Abstract
Aim
Angiotensin‐converting enzyme inhibitors (ACEIs) are widely prescribed for several cardiovascular indications. This study investigated patterns of ACEI use for various indications.
Methods
A descriptive, retrospective population‐based study was conducted using data from the UK Clinical Practice Research Datalink. Patients starting ACEIs (2007–2014) were selected and ACEI indications were retrieved from electronically recorded medical records. Stratified by indication, we distinguished between persistent and nonpersistent ACEI use, considering a 6‐month interval between two prescription periods as a maximum for persistent use. Five‐year persistence rates for various indications were calculated using the Kaplan–Meier method and compared in a log‐rank test. Nonpersistent users were subdivided into three groups: (i) stop; (ii) restart; and (iii) switch to an angiotensin II‐receptor blocker. Patients who received ACEIs for hypertension who switched to other classes of antihypertensive medications were further investigated.
Results
In total, 254 002 ACEI initiators were identified with hypertension (57.6%), myocardial infarction (MI; 4.2%), renal disease (RD; 3.7%), heart failure (HF; 1.5%), combinations of the above (17.2%) or none of the above (15.8%). Five‐year persistence rates ranged from 43.2% (RD) to 68.2% (MI; P < 0.0001). RD and HF patients used ACEIs for the shortest time (average 23.6 and 25.0 months, respectively). For the nonpersistent group, the percentage of switchers to angiotensin II‐receptor blockers ranged from 27.6% (RD) to 42.2% (MI) and the restarters ranged from 15.0% (HF) to 18.1% (group without indication).
Conclusions
Depending on the indication, there are various rates of ACEI nonpersistence. Patients with RD are most likely to discontinue treatment.
Keywords: ACE inhibitors, heart failure, hypertension, medication persistence, myocardial infarction, renal disease
What is Already Known about this Subject
Angiotensin‐converting enzyme inhibitors (ACEIs) are widely prescribed for several cardiovascular indications including hypertension, heart failure, myocardial infarction and renal failure.
Although ACEIs are usually prescribed as maintenance therapy, studies have shown a nonpersistence rate between 20 and 40%.
What this Study Adds
Using real‐world clinical practice data in a large UK population‐based study, we showed that the patterns of use for ACEIs vary among indications for initiation.
Patients who start ACEIs after a myocardial infarction are the most persistent users compared to those with hypertension and heart failure. Patients who start ACEIs for renal diseases are the least persistent group.
Introduction
http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1613 are one of the most frequently prescribed classes of medication. For instance, in 2013, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6339 (an ACEI) was the first antihypertensive medication with more than 24 million prescriptions dispensed in community pharmacies in the UK 1. ACEIs are commonly used to treat hypertension, heart failure (HF), myocardial infarction (MI) and renal disease (RD). It has been demonstrated that these drugs decrease cardiovascular disease morbidity and mortality, especially in patients with hypertension and HF 2, 3, 4. Studies on the use of all antihypertensive medications have consistently shown that ACEIs have the second lowest risk of discontinuation [lowest are http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=35] 5, 6, 7, 8, 9. Nonetheless, a substantial number of patients discontinue ACEI therapy, mainly because of adverse drug reactions (ADRs) 10. A US cohort study of >2200 outpatients who received ACEIs for the first time showed that 19% discontinued ACEIs due to ADRs (median follow‐up 336 days) 11. In a Dutch study on ACEI use based on a pharmacy drug‐dispensing database, Vegter et al. reported that approximately 24% of ACEI starters switched their therapy within the first 3 years, and 75% of this group switched to ARBs 12. In the UK, the percentage of ACEI switchers increased to >40% in a large population‐based cohort of newly diagnosed hypertensive patients including a subgroup of more than 36 000 ACEI starters with a maximum of 9 years of follow‐up 13.
No study has investigated whether persistence with ACEIs differs among indications. A prior UK study showed that patients with MI were less likely to stop β‐blocker therapy than patients with HF or angina pectoris 14. The aim of this study is to investigate whether the pattern of ACEI use differs by indication for persistence rate, stop, restart or switch to ARBs.
Methods
Setting
The data for this study were obtained from the Clinical Practice Research Datalink (CPRD), formerly known as General Practice Research Database, which contains computerized information from almost 700 UK primary care practices. At the time of this study, CPRD included clinical records of close to 12 million patients. Validity data and a detailed description of the CPRD have been described previously 15, 16.
The protocol for this study was reviewed and approved by the UK independent scientific advisory committee (ISAC), protocol number: 14_030R. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Study cohort
A descriptive, retrospective population‐based study was conducted with patients aged 45 years and older who initiated ACEI therapy between 1 January 2007 and 1 January 2014. To be eligible for the study, patient data had to include at least 12 months of valid prescription history before starting an ACEI and at least 6 months of valid prescription data after starting, so ACEI persistence could be evaluated. Assessment of validity was performed using general practitioner prescription data for any medication prescribed for the study participants.
Follow‐up
Subjects were followed through the study period, time of death or the date they moved outside the practice area. Subject mortality data are available through an established link between CPRD and the UK office for national statistics. The cohort entry date was the date of the patient's first ACEI prescription. To categorize patients according to the indication for ACEI initiation, we assessed whether they had a diagnosis (based on relevant Read codes in electronic medical records) of hypertension, HF, MI or RD prior to the cohort entry date or in the first year thereafter. Patients with more than one indication and patients for whom we could not retrieve any of the above indications within that period were classified in separate categories. The category of more than one indication was further subdivided based on the number and combination of indications (Supplementary Table S1). Both the average follow‐up time and duration of ACEI use were calculated for each indication.
Prescription patterns
According to the prescription data, starters with ACEIs were divided into two main categories.
Persistent group: Patients who started ACEIs and continued until the end of follow‐up. A maximum 6‐month time interval between two prescription periods was acceptable for this definition. This time interval has been shown to be a better indicator of ADRs in comparison to a 3‐month interval 10. Even if a patient is hospitalized (usually no longer than 1 month) or has a stock of the medication, they are expected to return for a refill of their prescription within this period.
-
Nonpersistent group: Patients who stopped receiving ACEI prescriptions for at least 6 months after the theoretical end date of their previous ACEI prescription. The discontinuation date was defined as the theoretical end date of the last ACEI prescription and calculated by dividing the quantity of prescribed medications by the number of daily doses. The nonpersistent group was further divided into three mutually exclusive subgroups according to the treatment pattern after ACEI discontinuation (Figure 1).
Stop group: Patients who stopped their ACEIs and never restarted by the end of the study period and also who had not started ARBs within 6 months after the theoretical end date of their last ACEI prescription.
Switch to ARBs group: Patients who stopped their ACEIs and started ARBs within 6 months after the theoretical end date of their last ACEI prescription. For patients with hypertension, a switch to another antihypertensive medication (β‐blocker, diuretic, calcium channel blocker or other antihypertensive such as α‐blocker, vasodilator or centrally acting antihypertensive) was also investigated.
Restart group: patients who stopped or switched their ACEIs according to the above definitions, but during the study follow‐up time, restarted ACEI therapy.
Figure 1.
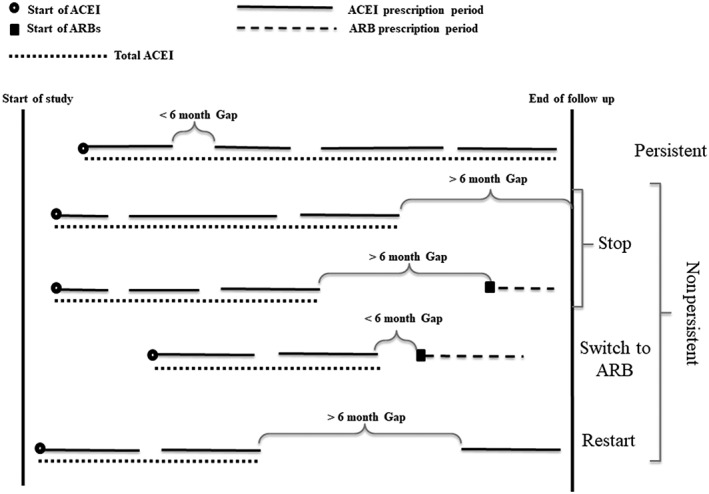
Definition of angiotensin‐converting enzyme inhibitor (ACEI) use patterns. ARB, angiotensin II‐receptor blocker
Statistical analyses
General characteristics for all ACEI starters were reported separately for each indication (hypertension, HF, MI, RD, more than one indication and none of the above). Five‐year persistence rates and the time to discontinuation among the various indications were calculated and compared using the Kaplan–Meier method and a log‐rank test, respectively. Patients who started with an ACEI and had a follow‐up time of <6 months were excluded, since these patients would have automatically been placed in the persistent group due to the definition of persistence. This result could have unrealistically increased the estimation of the proportion of persistent patients. To evaluate the influence of these exclusions, we performed a sensitivity analysis in which excluded patients were analysed once as persistent ACEI users and once as nonpersistent users. All statistical analyses were performed using SPSS 20 (IBM SPSS Statistics for Windows Version 20.0. Armonk, NY: IBM Corp).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 17, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 18, 19.
Results
There were 276 973 eligible patients who had started with an ACEI during the study period. A total of 22 971 patients (8.2%) were excluded from the main analyses due to a <6‐month follow‐up time. Table 1 presents the general characteristics of the remaining 254 002 patients (51.5% male) at the first date of ACEI prescription. Table 1 also includes the average follow‐up time, average duration of ACE inhibitor use and proportions of deceased patients during follow‐up; all were stratified by indication. The majority of participants had started with an ACEI because of hypertension (57.6%) and the smallest group for HF (1.5%). The patient group with more than one indication represented 17.2% of the sample, and 90.1% of these participants had hypertension as one indication. Patients who started an ACEI for HF and RD were approximately 9 years older than patients with an MI or hypertension.
Table 1.
General characteristics of included patients stratified by indications (n = 254 002 patients)
| Characteristics | Heart failure 1.5% | Hypertension 57.6% | Myocardial infarction 4.2% | Renal disease 3.7% | More than one indication 17.2% | None of the mentioned indications 15.8% | Total 100% |
|---|---|---|---|---|---|---|---|
| Mean age a (years) [SD], (SDM) | 72.1 [11.8] (−0.58) | 62.7 [11.0] (reference) | 64.1 [11.1] (−0.08) | 72.6 [11.0] (−0.63) | 73.4 [10.8] (−0.69) | 64.1 [11.5] (−0.08) | 65.3 [11.9] |
| Sex (% male) | 60.1% | 50.1% | 76.2% | 43.5% | 45.0% | 58.0% | 51.5% |
| Mean follow‐up a (months) [SD], (SDM) | 35.2 [20.9] (0.27) | 43.7 [22.2] (reference) | 39.2 [21.9] (0.14) | 41.8 [22.6] (0.05) | 43.7 [23.0] (0) | 39.1 [21.9] (0.14) | 42.6 [22.4] |
| Mean ACEI duration (months) [SD], (SDM) | 25.0 [21.5] (0.11) | 28.8 [25.1] (reference) | 30.5 [23.3] (−0.04) | 23.6 [23.3] (0.15) | 28.1 [25.1] (0.01) | 24.9 [23.2] (0.11) | 27.9 [24.7] |
| Death | 21.5% | 4.2% | 7.1% | 13.8% | 15.4% | 7.4% | 7.3% |
Recorded at the first angiotensin‐converting enzyme inhibitor (ACEI) prescription date
Minimum requirement of a 6‐month follow‐up after angiotensin‐converting enzyme inhibitor initiation
SD: standard deviation, SDM: standardized difference between means as compared to the hypertension group as the most common indication
The highest percentages of death were for patients with HF (21.5%) or more than one indication (15.4%). The mean duration of ACEI use was longest for those who had had an MI (30.5 months) and shortest for those with HF (25.0 months) or RD (23.6 months; Table 1).
Table 2 shows the patterns of ACEI use by indication. In the total study population, 60.3% of ACEI starters continued till the end of study follow‐up. For the 100 790 nonpersistent patients, 45.3% stopped their ACEI (did not switch to ARBs within 6 months and never restarted ACEIs), 37.1% switched to ARBs and 17.6% restarted their ACEIs after at least 6 months of discontinuation.
Table 2.
Patterns of angiotensin‐converting enzyme inhibitor use stratified by indication
| Indication (patients) | Pattern (n) % | |
|---|---|---|
| Heart failure ( n = 3762) | Persistent (2507) 66.6% | |
| Nonpersistent (1255) 33.4% | Stop (561) 44.7% | |
| Switch to ARB (506) 40.3% | ||
| Restart (188) 15.0% | ||
| Hypertension ( n = 146 275) | Persistent (88 632) 60.6% | |
| Nonpersistent (57 643) 39.4% | Stop a (24 206) 42.0% | |
| Switch to ARB (23 271) 40.4% | ||
| Restart (10 166) 17.6% | ||
| Myocardial infarction ( n = 10 639) | Persistent (7826) 73.6% | |
| Nonpersistent (2813) 26.4% | Stop (1200) 42.7% | |
| Switch to ARB (1187) 42.2% | ||
| Restart (426) 15.1% | ||
| Renal disease ( n = 9299) | Persistent (4727) 50.8% | |
| Nonpersistent (4572) 49.2% | Stop (2493) 54.5% | |
| Switch to ARB (1262) 27.6% | ||
| Restart (817) 17.9% | ||
| More than one indication ( n = 43 753) | Persistent (25 555) 58.4% | |
| Nonpersistent (18 198) 41.6% | Stop b (8399) 46.2% | |
| Switch to ARB (6650) 36.5% | ||
| Restart (3149) 17.3% | ||
| None of the mentioned indications ( n = 40 274) | Persistent (23 965) 59.5% | |
| Nonpersistent (16 309) 40.5% | Stop (8817) 54.1% | |
| Switch to ARB (4545) 27.9% | ||
| Restart (2947) 18.1% | ||
| Total ( n = 254 002) | Persistent (153 212) 60.3% | |
| Nonpersistent (100 790) 39.7% | Stop (45 676) 45.3% | |
| Switch to ARB (37 421) 37.1% | ||
| Restart (17 693) 17.6% | ||
Switched to calcium channel blockers (17.2%), diuretics (6.3%), combination of antihypertensives (5.0%), β‐blockers (3.6%) and other antihypertensives (0.1%).
Two subgroups: a) more than one indication including hypertension (90.5%) and b) not including hypertension (9.5%). Group A percent switched to calcium channel blockers (10.0%), diuretics (6.3%), combination of antihypertensives (3.3%), β‐blockers (3.2%) and other antihypertensives (0.3%).
ARB, angiotensin II‐receptor blocker
Patients who started an ACEI for MI had the highest probability of remaining on their initial ACEI treatment (73.6%). Patients who started an ACEI for RD were most likely to discontinue (49.2%). More than half (54.5%) of the nonpersistent patients with RD actually stopped and did not restart ACEIs or switch to ARBs. This was the highest percentage for this behaviour among all indications.
Study participants who switched from ACEIs to ARBs ranged from 27.6% (RD) to 42.2% (MI). Out of 24 206 patients with hypertension, who stopped their ACEI and did not restart or switch to ARBs, 17.2% switched to calcium channel blockers, which was the highest percentage, followed by a switch to diuretics (6.3%), a combination of antihypertensives (5.0%) or β‐blockers (3.6%). The same pattern was observed for patients with hypertension combined with other indications (10.0% switched to calcium channel blockers, 6.3% to diuretics, 3.3% to a combination of antihypertensives and 3.2% to β‐blockers).
Kaplan–Meier curves of ACEI use for various indications are presented in Figure 2. Five‐year persistence rates for indications included in this study were 68.2% (MI), 58.6% (HF), 56.4% (hypertension), 53.4% (no mentioned indication), 53.0% (more than one indication) and 43.2% (RD; log‐rank P < 0.0001).
Figure 2.
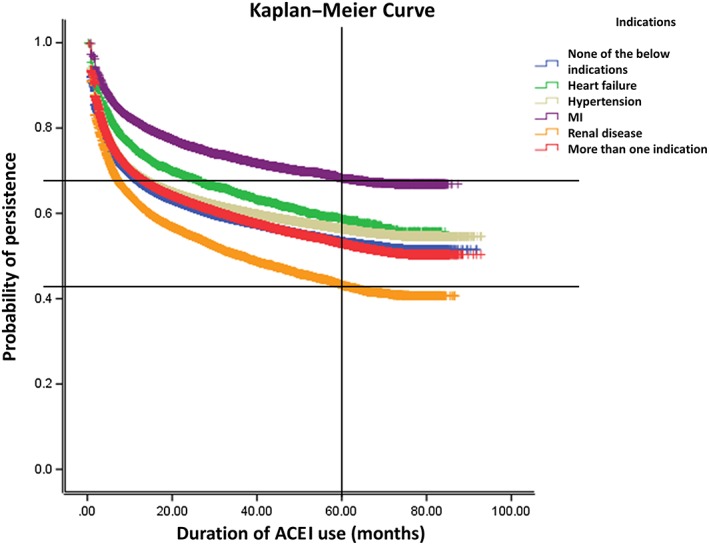
Comparison of nonpersistence rates of angiotensin‐converting enzyme inhibitor (ACEI) use for various indications
Sensitivity analyses, including the 22 971 patients with <6 months of follow‐up, changed the crude percentages for the nonpersistent patients for all indications. For example, in the MI group, the percentage of nonpersistent patients changed from 26.4% to 34.4% (excluded patients were included as nonpersistent patients) and to 23.6% (excluded patients were included as persistent patients). Detailed results of sensitivity analyses are presented in Supplementary Table S2.
Discussion
This study demonstrated that patterns of ACEI use and persistence differ among indications. Patients with RD discontinued their ACEI therapy more frequently and used ACEIs for a shorter time period than those with other indications. Five‐year nonpersistence rates ranged between 31.8% (MI) and 56.8% (RD).
Hypertension, RD and HF are three main indications of ACEIs previously studied for drug utilization patterns. Although ACEIs are well tolerated compared to other antihypertensive medications, the problem of poor persistence still exists for patients with hypertension. For example, a 1‐year discontinuation rate for http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6360 (ACEI) consumers in the USA and Australia with hypertension was reported to be >30% 20, 21.
Several sociodemographic factors have been shown to be associated with nonpersistence to antihypertensive therapy (e.g. sex, comedications, comorbidities and even demographic characteristics of patient geographic location) 22, 23, which can eventually result in poor clinical outcomes 24. A Dutch study showed that the putative ACEI‐related cough can affect patient compliance (20% higher compliance for patients without a putative cough); however, the precise cause of ACEI discontinuation could not be retrieved directly 25. In the early 2000s, two studies using the same population (Régie de l'assurance maladie du Québec administrative database) showed that among patients with hypertension (and specifically ACEI users), those patients who had many risk factors for cardiovascular events were more persistent with their drug therapy than patients with fewer risk factors 26, 27. Patients who start ACEIs for RD are more susceptible to adverse effects such as renal function deterioration or hyperkalaemia (in addition to the common side effect of coughing) because of a combination of drug action and disease complications. Therefore, it is not uncommon to recommend that patients with RD discontinue (permanently or temporarily) their ACEIs 28. It has also been shown that older age in patients with hypertension is associated with a higher risk of nonpersistence to ACEIs 29. In our study, the mean age of patients with RD was higher than patients with other conditions, which could potentially have influenced the higher nonpersistence rate in this group.
ACEIs are one of the main medications used in HF management and large population‐based studies have demonstrated that drug adherence is significantly associated with increased survival time in these patients 30. Recently, it has been shown in the USA that medication adherence for patients with HF decreases during the first few months after hospitalization 31. A 2015 French study showed that the pharmacological management of HF in elderly patients is not optimal 32; however, a 2015 systematic review of 17 studies (162 727 patients) found that older age alone is not related to the poor medical management in patients with HF 33. In our study, patients with HF were the third oldest group and had the highest mortality rate. This might explain the average time‐limited use of ACEI in this group.
Our study demonstrates that patients who start ACEIs for RD and HF have a higher probability of stopping and should have improved follow‐up and monitoring by health care providers to achieve the full benefit of ACEIs. We suggest either pharmacists or physicians contact patients who have discontinued relevant medication (specifically ACEIs) without clear justification, to improve persistence and thus, patient outcomes 34, 35.
More studies are needed to address the issue of whether or not ACEI discontinuation is inevitable or can be managed by a dose adjustment, addition of a new class of medication or other interventions.
The main strength of this population‐based study was the large number of patients who can be considered as representative of all ACEI starters in the UK. Valid data for at least 18 months (12 months before and 6 months after the first ACEI prescription) was an acceptable follow‐up time to identify new users and define usage and persistence patterns.
One of the limitations of this study was that the indications for ACEI use were based on electronic medical records registered by general practitioners. These diagnoses were not validated, so misclassifications cannot be ruled out. That said, a recent study compared CPRD codes for renal replacement therapy and decreased kidney function with external data sources in the UK (Health Survey for England and UK Renal Registry). The authors found an acceptable validity when comparing the prevalence of the abovementioned kidney diseases in the CPRD with external data sources 36. Another recent study could not find significant prognostic differences between patients with HF who were recorded in CPRD primary care data alone and those who were recorded both in hospital admission and primary care data 37. Unequal follow‐up time for all patients could potentially be another limitation. However, we tried to decrease the variation between patient follow‐up times by excluding patients with very short follow‐up times (<6 months of follow‐up after ACEI initiation).
In conclusion, this UK study demonstrated that for all patients with various indications for ACEIs a relatively high percentage will stop or switch their therapy, and the highest proportion of stoppers are patients with RD. The main cause of nonpersistence to ACEIs within these patient groups needs to be further investigated.
Competing Interests
There are no competing interests to declare.
This research was conducted as part of the PREDICTION‐ADR consortium (Personalisation of treatment In Cardiovascular disease through next generation sequencing in Adverse Drug Reactions); this is the Seventh Framework Programme of the European Union ‐Grant no. 602108. F.W.A. is supported by the UCL Hospitals NIHR Biomedical Research Centre. The authors would like to thank the principal investigators of PREDICTION‐ADR consortium for their contribution and support, particularly Colin N.A. Palmer (University of Dundee, Dundee, UK), Mia Wadelius (Uppsala University, Uppsala, Sweden), Alun McCarthy, (Pharmacogenomic Innovative Solutions Ltd, UK).
Contributors
S.H.M. performed the analysis and wrote the manuscript; P.C.S. managed the data; S.H.M., P.C.S., F.W.A., A.d.B. and A.H.M interpreted the results; F.W.A., A.d.B. and A.H.M. designed the research and critically revised the manuscript. S.H.M., P.C.S., F.W.A., A.d.B. and A.H.M have given final approval of the version to be published.
Supporting information
Table S1 Detailed retrieved diagnoses for patients who had more than one indication, the percentage of deceased patients in each category and patterns of angiotensin‐converting enzyme inhibitor use (n = 43 753 patients).
Table S2 Detailed results from the sensitivity analyses including the 22 971 patients with <6 months of follow‐up.
Mahmoudpour, S. H. , Asselbergs, F. W. , Souverein, P. C. , de Boer, A. , and Maitland‐van der Zee, A. H. (2018) Prescription patterns of angiotensin‐converting enzyme inhibitors for various indications: A UK population‐based study. Br J Clin Pharmacol, 84: 2365–2372. 10.1111/bcp.13692.
References
- 1. Croft K. Prescriptions dispensed in the community England 2003–13. Prescribing and Prim Care, Health and Social Care Information Centre; 9 July 2014.110 p. Available at http://www.hscic.gov.uk (last accessed Jan 2015).
- 2. Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al Effect of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on all‐cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta‐analysis. JAMA Intern Med 2014; 174: 773–785. [DOI] [PubMed] [Google Scholar]
- 3. Ferrari R, Boersma E. The impact of ACE inhibition on all‐cause and cardiovascular mortality in contemporary hypertension trials: a review. Expert Rev Cardiovasc Ther 2013; 11: 705–717. [DOI] [PubMed] [Google Scholar]
- 4. Khalil ME, Basher AW, Brown EJ Jr, Alhaddad IA. A remarkable medical story: benefits of angiotensin‐converting enzyme inhibitors in cardiac patients. J Am Coll Cardiol 2001; 37: 1757–1764. [DOI] [PubMed] [Google Scholar]
- 5. Bourgault C, Senecal M, Brisson M, Marentette MA, Gregoire JP. Persistence and discontinuation patterns of antihypertensive therapy among newly treated patients: a population‐based study. J Hum Hypertens 2005; 19: 607–613. [DOI] [PubMed] [Google Scholar]
- 6. Corrao G, Zambon A, Parodi A, Poluzzi E, Baldi I, Merlino L, et al Discontinuation of and changes in drug therapy for hypertension among newly‐treated patients: a population‐based study in Italy. J Hypertens 2008; 26: 819–824. [DOI] [PubMed] [Google Scholar]
- 7. Degli Esposti E, Sturani A, Di Martino M, Falasca P, Novi MV, Baio G, et al Long‐term persistence with antihypertensive drugs in new patients. J Hum Hypertens 2002; 16: 439–444. [DOI] [PubMed] [Google Scholar]
- 8. Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10‐year persistence with antihypertensive drugs. J Hypertens 2005; 23: 2101–2107. [DOI] [PubMed] [Google Scholar]
- 9. Mazzaglia G, Mantovani LG, Sturkenboom MC, Filippi A, Trifiro G, Cricelli C, et al Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens 2005; 23: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 10. Mahmoudpour SH, Asselbergs FW, de Keyser CE, Souverein PC, Hofman A, Stricker BH, et al Change in prescription pattern as a potential marker for adverse drug reactions of angiotensin converting enzyme inhibitors. Int J Clin Pharmacol 2015; 37: 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, et al An evaluation of risk factors for adverse drug events associated with angiotensin‐converting enzyme inhibitors. J Eval Clin Pract 2004; 10: 499–509. [DOI] [PubMed] [Google Scholar]
- 12. Vegter S, Nguyen NH, Visser ST, de Jong‐van den Berg LT, Postma MJ, Boersma C. Compliance, persistence, and switching patterns for ACE inhibitors and ARBs. Am J Manag Care 2011; 17: 609–616. [PubMed] [Google Scholar]
- 13. Burke TA, Sturkenboom MC, Lu SE, Wentworth CE, Lin Y, Rhoads GG. Discontinuation of antihypertensive drugs among newly diagnosed hypertensive patients in UK general practice. J Hypertens 2006; 24: 1193–1200. [DOI] [PubMed] [Google Scholar]
- 14. Kalra PR, Morley C, Barnes S, Menown I, Kassianos G, Padmanabhan S, et al Discontinuation of beta‐blockers in cardiovascular disease: UK primary care cohort study. Int J Cardiol 2013; 167: 2695–2699. [DOI] [PubMed] [Google Scholar]
- 15. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matcho A, Ryan P, Fife D, Reich C. Fidelity assessment of a clinical practice research datalink conversion to the OMOP common data model. Drug Saf 2014; 37: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simons LA, Ortiz M, Calcino G. Persistence with antihypertensive medication: Australia‐wide experience, 2004–2006. Med J Aust 2008; 188: 224–227. [DOI] [PubMed] [Google Scholar]
- 21. Elliott WJ, Plauschinat CA, Skrepnek GH, Gause D. Persistence, adherence, and risk of discontinuation associated with commonly prescribed antihypertensive drug monotherapies. J Am Board Fam Med 2007; 20: 72–80. [DOI] [PubMed] [Google Scholar]
- 22. Mancia G, Zambon A, Soranna D, Merlino L, Corrao G. Factors involved in the discontinuation of antihypertensive drug therapy: an analysis from real life data. J Hypertens 2014; 32: 1708–1715 discussion 16. [DOI] [PubMed] [Google Scholar]
- 23. Mahmoudpour SH, Baranova EV, Souverein PC, Asselbergs FW, de Boer A, Maitland‐van der Zee AH, et al Determinants of angiotensin‐converting enzyme inhibitor (ACEI) intolerance and angioedema in the UK clinical practice research datalink. Br J Clin Pharmacol 2016; 82: 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, et al Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens 2011; 29: 610–618. [DOI] [PubMed] [Google Scholar]
- 25. Vegter S, de Boer P, van Dijk KW, Visser S, de Jong‐van den Berg LT. The effects of antitussive treatment of ACE inhibitor‐induced cough on therapy compliance: a prescription sequence symmetry analysis. Drug Saf 2013; 36: 435–439. [DOI] [PubMed] [Google Scholar]
- 26. Gogovor A, Dragomir A, Savoie M, Perreault S. Comparison of persistence rates with angiotensin‐converting enzyme inhibitors used in secondary and primary prevention of cardiovascular disease. Value Health 2007; 10: 431–441. [DOI] [PubMed] [Google Scholar]
- 27. Perreault S, Lamarre D, Blais L, Dragomir A, Berbiche D, Lalonde L, et al Persistence with treatment in newly treated middle‐aged patients with essential hypertension. Ann Pharmacother 2005; 39: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 28. Sidorenkov G, Navis G. Safety of ACE inhibitor therapies in patients with chronic kidney disease. Expert Opin Drug Saf 2014; 13: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 29. Wong MC, Lau RK, Jiang JY, Griffiths SM. Discontinuation of angiotensin‐converting enzyme inhibitors: a cohort study. J Clin Pharm Ther 2012; 37: 335–341. [DOI] [PubMed] [Google Scholar]
- 30. Marzluf BA, Reichardt B, Neuhofer LM, Kogler B, Wolzt M. Influence of drug adherence and medical care on heart failure outcome in the primary care setting in Austria. Pharmacoepidemiol Drug Saf 2015; 24: 722–730. [DOI] [PubMed] [Google Scholar]
- 31. Sueta CA, Rodgers JE, Chang PP, Zhou L, Thudium EM, Kucharska‐Newton AM, et al Medication adherence based on part D claims for patients with heart failure after hospitalization (from the atherosclerosis risk in communities study). Am J Cardiol 2015; 116: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vorilhon C, Chenaf C, Mulliez A, Pereira B, Clerfond G, Authier N, et al Heart failure prognosis and management in over‐80‐year‐old patients: data from a French national observational retrospective cohort. Eur J Clin Pharmacol 2015; 71: 251–260. [DOI] [PubMed] [Google Scholar]
- 33. Krueger K, Botermann L, Schorr SG, Griese‐Mammen N, Laufs U, Schulz M. Age‐related medication adherence in patients with chronic heart failure: a systematic literature review. Int J Cardiol 2015; 184: 728–735. [DOI] [PubMed] [Google Scholar]
- 34. Brookhart MA, Patrick AR, Schneeweiss S, Avorn J, Dormuth C, Shrank W, et al Physician follow‐up and provider continuity are associated with long‐term medication adherence: a study of the dynamics of statin use. Arch Intern Med 2007; 167: 847–852. [DOI] [PubMed] [Google Scholar]
- 35. Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Effectiveness of interventions by community pharmacists to improve patient adherence to chronic medication: a systematic review. Ann Pharmacother 2005; 39: 319–328. [DOI] [PubMed] [Google Scholar]
- 36. Iwagami M, Tomlinson LA, Mansfield KE, Casula A, Caskey FJ, Aitken G, et al Validity of estimated prevalence of decreased kidney function and renal replacement therapy from primary care electronic health records compared with national survey and registry data in the United Kingdom. Nephrol Dial Transplant 2017; 32 (suppl_2): ii142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koudstaal S, Pujades‐Rodriguez M, Denaxas S, Gho JMIH, Shah AD, Yu N, et al Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population‐based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail 2017; 19: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Detailed retrieved diagnoses for patients who had more than one indication, the percentage of deceased patients in each category and patterns of angiotensin‐converting enzyme inhibitor use (n = 43 753 patients).
Table S2 Detailed results from the sensitivity analyses including the 22 971 patients with <6 months of follow‐up.


