Abstract
Porcine reproductive and respiratory syndrome (PRRS) is a widespread economically devastating disease caused by PRRS virus (PRRSV). First recognized in the late 1980s, PRRSV is known to undergo somatic mutations and high frequency viral recombination, which leads to many diverse viral strains. This includes differences within viral virulence factors, such as the viral ovarian tumor domain (vOTU) protease, also referred to as the papain-like protease 2. These proteases down-regulate innate immunity by deubiquitinating proteins targeted by the cell for further processing and potentially also acting against interferon stimulated genes (ISGs). Recently, vOTUs from vaccine derivative Ingelvac PRRS® modified live virus (MLV) and the highly pathogenic PRRSV strain JXwn06 were biochemically characterized, revealing a marked difference in activity toward K63 linked polyubiquitin chains and a limited preference for interferon-stimulated gene product 15 (ISG15) substrates. To extend our research, the vOTUs from NADC31 (low virulence) and SDSU73 (moderately virulent) were biochemically characterized using a myriad of ubiquitin and ISG15 related assays. The K63 polyubiquitin cleavage activity profiles of these vOTUs were found to track with the established pathogenesis of MLV, NADC31, SDSU73, and JXwn06 strains. Fascinatingly, NADC31 demonstrated significantly enhanced activity toward ISG15 substrates compared to its counterparts. Utilizing this information and strain differences within the vOTU encoding region, sites were identified that can modulate K63 polyubiquitin and ISG15 cleavage activities. This information represents the basis for new tools to probe the role of vOTUs in the context of PRRSV pathogenesis.
Keywords: Ub, ISG15, PRRSV, vOTU, nsp2
Table of Contents Graphic
Porcine reproductive and respiratory syndrome (PRRS) is a devastating swine disease that was first recognized in the United States in the late 1980s and has since rapidly spread around the world.1–6 PRRS is considered one of the most economically significant porcine diseases worldwide as it causes increased mortality and reduced growth performance in swine, which has resulted in a loss of over $600 million annually for the United States alone.7,8 The causative agent of PRRS is positive-sense single-stranded RNA virus (PRRSV) belonging to the family Arteriviridae, order Nidovirales.
PRRSV has consistently caused outbreaks with new strains frequently emerging due to high rates of genetic recombination and mutations.9–11 PRRSV strains are characterized into two distinct genetic types: Type 1 or European (prototype strain Lelystad) and Type 2 or North American (prototype strain VR-2332). The substantial sequence and behavioral differences between the two PRRSV types supports the conclusion that their evolution occurred independently and on separate continents initially.12,13 Thus far, Type 2 PRRSV strains are classified into at least nine distinct lineages based on a comprehensive comparison of open reading frame 5 (ORF5).10,14 Currently, multiple Type 2 strains of varying virulence have been genetically characterized and share around 80% sequence identity across their genome9,15–17 (Figure 1a). Of these, the first North American PRRSV strain (VR-2332) was recorded in 1987, but the strain was not identified until 1992.18,19 This index strain was used as a basis for avirulent modified-live virus (MLV) vaccine strain Ingelvac PRRS®.18,19 Subsequently and periodically, newer and moderately more virulent Type 2 strains have emerged in the United States, such as SDSU73, MN184, and NADC30.9,20 In contrast, PRRSV strain JXwn06 belongs to a group embodying the other end of the pathogenicity spectrum. Originally isolated from a Chinese in 2006, due to the large number of porcine deaths associated with its outbreak, JXwn06 formed the basis for a subgroup of PRRSV Type 2 strains now designated as highly pathogenic PRRSV (HP-PRRSV).15,21 Pertinent to this study, strain SDSU73 was first isolated in 1996 in Iowa and was of special interest as it was more virulent than previous United States strains at the time.22 Isolated in 2008, strain NADC31 has been shown to less virulent in swine than SDSU73 and HP-PRRSV JXwn06. 9,23 Put together, the rapidly evolving nature of PRRSV along with its economic impact and wide variability in pathogenicity has underscored a need to better understand potential and complex virulence factors within these different emerging strains.
Fig. 1. PRRSV vOTU sequence alignment, phylogenetic analysis, and ISG15 sequence alignment.
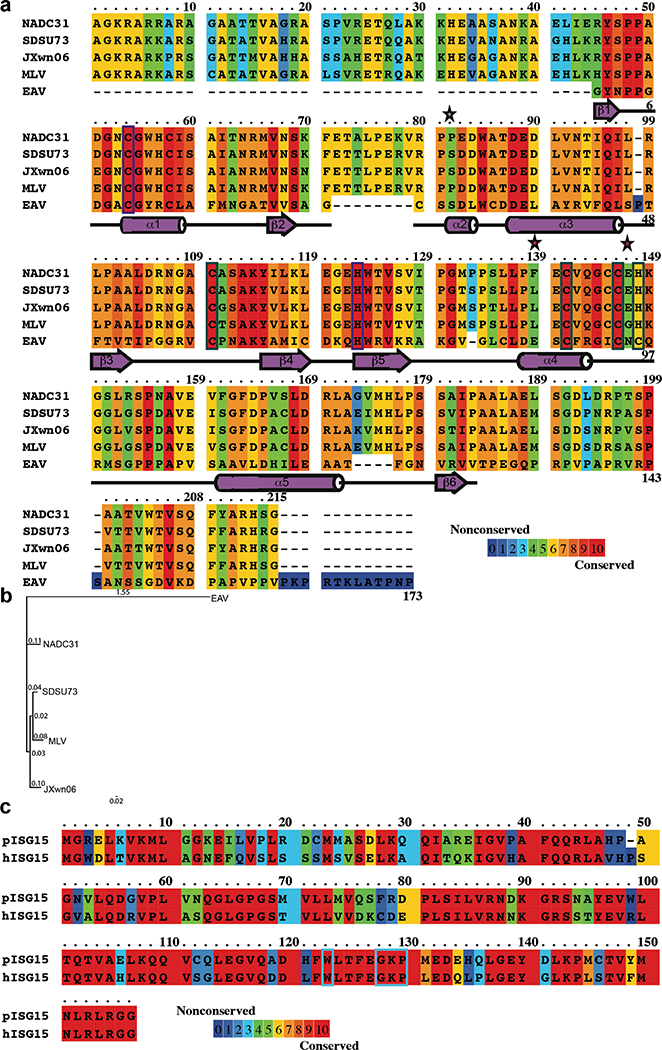
(a) The alignment shows sequence similarity as a 10-color scale from royal blue to red with a royal blue background representing a nonconserved residue and a red background denoting a conserved residue. Catalytic and zinc-binding residues are boxed in dark purple and dark green, respectively. The secondary structure predicted by DSSP is shown for EAV PLP2 as deep lavender arrows and cylinders representing the β-sheets and α-helices. The colored stars indicate residues mutated in this study with a light blue star denoting a mutation affecting diubiquitin activity and bright pink stars signifying mutations influencing ISG15 activity. (b) The phylogenetic analysis was completed using the Geneious Tree Builder application, 60 Neighbor Joining tree builder method with 500 bootstrapping replicates, and Jukes-Cantor distance model. (c) The sequence alignment between human and porcine ISG15 is shown using a 10-color scale from royal blue to red with a royal blue background representing a nonconserved residue and a red background denoting a conserved residue. Residues that potentially are involved in interactions between the ISG15 and the PRRSV vOTU are boxed in light blue.
Typical PRRSV pathogenesis includes viremia after 6–12 h and continued viral shedding up to 157 days.24 Several groups have shown that the host immune response is greatly compromised with PRRSV, which likely gives rise to the slow viral clearance and immunosuppression.25–27 More specifically, the innate immune system has often been observed to be severely dysregulated via disruptions within the Type I interferon (IFN-I) associated pathways. The innate immunity is considered the first line of defense for the host against a viral infection and is responsible for the production of cytokines, IFNs, IFN-stimulated genes (ISGs), and an inflammatory response through the NF-κB pathway.28–31 Within the past few years, a number of nonstructural (nsp) proteins of PRRSV have been implicated as potential virulence factors for their influence on innate immune down regulation including nsp1, nsp2, nsp9, and nsp11. 32–36 Of these, nsp2 has been particularly highlighted as playing a key role not only in genomic replication but also to assist with viral evasion within host cells.9,34,37,38
Nsp2 is encoded within ORF1a and is the most variable region within the PRRSV genome.39,40 Nsp2 is cleaved from the ORF1a polyprotein via two papain-like proteases (PLPs): the nsp1β protease (PLP1β) at its N-terminus and the nsp2 protease (PLP2) at its C-terminus.39 Interestingly, the PLP2 domain not only cleaves the polyprotein between nsp2 and nsp3 at a conserved GG dipeptide sequence but according to bioinformatics studies, falls into a larger family of mammalian proteins known as ovarian tumor domain (OTU) proteases.41–44 The viral ovarian tumor domain (vOTU) region from Type 2 PRRSV isolates can display an approximate 80.5% identity, which is drastically higher when compared to a related arterivirus, equine arteritis virus (EAV), at approximately 21% identity (Figure 1b). As with other vOTUs, such as those originating within the Nairoviradae family, PRRSV vOTUs have been observed to reverse post-translational modification of proteins by ubiquitin (Ub).6,43,45,46 Polyubiquitination of host proteins is essential in the regulation of several innate immune pathways and are formed via an isopeptide bond between two or more Ub domains at one of seven different lysine positions available on Ub (K6, 11, 27, 29, 33, 48, and 63) as well as its N-terminus (linear).47,48
Recently, vOTUs originating from HP-PRRSV strain JXwn06 and vaccine derivative Ingelvac PRRS® MLV were shown to have a preference for K11, K48, and K63-linked polyubiquitin chains.15 Previously, K63-linked polyubiquitination has been implicated in the RIG-I/MAVS pathway among others with K48-linked poly-Ub being observed in the NF-kB pathway triggering IκB degradation, and K11-linked poly-Ub recently has been linked to TNF signaling.26,27,30,31,38,49–52 Intriguingly, the more pathogenic JXwn06 vOTU was shown to have a markedly enhanced ability to cleave K63-linked polyubiquitin, approximately 40-fold higher than that of the MLV vOTU.15 With K63-linked poly-Ub tied to innate immune response regulation, the unique greater ability of JXwn06 vOTU to turn over K63-linked was proposed to play a significant role in heightened disease severity.15 Beyond their activities as deubiquitinases, a previous report using a vOTU from an unidentified PRRSV strain also suggested that PRRSV vOTUs may also reverse modification of innate immune signaling proteins by Ub-like interferon-stimulated gene product 15 (ISG15; Figure 1c).31,34,35 However, similar activity has not been observed for vOTUs originating from PRRSV JXwn06 and MLV strains.15
Here, we present the biochemical characterization of the vOTU activity from two additional Type 2 strains, NADC31 and SDSU73, as well as a comparison of them within the context of the deubiquitinase linkage profiles of vOTUs originating from JXwn06 and MLV. Through these studies, we have also revealed the first PRRSV vOTU, originating from NADC31 found to have a marked increase in deISGylase activity over previously evaluated PRRSV vOTUs. Combining this data along with sequence alignments and structural information from a vOTU encoded by the related arterivirus EAV, sites within the vOTU that in part drive PRRSV vOTU preferences for ISG15 and K63 polyubiquitin linkages were identified. This information not only provides greater insight into the substrate variance of vOTUs within Type 2 PRRSV vOTUs but also is a foundation for new tools in the prediction of vOTU activities within other PRRSV strains as well as to tease out the relative impact of vOTUs to other PRRSV virulence factors.
Results and Discussion
Biochemical Characterization of PRRSV Strain SDSU73 and NADC31 vOTU Domains
The vOTU domain of PRRSV has been observed to play a key role in the suppression of the innate immune system in cultured cells.9,38,53 Although several different strains of Type 2 PRRSV have been studied in cellular systems and animals, limited data exists on the impact of strain to strain variances within individual virulence factors such as PRRSV vOTUs.9,23,38,53 Recently, the first biochemical insights into PRRSV vOTUs acting as deubiquitinating enzymes were revealed for PRRSV low pathogenic strain MLV PRRSV vOTU and Asian HP-PRRSV JXwn06.15 Albeit a narrow sampling of PRRSV vOTUs, noticeable differences in preference were observed among polyubiquitin linkages. To provide a better understanding of these differences within PRRSV, vOTUs were obtained from PRRSV strains NADC31 and SDSU73. Strain selection was based on the fact that these two strains exhibited pathogenicity that fell in between those exhibited by HP-PRRSV JXwn06 and MLV. To investigate the kinetics for these vOTUs, biochemical data for substrate specificity and activity was assessed utilizing Ub, human ISG15 (hISG15), and a short peptide substrate. The peptide substrate is comprised of the amino acid sequence RLRGG, which is the shared C-terminal recognition sequence between Ub and ISG15 and used to test intrinsic catalytic activity. The C-terminus of the three substrates is conjugated with an amino-4-methylcoumarin (AMC) fluorophore, and the release of this fluorophore is monitored over time to obtain the rate and substrate preference (Figure 2).
Fig. 2. vOTU cleavage of Ub, ISG15, and peptide AMC conjugates.
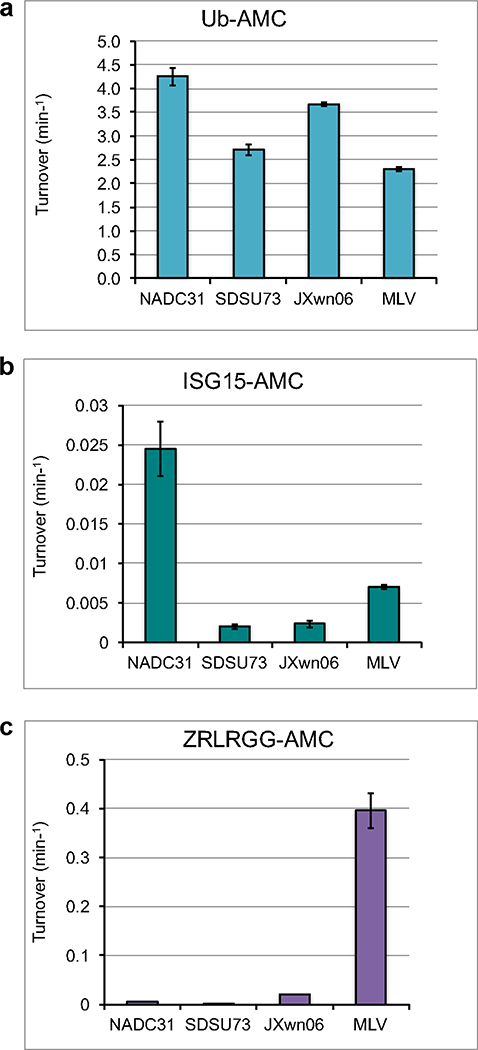
The cleavage activities of vOTUs from PRRSV strains NADC31, SDSU73, JXwn06, 15 and MLV15 for 1 μM Ub-AMC (a), 1 μM ISG15-AMC (b), and 50 μM ZRLRGG-AMC (c) were analyzed. The error bars indicate standard deviations from the mean.
The peptide activity for vOTUs from NADC31 and SDSU73 resembled the lower activity seen for JXwn06 with activity at 0.0048 ± 0.0001 and 1.57 ± 0.21 10−5 molecules/min, respectively (Figure 2c). PRRSV vOTUs originating from strains NADC31 and SDSU73 possess a clear ability to cleave Ub-AMC at 4.25 ± 0.18 and 2.56 ± 0.093 molecules/min respectively. This is consistent with other characterized PRRSV vOTUs, JXwn06 at 3.67 ± 0.04 and MLV at 2.30 ± 0.04 molecules/min (Figure 2a). When the vOTUs from NADC31 and SDSU73 are compared to their vOTUs counterparts, such as the nairoviruses Crimean-Congo hemorrhagic fever virus (CCHFV), Dugbe virus, and Erve virus (ERVEV), and tymovirus prototypical member turnip yellow mosaic virus (TYMV), their deubiquitinating activity is closer to those of nairovirus deubiquitinases that can turn over up to 27 molecules/min than that of TYMV vOTU at 0.03 molecules/min when assessed at similar substrate concentrations.54 Previously, relatively low deISGylase activity was observed for vOTUs from JXwn06 and MLV.15 DeISGylase activity of vOTU from SDSU73 followed this trend with extremely low activity of 0.002 ± 0.0003 molecules/min, which is over 3 orders of magnitude times less than its Ub-AMC activity (Figure 2b). Intriguingly, when the NADC31 activity was assessed for hISG15-AMC, there was an order of magnitude increase, to 0.025 ± 0.003 min−1, when compared to the other three strains (Figure 2b). Although compared to other vOTU deISGylases this level of activity is on the lower end, this relatively dramatic increase suggests that deISGylase activity may vary from strain to strain. This may reconcile while despite low deISGylase activity observed in vOTUs from JXwn06 and MLV, other reports suggested PRRSV vOTUs to be deISGylases.31
Diubiquitin Specificity
Even though SDSU73 and NADC31 closely resembled the Ub-AMC activity seen for the previous PRRSV vOTUs, differences may exist in the activity toward the different di-Ub linkages. As host protein substrates are typically not monoubiquitinated and instead undergo polyubiquitination events, viral protease activity toward these substrates is likely more relevant to their impact on host immune signaling pathways. As a result, vOTUs from SDSU73 and NADC31 were assessed against a di-Ub panel containing the eight types of polyubiquitin linkages47,48 (Figure 3). PRRSV vOTU from SDSU73 has relatively high activity toward K63-linked di-Ub followed by robust activity for K48-linked di-Ub and lesser activities for K6 and K33-linked di-Ubs. This differs for the PRRSV vOTU originating from NADC31. This protease preferred K11-linked diubiquitin (di-Ub) followed by a modest preference of K63-linked di-Ub over its K48-linked counterpart. Also, minor activity for K6-linked di-Ub was observed.
Fig. 3. Cleavage assays of PRRSV strain vOTU polyubiquitination linkage specificity.
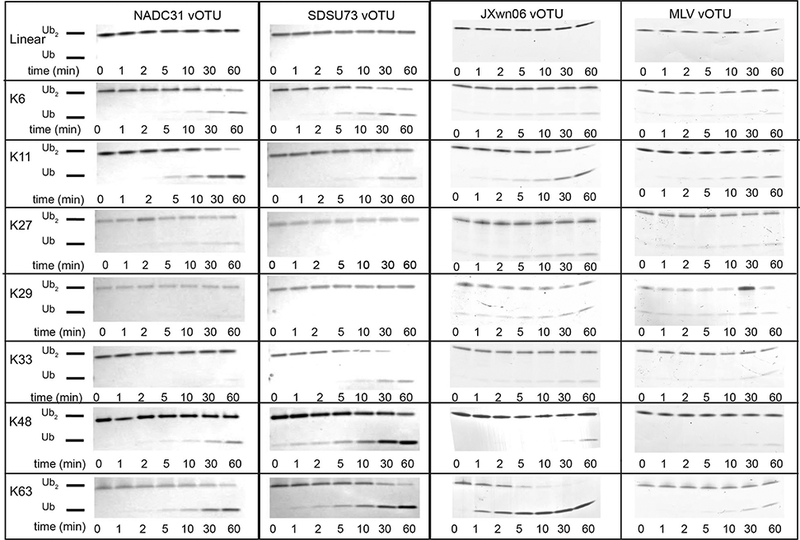
di-Ub linkage (10 μM) was incubated with 500 nM vOTU from NADC31, SDSU73, JXwn06,15 and MLV15 at 37 °C for 1 h with samples taken at the indicated time points. The samples were inactivated by heating at 95 °C for 5 min and thereafter analyzed on a 10 % Mini-Protean Tris-Tricine precast gel (Bio-Rad) and visualized by staining with Coomassie blue.
To take a more quantitative approach in assessing di-Ub activity, di-Ub FRET substrates were utilized to monitor cleavage of K11, K48, and K63 (Figure 4). The di-Ub panel results mirrored those of the di-Ub FRET cleavage assay results for the vOTU from NADC31, showing NADC31 to have the highest activity toward K11-linkages followed by K63-and K48-linkages. In the case of the vOTU from SDSU73, activity toward K11- and K63-linkages were in line with to those seen in the di-Ub panel. However, this protease’s di-Ub FRET activity for the K48-linkage substrate was unusually low, likely suggesting that the position of the FRET pairs interfered with the substrate-protease interaction.
Fig. 4. vOTU preference for FRET poly-Ub linkage substrates.
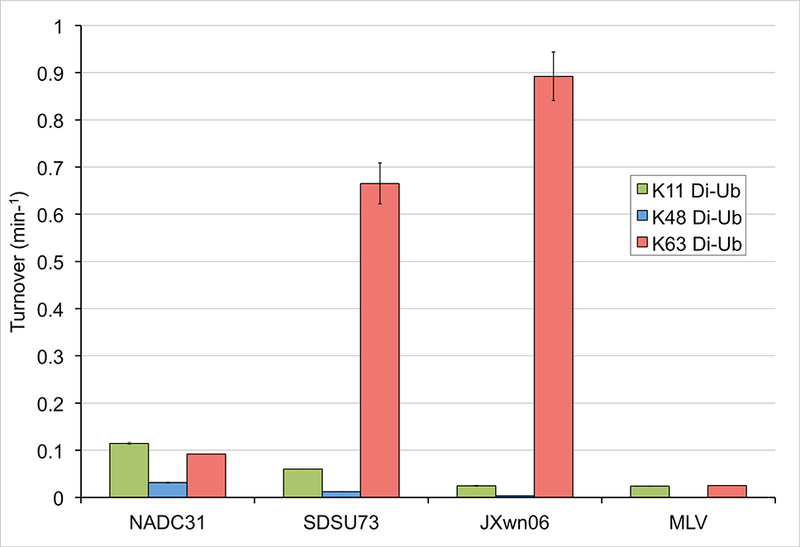
Cleavage activity for K63-linked (salmon pink), K48-linked (blue), and K11-linked (green). Determination of turnover values was based on the increase in emission upon cleavage of 1 μM di-Ub in the presence of the vOTU from NADC31, SDSU73, JXwn06,15 and MLV.15
A comparison of the di-Ub cleavage profiles of vOTUs from NADC31 and SDSU73 with those originating from JXwn06 and MLV suggests that robust PRRSV vOTU activities are largely confined to K63-, K48-, and K11-linkages. Not surprisingly for a virus that is attempting to evade host immunity, these linkages have been heavily implicated in regulation of host immunity. Specifically, K48-linked poly-Ub is responsible for the degradation of inhibitor of nuclear factor κΒ (IκΒα), which is required for the activation of the NF-kB pathway and associated inflammatory response.26 K63-linked poly-Ub has been tied to the induction of the Type I IFN innate immune response and directly involved in the antiviral signaling through RIG-I/MAVS. Lastly, K11-linked poly-Ub has been observed to be involved in TNF signaling within the NF-kB pathway.50 However, their preferences among these linkages can vary depending on from which strain they originate. For instance, the vOTU within NADC31 appears to have evolved toward disrupting K11-linkage mediated pathways instead of those mediated by K48- and K63-linkages. This differs from the vOTU originating from SDSU73 that appears to more closely mirror the polyubiquitin activities profile of JXwn06 with the exception of more robust activity toward K48 linkages. Previously, the vOTU from HP-PRRSV showed a significantly heightened ability to process K63-linkages over that of the vOTU from the avirulent PRRSV MLV, suggesting that this may play at least a part in the increased pathogenicity observed for HP-PRRSV.55 When the K63-linkage cleavage activities of SDSU73 and NADC31 are taken into context with their reported virulence, this finding appears to further support this assertion.
Potential Interaction Site between K63-Linked di-Ub and PRRSV vOTUs
In order to gain the first insight into the potential molecular driving forces behind the high K63-linked di-Ub activity and PRRSV vOTUs, a structural model of the substrate-protease interaction was created using Modeller.56 The known EAV PLP2 domain (PDB: 4IUM) was used as the structural basis57 (Figure S1a). Using the bound mono-Ub as an anchor point, K63-linked di-Ub (PDB: 3H7P) was mated to the PRRSV model active site with the distal Ub monomer adjusted to place the isopeptide bond within the PRRSV homology model active site58 (Figure 5a). Naturally as a homology model, the model could not provide definitive interactions between the two proteins. As a result, this model was utilized to identify potential surfaces on PRRSV vOTUs where interactions may occur. With the mono-Ub activity between the strains largely comparable, focus was placed on regions where the distal Ub of the K63-linked di-Ub moiety would potentially interact. When the deubiquitinase activities of the vOTUs originating from PRRSV strains SDSU73, NADC31, JXwn06, and MLV for K63-linkages were taken into account as well as strain-strain polymorphisms within those regions, position 82 particularly stood out. Specifically, residue 82 is a serine in SDSU73 and JXwn06 while a proline in NADC31 and MLV. Upon closer examination, S82 could potentially be involved in several electrostatic interactions with the distal domain of K63-linked di-Ub (Figure 5b). To examine this position’s influence on PRRSV vOTUs activity toward 63-linkages, a S82P point mutation was made in both SDSU73 and JXwn06 and the activity for Ub-AMC and K63-linked di-Ub FRET was assessed. As expected, K63-linked di-Ub FRET activity was reduced in both the S82P SDSU73 and JXwn06 mutants from 0.67 ± 0.043 min−1 to 0.54 ± 0.0011 molecule/min and 0.89 ± 0.051 molecule/min to 0.10 ± 0.0015 molecule/min, respectively (Figure 5c). However, this was accompanied by a modest decrease in mono-Ub seen in the JXwn06 S82P mutant. This suggests that increased rigidity of the proline in the loop may also indirectly influence catalytic function or the binding of nearby proximal ubiquitin (Figure 5d). To further validate this mutant’s effect on the ability of these two proteases to cleave K63-linkages, the K63-linked di-Ub cleavage assay was performed. Mirroring the K63-linkage FRET results for both S82P mutants, a significant reduction in K63 di-Ub activity was observed with neither mutant able to significantly cleave K63 di-Ub after 60 min (Figure 6a,b). To probe this position’s role further, a P82S mutation was made in MLV in order to see if K63-linkage activity could be knocked-in. Excitingly, the P82S MLV mutant showed an order of magnitude increase in K63 di-Ub FRET activity going from 0.025 ± 0.0001 molecule/min to 0.21 ± 0.016 molecule/min at the expense of a decrease in mono-Ub activity (Figure 5c,d). The increase of K63-linkage activity for P82S MLV was also reflected in the di-Ub cleavage assay. Naturally, differences within activity toward K63-linkages between vOTUs originating from SDSU73 and JXwn06 do exist suggesting other residues may also be involved. However, serine being at position 82 within the vOTUs from JXwn06 and SDSU73 appears to be at least in part responsible for the heightened activity of these proteases for K63-linkages. As a result, this position may not only offer a hallmark to those vOTUs that possess this heightened activity, but also point toward the first molecular tool at untangling the influence of vOTU mediated K63-linked polyubiquitin cleavage.
Figure 5. Model of PRRSV SDSU73 vOTU’s interaction with di-Ub K63.
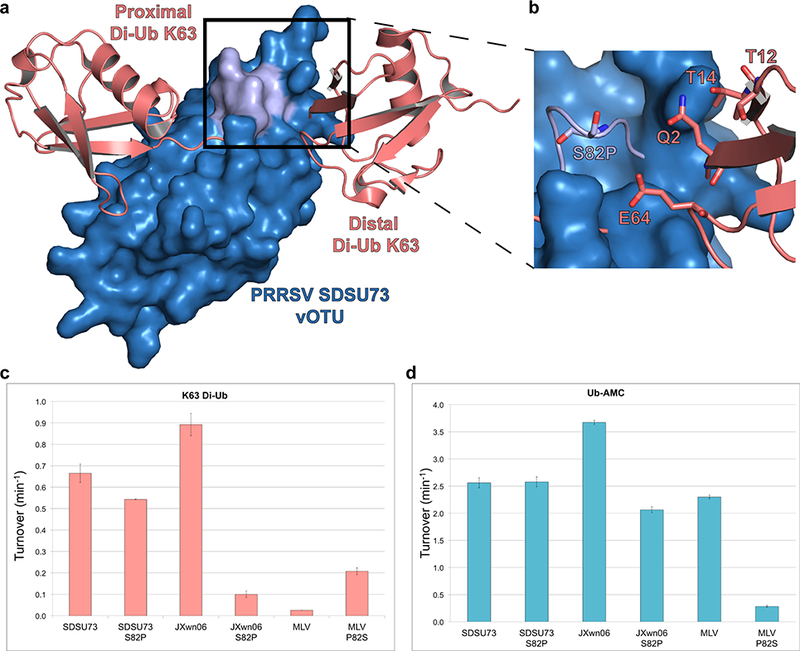
(a) A surface rendering of a homology model for PRRSV SDSU73 vOTU (blue), built from EAV PLP2 (PDB: 4IUM), and the potential interactions with di-Ub linked K63 (PDB: 3H7P; salmon). The possible interface between the vOTU and distal di-Ub K63 is shaded in light purple. (b) A zoomed in view of the potential interaction between PRRSV SDSU73 vOTU and the distal di-Ub K63. Residues potentially involved in the interaction have been rendered as sticks. (c,d) Enzyme data, presented as turnover (min−1), for the different wild-type PRRSV vOTU strains and their associated mutations for FRET di-Ub K63 and Ub-AMC respectively. The enzyme data of these strains and mutants was compared to PRRSV strains JXwn06,15 and MLV.15
Figure 6. Cleavage assays of PRRSV strain vOTU mutants’ polyubiquitination linkage specificity toward K63-linked di-Ub.
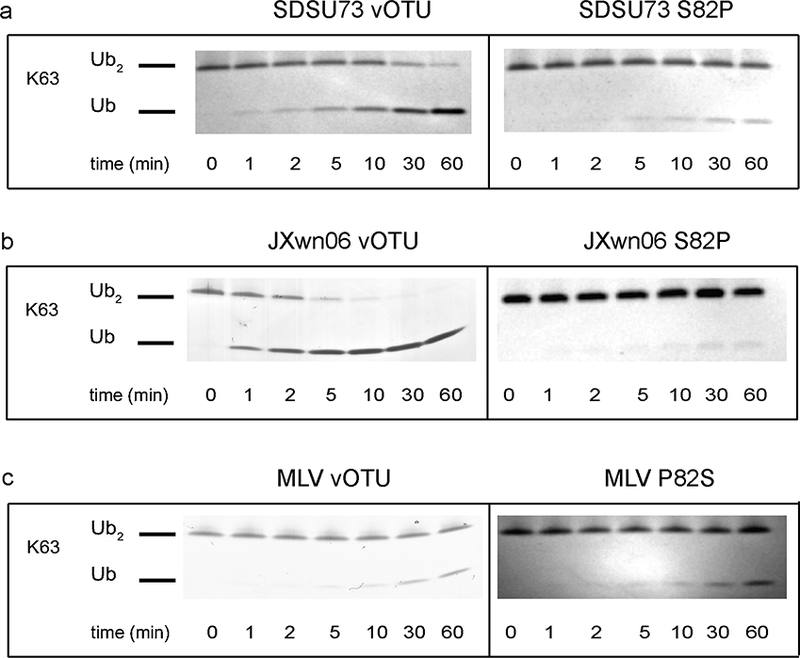
di-Ub linkage (10 μM) was incubated with 500 nM vOTU from mutants of SDSU73, JXwn06, and MLV at 37 °C for 1 h with samples taken at the indicated time points. The samples were inactivated by heating at 95 °C for 5 min and thereafter analyzed on a 10 % Mini-Protean Tris-Tricine precast gel (Bio-Rad) and visualized by staining with Coomassie blue. The cleavage assays of the mutants were then compared to the K63-linked di-Ub cleavage assays of the wild-type strains, SDSU73, JXwn06,15 and MLV.15
Potential Interaction Site between ISG15 and PRRSV vOTUs
Using a similar approach to identify the S82P polymorphism link to heightened K63-linked cleavage activity of PRRSV vOTUs, the identity of molecular drivers for NADC31 vOTU’s elevated deISGylase activity was sought. Specifically, a homology model, generated using Modeller,56 of PRRSV vOTU from NADC31 bound to Ub was generated utilizing the X-ray structure of the EAV PLP2 domain bound to Ub (PDB: 4IUM)57 (Figure S1b). Using the bound Ub as an anchor point for the C-terminal domain of ISG15, hISG15 (PDB: 1Z2M) was overlaid.59 This provided a perspective on regions that potentially could influence deISGylase activity. The resulting placement of hISG15 placed the N-terminal domain away from the proteases suggesting that interactions with the protease were likely limited to the C-terminal domain. Taking into account the heightened deISGylase activity of NADC31 vOTU and polymorphisms located along the potential protease-ISG15 interface, two potential positions within PRRSV vOTUs were highlighted as potential molecular drivers of deISGylase activity. Specifically, positions 139 and 147, which are located in predicted loop regions surrounding an a-helix (Figures 1 and 7a). The former is a leucine residue in vOTUs originating from MLV, JXwn06, and SDSU73 but a much bulkier phenylalanine residue in NADC31 vOTU. For the 147 position, MLV vOTU has a flexible glycine whereas vOTUs from the other three strains had a glutamate at this position. To probe the influence of these positions on deISGylase activity, F139L/E174G was introduced within the vOTU form NADC31. These mutations noticeably reduced NADC31 vOTU’s deISGylase activity, to that reminiscent of the other three PRRSV vOTUs. However, there was no apparent impact on its deubiquitinase activity, illustrating that removal of deISGylase activity did not come due to a broad decrease in catalytic function. Additionally, utilizing a L139F mutation within the vOTU from JXwn06 did result in a detectable increase in deISGylase activity and a subsequent mutation of E147G did reverse some of those gains (Figure 7c). Interestingly, these changes were much more modest than those observed in the NADC31 vOTU mutants and came at the expense of JXwn06 vOTU’s deubiquitinating activity. As a result, for JXwn06 vOTU, this represented more of a switch in preference toward ISG15 than a straightforward gain of deISGylase activity. In addition, the residues within hISG15 that are suspected of forming the potential protease-ISG15 interface are conserved between that of hISG15 and porcine ISG15 (pISG15) (Figure 1c). This suggests that the lowered deISGylation activity observed with the NADC31 Votu mutants against hISG15 could also be a representation of what would be observed in the natural host. Put together, the presence of phenylalanine at position 139 and glutamate at position 147 appear to be a key driver to NADC31 vOTU’s elevated deISGylase activity suggesting that they could be potential hallmarks of similar activity in other vOTUs.
Figure 7. Model of PRRSV NADC31 vOTU’s interaction with hISG15.
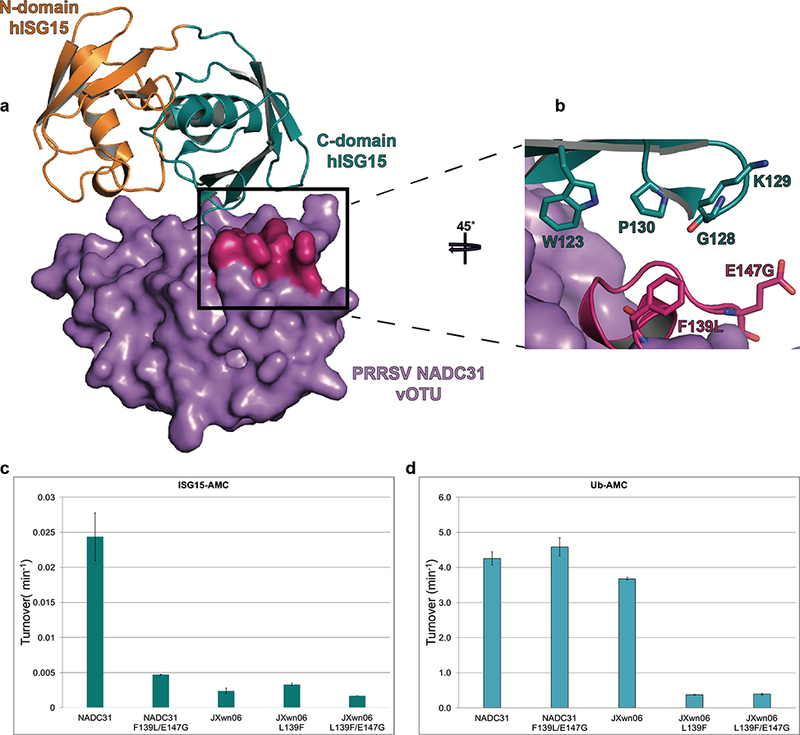
(a) A surface rendering of a homology model for PRRSV NADC31 vOTU (purple), built from EAV PLP2 (PDB 4IUM), and the potential interactions with hISG15 (PDB 1Z2M; C-terminal domain in teal and N-terminal domain in orange). The possible interface between the vOTU and ChISG15 is shaded in magenta. (b) A zoomed in view of the potential interaction between PRRSV NADC31 vOTU and the ChISG15. Residues potentially involved in the interaction have been rendered as sticks. (c,d) Enzyme data, presented as turnover (min−1), for the different wild-type PRRSV vOTU strains and their associated mutations for different AMC conjugated substrates. The enzyme data of these strains and mutants was compared to PRRSV strain JXwn06.15
Conclusion
In summary, two Type 2 PRRSV vOTUs, NADC31 (low virulence) and SDSU73 (moderate virulence), were biochemically characterized and compared to PRRSV vOTUs originating from the avirulent vaccine derivative Ingelvac PRRS® MLV and the highly pathogenic PRRSV strain JXwn06. Although vOTUs comprise only one virulence factor within PRRSV, the di-Ub specificity studies of these two newly characterized vOTUs further supported the correlation between these proteases preference for K63-linked poly-Ub and the individual PRRSV stain’s reported virulence. This suggests that removal of K63-linked poly-Ub, which is directly involved in the induction of the Type I IFN innate immune response, may play a key role in the pathogenicity of PRRSV. Unexpectedly, the vOTU originating from the PRRSV NADC31 strain was observed to have noticeably higher deISGylase activity then those originating from other strains. This highlights that deISGylase activity may also vary strain-to-strain reconciling a disconnect in earlier studies.15,41. Utilizing the wealth of biochemical data pertaining to vOTUs from NADC31, SDSU73, JXwn06, and MLV along with PRRSV homology models, a specific amino acid, S82, was implicated to considerably contribute to the robust preference of certain PRRSV vOTUs for immunologically important K63 poly-Ub moieties. Similarly, amino acids, E139 and F147, were observed to play a role in relatively robust deISGylase activity in the vOTU originating from NADC31. Naturally, this provides the first insight into the molecular underpinnings of PRRSV vOTU substrate preference potentially enhancing the ability to predict vOTU substrate preference. In addition, this provides the foundation for the development of the first molecular tools to begin unraveling the contributions of PRRSV vOTUs to PRRSV pathogenicity, specifically, the processing of K63 poly-Ub moieties as well as the possible contributions of PRRSV vOTU deISGylase activity to viral evasion.
Experimental Methods
Expression and Purification of vOTU Domains Originating from PRRSV JXwn06, MLV, NADC31, and SDSU73
Constructs for the vOTU domains originating from PRRSV NADC31 and SDSU73 were constructed and expressed as previously described for the PRRSV vOTU from JXwn06 and MLV.15 Briefly, PRRSV vOTUs from JXwn06, MLV, NADC31, and SDSU73 were transformed into E. coli BL21 (DE3) competent cells (New England Biolabs) via heat shock. The cells were grown to OD600 of 0.6 at 37 °C in LB broth supplemented with 100 μg/mL ampicillin. Expression was induced with 1 mM IPTG and incubated for an additional 12 h at 18 °C. Cells were harvested via centrifugation at 6000g for 10 min and stored at −80 °C for subsequent purification. For purification, the cell pellets were resuspended in Buffer A (5 M guanidine, 500 mM NaCl, 100 mM Tris [pH 7.5], 10 % (v/v) glycerol), supplemented with lysozyme, for 30 min at 4 °C. The cells were then placed on ice and lysed by sonication at 80% power with a 50% duty cycle for 4 rounds, each 2 min in length. The lysate was then centrifuged at 70600g for 30 min at 4 °C with the supernatant being removed and filtered for further purification. The filtered supernatant was subjected to high density nickel agarose beads (Gold Biotechnology, Olivette, MO) pre-equilibrated with cold Buffer A. The column was washed with 5 CV of 30 mM imidazole in Buffer A. The protein was eluted from the column utilizing 300 mM imidazole in Buffer A, which was then dialyzed overnight in Buffer B (1 M L-Arg, 100 mM NaCl, 100 mM Tris [pH 7.5], 0.1 mM ZnCl2) at 4 °C. The protein was dialyzed for a second time into Buffer C (300 mM NaCl, 20 mM Tris [pH 7.5], 0.1 mM ZnCl2, 2 mM DTT, and 5% (v/v) glycerol) for a minimum of 12 h at 4 °C, and then concentrated to ~1 mL. The protein was further purified by size-exclusion chromatography using a Superdex 200 column (GE Healthcare, Pittsburgh, PA) equilibrated with Buffer D (150 mM NaCl, 10 mM HEPES [pH 7.5], 0.1 mM 0.1 mM ZnCl2, 2 mM DTT). The purified protein was collected and used for subsequent assays.
Generation of PRRSV vOTU Mutants
Utilizing the manufacturer’s protocol for the QuickChange Lightening site-directed mutagenesis kit (Agilent Technologies Inc.), mutations were introduced into the PRRSV vOTU domains. The resulting plasmids were transformed into E. coli NEB-5α cells by heat shock, confirmed by sequencing and subsequently transformed into BL21 (DE3) cells for further expression and purification.
Analysis of PRRSV vOTU Specific Enzymatic Activity
To assess purified wild-type and mutant PRRSV vOTUs activity, fluorescence assays were performed as described previously.15 Briefly, purified PRRSV vOTUs were tested against Ub, ISG15, and Z-RLRGG peptide conjugated to 7-amino-4-methyl-coumarin (AMC) and di-Ub fluorescence resonance energy transfer (FRET) linkage substrates K11, K48, and K63 (Boston Biochem, MA). Assays were performed in duplicate as a 50 ∝1 reaction in Buffer E (100 mM NAC1, 50 mM HEPES [pH 7.5], 0.01 mg/mL bovine serum albumin (BSA), 5 mM DTT]. For assays with Ub- and ISG15-AMC, the substrates were present at 1 μM, the ZRLRGG-AMC was present at 50 μΜ, and the enzyme was present at 4 nM, 1 μΜ, and 4 μΜ respectively. The turnover rates were determined by monitoring the increase in fluorescence of AMC upon cleavage from the substrates. The di-Ub FRET assays were performed in a similar fashion with the substrates present at 1 μΜ and the enzyme present at 50 nM. The turnover was determined by monitoring the increase in fluorescence resulting from the FRET TAMRA/QXL pair being separated.
di-Ub Cleavage Assays
di-Ub linkages K6, K11, K29, K33, K48, and K63 and N-terminal linear forms were purchased from Boston Biochem, MA, and K27 was from Ubiquigent, Dundee, UK, in order to perform cleavage assays as previously described.15,54 For the 70 μL assay performed in Buffer F (100 mM NaCl, 50 mM HEPES, [pH 7.5], and 2 mM DTT), the substrates were present at 10 μΜ and incubated at 37 °C with each PRRSV vOTU present at 500 nM. The reactions were stopped at 7 different time points (0, 1, 2, 5, 10, 30, and 60 min) by mixing 9 μL of sample with 9 μL of 2 X SDS-Tricine sample buffer and boiled for 5 min at 95 °C. Samples were subsequently analyzed by SDS-PAGE on 10–20% Mini-Protean Tris-Tricine precast gels (Bio-Rad, Hercules, CA).
Supplementary Material
Footnotes
Publisher's Disclaimer: Disclaimer: Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
References
- 1.Albina E Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet. Microbiol 1997, 55, 309–316 10.1016/S0378-1135(96)013223. [DOI] [PubMed] [Google Scholar]
- 2.Guo B; Lager KM; Schlink SN; Kehrli ME Jr.; Brockmeier SL; Miller LC; Swenson SL; Faaberg KS Chinese and Vietnamese strains of HP-PRRSV cause different pathogenic outcomes in United States high health swine. Virology 2013, 446, 238–250 10.1016/j.virol.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Neumann EJ; Kliebenstein JB; Johnson CD; Mabry JW; Bush EJ; Seitzinger AH; Green AL; Zimmerman JJ Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc 2005, 227, 385–392 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- 4.Wu J; Li J; Tian F; Ren S; Yu M; Chen J; Lan Z; Zhang X; Yoo D; Wang J Genetic variation and pathogenicity of highly virulent porcine reproductive and respiratory syndrome virus emerging in China. Arch. Virol 2009, 154, 1589–1597 10.1007/s00705-009-0478-6. [DOI] [PubMed] [Google Scholar]
- 5.Li BX; Ge JW; Li YJ Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology 2007, 365, 166–172 10.1016/j.virol.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akutsu M; Ye Y; Virdee S; Chin JW; Komander D Molecular basis for ubiquitin and ISG15 cross-reactivity in viral ovarian tumor domains. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 2228–2233 10.1073/pnas.1015287108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtkamp D, and Kliebenstein J (2011) Industry Study: Assessment of the economic impact of Porcine Reproductive and Respiratory Syndrome Virus on U.S. Pork Producers; https://www.pork.org/wp-content/uploads/2011/08/10-158-HOLTKAMP-ISU.pdf.
- 8.Holtkamp DJ; Kliebenstein JB; Neumann EJ; Zimmerman JJ; Rotto HF; Yoder TK; Wang C; Yeske PE; Mowrer CL; Haley CA Assessment of the economic impact of porcine reproductive and respiratorysyndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar]
- 9.Brockmeier SL; Loving CL; Vorwald AC; Kehrli ME Jr.; Baker RB; Nicholson TL; Lager KM; Miller LC; Faaberg KS Genomic sequence and virulence comparison of four Type 2 porcine reproductive and respiratory syndrome virus strains. Virus Res. 2012, 169, 212–221 10.1016/j.virusres.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Shi M; Lam TT; Hon CC; Murtaugh MP; Davies PR; Hui RK; Li J; Wong LT; Yip CW; Jiang JW; Leung FC Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol 2010, 84, 8700–8711 10.1128/JVI.02551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappes MA; Faaberg KS PRRSV structure, replication and recombination: Origin of phenotype and genotype diversity. Virology 2015, 479–480 475–486, 10.1016/j.virol.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelsen CJ; Murtaugh MP; Faaberg KS Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol 1999, 73, 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Lobo FJ; Diez-Fuertes F; Segales J; Garcia-Artiga C; Simarro I; Castro JM; Prieto C Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Vet. Microbiol 2011, 154, 58 10.1016/j.vetmic.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Shi M; Lemey P; Singh Brar M; Suchard MA; Murtaugh MP; Carman S; D’Allaire S; Delisle B; Lambert ME; Gagnon CA; Ge L; Qu Y; Yoo D; Holmes EC; Chi-Ching Leung F The spread of type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in North America: a phylogeographic approach. Virology 2013, 447, 146–154 10.1016/j.virol.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Deaton MK; Spear A; Faaberg KS; Pegan SD The vOTU domain of highly-pathogenic porcine reproductive and respiratory syndrome virus displays a differential substrate preference. Virology 2014, 454–455 247–253, 10.1016/j.virol.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y; Kim DY; Ropp S; Steen P; Christopher-Hennings J; Nelson EA; Rowland RR Heterogeneity in Nsp2 of European-like porcine reproductive and respiratory syndrome viruses isolated in the United States. Virus Res. 2004, 100, 229–235 10.1016/j.virusres.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Grebennikova TV; Clouser DF; Vorwald AC; Musienko MI; Mengeling WL; Lager KM; Wesley RD; Biketov SF; Zaberezhny AD; Aliper TI; Nepoklonov EA Genomic characterization of virulent, attenuated, and revertant passages of a North American porcine reproductive and respiratory syndrome virus strain. Virology 2004,321, 383–390 10.1016/j.virol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Collins JE; Benfield DA; Christianson WT; Harris L; Hennings JC; Shaw DP; Goyal SM; McCullough S; Morrison RB; Joo HS et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest 1992, 4, 117–126 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- 19.Opriessnig T; Halbur PG; Yoon KJ; Pogranichniy RM; Harmon KM; Evans R; Key KF; Pallares FJ; Thomas P; Meng XJ Comparison of molecular and biological characteristics of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Ingelvac PRRS MLV), the parent strain of the vaccine (ATCC VR2332), ATCC VR2385, and two recent field isolates of PRRSV. J. Virol 2002, 76, 11837–11844 10.1128/JVI.76.23.11837-11844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J; Wang Y; Faaberg KS Complete genome analysis of RFLP 184 isolates of porcine reproductive and respiratory syndrome virus. Virus Res. 2006, 122, 175–182 10.1016/j.virusres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Li L; Zhao Q; Ge X; Teng K; Kuang Y; Chen Y; Guo X; Yang H Chinese highly pathogenic porcine reproductive and respiratory syndrome virus exhibits more extensive tissue tropism for pigs. Virol. J 2012, 9, 203 10.1186/1743-422X-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mengeling WL; Lager KM; Vorwald AC Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am. J. Vet. Res 1998, 59, 1540–1544. [PubMed] [Google Scholar]
- 23.Guo B; Lager KM; Henningson JN; Miller LC; Schlink SN; Kappes MA; Kehrli ME Jr.; Brockmeier SL; Nicholson TL; Yang HC; Faaberg KS Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013, 435, 372–384 10.1016/j.virol.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wills RW; Zimmerman JJ; Yoon KJ; Swenson SL; Hoffman LJ; McGinley MJ; Hill HT; Platt KB Porcine reproductive and respiratory syndrome virus: routes of excretion. Vet. Microbiol. 1997, 57, 69–81 10.1016/S0378-1135(97)00079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkalay I; Yaron A; Hatzubai A; Orian A; Ciechanover A; Ben-Neriah Y Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. U. S. A 1995, 92, 10599–10603 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z; Hagler J; Palombella VJ; Melandri F; Scherer D; Ballard D; Maniatis T Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995, 9, 1586–1597 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 27.Zeng W; Sun L; Jiang X; Chen X; Hou F; Adhikari A; Xu M; Chen ZJ Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 2010,141,315–330 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beura LK; Sarkar SN; Kwon B; Subramaniam S; Jones C; Pattnaik AK; Osorio FA Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation. Journal of Virology 2010, 84, 1574–1584 10.1128/JVI.01326-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y; Treffers EE; Li Y; Tas A; Sun Z; van der Meer Y; de Ru AH; van Veelen PA; Atkins JF; Snijder EJ; Firth AE Efficient −2 frameshifting by mammalian ribosomes to synthesize an additional arterivirus protein. Proc. Natl. Acad. Sci. U. S. A 2012, 109, E2920–2928 10.1073/pnas.1211145109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song C; Krell P; Yoo D Nonstructural protein 1alpha subunit-based inhibition of NF-kappaB activation and suppression of interferon-beta production by porcine reproductive and respiratory syndrome virus. Virology 2010, 407, 268–280 10.1016/j.virol.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Sun Z; Chen Z; Lawson SR; Fang Y The cysteine protease domain of porcine reproductive and respiratory syndrome virus nonstructural protein 2 possesses deubiquitinating and interferon antagonism functions. Journal of Virology 2010, 84, 7832–7846 10.1128/JVI.00217-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappes MA; Miller CL; Faaberg KS Porcine reproductive and respiratory syndrome virus nonstructural protein 2 (nsp2) topology and selective isoform integration in artificial membranes. Virology 2015,481,51–62 10.1016/j.virol.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Li C; Zhuang J; Wang J; Han L; Sun Z; Xiao Y; Ji G; Li Y; Tan F; Li X; Tian K Outbreak Investigation of NADC30-Like PrRSV in South-East China. Transboundary Emerging Dis. 2016, 63, 474–479 10.1111/tbed.12530. [DOI] [PubMed] [Google Scholar]
- 34.Wang FX; Song N; Chen LZ; Cheng SP; Wu H; Wen YJ Non-structural protein 2 of the porcine reproductive and respiratory syndrome (PRRS) virus: A crucial protein in viral pathogenesis, immunity and diagnosis. Res. Vet. Sci 2013, 95, 1 10.1016/j.rvsc.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y; Han M; Kim C; Calvert JG; Yoo D Interplay between interferonmediated innate immunity and porcine reproductive and respiratory syndrome virus. Viruses 2012, 4, 424–446 10.3390/v4040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo D; Song C; Sun Y; Du Y; Kim O; Liu HC Modulation of host cell responses and evasion strategies for porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 48–60 10.1016/j.virusres.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allende R; Lewis TL; Lu Z; Rock DL; Kutish GF; Ali A; Doster AR; Osorio FA North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol 1999, 80 (Pt 2), 307–315 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- 38.van Kasteren PB; Beugeling C; Ninaber DK; Frias-Staheli N; van Boheemen S; Garcia-Sastre A; Snijder EJ; Kikkert M Arterivirus and nairovirus ovarian tumor domain-containing Deubiquitinases target activated RIG-I to control innate immune signaling. J. Virol 2012, 86, 773–785 10.1128/JVI.06277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faaberg KS; Han J; Wang Y Molecular dissection of porcine reproductive and respiratory virus putative nonstructural protein 2. Adv. Exp. Med. Biol 2006, 581, 73–77 10.1007/978-0-387-33012-99__11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kappes MA; Miller CL; Faaberg KS Highly divergent strains of porcine reproductive and respiratory syndrome virus incorporate multiple isoforms of nonstructural protein 2 into virions. J. Virol 2013, 87,13456–13465 10.1128/JVI.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frias-Staheli N; Giannakopoulos NV; Kikkert M; Taylor SL; Bridgen A; Paragas J; Richt JA, Rowland RR; Schmaljohn CS; Lenschow DJ; Snijder EJ; Garcia-Sastre A; Virgin HW t. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe 2007, 2, 404–416 10.1016/j.chom.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han J; Rutherford MS; Faaberg KS The porcine reproductive and respiratory syndrome virus nsp2 cysteine protease domain possesses both trans- and cis-cleavage activities. Journal of Virology 2009, 83, 9449–9463 10.1128JVI.0083409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mielech AM; Chen Y; Mesecar AD; Baker SC Nidovirus papain-like proteases: multifunctional enzymes with protease, deubiquitinating and deISGylating activities. Virus Res. 2014,194,184–190 10.1016/j.virusres.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziebuhr J; Snijder EJ; Gorbalenya AE Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol 2000, 81, 853–879 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 45.Mielech AM; Deng X; Chen Y; Kindler E; Wheeler DL; Mesecar AD; Thiel V; Perlman S; Baker SC Murine coronavirus ubiquitin-like domain is important for papain-like protease stability and viral pathogenesis. J. Virol 2015, 89, 4907–4917 10.1128/JVI.00338-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mielech AM; Kilianski A; Baez-Santos YM; Mesecar AD; Baker SC MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology 2014, 450–45164–70, 10.1016/j.virol.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulathu Y; Komander D Atypical ubiquitylation - the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol 2012, 13, 508–523 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- 48.Komander D; Clague MJ; Urbe S Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol 2009, 10, 550–563 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 49.Castaneda CA; Kashyap TR; Nakasone MA; Krueger S; Fushman D Unique structural, dynamical, and functional properties of k11-linked polyubiquitin chains. Structure 2013, 21, 1168–1181 10.1016/j.str.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dynek JN; Goncharov T; Dueber EC; Fedorova AV; Izrael-Tomasevic A; Phu L; Helgason E; Fairbrother WJ; Deshayes K; Kirkpatrick DS; Vucic D c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J.2010, 29, 4198–4209 10.1038/emboj.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skaug B; Jiang X; Chen ZJ The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem.2009,78,769–796 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 52.Wang D; Fan J; Fang L; Luo R; Ouyang H; Ouyang C; Zhang H; Chen H; Li K; Xiao S The nonstructural protein 11 of porcine reproductive and respiratory syndrome virus inhibits NF-kappaB signaling by means of its deubiquitinating activity. Mol. Immunol 2015, 68, 357–366 10.1016/j.molimm.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Z; Chen Z; Lawson SR; Fang Y The cysteine protease domain of porcine reproductive and respiratory syndrome virus nonstructural protein 2 possesses deubiquitinating and interferon antagonism functions. J. Virol 2010, 84, 7832–7846 10.1128/JVI.00217-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capodagli GC; Deaton MK; Baker EA; Lumpkin RJ; Pegan SD Diversity of Ubiquitin and ISG15 Specificity among Nairoviruse—s’’ Viral Ovarian Tumor Domain Proteases. Journal of Virology 2013, 87, 3815–3827 10.1128/JVI.03252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou L; Zhang J; Zeng J; Yin S; Li Y; Zheng L; Guo X; Ge X; Yang H The 30-amino-acid deletion in the nsp2 of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China is not related to its virulence. Journal ofVirology 2009, 83, 5156–5167. 10.1128/JVI.02678-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sali A; Blundell TL Comparative Protein Modeling by Satisfaction of Spatial Restraints. J. Mol. Biol 1993, 234, 779–815 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 57.van Kasteren PB; Bailey-Elkin BA; James TW; Ninaber DK; Beugeling C; Khajehpour M; Snijder EJ; Mark BL; Kikkert M Deubiquitinase function of arterivirus papain-like protease 2 suppresses the innate immune response in infected host cells. Proc. Natl. Acad. Sci. U. S. A 2013, 110, E838–847 10.1073/pnas.1218464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weeks SD; Grasty KC; Hernandez-Cuebas L; Loll PJ Crystal structures of Lys-63-linked tri- and di-ubiquitin reveal a highly extended chain architecture. Proteins: Struct., Funct., Genet 2009, 77, 753–759 10.1002/prot.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narasimhan J; Wang M; Fu Z; Klein JM; Haas AL; Kim JJ Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J. Biol. Chem 2005, 280, 27356–27365 10.1074/jbc.M502814200. [DOI] [PubMed] [Google Scholar]
- 60.Kearse M; Moir R; Wilson A; Stones-Havas S; Cheung M; Sturrock S; Buxton S; Cooper A; Markowitz S; Duran C; Thierer T; Ashton B; Mentjies P; Drummond A Geneious Basic: an integrated and extendable desktop software platform for Bioinformatics 2012, 28, 1647–1649 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


