Abstract
Aims
Flucloxacillin dosing may be guided by measurement of its total plasma concentrations. Flucloxacillin is highly protein bound with fraction unbound in plasma (fu) of around 0.04 in healthy individuals. The utility of measuring unbound flucloxacillin concentrations for patients outside the intensive care unit (ICU) is not established. We aimed to compare flucloxacillin fu in non‐ICU hospitalised patients against healthy volunteers, and to examine the performance of a published model for predicting unbound concentrations, using total flucloxacillin and plasma albumin concentrations.
Methods
Data from 12 healthy volunteers (248 samples) and 47 hospitalized patients (61 samples) were examined. Plasma flucloxacillin concentrations were measured using a validated liquid chromatography–tandem mass spectrometry method. Flucloxacillin fu for the two groups was compared using a generalized estimating equation model to account for clustered observations. The performance of the single protein binding site prediction model in hospitalized patients was compared with measured unbound concentrations using Bland–Altman plots.
Results
The median (range) flucloxacillin fu for healthy (median albumin 45 g l–1) and hospitalized individuals (median albumin 30 g l–1) were 0.04 (0.02–0.07) and 0.10 (0.05–0.37), respectively (P < 0.0001). The prediction model underpredicted unbound flucloxacillin concentrations with a mean bias (95% limits of agreement) of –54% (–137%, +30%).
Conclusions
The flucloxacillin fu values observed in our cohort of hospitalized patients had a wide range and were greater than those of healthy individuals. Unbound flucloxacillin plasma concentrations were predicted poorly by the model. Instead, unbound concentrations should be measured to guide dosing.
Keywords: biological models, drug monitoring, flucloxacillin, protein binding
What is Already Known about this Subject
Flucloxacillin is highly protein bound with the reported fraction unbound (fu) of 0.04.
Unbound flucloxacillin concentrations represent the active portion of total concentrations.
Predicted unbound flucloxacillin concentrations poorly reflect measured unbound flucloxacillin concentrations in intensive care unit (ICU) patients.
What this Study Adds
Support from a large dataset (248 samples) of an fu of flucloxacillin in healthy individuals.
Demonstrates that the flucloxacillin fu of non‐ICU hospitalized patients are substantially higher than those of healthy individuals.
Predicted unbound flucloxacillin concentrations poorly reflect measured unbound concentrations in non‐ICU hospitalized patients.
Introduction
Flucloxacillin is a key β‐lactam antibacterial agent used to treat infections caused by susceptible organisms including methicillin susceptible Staphylococcus aureus (MSSA). It is highly protein bound, with the fraction unbound (fu) reported to be 0.04 1. Flucloxacillin fu is increased in the setting of hypoalbuminaemia, renal impairment and concomitant drugs such as benzodiazepines that compete for the protein binding site 2.
The dosing of flucloxacillin may be guided by measuring its plasma concentrations 3. This is primarily to ensure adequate concentrations are achieved when treating severe infections such as MSSA bacteraemia, which is associated with 20% mortality at 30 days following diagnosis 4. For this purpose, the total flucloxacillin concentration has been used to estimate the unbound concentration, which is responsible for bacterial killing. However, in patients in a tertiary referral intensive care unit (ICU; 11 plasma samples), unbound concentrations were underestimated when calculated using total concentrations and a fixed fu of 0.07, with a mean (95% confidence interval) bias of –57% (–130%, 16.5%) 5. The bias and imprecision of this approach for estimating unbound flucloxacillin concentrations has not been investigated in hospitalized patients outside the ICU. Further, more sophisticated models for estimating unbound drug concentrations using plasma concentrations of the relevant binding protein (e.g. albumin) and total drug concentrations have been proposed 6. Hence, we aimed to compare flucloxacillin protein binding in hospitalized patients outside of the ICU with that of healthy volunteers. We also aimed to assess the performance of a single binding site model for predicting unbound flucloxacillin concentrations from total concentrations.
Methods
Study design and participants
This was an observational study using data from healthy volunteers, and hospitalized patients at Christchurch Hospital (Christchurch, New Zealand) collected during 2014 and 2015. Two sources of data were employed. Data from hospitalized patients aged at least 18 years were acquired by extracting all measured flucloxacillin concentrations from the local hospital electronic health record. Flucloxacillin was measured in these patients as part of routine clinical care. Healthy volunteer data were from a separate local study of the interaction between food and flucloxacillin (Australian New Zealand Clinical Trials Registry, ACTRN12617001046392). Total and unbound flucloxacillin concentrations that were below the lower limit of quantification (LLOQ, 0.2 mg l–1 and 0.005 mg l–1, respectively) were excluded, as were samples taken while the patient was in an ICU. Data collected for each individual included measured total and unbound plasma flucloxacillin concentrations, indication for flucloxacillin, dosing regimen used, age, sex, plasma albumin concentration and estimated glomerular filtration rate (eGFR, calculated using the Chronic Kidney Disease Epidemiology Collaboration equation) 7. The New Zealand Health and Disability Ethics Committees (Ministry of Health, Wellington, New Zealand) approved the present study as an outcomes analysis that did not require detailed ethics review.
Prediction of unbound flucloxacillin concentration
Flucloxacillin is thought to bind predominantly to albumin 8, in a 1:1 ratio 9. Hence, we employed a single site protein binding model as proposed by Musteata 6 to predict unbound flucloxacillin concentrations (pC u) using total flucloxacillin concentrations (C t), plasma albumin concentrations (P) and an assumed binding constant (K) of 27 180 l mol–1 9:
All values were converted to molar units for use in this equation. As an example, a patient with C t = 20 mg l–1 (44.1 μmol l–1) and P = 40 g l–1 (601.5 μmol l–1) has a pC u = 1.2 mg l–1 (2.7 μmol l–1), and hence an fu = 0.06. The pC u was only calculable for patients with a measured plasma albumin concentration at the time of flucloxacillin use.
Flucloxacillin laboratory analysis
Total and unbound plasma flucloxacillin concentrations were measured using a validated liquid chromatography‐mass spectrometry (LC–MS/MS) method 10. The unbound fraction of flucloxacillin was isolated by ultrafiltration (2600 g for 30 min at 37°C) using Centrifree Ultrafiltration Devices (Merck Millipore Ltd., Tullagreen, Carrigtwohill, Co. Cork, Ireland). Briefly, for the total flucloxacillin range of 0.2–100 mg l–1, the intra‐ and interday bias and CV were ≤6.8% and ≤7.8%, respectively. Similarly, for the unbound flucloxacillin range of 0.005–10 mg l–1, the intra‐ and interday bias and CV were ≤7.6% and ≤7.3%, respectively. All samples were analysed at Canterbury Health Laboratories (Christchurch, New Zealand).
Statistical analysis
Scatter plots and summary statistics of the pooled observations for the patient and healthy volunteer groups were used to describe the relationships between measured total and unbound flucloxacillin concentrations. As many individuals had more than one flucloxacillin concentration measured, a generalized estimating equations (GEE) linear model was used to account for this clustered structure and thus obtain unbiased marginal parameter estimates. The model was specified with an exchangeable working correlation matrix, and robust standard error estimation was applied to account for potential misspecification 11. In this model, mean fu was compared between the healthy volunteer and patient groups using the Wald test.
The Bland–Altman plot was used to compare measured and predicted unbound flucloxacillin concentrations for hospitalized patients, and to identify any predicted concentrations that were more than ±15% different from those measured. This is an arbitrary threshold but is used by the Food and Drug Administration as a marker of assay quality 12.
A scatter plot for predicted vs. measured unbound flucloxacillin concentrations in hospitalized patients was employed to identify incongruent samples. For this analysis, we assumed that all samples were trough flucloxacillin concentrations, and that the clinical target was to achieve unbound concentrations with a 100% time above the cloxacillin epidemiological cut‐off (ECOFF) of 0.5 mg l–1 13. The ECOFF is the upper limit of the minimum inhibitory concentration of wildtype MSSA. The ECOFF for flucloxacillin has not been reported, and instead we used that for cloxacillin as it has similar activity against MSSA as flucloxacillin 14. Further, a target of four times the minimum inhibitory concentration (2 mg l–1) has been advocated and used by some clinicians 3. Incongruent samples included those where: (i) the predicted unbound concentration exceeded the target concentration, but the measured unbound concentration was below the target (false reassurance from predicted concentration that dosing is adequate); and (ii) the predicted concentration was below target, but the measured concentration exceeded the target (leading to unnecessary dose escalation based on predicted concentration). This was performed for both 0.5 and 2 mg l–1. Sensitivity analysis was conducted for both the Bland–Altman plot, and scatter plot for incongruent samples, by using a fixed fu of 0.04 to predict unbound concentrations from total concentrations.
Statistical analysis was performed using GraphPad Prism (version 7.04 for Windows, GraphPad Software, La Jolla, CA, USA, http://www.graphpad.com) and R (version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/) implemented the RStudio environment (version 1.0.136, RStudio Team (2016), RStudio, Inc., Boston, MA, http://www.rstudio.com/). GEEs were fitted using the geepack package for R 15.
Results
Data from 12 healthy volunteers (248 pairs of measured total and unbound flucloxacillin concentrations) and 47 hospitalized patients (65 pairs, of which four were excluded because the total concentrations were below LLOQ) were examined (Table 1, Figure 1). All patients were undergoing treatment for MSSA bacteraemia.
Table 1.
Demographic characteristics at time of sampling
| Baseline characteristics | Healthy volunteers | Hospitalized patients | Hospitalized patients with albumin measured |
|---|---|---|---|
| Individuals | |||
| n | 12 | 47 | 29 |
| Age, y, median (range) | 26 (21–38) | 68 (18–87) | 68 (27–87) |
| Male/female | 7/5 | 33/14 | 20/9 |
| Flucloxacillin dosing, mg | 1000 single dose | 1000–2000 per 6 h | |
| Measured flucloxacillin concentrations | |||
| n | 248 | 61 | 32 |
| Total, mg l –1 , median (range) | 7.89 (0.20–74.10) | 24.70 (0.49–83.00) | 21.35 (0.49–83.00) |
| Unbound, mg l –1 , median (range) | 0.28 (0.0047–2.78) | 2.07 (0.04–30.30) | 2.58 (0.04–30.30) |
| f u , median (range) | 0.04 (0.02–0.07) | 0.10 (0.05–0.37) | 0.18 (0.05–0.37) |
| Albumin concentration, g l –1 , median (range) | 45 (40–48) | 30 (22–42) | |
| eGFR, ml min –1 1.73 m –2 , median (range) | 92 (77–120) | 76 (28–127) for 43 samples | 74 (28–117) |
eGFR, estimated glomerular filtration rate, fu, fraction unbound
Figure 1.
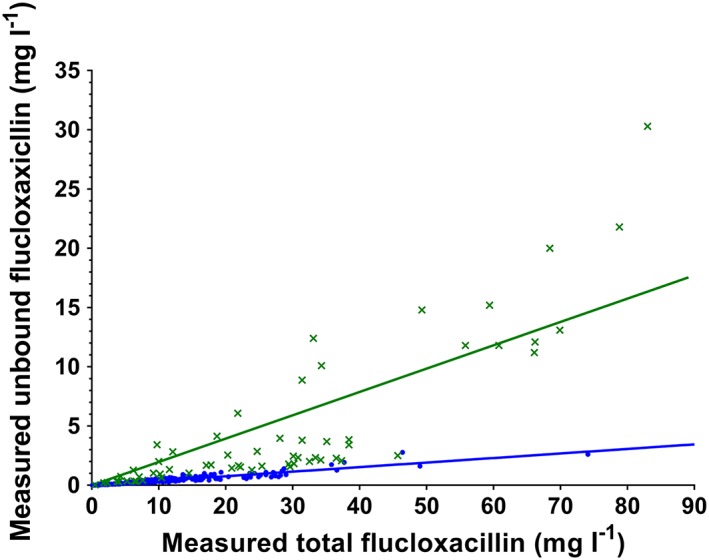
Scatter plots for measured unbound vs. total flucloxacillin concentrations. Ct, total concentration; Cu, unbound concentration. Blue linear regression line and data points, healthy volunteers; green linear regression line and data points, hospitalized patients
The median flucloxacillin fu was higher in hospitalized (0.10) than in healthy individuals (0.04). Using the GEE model, there was strong evidence that the mean fu was higher in the hospitalized patients than in the healthy individuals (0.14 vs. 0.04, mean difference 0.10, 95% confidence interval 0.075–0.13, P < 0.0001).
Predicted unbound concentrations were calculable using the single binding site model for 32/61 (52%) flucloxacillin samples in hospitalized patients. The resulting Bland–Altman plot (Figure 2A) showed that predicted unbound concentrations were mostly lower than measured concentrations, with a mean bias (95% limits of agreement) of –54% (–137%, +30%). The bias in the majority (88%, 28/32) of these predicted unbound concentrations exceeded ±15%. Predicted unbound concentrations calculated using a fixed fu of 0.04 had a mean bias (95% limits of agreement) of –111% (–191%, –32%) with the bias in all values exceeding ±15%.
Figure 2.
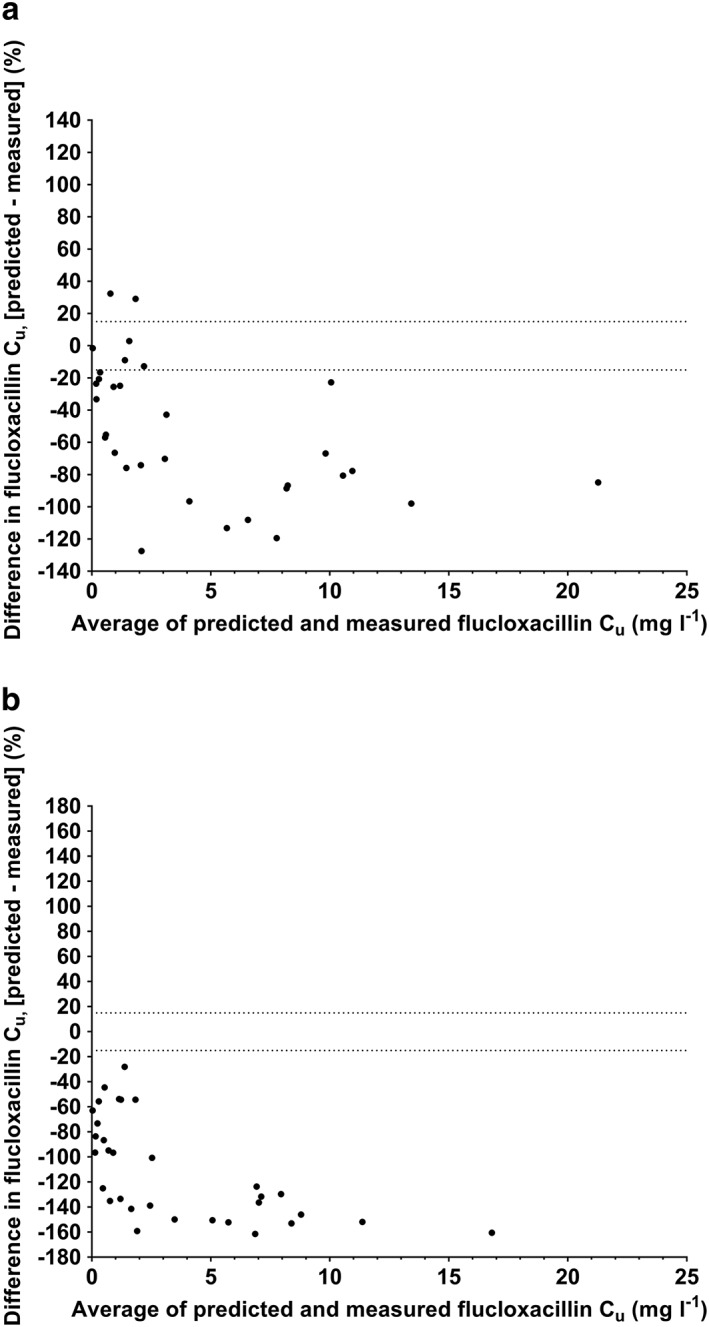
Bland–Altman plots of predicted unbound flucloxacillin concentrations using single binding site prediction model (A) and fixed fu of 0.04 (B) compared with measured unbound flucloxacillin concentrations in hospitalized patients. Cu, unbound concentration. Dotted horizontal lines represent +15% and –15% difference
The scatter plots of predicted against measured unbound concentrations in hospitalized patients showed that for a target of 0.5 mg l–1, predicted unbound concentrations were incongruent for two and nine of 32 for the single site binding model and fixed fu of 0.04 model, respectively. For a target of 2 mg l–1, predicted unbound concentrations were incongruent for four and 10 of 32 for the single site binding model and fixed fu of 0.04 model, respectively. All incongruent predictions, except one, underestimated measured concentrations.
Discussion
This observational study demonstrates the value of measuring, rather than predicting, unbound flucloxacillin concentrations in hospitalized patients with MSSA bacteraemia outside the ICU. In these patients, predicted unbound flucloxacillin concentrations using the single site protein binding model typically underestimated measured values, and the median flucloxacillin fu of 0.10 was >2‐fold that of healthy volunteers (median of 0.04). Further, fu in all of the hospitalized patients (range 0.05, 0.37) exceeded the median healthy value. Hence, in comparison to measured unbound concentrations, the use of predicted concentrations is expected to lead to unnecessary flucloxacillin dose escalation. Similarly, if a dosing strategy consisting of a large initial dose and then back‐titration according to unbound concentrations were employed, the use of predicted concentrations may lead to continued high doses that are not needed. Using the literature fu = 0.04 to derive target total concentrations that err on the side of favouring efficacy, the unbound concentrations of 0.5 and 2 mg l–1 correspond to total concentrations of 12.5 and 50 mg l–1, respectively. As flucloxacillin has a high therapeutic index, it could be argued that unnecessarily high doses are unlikely to lead to harm. However, seizures are a concerning toxicity from β‐lactams at high concentrations 16.
At our institution, the unbound flucloxacillin concentration is now a standard clinical assay with a turnaround time of 3–24 h 10. However, for a given drug, total drug concentrations, instead of unbound concentrations, are often measured because of methodological ease and lower cost 17. When used as a surrogate of drug effect, total concentrations need to accurately reflect unbound concentrations to be useful clinically 6, 17, 18. A simple approach is to multiply measured total concentrations by a fixed reference fu value. However, the reference fu is typically established in healthy individuals, instead of in the target population for the drug 5. Some patients in our cohort with MSSA bacteraemia may have had comorbidities such as acute kidney injury or chronic liver disease, which may affect binding affinity and binding protein concentrations, respectively. In these settings, the simple approach is expected to poorly predict actual unbound concentrations. Hence, we employed an alternative approach recently proposed by Musteata 6, which can account for binding affinity and binding protein concentrations. However, our application of this approach was limited by the requirement for an accurate estimate of the binding constant for the individual patient. In the absence of data correlating the binding affinity with covariates such as severity of acute kidney injury, we used a fixed binding constant, which is likely to have contributed to the differences we found between predicted and measured flucloxacillin concentrations in the hospitalized patients.
Our study has a number of limitations. The number of hospitalized patients (47) and associated flucloxacillin samples (61) is not large. However, we are not aware of any larger published cohorts. Further, all patients had flucloxacillin for MSSA bacteraemia and our results may not be generalizable to other infections, such as skin and soft tissue infections without bacteraemia. The exclusion of patient samples below the LLOQ may have biased the distribution of fu values in this group, but it is unclear what impact this would have on our findings. We did not account for covariates of binding affinity in the prediction model, thus limiting its performance. Finally, the analysis regarding erroneous decisions resulting from the use of predicted unbound flucloxacillin concentrations is based on the assumption that samples were taken at the appropriate time point in the dosing interval. Prospective studies, such as randomized controlled trials, are required to quantify the clinical benefit of using unbound flucloxacillin for therapeutic drug monitoring.
In conclusion, flucloxacillin protein binding is significantly reduced in hospitalized patients even outside of critical care. Use of predicted unbound flucloxacillin concentrations in a single site binding model significantly underestimated measured concentrations, potentially leading to unnecessary dose escalation. Where available, unbound flucloxacillin should be measured to inform clinical decisions in hospitalized patients with MSSA bacteraemia.
Competing Interests
There are no competing interests to declare.
The authors thank Jared K. Green for helping to establish the MSSA bacteraemia bundle of care as routine clinical management at Christchurch Hospital, which included sampling for flucloxacillin concentrations.
Chin, P. K. L. , Drennan, P. G. , Gardiner, S. J. , Zhang, M. , Dalton, S. C. , Chambers, S. T. , and Begg, E. J. (2018) Total flucloxacillin plasma concentrations poorly reflect unbound concentrations in hospitalized patients with Staphylococcus aureus bacteraemia. Br J Clin Pharmacol, 84: 2311–2316. 10.1111/bcp.13673.
References
- 1. Bennett JE, Dolin R, Blaser MJ. Principles and practice of infectious diseases: Elsevier Health Sciences, 2014.
- 2. Grandison MK, Boudinot FD. Age‐related changes in protein binding of drugs: implications for therapy. Clin Pharmacokinet 2000; 38: 271–290. [DOI] [PubMed] [Google Scholar]
- 3. Wong G, Brinkman A, Benefield RJ, Carlier M, De Waele JJ, El Helali N, et al An international, multicentre survey of beta‐lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother 2014; 69: 1416–1423. [DOI] [PubMed] [Google Scholar]
- 4. Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR, et al Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust 2009; 191: 368–373. [DOI] [PubMed] [Google Scholar]
- 5. Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, et al Protein binding of beta‐lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 2013; 57: 6165–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musteata FM. Calculation of normalized drug concentrations in the presence of altered plasma protein binding. Clin Pharmacokinet 2012; 51: 55–68. [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roder BL, Frimodt‐Moller N, Espersen F, Rasmussen SN. Dicloxacillin and flucloxacillin: pharmacokinetics, protein binding and serum bactericidal titers in healthy subjects after oral administration. Infection 1995; 23: 107–112. [DOI] [PubMed] [Google Scholar]
- 9. Seedher N, Agarwal P. Interaction of some isoxazolyl penicillins with human serum albumin. Aust J Biol Sci 2006; 6: 167–172. [Google Scholar]
- 10. Zhang M, Moore G, Everts R, Begg E. Determination of total and free concentrations of Flucloxacillin and cefazolin in human plasma by liquid chromatography/tandem mass spectrometry. J Anal Bioanal Tech 2014; 5: 2. [Google Scholar]
- 11. Liang K‐Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73: 13–22. [Google Scholar]
- 12. US Food and Drug Administration . Guidance for industry: bioanalytical method validation. 2001.
- 13. The European committee on antimicrobial susceptibility testing. Breakpoint tables for interpretation of MICs and zone diameters, version 8.0. In, 2018.
- 14. Sutherland R, Croydon EA, Rolinson GN. Flucloxacillin, a new isoxazolyl penicillin, compared with oxacillin, cloxacillin, and dicloxacillin. Br Med J 1970; 4: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. Journal of Statistical Software 2006; 15: 1–11. [Google Scholar]
- 16. Sutter R, Ruegg S, Tschudin‐Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology 2015; 85: 1332–1341. [DOI] [PubMed] [Google Scholar]
- 17. Musteata FM. Monitoring free drug concentrations: challenges. Bioanalysis 2011; 3: 1753–1768. [DOI] [PubMed] [Google Scholar]
- 18. Schalkwijk S, Greupink R, Burger D. Free dug concentrations in pregnancy: bound to measure unbound? Br J Clin Pharmacol 2017; 83: 2595–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]


