA adverse events reporting system (FAERS), and (ii) to investigate the association between the risk for reporting diabetes and the occupancy on neurotransmitter receptors and transporters.
Methods
Case/non‐case study of the FAERS database
A case/non‐case study was conducted using spontaneous reports submitted in FAERS between the first quarter of 2004 and the second quarter of 2017 (2017Q2). FAERS is a spontaneous adverse events database, consisting of individual safety reports, collected by the pharmaceutical industry, health professionals and consumers, mainly from the United States. The data consists of administrative information, patient demographics, adverse events, information about drug therapy, patient outcomes and type of reporter 10. Data extraction was performed using the OpenVigil2.1‐MedDRA, an open pharmacovigilance data extraction, mining and analysis tool of the FAERS database. OpenVigil2.1‐MedDRA operates only on cleaned FDA data, that is to say deleting most duplicates or reports with missing data 11. To ascertain a minimum quality of data, further data cleaning was performed, and reports with residual miss‐mapping, errors or unknown drug name, age, gender and reporting year were excluded. Reports involving only adults were included.
In FAERS, adverse events are coded using the MedDRA ontology. Reports with diabetes were identified using the narrow scope of the standardized MedDRA query (SMQ) ‘hyperglycaemia/new‐onset diabetes mellitus’, which is more specific for new‐onset diabetes mellitus compared to the broad scope which includes less specific terms, such as ‘weight increased’. All the other events were defined as non‐cases. All reports including one or more of 22 FDA‐approved antidepressants (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=200, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7135, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7547, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2398, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2399, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7158, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1225, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=202, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7177, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=203, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7189, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=357, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2402, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7436, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7241, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7247, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2404, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4790, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4798, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=213, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7317, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7321) as suspected, interacting or concomitant were extracted. Non‐FDA approved antidepressants (e.g. agomelatine, reboxetine) were not included because they would suffer from underreporting rates in FAERS. Recently FDA‐approved antidepressants, such as levomilnacipran, vortioxetine and vilazodone, were not included, in order to avoid the reporting patterns during the first marketing years (studied period until the second quarter of 2017). Finally, monoamine oxidase inhibitors, a sparsely used category of antidepressants with different mechanism of action (enzyme inhibitors), which have been associated with hypoglycaemia 12,were also excluded. Additional extracted data were identity numbers of the report, gender, age, reporting year and associated drugs, while the source of the report was not available for extraction.
Concomitant medication could confound the results 13, thus a number of drugs were excluded (Table S1). Reports with antidiabetics were excluded in order to avoid reverse causality, since they indicate pre‐existing, but not incident, diabetes. Antipsychotics were excluded as they have been associated with diabetes and important notoriety bias has been considered for clozapine and olanzapine in FAERS 4. Along with antipsychotics, reports with a combination of antidepressants were also not included, to avoid the competition bias of the former and parallel targeting on neurotransmitter receptors and transporters. Concomitant use of hyperglycaemic drugs was also previously defined as a potential confounder 14, 15, 16. The risk for reporting diabetes was also adjusted for the further possible confounders: age, gender, reporting year and use of hyperglycaemic drugs.
Pharmacodynamic data
Receptor occupancy was selected to quantitate receptor‐mediated mechanisms 4 and it was calculated using the pharmacological receptor theory 4, 17: Occupancy (%) = 100 * [Cr]/(Ki + [Cr]). The inhibitory constants (Ki [nM]) for 23 human transporters (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=928, NET, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=927) or receptors (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=6, 5‐HT2C, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=68, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=11, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=12 serotonin receptors, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=4 adrenoceptors, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=13 , http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=14 , M3 , http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=16 , http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=17 muscarinic receptors, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=214 , http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=215 , http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=216 , http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=217 dopamine receptors and H1) were extracted using the PDSP 18 and IUPHAR/BPS 19 databases, following this hierarchy based on availability. The mean value was calculated when more than one datum was available for one receptor. The mean Ki values of alpha adrenoceptors were calculated regardless of receptor subtypes. The total blood drug concentration (C max [ng ml−1]) was estimated using the maximum point of the therapeutic reference range reported in the Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology 20. Nefazodone was not included in the former guidelines and its total blood concentration was extracted from a pharmacokinetic study on healthy male volunteers 21. Τhe unbound drug fraction (Fu) was extracted from the Drugbank 22 and the molecular weights (M r [g mol−1]) of antidepressants extracted from the IUPHAR database 19. The unbound blood concentration (Cr [nM]) was estimated using the equation Cr = 1000 * Fu * C max/M r.
Statistical analysis
Descriptive analysis
Study population characteristics were compared between cases and non‐cases using the Mann–Whitney U‐test for age and chi‐squared for the categorical variables (age groups, gender, use of hyperglycaemic drugs and reporting year).
Disproportionality analysis
The association with reporting diabetes was expressed by the reporting odds ratio (ROR), which estimates the frequency of diabetes co‐reported with the tested drugs compared with the other drugs. Disproportionality signals were detected when the number of reports was higher than three and ROR – 95% CI was greater than one 10. Apart from the crude ROR (cROR), the adjusted ROR (aROR) was calculated using multivariate logistic regression analysis including as covariates potential confounding factors as categorical variables, i.e. age, gender, reporting year and use of hyperglycaemic. For each antidepressant, the cROR and aROR were calculated to compare the proportion of diabetes with this individual antidepressant to the proportion of diabetes with the other antidepressants.
Relationship between disproportionality analysis and receptor occupancy
Pearson's correlation coefficients (r) between the aROR for diabetes and the degree of occupancy on each receptor investigated the relationship between occupancy and the risk for reporting diabetes. Antidepressants with less than three reports with diabetes were excluded, as well as degrees of occupancy less than 0.1% from the analysis 8. We assumed that the higher the degree of occupancy, the higher the risk for reporting diabetes, expressed as aROR for diabetes. In order to minimize the risk of type I error from multiple comparisons, the alpha level was adjusted using the Bonferroni correction, due to multiple correlations, i.e. alpha level = 0.05/21 = 0.00238 (21 is the number of correlations, one for each receptor; 5‐HT3 and D4 were excluded due to lack of data) 23. For significant correlations, the relationship between the risk for reporting diabetes and occupancy on receptors was further investigated with univariate linear regression. The dependent variable was the aROR for diabetes and the exploratory variable was receptor occupancy. All analyses were conducted in R version 3.3.2 24.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 19, and follow The Concise Guide to PHARMACOLOGY 2017/18 25, 26, 27.
Results
Study population and receptor occupancy
Of the total of 4 704 663 reports submitted in FAERS between 2004 and 2017Q2, the final sample consisted of 136 028 reports concerning the 22 antidepressants after the aforementioned data cleaning and exclusions (Figure 1). Diabetes was identified in 1610 reports, which is 1.18% of the final sample. The demographics and medical characteristics are displayed in Table 1. The age of cases was greater than non‐cases (P < 0.001), whereas no difference in gender was observed and the female‐to‐male ratio was about 2.26. Use of hyperglycaemic drugs was associated with cases (P < 0.001). Difference in reporting year was observed (P < 0.001), and reports regarding cases were submitted in earlier years. The pharmacodynamic profile of the antidepressants is presented in Figure 2 and Table S2.
Figure 1.
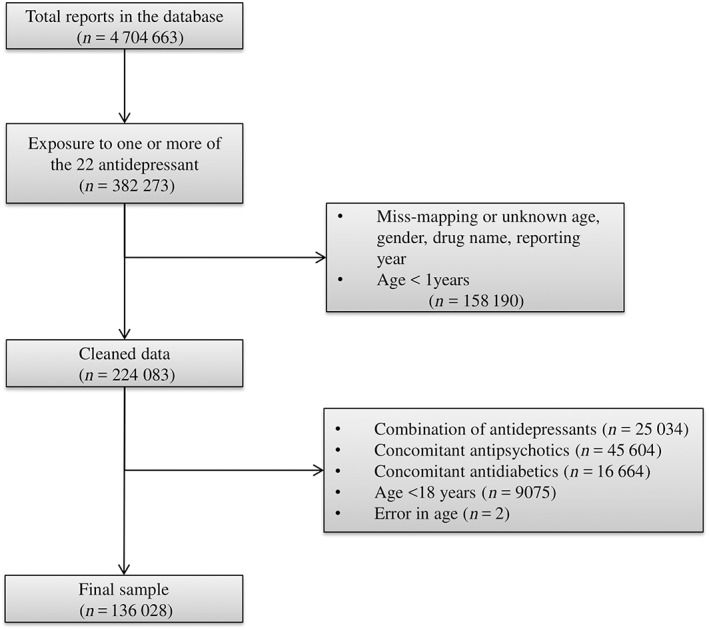
Flow diagram of reports. The total number of reports in the database of FAERS (extracted from OpenVigil2.1‐MedDRA) was 4 704 663, of which 382 273 involved one or more of the tested antidepressants. Further data cleaning and exclusion of reports involving patients less than 18 years, and the combination of antidepressants, antidiabetics or antipsychotics concluded in the final sample of 136 028 reports
Table 1.
Population characteristics
| Cases (n = 1610) | Non‐cases (n = 134 418) | P‐value | |
|---|---|---|---|
| Age | |||
| Mean [SD] | 53.71 [14.2] | 51.07 [16.7] | <0.001 |
| Median | 53 | 51 | |
| 18–30 (%) | 91 (5.65) | 16 727 (12.44) | <0.001 |
| 31–45 (%) | 379 (23.54) | 34 751 (25.85) | |
| 46–65 (%) | 838 (52.05) | 56 428 (41.98) | |
| >65 (%) | 302 (18.76) | 26 512 (19.72) | |
| Gender | |||
| Female (%) | 1083 (67.27) | 93 157 (69.3) | 0.08 |
| Concomitant drugs | |||
| Hyperglycemic drugs (%) | 642 (39.88) | 30 570 (22.74) | <0.001 |
| Reporting year | |||
| 2004–2008 (%) | 697 (43.29) | 45 072 (33.53) | <0.001 |
| 2009–2012 (%) | 647 (40.19) | 48 844 (36.34) | |
| 2013–2017 (%) | 266 (16.52) | 40 502 (30.13) | |
P‐values from Mann–Whitney U test for age and chi‐squared for the categorical variables
Figure 2.
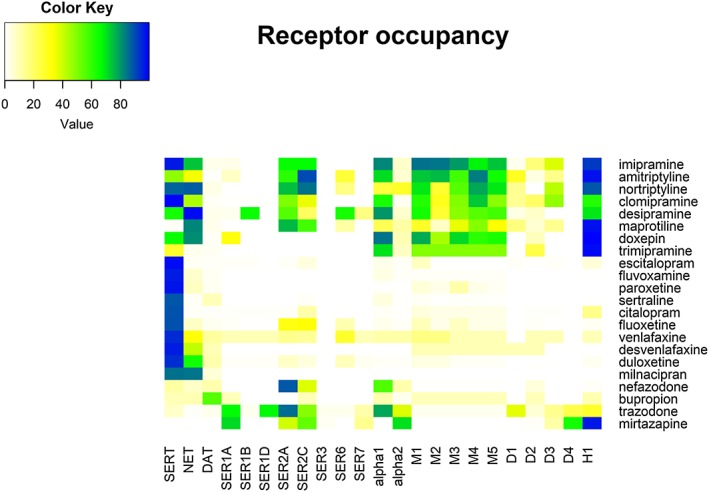
Occupancy of antidepressants on human transporters and receptors. The occupancy was calculated using the pharmacological receptor theory, i.e. occupancy = 100 * [Cr]/([Cr] + Ki). The inhibitory constant (Ki) was extracted from PDSP and IUPHAR databases and the unbound concentration (Cr) was calculated using the unbound fraction and the maximum therapeutic reference range, except for nefazodone. Colour key displays the degrees of occupancy (%)
Disproportionality analysis
The number of cases and the aROR for diabetes for antidepressants are presented in Figure 3 and Table S3. Disproportional signals were identified for six antidepressants, with aROR [95% CI] for diabetes: 2.01 [1.41–2.87] for nortriptyline, 1.97 [1.31–2.97] for doxepin, 1.82 [1.09–3.06] for imipramine, 1.47 [1.29–1.68] for sertraline, 1.33 [1.04–1.69] for mirtazapine, and 1.31 [1.09–1.59] for amitriptyline. Bupropion, citalopram, paroxetine, trazodone and desvenlafaxine were less frequently (aROR<1) associated with diabetes, while clomipramine, venlafaxine, duloxetine, fluoxetine, escitalopram, nefazodone, fluvoxamine and milnacipran were not associated with diabetes. Trimipramine, desipramine and maprotiline had less than three reports with diabetes.
Figure 3.
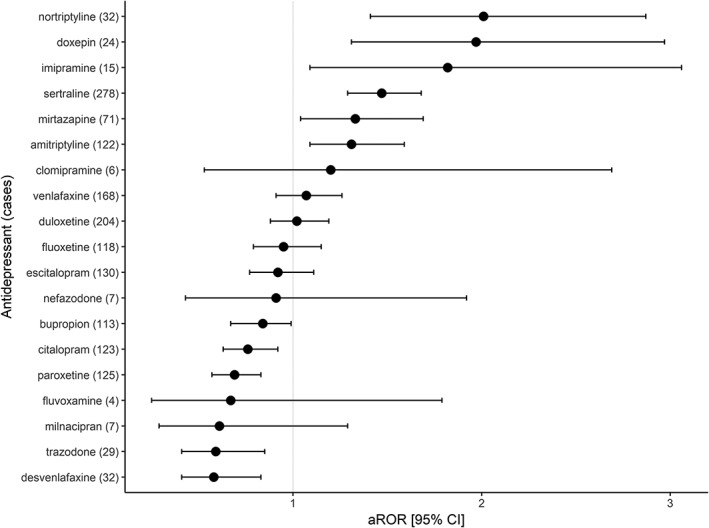
Disproportionality analysis in FAERS for the association between diabetes and individual antidepressants. The differential risk for reporting diabetes of individual antidepressants in comparison to the other antidepressants was quantified as adjusted reporting odds ratio (aROR). The ROR was adjusted to age, gender, reporting year, and use of hyperglycaemic drugs in multivariate logistic regression. Trimipramine, maprotiline, desipramine had less than three reports, so they are not presented
Relationship between the risk for diabetes and the occupancy on receptors/transporters
Pearson's r between aROR for diabetes and occupancy along with their P‐values are displayed in Figure 4. Significant correlation coefficients r were observed for the muscarinic receptors, M1 (r = 0.81), M3 (r = 0.81), M4 (r = 0.75), M5 (r = 0.82) and for the histamine H1 (r = 0.75). Strong but not significant r after adjustment to Bonferroni correction was observed for D3 (r = 0.70), as well as moderate r for NET (r = 0.57), 5‐HT2C (r = 0.59) and M2 (r = 0.65). Univariate linear regression for the associated muscarinic receptors and H1 are displayed in Figure 5. The relationship between aROR and the occupancy on the associated muscarinic receptors and H1 seems to be linear, but sertraline might have affected the shape of the line. Sertraline was associated with diabetes but has low degrees of occupancy on both muscarinic and histamine receptors. Excluding sertraline, the correlation coefficients increased for all the associated receptors with M1 (r = 0.89), M3, (r = 0.88), M4 (r = 0.82), M5 (r = 0.89) and H1 (r = 0.82).
Figure 4.
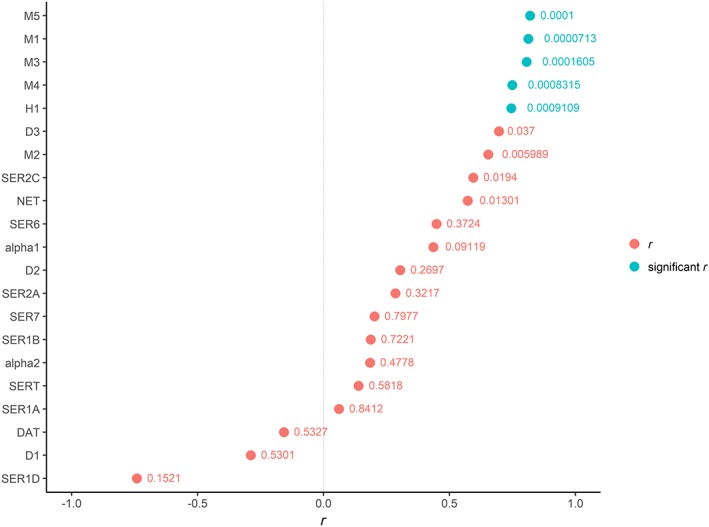
Pearson's correlation coefficients (r) and their P‐values of the relationship between aROR for diabetes and the occupancy on each receptor/transporter. The receptors 5‐HT3 and D4 were not analysed due to lack of pharmacodynamic data. P‐values were adjusted to Bonferroni correction due to multiple correlations (21) to 0.00238. Significant correlations were observed for occupancies on the muscarinic receptors, except for M2, and the histamine H1 receptor. Degrees of occupancy less than 0.1% were excluded, as well as antidepressants with less than three reports with diabetes
Figure 5.
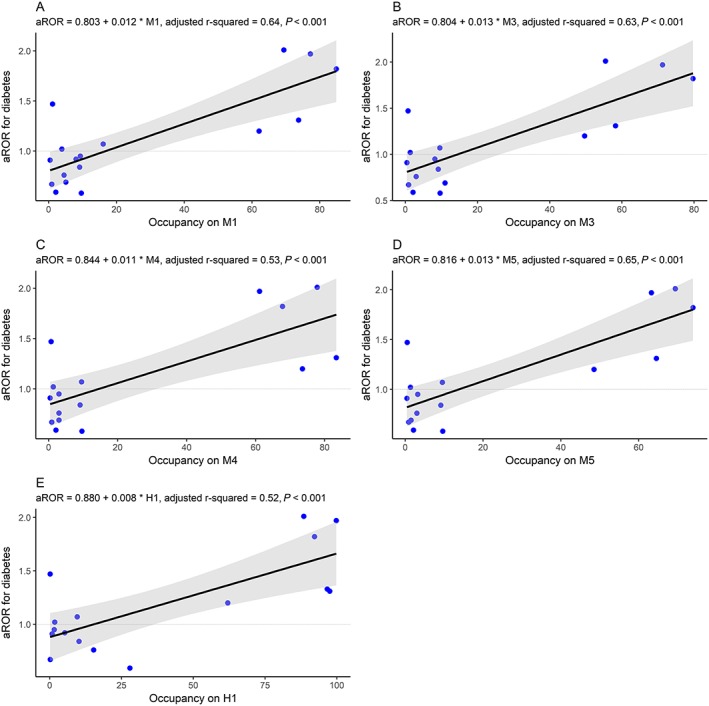
Univariate linear regression models of the relationship between the adjusted reporting odds ratio (aROR) for diabetes and the occupancy on (A) M1, (B) M3, (C) M4, (D) M5 and (E) H1 of individual antidepressants. The line demonstrates the linear regression model and the grey area demonstrates the 95% CI of the linear regression model. Degrees of occupancy less than 0.1% were excluded, as well as antidepressants with less than three reports with diabetes
Discussion
This study investigated the risk for diabetes of individual antidepressants and its relationship with their pharmacodynamic profile using a combined pharmacovigilance‐pharmacodynamic approach. The risk for reporting diabetes was estimated with disproportionality analysis of the FAERS database. Individual antidepressants seem to carry differential risk for diabetes. Most of the tricyclic antidepressants, i.e. imipramine, amitriptyline, nortriptyline and doxepin, as well as sertraline and mirtazapine, were associated with diabetes in comparison to the other antidepressants. The risk for diabetes seems to correlate with the pharmacodynamic profile of antidepressants. Higher degrees of occupancy on H1 and muscarinic receptors, except for M2, seem to be related with higher risk for diabetes. However, these receptor mechanisms could not explain the disproportionate signal for sertraline.
The better‐studied antipsychotics can provide insights on receptor mechanisms of glucose intolerance. Antipsychotics seem to interfere with central and peripheral metabolic regulation and they could promote obesity, insulin resistance and beta cell dysfunction. Central energy balance centres express 5‐HT2C and H1 receptors, and their antagonism could induce weight gain as well as disturbances in peripheral flow of autonomic nervous system and glucose intolerance. Pancreatic islets seem to express muscarinic, serotonergic receptors and serotonin transporter, and their inhibition could disrupt insulin secretion and glucose handling. Adrenergic and dopaminergic signalling may also play important roles in metabolic side effects. Τhe net result is a complex interplay between central and peripheral mechanisms 3, 28. In accordance with the above mechanisms, a positive correlation between the risk for diabetes of antidepressants and degrees of occupancy on H1 and muscarinic receptors were observed in FAERS. Additionally, non‐significant moderate to strong correlations were also identified for NET, 5‐HT2C and D3.
Our results are in agreement with previous studies, which associate the risk for diabetes with the pharmacodynamic profile of antidepressants. A cross‐sectional study of patients with bipolar disorders suggested that antidepressants with strong H1 antagonistic properties, but not in general antidepressants, are associated with metabolic syndrome. An inverse association between the inhibitory constant (Ki) for H1 and prevalence of metabolic syndrome was also observed 6. In addition, a case–control study suggested that antidepressants with antagonistic properties on M3 are associated with incident type 2 diabetes 7. A disproportionality analysis in the pharmacovigilance database of the WHO has also suggested that antidepressants might associate with diabetes, with a more pronounced risk for antidepressants with affinities to H1, 5‐HT2C and NET 5. These studies used pharmacodynamic classifications of antidepressants to investigate receptor mechanisms, rather than assessing directly the role of the pharmacodynamic profile. The direct combination of pharmacovigilance with pharmacodynamic data has been proposed to study receptor mechanisms of adverse events in safety reports databases. This approach has been applied in VigiBase to study drug‐induced arrhythmias 29, antipsychotic‐induced diabetes 4, antipsychotic‐induced movement disorders 8 and cardiac failure induced by protein kinase inhibitors 9. Herein, we used a similar approach in FAERS, which can also be used to study receptor mechanisms of adverse events 30.
Antagonism of H1 seems to explain the association between imipramine, amitriptyline, nortriptyline, doxepin and mirtazapine with diabetes, as these antidepressants have the highest degrees of occupancy on H1. Except for mirtazapine, these antidepressants also have high degrees of occupancy on muscarinic receptors, but without displaying marked selectivity among M1 to M5 receptors. Due to the small number of antidepressants, multivariable analysis could not be utilized to assess the effect of both receptor systems. Other antidepressants with high occupancies on H1, such as trimipramine, maprotiline and desipramine, had small and potentially insufficient numbers of reports in order to raise disproportionality signals. The role of 5‐HT2A/C antagonism has also been suggested in psychotropic‐induced diabetes, especially in antipsychotics 3, 4. However, a significant correlation was not observed, which is also supported by the fact that nefazodone and trazodone were not associated with diabetes. Nefazodone and trazodone have relatively high degrees of occupancy on 5‐HT2A/C, but they lack high degrees of occupancy on H1 and muscarinic receptors. Despite the proposed role of NET inhibition 5, milnacipran and duloxetine were also not associated with diabetes. These drugs have relatively high degrees of occupancy on NET but they also lack occupancy on H1 or muscarinic receptors.
On the other hand, the SSRI sertraline was associated with diabetes, despite its low degrees of occupancy on H1, muscarinic or even 5‐HT2C and NET. As a result, other mechanisms cannot be excluded. The role of SERT inhibition seems to be complex. Short‐term treatment with SERT inhibitors may be linked to weight loss 31, probably due to increased extracellular serotonin and indirect stimulation of 5‐HT1B and 5‐HT2C in energy balance centres, which promote satiety states 32. However, long‐term treatment may be linked to weight gain and glucose intolerance 31, probably due to compensatory mechanisms and/or pancreatic SERT inhibition. At least some antidepressants, i.e. fluoxetine, paroxetine and clomipramine, may also have direct oxidative and cytotoxic effects on pancreatic beta cells, independent of SERT inhibition 33. Furthermore, a placebo‐controlled randomized trial in depressed non‐human primates suggested that chronic administration of sertraline decreases adiponectin, independently of effects on adiposity. Decreased levels of adiponectin could promote insulin resistance and atherosclerosis 32.
The present study has certain limitations. Disproportionality analysis is a statistical method used to identify potential significant drug–event combinations, highlighting combinations which need further clinical validation 10, 13. It cannot remove the absolutely necessary step of a careful clinical causality assessment, thus, association found is not causation. There might be other reasons leading to an association and thus confounding the results: confounding by indication, by underlying diseases and by concomitant drugs. Regarding a possible indication bias, tricyclic antidepressants are considered for second‐line treatment in depression 34, while metabolic dysregulation has been associated with chronicity of illness and poor response to antidepressants 35. However, there is evidence of the biological plausibility of tricyclic antidepressant‐induced glucose dysregulation; a randomized double‐blind study suggested that nortriptyline had direct hyperglycaemic effects in comparison to placebo in diabetic patients with active major depression 36. A further limitation of our study was that pharmacovigilance databases, like FAERS, are mostly based on spontaneous reports and the quality of information may be suboptimal leading to case misclassification bias, while underreporting still remains a major concern of spontaneous adverse drug reactions reporting systems 37, 38. Nevertheless, reporting rates are suggested to be similar in drugs of the same therapeutic class 39, such as antidepressants. The estimation of occupancy on receptors also has inherent limitations. Since concentrations of drugs in the cerebrospinal fluid are scarcely available, total blood concentrations were used to estimate occupancy on receptors, such as those presented in therapeutic reference ranges of recommended doses of antidepressants 4. In addition, the occupancy on receptors based on the pharmacological receptor theory is not directly associated with the degree of pharmacological activity 8, 17. However, in clinical setting, the action of the selected antidepressants is explained mostly by antagonistic properties on receptors and transporters. Finally, regarding the linear regression analysis, the moderate number of drugs prohibit multivariate analysis and could limit the statistical power 40. Despite these limitations, the major strength of this study is the large number of reports exposed to antidepressants (136 028) and the attempts to minimize confounding and bias on the available data.
In conclusion, our results suggest a disproportionality signal of antidepressant‐induced diabetes with plausible pharmacological mechanism being the antagonism on muscarinic and H1 receptors, but other mechanisms should also be expected. Taking into account the clinical and pharmacological aspects of this association, an appropriate causality assessment is needed to validate it and to proceed to further investigation through post‐marketing long‐term safety studies as appropriate.
Competing Interests
There are no competing interests to declare.
The authors would like to thank Pantazis Vouzaxakis, electrical and computer engineer at the School of Engineering, Aristotle University of Thessaloniki, who helped with data cleaning in R.
Contributors
S.S. was responsible for protocol development, data collection and analysis and manuscript drafting. G.P. was responsible for protocol development, data analysis and manuscript editing.
Supporting information
Table S1 Concomitant drugs. Reports with concomitant antipsychotics (first or second generation) or combination of antidepressant drugs were excluded from further analysis, due to targeting the same receptors as well as for the notoriety bias of some antipsychotics in FAERS. Reports with concomitant antidiabetic medication were excluded, since they indicate pre‐existing diabetes, rather than incident diabetes. Use of hyperglycaemic was included as covariates in logistic regression to adjust the reporting odds ratio for diabetes.
Table S2 Occupancies of antidepressants on human neurotransmitter receptors and transporters. The pharmacodynamic profile of antidepressants is presented as the inhibitory constant (Ki [nM]). Ki values were extracted from PDSP and IUPHAR databases, following this hierarchy on availability. When more than one value was available for one receptor, the mean value was calculated. Ki values for adrenoceptors alpha1 and alpha2 were calculated, regardless of receptor subtype. The total blood concentration of antidepressants (C max [ng ml−1]) was estimated using the maximum point of therapeutic reference range according to the AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology and Dockens et al. for nefazodone. The unbound blood concentration of antidepressants (Cr [nM]) was estimated using the unbound fraction (Fu) and the molecular weight (M r [g mol−1]) with the equation: Cr = 1000 * Fu * C max/M r. The values of Fu were extracted from the Drugbank database and the molecular weights from the IUPHAR database. The occupancy on receptors was estimated using the pharmacological receptor theory with the equation: Occupancy (%) = 100 * Cr/(Cr + Ki).
Table S3 Disproportionality analysis in FAERS for antidepressants and diabetes. Diabetes cases were identified with the narrow scope standardized MedDRA query ‘Hyperglycemia/new‐onset diabetes’. Both the crude and the adjusted ROR are presented. ROR was adjusted to age, gender, reporting year and use of hyperglycaemic drugs. Reports involving patients under the age of 18, concomitant use of antidiabetics, antipsychotics or combination of antidepressants were excluded.
Siafis, S. , and Papazisis, G. (2018) Detecting a potential safety signal of antidepressants and type 2 diabetes: a pharmacovigilance‐pharmacodynamic study. Br J Clin Pharmacol, 84: 2405–2414. 10.1111/bcp.13699.
References
- 1. Salvi V, Grua I, Cerveri G, Mencacci C, Barone‐Adesi F. The risk of new‐onset diabetes in antidepressant users – a systematic review and meta‐analysis. PloS one 2017; 12: e0182088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care 2013; 36: 3337–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siafis S, Tzachanis D, Samara M, Papazisis G. Antipsychotic drugs: from receptor‐binding profiles to metabolic side effects. Current Neuropharmacol 2017. 10.2174/1570159X15666170630163616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montastruc F, Palmaro A, Bagheri H, Schmitt L, Montastruc J‐L, Lapeyre‐Mestre M. Role of serotonin 5‐HT2C and histamine H1 receptors in antipsychotic‐induced diabetes: a pharmacoepidemiological‐pharmacodynamic study in VigiBase. Eur Neuropsychopharmacol 2015; 25: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 5. Derijks HJ, Meyboom RH, Heerdink ER, De Koning FH, Janknegt R, Lindquist M, et al The association between antidepressant use and disturbances in glucose homeostasis: evidence from spontaneous reports. Eur J Clin Pharmacol 2008; 64: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvi V, Barone‐Adesi F, D'Ambrosio V, Albert U, Maina G. High H1‐affinity antidepressants and risk of metabolic syndrome in bipolar disorder. Psychopharmacology (Berl) 2016; 233: 49–56. [DOI] [PubMed] [Google Scholar]
- 7. Tran YH, Schuiling‐Veninga CCM, Bergman JEH, Groen H, Wilffert B. Impact of muscarinic M3 receptor antagonism on the risk of type 2 diabetes in antidepressant‐treated patients: a case‐controlled study. CNS Drugs 2017; 31: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen TT, Pariente A, Montastruc JL, Lapeyre‐Mestre M, Rousseau V, Rascol O, et al An original pharmacoepidemiological‐pharmacodynamic method: application to antipsychotic‐induced movement disorders. Br J Clin Pharmacol 2017; 83: 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patras de Campaigno E, Bondon‐Guitton E. Identification of cellular targets involved in cardiac failure caused by PKI in oncology: an approach combining pharmacovigilance and pharmacodynamics. Br J Clin Pharmacol 2017; 83: 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci 2013; 10: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Böhm R, von Hehn L, Herdegen T, Klein H‐J, Bruhn O, Petri H, et al OpenVigil FDA – inspection of US American adverse drug events pharmacovigilance data and novel clinical applications. PloS One 2016; 11: e0157753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hennings JM, Schaaf L, Fulda S. Glucose metabolism and antidepressant medication. Curr Pharm Des 2012; 18: 5900–5919. [DOI] [PubMed] [Google Scholar]
- 13. Faillie J‐L. Les études cas–non cas: principe, méthodes, biais et interprétations. Therapie 2018; 73: 247–255. [DOI] [PubMed] [Google Scholar]
- 14. Goncalves P, Araujo JR, Martel F. Antipsychotics‐induced metabolic alterations: focus on adipose tissue and molecular mechanisms. Eur Neuropsychopharmacol 2015; 25: 1–16. [DOI] [PubMed] [Google Scholar]
- 15. Robert DB, Sarah MS, Sarah KG. Risk of new‐onset diabetes associated with statin use. SAGE Open Med 2015; 3: 2050312115605518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rehman A, Setter SM, Vue MH. Drug‐induced glucose alterations Part 2: Drug‐induced hyperglycemia. Diabetes Spectr 2011; 24: 234–238. [Google Scholar]
- 17. Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci 2004; 25: 186–192. [DOI] [PubMed] [Google Scholar]
- 18. Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, et al Automated design of ligands to polypharmacological profiles. Nature 2012; 492: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2017; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, et al Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry 2018; 51: 9–62. [DOI] [PubMed] [Google Scholar]
- 21. Dockens RC, Greene DS, Barbhaiya RH. Assessment of pharmacokinetic and pharmacodynamic drug interactions between nefazodone and digoxin in healthy male volunteers. J Clin Pharmacol 1996; 36: 160–167. [DOI] [PubMed] [Google Scholar]
- 22. Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 2018; 46: D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtin F, Schulz P. Multiple correlations and Bonferroni's correction. Biol Psychiatry 1998; 44: 775–777. [DOI] [PubMed] [Google Scholar]
- 24. R Core Team . R: A Language and Environment for StatisticalComputing, version, 3.3.2 edn. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 25. Alexander SP, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alexander SP, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 2017; 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander SP, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 2017; 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amato D, Beasley CL, Hahn MK, Vernon AC. Neuroadaptations to antipsychotic drugs: insights from pre‐clinical and human post‐mortem studies. Neurosci Biobehav Rev 2017; 76: 317–335. [DOI] [PubMed] [Google Scholar]
- 29. De Bruin ML, Pettersson M, Meyboom RH, Hoes AW, Leufkens HG. Anti‐HERG activity and the risk of drug‐induced arrhythmias and sudden death. Eur Heart J 2005; 26: 590–597. [DOI] [PubMed] [Google Scholar]
- 30. Maciejewski M, Lounkine E. Reverse translation of adverse event reports paves the way for de‐risking preclinical off‐targets. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersohn F, Schade R, Suissa S, Garbe E. Long‐term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry 2009; 166: 591–598. [DOI] [PubMed] [Google Scholar]
- 32. Silverstein‐Metzler MG, Shively CA, Clarkson TB, Appt SE, Carr JJ, Kritchevsky SB, et al Sertraline inhibits increases in body fat and carbohydrate dysregulation in adult female cynomolgus monkeys. Psychoneuroendocrinology 2016; 68: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elmorsy E, Al‐Ghafari A, Helaly ANM, Hisab AS, Oehrle B, Smith PA. Editor's highlight: therapeutic concentrations of antidepressants inhibit pancreatic beta‐cell function via mitochondrial complex inhibition. Toxicol Sci 2017; 158: 286–301. [DOI] [PubMed] [Google Scholar]
- 34. Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 2015; 29: 459–525. [DOI] [PubMed] [Google Scholar]
- 35. Vogelzangs N, Beekman AT, van Reedt Dortland AK, Schoevers RA, Giltay EJ, de Jonge P, et al Inflammatory and metabolic dysregulation and the 2‐year course of depressive disorders in antidepressant users. Neuropsychopharmacology 2014; 39: 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, et al Effects of nortriptyline on depression and glycemic control in diabetes: results of a double‐blind, placebo‐controlled trial. Psychosom Med 1997; 59: 241–250. [DOI] [PubMed] [Google Scholar]
- 37. Montastruc JL, Sommet A, Lacroix I, Olivier P, Durrieu G, Damase‐Michel C, et al Pharmacovigilance for evaluating adverse drug reactions: value, organization, and methods. Joint Bone Spine 2006; 73: 629–632. [DOI] [PubMed] [Google Scholar]
- 38. Macia‐Martinez MA, de Abajo FJ, Roberts G, Slattery J, Thakrar B, Wisniewski AF. An empirical approach to explore the relationship between measures of disproportionate reporting and relative risks from analytical studies. Drug Saf 2016; 39: 29–43. [DOI] [PubMed] [Google Scholar]
- 39. Pierfitte C, Bégaud B, Lagnaoui R, Moore ND. Is reporting rate a good predictor of risks associated with drugs? Br J Clin Pharmacol 1999; 47: 329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneider A, Hommel G, Blettner M. Linear regression analysis: part 14 of a series on evaluation of scientific publications. Dtsch Arzteblatt Int 2010; 107: 776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Concomitant drugs. Reports with concomitant antipsychotics (first or second generation) or combination of antidepressant drugs were excluded from further analysis, due to targeting the same receptors as well as for the notoriety bias of some antipsychotics in FAERS. Reports with concomitant antidiabetic medication were excluded, since they indicate pre‐existing diabetes, rather than incident diabetes. Use of hyperglycaemic was included as covariates in logistic regression to adjust the reporting odds ratio for diabetes.
Table S2 Occupancies of antidepressants on human neurotransmitter receptors and transporters. The pharmacodynamic profile of antidepressants is presented as the inhibitory constant (Ki [nM]). Ki values were extracted from PDSP and IUPHAR databases, following this hierarchy on availability. When more than one value was available for one receptor, the mean value was calculated. Ki values for adrenoceptors alpha1 and alpha2 were calculated, regardless of receptor subtype. The total blood concentration of antidepressants (C max [ng ml−1]) was estimated using the maximum point of therapeutic reference range according to the AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology and Dockens et al. for nefazodone. The unbound blood concentration of antidepressants (Cr [nM]) was estimated using the unbound fraction (Fu) and the molecular weight (M r [g mol−1]) with the equation: Cr = 1000 * Fu * C max/M r. The values of Fu were extracted from the Drugbank database and the molecular weights from the IUPHAR database. The occupancy on receptors was estimated using the pharmacological receptor theory with the equation: Occupancy (%) = 100 * Cr/(Cr + Ki).
Table S3 Disproportionality analysis in FAERS for antidepressants and diabetes. Diabetes cases were identified with the narrow scope standardized MedDRA query ‘Hyperglycemia/new‐onset diabetes’. Both the crude and the adjusted ROR are presented. ROR was adjusted to age, gender, reporting year and use of hyperglycaemic drugs. Reports involving patients under the age of 18, concomitant use of antidiabetics, antipsychotics or combination of antidepressants were excluded.


