Abstract
Aims
Recreational use of novel psychoactive substance (NPS) has become increasingly common. We aimed to assess the association of national legislation and local trading standards activity with hospital presentations.
Methods
We established observational cohorts of patients with recreational drug toxicity presenting to Edinburgh Royal Infirmary and dying with detectable recreational drugs in Edinburgh. We assessed associations with two temporary class drug‐orders (April 2015: methylphenidates, Nov 2015: methiopropamine), the Psychoactive Substances Act (June 2016), and trading standards forfeiture orders (October 2015).
Results
The methylphenidate temporary class drug‐order was associated with rapid 46.7% (P = 0.002) and 21.0% (P = 0.003) reductions in presentations and admissions, respectively, for NPS drug toxicity, comparing 12 months before with 6 months after. The change was greatest for ethylphenidate toxicity (96.7% reduction in admissions, P < 0.001) that was partly offset by a tripling in synthetic cannabinoid receptor agonist cases (P < 0.001) over the next 6 months. This increase reversed following trading standards activity removing all NPS drugs from local shops in October 2015, associated with 64.3% (P < 0.001) and 83.7% (P < 0.001) reductions in presentations and admissions, respectively, for all NPS drugs over the next 12 months. The effect was sustained and associated with a reduced postmortem detection of stimulant NPS drugs. The two interventions prevented an estimated 557 (95% confidence interval 327–934) NPS admissions during 2016, saving an estimated £303 030 (£177 901–508 133) in hospital costs.
Conclusions
We show here that drug legislation and trading standards activity may be associated with effective and sustained prevention. Widespread adoption of trading standards enforcement, together with focused legislation, may turn the tide against these highly‐damaging drugs.
Keywords: clinical epidemiology < epidemiology, clinical toxicology < toxicology, forensic toxicology < toxicology
What is Already Known about this Subject
The use of novel psychoactive substances (NPS, legal highs) as recreational drugs is a major source of drug harm in the UK.
The government has used temporary control orders and more recently the Psychoactive Substances Act to control their use and the harms; local councils have used trading standard actions to prevent shops selling NPS.
There is currently little evidence on the effect of these interventions on NPS induced harm and switching to other drugs
What this Study Adds
This study shows that the ethylphenidate temporary control order was associated with an almost complete disappearance of admissions with reported ethylphenidate toxicity
Subsequent trading standards activity, removing all NPS from local shops, was associated with a sustained >80% reduction in NPS admissions to hospital over the next year.
Such a combined approach, spread across the UK, could offer major health benefits and save the health service millions of pounds per year.
Introduction
The use of novel psychoactive substances (NPS), sometimes previously referred to as legal highs, is a recent phenomenon in the UK and worldwide 1, 2, 3. NPS drugs are commonly stimulants (based on an http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4804 structure) 4, 5, highly potent synthetic cannabinoid receptor agonists (SCRA) 5, 6, or novel high potency benzodiazepines such as diclazepam or etizolam 7, 8. They are bought online or from high street head shops, sometimes sold as research chemicals labelled not for human consumption 2, 3, or as common illicit drugs. Serious complications and deaths can occur 9. Stimulants can cause agitation, seizures, hyperthermia and, rarely, multiorgan failure 4, 10, while SCRAs can cause tachycardia, agitation, psychosis, and, rarely, stroke, seizures, myocardial infarction or rhabdomyolysis 6, 10, and novel benzodiazepines cause coma and respiratory arrest at low doses 8.
Most NPS were not controlled by the Misuse of Drugs Act (1971). Instead, piecemeal and temporary actions [reclassifications and temporary class drug orders (TCDOs)] were enacted to control specific substances or classes 11. Such actions, both in the UK and overseas, have been associated with reductions in use 9, 12. However, reported studies have been of poor design and the overall evidence for effect from interventions is weak 12. Furthermore, NPS producers have usually stayed ahead of these actions by making minor revisions of chemical structure, creating new uncontrolled compounds 9, 13, 14. In response, the UK government enacted legislation in May 2016 that specifically addressed this problem by controlling all psychoactive substances, regardless of chemical structure 15. The effectiveness of this intervention is uncertain.
Edinburgh has been affected by multiple waves of NPS toxicity over the last 8 years, from mephedrone in 2009 16, to desoxypipradrol in 2010 17, to ethylphenidate in 2014. Ethylphenidate was a particular problem, causing a marked increase in deaths, hospital admissions, and complications (including severe agitation, psychosis, abscesses, skin ulcers and endocarditis), often in people who previously used heroin 18, 19, 20. To investigate the impact of legislative and local trading standards actions on NPS associated harms, we report on the number of patients presenting and being admitted to the specialist toxicology ward in Edinburgh.
Methods
Anonymous data for audit of management of recreational drug toxicity are routinely and prospectively collected on a secure database held within the clinical toxicology unit of the Royal Infirmary of Edinburgh. Data on patients presenting to the Emergency Departments and discharged, without admission to the toxicology units, were also recorded. The use of these anonymous unlinked databases for audit has approval of the data protection officer/Caldicott Guardian of NHS Lothian Health Board. Toxicology data on fatalities is generated and stored by the Department of Forensic Medicine and Science, University of Glasgow, on behalf of the Crown Office and Procurator Fiscal Service. Interrogation of this data was approved by the West of Scotland NHS REC (17/WS/0037).
Patient involvement
Patients were not involved in formulating the research question or in designing and conducting the research.
Hospital presentations and admissions
Consecutive patients presenting to the emergency department of the Royal Infirmary of Edinburgh with a history of recreational drug toxicity over 33 months, from 1 April 2014 until 31 December 2016, were included in this study. Patients who took recreational drugs for self‐harm were excluded. Patients admitted to the toxicology ward or other hospital wards, including the intensive care unit, were seen by the toxicology nursing and medical staff and their data prospectively recorded. Patients discharged from the emergency department, without admission, were identified by retrospective review of medical notes. Data were entered into a standard database.
For admitted patients, the drugs were identified by toxicology staff, based on careful questioning about history as well as the toxidrome produced. They were grouped into the following categories: NPS, conventional opioids, conventional stimulants (including amphetamine, methamphetamine, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4574 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2286), non‐NPS benzodiazepines, and other recreational drugs (including ketamine, γ‐hydroxybutyric acid, γ‐butyrolactone, cannabis, gabapentin and pregabalin). NPS drugs were subdivided into reported ethylphenidate, other stimulant (not identified as ethylphenidate), SCRA, sedative (probably benzodiazepines), hallucinogenic, or unknown groups, based on history and local epidemiology (from both drug seizures and laboratory analysis) 10. Urine toxicology screens were not routinely done. For patients discharged without admission, the drug was identified through retrospective review of the notes.
Fatal cases
All sudden, unexplained or suspicious deaths in Scotland are investigated by the Crown Office and Procurator Fiscal Service. Where appropriate, this includes toxicological analysis of blood and urine samples for a broad spectrum of drugs including NPS, carried out by the department of Forensic Medicine and Science at the University of Glasgow. All cases were tested for alcohol and underwent an enzyme‐linked immunosorbent assay‐based classic drugs of abuse screen and gas chromatography–mass spectrometry (with a mixed‐mode solid phase extraction) analysis for: (i) drugs of abuse initially identified by enzyme‐linked immunosorbent assay; (ii) 10 benzodiazepines (including etizolam and phenazepam); (iii) paracetamol; and (iv) a drugs screen including around 61 known compounds, both prescribed and illicit. Unknowns picked up by gas chromatography–mass spectrometry were compared with a library of compounds for identification. Where there was prior intelligence [for example if specific drugs were found at the scene or common local use of a particular NPS was identified (e.g. ethylphenidate 19)], further focused testing was included. Due to the wide variation of chemical characteristics of drugs and their changing availability it was not possible to test every case for every drug; however, each case was reviewed individually to ensure all relevant tests were included.
Anonymized data were collected for all deaths with detectable recreational drugs in the Edinburgh fiscal area (covering the City of Edinburgh and its suburbs). Many cases showed polydrug use.
Interventions
Five public health measures were implemented during the study period to curb the availability of NPS. On 10 April 2015, five http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7236‐based NPS drugs including ethylphenidate were controlled by a TCDO; two additional methylphenidate‐based NPS were added to the TCDO in June 2015 21. Methiopropamine was controlled by TCDO on 27 November 2015. The Psychoactive Substances Act (2016) came into effect on 26 May 2016, prohibiting the supply of any substance intended for human consumption capable of producing a psychoactive effect.
On 16 October 2015, trading standards officials obtained forfeiture orders for NPS from the Sheriff Court in Edinburgh, with the backing of Police Scotland 22. This resulted in eight head shops forfeiting their NPS supplies to the council.
Statistical analysis
We hypothesized before the methylphenidate TCDO that it might be associated with a change in the presentation of NPS patients to hospital; however, we used the null hypothesis for our formal statistical testing. The initial analysis was performed using Graphpad Prism v7; categorical variables were compared using χ2 tests, continuous data with a two‐tailed Mann–Whitney test. However, this analysis could not account for underlying secular changes occurring before the interventions. There was statistical evidence of over‐dispersion in the Poisson regression models, and therefore we used negative binomial regression in Stat 14 (StataCorp, College Station, TX, USA) to compare rates after the legislative (April 2015) and trading standard (October 2015) changes with those predicted based on pre‐ban trends. We calculated rate ratios (and the change in the number of admissions) for each month compared to predicted rates based on extrapolated trends before the legislation (May 2014 to March 2015). Negative binomial regression models included a single trend term for calendar month and a dummy variable for each of the postlegislative months (21 dummy variables: April 2015 to December 2016). We could not estimate the effect on presentations because the highly variable numbers during the 12 months preceding the TCDO made it difficult to establish a baseline for the analysis.
Cost estimation
We estimated cost benefits from the reduced admissions from an NHS perspective using NHS financial year 2015–16 reference costs (HRG4) (https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016). For the cost of admissions, supplemental to presentations, we used the PA50Z [Ingestion poisoning] in‐patient episode cost (£544.04).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries, where available, in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 23, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.
Results
Over the 33 months of this study, there were 4513 presentations {median 137 [interquartile range (IQR) 112–158] per month} and 1912 admissions [median 61 (IQR 41–74) per month] to the Royal Infirmary of Edinburgh for recreational drug use. Of these, 935 [20.7%, median 26 (IQR 9–48) per month) presentations and 605 (31.6%, median 19 (IQR 4–31) per month] admissions were categorized as due to alleged NPS misuse.
Effect on NPS hospital presentations and admissions
The methylphenidate‐based NPS TCDO (April 2015) was associated with a rapid fall in presentations (reduction of 46.7%, P = 0.002) and admissions (reduction of 21.0%, P = 0.003) for all NPS toxicity, comparing the 12 months before with the 6 months after the action (up to the trading standards action; Figures 1 and 2; Table 1). The median (IQR) number of ethylphenidate NPS admissions fell from 15 (13–18) per month before the TCDO to 1 (0–2) per month after, a reduction of 96.7% (P < 0.001), while median monthly number of SCRA admissions tripled from 3 (2–5) before the TCDO to 10 (8–13) afterwards (P < 0.001). There was no apparent replacement with conventional drugs (Figure 1).
Figure 1.
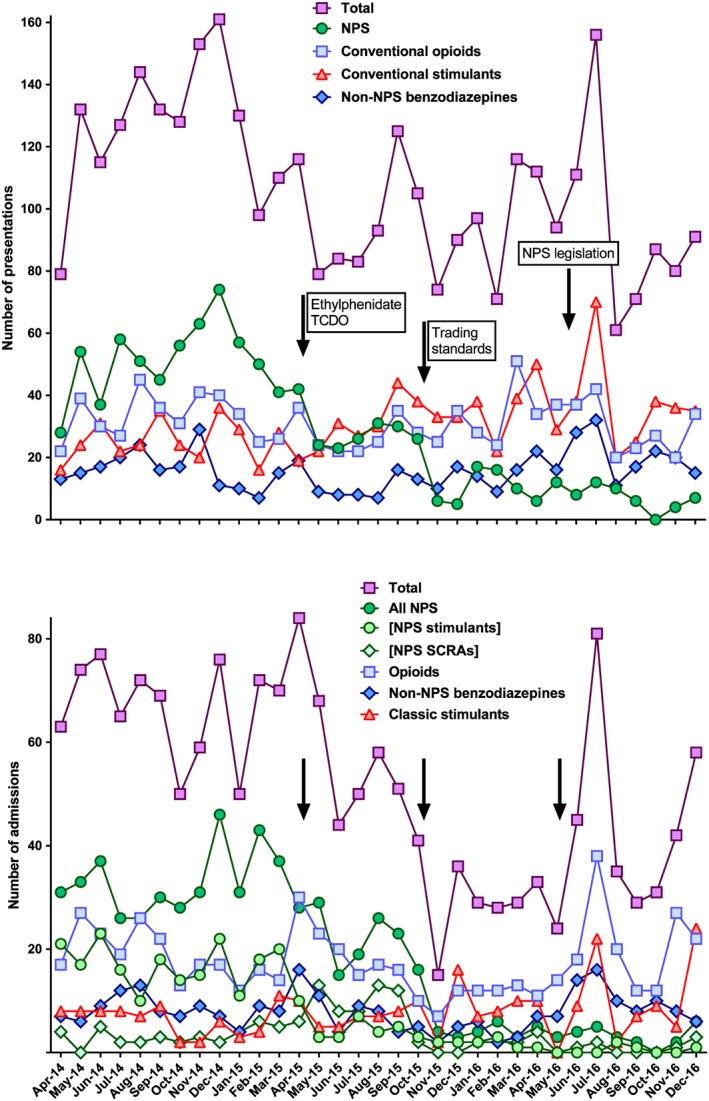
Numbers of presentations (top) and admissions (bottom) with recreational drug toxicity to the Royal Infirmary of Edinburgh between April 2014 and December 2016, related to recreational drug legislation and trading standard enforcement. All novel psychoactive substances (NPS) are grouped together in the presentations section (top). In the admissions section, where the NPS used was subclassified by clinical examination and careful history taking, NPS are further subdivided into NPS stimulants (such as ethylphenidate) and NPS synthetic cannabinoid receptor agonists. The number of all NPS admissions fell sharply in April 2015 after the ethylphenidate temporary class drug order and again in October 2015 after the trading standards action. Review of the admissions data shows that the initial fall was due to an almost complete reduction in stimulant NPS admissions. The stimulant NPS were replaced by NPS synthetic cannabinoid receptor agonist admissions for 6 months before these too were markedly reduced after October 2015.
Figure 2.
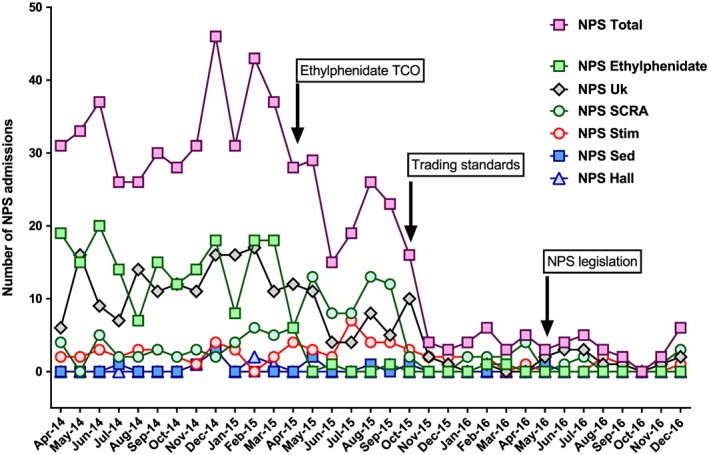
Numbers of admissions with NPS toxicity to the Royal Infirmary of Edinburgh between April 2014 and December 2016.In this figure, the NPS admissions are broken down into further subsections showing the initial high number of NPS ethylphenidate and NPS unknown (probably ethylphenidate) admissions. NPS total: all admissions with NPS toxicity; NPS E: ethylphenidate; NPS Uk: unknown NPS drug; NPS SC: synthetic cannabinoid receptor agonist; NPS Stim: stimulant NPS (not ethylphenidate); NPS Sed: sedative NPS; NPS Hall: hallucinogenic NPS.
Table 1.
Changes in median (interquartile range) monthly admissions and presentations for novel psychoactive substance drug toxicity, related to interventions
| NPS | Pre | Post | P value | |
|---|---|---|---|---|
| TCDO April 2015 | April 2014 to March 2015 | April 2015 to September 2015 | ||
| Presentations | 52.5 (42–58; n = 12) | 28 (24–34; n = 6) | 0.002 | |
| Admissions | 31 (29–37; n = 12) | 24.5 (18–28; n = 6) | 0.003 | |
| Trading standards October 2015 | April 2015 to September 2015 | October 2015 to September 2016 | ||
| Presentations | 28 (24–34; n = 6) | 10 (6–15; n = 12) | <0.001 | |
| Admissions | 24.5 (18–28; n = 6) | 4 (3–5; n = 12) | <0.001 | |
| PSA May 2016 | Nov 2015 to April 2016 | May 2016 to December 2016 | ||
| Presentations | 8 (6–16; n = 6) | 7.5 (5–12; n = 8) | 0.591 | |
| Admissions | 4 (3–5; n = 6) | 3 (2–5; n = 8) | 0.257 |
PSA, Psychoactive Substances Act; TCDO, temporary class drug order
The trading standards activity (October 2015) was associated with a further rapid reduction in all NPS presentations and admissions. Comparing the 6 months before the activity to the subsequent 12 months, the median monthly number of NPS presentations and admissions fell from 28.0 and 24.5 to 10.0 and 4.0 per month, falls of 64.3% (P < 0.001) and 83.7% (P < 0.001), respectively (Table 1). The fall in presentations was sustained with medians of only 6.5 (IQR 3–11) and 2.5 (2–5) alleged NPS presentations and admissions per month, respectively, during the last 6 months of 2016 (Figure 2).
Methiopropamine was not recognized to be a clinical problem in Edinburgh. Therefore, the TCDO (November 2015) was not associated with any apparent effect on NPS admissions (Figure 1).
The Psychoactive Substances Act (May 2016) was associated with non‐significant reductions in presentations and admissions for NPS toxicity: comparing the 6 months before with the 8 months after (until the end of 2016) revealed reductions of 6.3% (P = 0.591) in presentations and 25.0% (P = 0.257) in admissions (Figures 1 and 2).
Effect on hospital presentations and admissions for conventional recreational drugs
It was difficult to detect any associated change in presentations for conventional recreational drug use immediately after each intervention, probably due to their rapid sequence. However, comparing the first 12 months of the study period (before the methylphenidate TCDO) with the last 12 months of the study period (starting 2 months after trading standards action), we found a significant increase (54.2%, P = 0.0103) in conventional stimulant presentations but not admissions (Table 2). By contrast, no significant effect in the number of presentations or admissions for conventional opioids, non‐NPS benzodiazepines or unknown/other recreational drugs was seen (Table 2). There was a small increase in admissions, but not presentations, for opioid toxicity in the three months immediately after the methylphenidate TCDO that was not sustained (Figure 2).
Table 2.
Changes in median (interquartile range) monthly admissions and presentations for non‐novel psychoactive substance (NPS) drug toxicity for the 12 months before the methylphenidate temporary class drug order (April 2014 to March 2015) and 12 months of 2016 (January to December 2016)
| Drug class | Pre | Post | P value | |
|---|---|---|---|---|
| Conventional opioids | Presentations | 32.5 (26–40; n = 12) | 31 (23–37; n = 12) | P = 0.560 |
| Admissions | 17 (15–23; n = 12) | 13.5 (12–22; n = 12) | P = 0.282 | |
| Conventional stimulants | Presentations | 24 (21–31; n = 12) | 37 (26–39; n = 12) | P = 0.010 |
| Admissions | 7.5 (3–8; n = 12) | 8.5 (5–10; n = 12) | P = 0.294 | |
| Non‐NPS benzodiazepines | Presentations | 15.5 (12–19; n = 12) | 16.5 (14–22; n = 12) | P = 0.433 |
| Admissions | 8 (7–9; n = 12) | 7.5 (6–10; n = 12) | P = 0.743 | |
| Other/unknown | Presentations | 29.5 (26–30; n = 12) | 31 (28–39; n = 12) | P = 0.153 |
| Admissions | 5.5 (2–8; n = 12) | 7 (5–9; n = 12) | P = 0.160 |
Looking at admissions for the different drug groups, there was little difference in the median age before the April 2015 intervention vs. after the October 2015 intervention: NPS 33 vs. 32 years, P = 0.1209; opioids 36 vs. 35 years, P = 0.8030; non‐NPS benzodiazepines 34 vs. 34 years, P = 0.8995; classical stimulants 26 vs. 23 years, P = 0.0101. There was no statistically significant change in the proportion of male to female patients after the interventions compared to the period before April 2015.
Deaths associated with recreational drugs
The interventions were associated with little effect on the number of deaths with any NPS detected, with 26 and 19 deaths in 2014 and 2016, respectively (Figure 3). However, the number of ethylphenidate and methiopropamine postmortem detections fell markedly, with 20 and six cases up to June 2015 vs. none of either in 2016. Instead, the NPS detected in fatal cases during 2016 were novel benzodiazepines (15 etizolam and four diclazepam ). In contrast to the small fall in NPS detections, there were modest increases in detection of opioids (132 vs. 171), classic stimulants (13 vs. 36), and other drugs (61 vs. 90) from 2014 to 2016. The number of cases with non‐NPS benzodiazepines fell (146 vs. 132).
Figure 3.
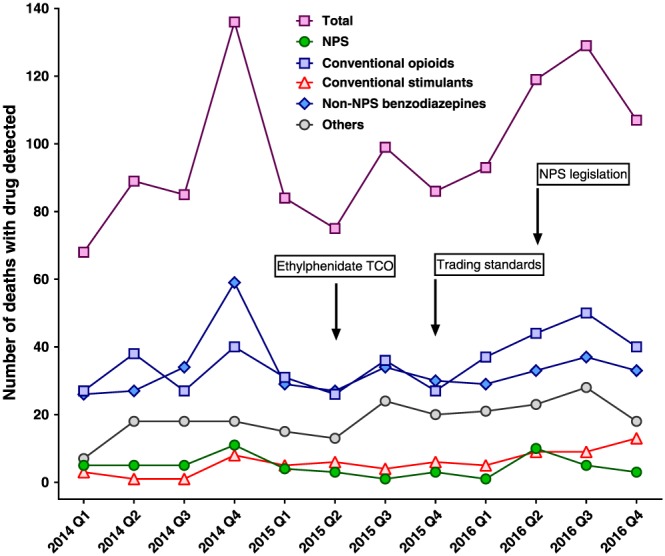
Number of deaths in Edinburgh region with recreational drugs detected in plasma or urine analysis by quarter
Estimated effect of the methylphenidate TCDO and trading standards activity on admissions
Comparing the 12 months before the methylphenidate TCDO to the last 12 months of the study period, there were an estimated 557 (95% confidence interval 327–934) fewer admissions than expected in 2016 if none of the interventions had occurred (43 compared to the expected 603). Using NHS costings for 2015–16, the interventions were associated with an estimated saving of £303 030 (95% confidence interval £177 901–508 133) on admissions for NHS Lothian in Edinburgh during 2016.
Discussion
Our study has shown an association between the methylphenidate‐based NPS TCDO and a rapid and sustained fall in ethylphenidate‐related harms in Edinburgh. The fall in ethylphenidate admissions was soon followed by modestly increased use of SCRAs. Subsequent trading standards activity to remove NPS from local shops was associated with a rapid reduction in all NPS presentations to hospital that was sustained for >1 year. This was associated with a 40% increase in presentations and admissions following classic stimulant use, but not opioids, non‐NPS benzodiazepines or other recreational drugs. The effect has now been sustained for 30 months (from Nov 2015 until now [May 2018]) with fewer than three NPS cases per month being admitted over the last 6 months (Pettie, unpublished results). This article adds to the existing modest evidence for the effect of public health NPS interventions 9, 12.
The reduction associated with the TCDO and trading standards action was surprising since NPS are widely available on the web and it was presumed that sales and use would continue from the web after removal of NPS drugs from local shops 13, 24. That this did not appear to happen instead suggests that the use of these drugs, at least in Edinburgh, was driven by sales of NPS in local shops, not via the web. Discussions with our patients and with colleagues in public health suggested that the life of some NPS users was too chaotic for them to use web‐based sales and that easy access drove new users to NPS drugs who did not then continue to use them when access became more difficult. Despite previous evidence 18, 19 that heroin users were attracted to NPS use by their availability, there was no marked increase in the number of hospital admissions or in deaths from opioid toxicity in the period following the interventions as might have occurred if users went back to their usual doses of heroin for which they had lost tolerance, as was predicted might occur (http://www.nedac.co.uk/news.htm).
There have been no previous reported studies looking in general at the association of trading standard activity or TCDOs on presentations to hospital with NPS toxicity in the UK. One study looking at the number of phone calls and TOXBASE accesses for methoxetamine after its TCDO in April 2012, noting a marked fall in the following months 25. Retrospective studies in Edinburgh found a reduction in Streptococcus pyogenes and Staphylococcus aureus skin infections 26, and lower reported use of ethylphenidate by psychiatric patients 27, after the TCDO and trading standards action. A comprehensive review of the international literature to 2016 reported a series of methodologically weak studies presenting similar before and after data for bans of particular NPS 12. These studies all showed a general decline in incidence after bans 12. Unfortunately, the difficulty of performing a randomized controlled study of a public health intervention for NPS control limits the ability to produce high quality effectiveness data.
Our data suggest that trading standards enforcement may be particularly effective at sustainably reducing NPS use and harms. Implementing trading standards actions across the country, if productive of similar effects, may reduce direct hospital costs by millions of pounds. It was not possible to look at the effects of the Psychoactive Substances Act because the rate of NPS drug presentations/admissions had already fallen by May 2016 to low levels following the TCDO and trading standards activity. Any associated effect of the methiopropamine TCDO could not be detected because, although found postmortem for six Edinburgh cases in 2014–15, it was not recognized as a clinical problem and therefore not recorded in the hospital database.
This paper is limited by the data being from only one hospital in one part of the UK. Edinburgh has been badly hit by NPS toxicity over the last decade, with the very large numbers of ethylphenidate cases being specifically recognized in the text of the methylphenidate TCDO 21. Prospective audit of admitted patients provided data from before and after the actions to be compared using identical methodology. Admission of patients to one of the UK's specialist toxicology wards meant that effort was made to identify the drug involved from history, clinical syndrome, and knowledge of local epidemiology (from police seizures and laboratory analysis). However, anonymous collection of routine audit data did not allow for collection of data on comorbidity, particularly psychiatric history.
A second limitation is the lack of laboratory proven identification of the drug involved for each patient. Since identification of the drug would not have assisted clinical management, and the turnaround for analysis too long to be clinically useful, such analysis would have required setting up a research study and seeking funds for the analysis – both of which would have taken time and hindered the collection of prospective data before the first intervention. Although the precise recreational drug involved cannot be identified for sure, there were clear changes in the toxidromes of patients presenting over time consistent with the switch from ethylphenidate to SCRAs, and then away from SCRAs. Furthermore, the interventions were associated with a near complete reduction in all cases of NPS toxicity, making the identification of the responsible compounds moot. The history‐based data from the clinical cases were similar to the forensic toxicology analysis‐derived data being performed on patients dying from suspected overdose in Edinburgh.
A third limitation was the short interval between actions, with several interventions following each other in quick succession, reducing the amount of data between interventions available with which to analyse associated effects. The periods between interventions also varied meaning that we had to use different duration data for comparisons. Since we preferred to use all the data available, we did not drop data to make the durations of similar length. The constantly changing epidemiology of NPS drug use also meant that it was difficult to identify a baseline or underlying secular change for presentations before the first intervention.
In conclusion, TCDOs and trading standards activity were associated with a remarkable and sustained reduction in the number of presentations and admissions for NPS toxicity in Edinburgh. If causal, such actions implemented across the UK may be able to turn the tide against these highly damaging drugs. It seems unlikely that controlled public health studies will occur in the future; further observational studies may provide more data on the possible causal relationship between such interventions and reducing harm from NPS.
Competing Interests
There are no competing interests to declare.
We thank the nursing and medical staff of the toxicology ward and emergency medicine department of the Royal Infirmary of Edinburgh for their support with data collection, in particular Euan Sandilands, Arvind Veiraiah and Jonathan Wraight, as well as Simon Thomas, Simon Hill and Nick Bateman for critical comments on the manuscript. The post‐mortem data is published with kind permission from the Crown Office and Procurator Fiscal Service.
The study received no direct funding. D.W.K. was an Economic and Social Research Council (UK) postdoctoral fellow (ES/P009735/1).
Contributors
J.P., M.D. and J.D. set up the prospective database of recreational drug patients. J.P., A.B., M.D., K.O., R.G., D.S. and K.E. extracted the data. H.T. extracted the forensic toxicology data. M.E. and D.K. analysed the data. M.E. wrote the first draft of the paper; all authors then critically revised it.
M.E. is the guarantor for the study. He had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Pettie, J. , Burt, A. , Knipe, D. W. , Torrance, H. , Dow, M. , Osinski, K. , Greig, R. , Sabatini, D. , Easterford, K. , Dear, J. , and Eddleston, M. (2018) New drug controls and reduced hospital presentations due to novel psychoactive substances in Edinburgh. Br J Clin Pharmacol, 84: 2303–2310. 10.1111/bcp.13672.
References
- 1. Advisory Council on the Misuse of Drugs . Consideration of the novel psychoactive substances (‘legal highs’). London, 2011.
- 2. Stephenson G, Richardson A. New psychoactive substances in England. A review of the evidence. London: Crime and Policing Analysis Unit, Home Office Science. 2014.
- 3. Gilani F. Novel psychoactive substances: the rising wave of 'legal highs. Br J Gen Pract 2016; 66: 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila) 2011; 49: 705–719. [DOI] [PubMed] [Google Scholar]
- 5. Liechti M. Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signaling. Swiss Med Wkly 2015; 145: w14043. [DOI] [PubMed] [Google Scholar]
- 6. Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila) 2016; 54: 1–13. [DOI] [PubMed] [Google Scholar]
- 7. Moosmann B, King LA, Auwarter V. Designer benzodiazepines: a new challenge. World Psychiatry 2015; 14: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoiseth G, Tuv SS, Karinen R. Blood concentrations of new designer benzodiazepines in forensic cases. Forensic Sci Int 2016; 268: 35–38. [DOI] [PubMed] [Google Scholar]
- 9. Helander A, Backberg M. New psychoactive substances (NPS) ‐ the Hydra monster of recreational drugs. Clin Toxicol (Phila) 2017; 55: 1–3. [DOI] [PubMed] [Google Scholar]
- 10. Tracy DK, Wood DM, Baumeister D. Novel psychoactive substances: types, mechanisms of action, and effects. BMJ 2017; 356: i6848. [DOI] [PubMed] [Google Scholar]
- 11. DrugScope . Business as usual? A status report on new psychoactive substances (NPS) and ‘club drugs’ in the UK. London, 2014.
- 12. Meader N, Mdege N, McCambridge J. The public health evidence‐base on novel psychoactive substance use: scoping review with narrative synthesis of selected bodies of evidence. J Public Health (Oxford, England) 2018; 10.1093/pubmed/fdy016 [DOI] [PubMed] [Google Scholar]
- 13. Faculty of Addictions Psychiatry of the Royal College of Psychiatrists . One new drug a week. Why novel psychoactive substances and club drugs need a different response from UK treatment providers. London, 2014.
- 14. Sellers EM. Deconstructing designer drugs. Clin Pharmacol Ther 2017; 101: 167–169. [DOI] [PubMed] [Google Scholar]
- 15. Home Office . Psychoactive substances act 2016. London: Home Office, 2016. [Google Scholar]
- 16. James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, et al Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emerg Med J: EMJ 2011; 28: 686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray DB, Potts S, Haxton C, Jackson G, Sandilands EA, Ramsey J, et al 'Ivory wave' toxicity in recreational drug users; integration of clinical and poisons information services to manage legal high poisoning. Clin Toxicol (Phila) 2012; 50: 108–113. [DOI] [PubMed] [Google Scholar]
- 18. Lafferty C, Smith L, Coull A, Shanley J. The experience of an increase in the injection of ethylphenidate in Lothian April 2014‐March 2015. Scott Med J 2016; 61: 74–83. [DOI] [PubMed] [Google Scholar]
- 19. Parks C, McKeown D, Torrance HJ. A review of ethylphenidate in deaths in East and West Scotland. Forensic Sci Int 2015; 257: 203–208. [DOI] [PubMed] [Google Scholar]
- 20. Stanley JL, Mogford DV, Lawrence RJ, Lawrie SM. Use of novel psychoactive substances by inpatients on general adult psychiatric wards. BMJ Open 2016; 6: e009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Home Office . Two new "legal highs" to be banned under temporary order. London: Home Office, 2015. [Google Scholar]
- 22. The City of Edinburgh Council . Council cracks down on the sale of psychoactive substances. Edinburgh, 2015.
- 23. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shapiro H, Daly M. Highways and buyways: a snapshot of UK drug scenes 2016. DrugWise, 2017.
- 25. Hill SL, Harbon SC, Coulson J, Cooper GA, Jackson G, Lupton DJ, et al Methoxetamine toxicity reported to the National Poisons Information Service: clinical characteristics and patterns of enquiries (including the period of the introduction of the UK's first temporary class drug order). Emerg Med J: EMJ 2014; 31: 45–47. [DOI] [PubMed] [Google Scholar]
- 26. Yeung A, Weir A, Austin H, Morrison K, Inverarity D, Sherval J, et al Assessing the impact of a temporary class drug order on ethylphenidate‐related infections among people who inject drugs in Lothian, Scotland: an interrupted time‐series analysis. Addiction 2017; 112: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 27. Bennett KH, Hare HM, Waller RM, Alderson HL, Lawrie S. Characteristics of NPS use in patients admitted to acute psychiatric services in Southeast Scotland: a retrospective cross‐sectional analysis following public health interventions. BMJ Open 2017; 7: e015716. [DOI] [PMC free article] [PubMed] [Google Scholar]


