Summary
Objective
Pretreatment with lithium (Li) is associated with an increased residence time of radioactive iodine (RAI) in differentiated thyroid cancer (DTC) metastases. There are no data translating this observation into long-term outcomes. The study goal was to compare the efficacy of three methods of preparation for RAI therapy in metastatic DTC – thyroid hormone withdrawal (THW), THW with pre-treatment with Li (THW+Li), and recombinant human TSH (rhTSH).
Design/Patients/Measurements
We performed a cohort study comparing overall survival (OS) and progression-free survival (PFS) between the three groups: THW(n=52), THW+Li (n=41) and rhTSH (n=42). Kaplan-Meier analyses were performed to compare OS and PFS between the groups. Cox proportional hazards regression model with a stepwise variable selection was performed to study the contribution of age, gender, histology, TNM status, a location of distant metastases and RAI dose.
Results
During the follow up of median 5.1 (IQR=3.0–8.1) years, 52% of patients had disease progression and 12.6% died. Although THW+Li group was characterized by the longest OS (p=0.007), only age (HR 1.05, CI 1.01–1.09, p=0.01) and widespread disease (HR3.8, CI 1.2–11.8, p=0.02) were found to affect OS in a multivariate model. There was no difference in PFS between the groups (p=0.47). Presence of distant metastases limited to the lungs only was associated with longer PFS (PFS HR 0.35, CI 0.20–0.60, p=0.0002).
Conclusion
The older age is associated with shorter OS, while disease burden affects OS and PFS in patients with metastatic thyroid cancer. The method of preparation for RAI therapy does not affect the outcome.
Keywords: thyroid cancer, lithium, radioactive iodine, dosimetry
Introduction
The routine management of patients with differentiated thyroid cancer (DTC) presenting with distant metastases consists of total thyroidectomy with or without lymph node dissection, as appropriate, followed by therapy with radioiodine (RAI). RAI dosage could be administered as either (1) empiric fixed dose, which for high risk patients with distant metastases, varies between 100 to 200 mCi or (2) based on dosimetry calculations enabling therapy with the maximum safe activity not exceeding 200 rads to the bone marrow 1–3. The dosimetry-based individualized approach is especially important for older patients and children in whom empiric activity might exceed safe radiation limits 4,5. Administration of RAI requires thyrotropin (TSH) stimulation which may be achieved by two possible methods: (1) thyroid hormone withdrawal (THW) to provoke endogenous TSH elevation or (2) exogenous stimulation by recombinant human TSH (rhTSH).
While rhTSH is approved and widely used for thyroid remnant ablation, there is still a controversy regarding its use in preparation for RAI treatment of distant metastases. In fact, current 2015 American Thyroid Association guidelines state that “in patients with ATA high risk DTC with attendant higher risks of disease-related mortality and morbidity, more controlled data from long-term outcome studies are needed before rhTSH preparation for RAI adjuvant treatment can be recommended. (No recommendation, Insufficient evidence)” 1. The potential benefit of using rhTSH-aided RAI treatment is to avoid the effects of hypothyroidism that might be poorly tolerated especially by older individuals or patients with comorbidities. The additional advantage is more rapid whole-body clearance of RAI after rhTSH, which results in a lower total-body, bone-marrow, and gastrointestinal radiation exposure for a given administered activity 6. On the other hand, there are several studies showing lower RAI uptake in metastatic lesions after preparation with rhTSH compared with THW 7–10. Therefore, rhTSH-aided therapy for metastatic thyroid cancer is currently not FDA approved, and its administration is performed off label or via compassionate use program.
Treatment with lithium has been associated with increased uptake and residence time of RAI in metastatic lesions 11. The initial pilot study involving 15 patients with thyroid cancer metastases, performed in the National Institutes of Health, revealed that the mean lithium-induced increase in the biological retention half-life in tumors was 50% and occurred in at least 1 lesion in each patient leading to increased accumulation of RAI in metastatic lesions by an average of 2.3 +/− 0.6 times 11. The potential translation of these findings to the effect on patients’ outcomes is unknown. Therefore, current 2015 American Thyroid Association guidelines state that the data are insufficient to recommend lithium therapy as an adjunct to RAI treatment 1. These unknowns formed the rationale for our study, which compared the relative efficacy of three methods of preparation for RAI therapy of metastatic DTC: 1) THW; 2) THW with pre-treatment with therapeutic dose of lithium for 7 days; and 3) rhTSH.
Methods
Study design and population
We performed a cohort study at the National Institute of Health (NIH) including patients with DTC presenting with RAI avid distant metastases, who after total thyroidectomy underwent repeated dosimetry-based RAI therapies under the preparation with THW and administration of lithium carbonate for 7 days before RAI, and followed for median 5.7 (3.5–9.5) years. We retrospectively analyzed the medical records of this cohort and compared it to control groups of retrospective cohorts of patients with metastatic DTC treated and monitored at Medstar Washington Hospital Center (WHC), Washington DC between 1996 to 2016 and fulfilling the following inclusion criteria: 1) confirmed diagnosis of DTC after total or near total thyroidectomy; 2) evidence of iodine-avid distant metastases based on I131 whole body scans (WBS) 3) full quantitative dosimetry studies provided before treatment with RAI enabling treatment with maximum safe RAI dose not exceeding 200 rads to the bone marrow and 80 mCi 48h retention in the lungs; 4) at least one complete follow-up examination including suppressed and/or stimulated Tg levels, ultrasound of the neck, I123 or I131 whole body scan, chest CT, or other imaging techniques as appropriate performed every 6–12 months after treatment. Patients with evidence of RAI-non-avid disease were excluded from the study. The study protocol was approved by the Institutional Review Boards of NIDDK/NIAMS and Medstar WHC.
Exposures
The NIH cohort was prepared for each radioactive iodine treatment with thyroid hormone withdrawal for 6 weeks - with triiodothyronine supplementation for the first 4 weeks, and no thyroid hormone therapy and low iodine diet for the last 2 weeks of this period. The patients were admitted to the hospital for 7–10 days and treated with lithium carbonate 600 mg orally as a loading dose followed by 300 mg po BID with dose adjustments to target serum Li level of 0.6–1.2 mEq/ml. Lithium levels were measured daily. Therapeutic RAI dosage not exceeding a maximum tolerated activity (MTA) was determined by a standard dosimetry protocol 12–14. Briefly, calculation of MTA was based on measurements of kinetics of a tracer-prescribed activity of 131I [1–2 mCi) distributed in two compartments: 1) β-component due to activity in the blood; and 2) the γ-ray contribution from activity throughout the whole body. After administration of the tracer activity of 131I, heparinized blood samples were obtained at 2–4, 24, 48, 72, 96 h to assess β-radiation and WBSs were performed at approximately the same time points to measure γ-radiation 12,13. The MTA was calculated by a radiation physicist using a standard formula 12,13 and the patients were treated with the MTA of RAI corrected by 10–20%. The post-therapy scan was performed approximately one week following the therapeutic dose of 131I.
The control groups were prepared for RAI therapy with either rhTSH (rhTSH group) 0.9 mg i.m on 2 consecutive days or 4–6 weeks of thyroid hormone withdrawal (THW group) and underwent similar dosimetry calculations of MTA before rhTSH- or THW-aided RAI therapy 14.
All patients adhered to a low iodine diet for at least 2 weeks prior to the treatment with the goal urine iodine of <100mcg/l.
Covariates
Information on age at diagnosis, gender, histology subtype, TNM status, number of metastatic lymph nodes involved, completeness of surgical resection, and RAI dose was extracted from medical records. Distant metastases were categorized into: (1) only micronodular pulmonary metastases, (2) pulmonary micro- and macro-metastases without other organs involved, (3) bone metastases (including spine) with or without pulmonary metastases, and (4) multiple organ involvement with additional metastatic foci in the skin, brain, pleura, etc.
Outcomes
The primary outcomes were overall survival (OS) and progression free survival (PFS). PFS was defined as time from initial diagnosis to the first evidence of structural disease progression, defined per RECIST 1.1 criteria as at least a 20% increase in the sum of three diameters of target lesions taking as a reference the smallest sum on the study and an absolute increase of at least 5 mm when a total sum is very small or appearance of one or more new lesions 15,16. To objectively assess overall survival the individual medical records of patients with thyroid cancer who died during follow-up were reviewed.
The secondary outcome was the best overall response to treatment, which according to 2015 ATA guidelines was categorized as : 1) excellent response (ER) - negative imaging and suppressed Tg <0.2 ng/mL or stimulated Tg < 1 ng/mL, 2) biochemical incomplete response (BIR) - negative imaging and suppressed Tg > 1 ng/mL or stimulated Tg > 10 ng/mL or rising anti-Tg Ab levels, 3) structural incomplete response (SIR) -structural or functional evidence of disease with any Tg level+/− Tg Ab, 4) indeterminate response (IR) - non-specific findings on imaging studies, faint uptake in thyroid bed on RAI scanning, non-stimulated Tg detectable, but less than 1 ng/mL, stimulated Tg detectable, but less than 10 ng/mL or Tg antibodies stable or declining in the absence of structural or functional disease1.
Statistical analysis
The baseline demographics and clinical characteristics were summarized using either median with interquartile range (IQR) or proportions as appropriate and were compared between the groups using Kruskal-Wallis test for continuous variables and Chi-square tests for categorical variables. Kaplan-Meier survival analyses were performed to compare time to progression (Progression Free Survival - PFS) and death (Overall Survival - OS) between the groups. Cox proportional hazards regression model was performed to study the contribution of age, gender, histology, TNM status, location of distant metastases and RAI dose with a stepwise variable selection for PFS and OS respectively. Estimated Hazard ratios (HR) with corresponding 95% confidence intervals (CI) were reported using the final model. All analyses were two-tailed tests based on α = 0.05 and conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Out of 141 patients with metastatic DTC, 6 patients with initially positive RAI uptake in metastatic lesions, had subsequently developed RAI non-avid metastatic disease. These patients were excluded from the study. Diagnostic and/or post-treatment I131 WBS performed during follow up confirmed RAI-avid disease in all remaining patients. The study cohort consisted of 135 DTC patients (82 women, 53 men) with RAI-avid distant metastases, treated with median cumulative RAI activity of 419 (IQR= (245–755) mCi given in 1–8 divided dosages and prepared for RAI with either THW (n=52), THW+Li (n=41) or rhTSH (n=42). There was no difference in a gender breakdown, tumor size and presence of gross extrathyroidal extension (Table 1). The rhTSH group was characterized by the oldest age at diagnosis, the highest prevalence of follicular thyroid cancer and bone metastases as well as the lowest prevalence of disease confined to the lungs only, while the THW+Li group was characterized by the highest prevalence of tall cell variant of papillary thyroid cancer. Both rhTSH and THW+Li groups were characterized by similar, and higher than THW group, prevalence of widespread disease with multiple organ involvement (Table 1). The duration of follow up was similar between the study groups (Table 1). During follow up, THW+Li group received the highest cumulative RAI dosage (Table 1). The highest cumulative dosage most likely relates to the largest number of repeated yearly RAI therapies - median of 4 (p<0.001), (Table 1).
Table 1.
Baseline characteristics of the study groups.
| Baseline Characteristics | Overall (n=135) | THW (n=52) | THW+Li (n=41) | rhTSH (n=42) | P-value# |
|---|---|---|---|---|---|
| Age at Diagnosis (median, IQR) years | 50 (33–62) | 49 (33–62) | 39 (32–53) | 53 (48–68) | < 0.001 |
| Gender (no. of patients, %) | |||||
| Female | 82 (60.7) | 31 (59.6) | 28 (68.3) | 23 (54.8) | 0.440 |
| Histology (no. of patients, %) | < 0.010 | ||||
| Follicular Thyroid Cancer | 22 (16.3%) | 4 (7.5%) | 6 (14.6%) | 12 (28.6%) | |
| Hurtle Cell Thyroid Cancer | 9 (6.7%) | 6 (11.3%) | 0 (0.0%) | 3 (7.1%) | |
| Foci of Poorly Differentiated Thyroid Cancer | 8 (5.9%) | 4 (7.5%) | 3 (7.3%) | 1 (2.4%) | |
| Papillary Thyroid Cancer (Tall Cell) | 13 (9.6%) | 2 (3.8%) | 10 (24.4%) | 1 (2.4%) | |
| Papillary Thyroid Cancer (Follicular Variant) | 25 (18.5%) | 9 (17.0%) | 10 (24.4%) | 7 (16.7%) | |
| Papillary Thyroid Cancer (Classic) | 58 (43.0%) | 28 (52.8%) | 12 (29.3%) | 18 (42.9%) | |
| Tumor Size (median, IQR) cm | 3.5 (2.0–5.0) | 3.7 (2.9–5.25) | 3.7 (2.1 –5) | 2.7 (1.9–5.5) | 0.770 |
| Metastases (no. of patients, %) | |||||
| Pulmonary micro-metastases (< 1.0 cm) only | 44 (32.6) | 24 (46.2) | 12 (29.3) | 8 (19.0) | 0.018 |
| Pulmonary metastases only | 63 (46.7) | 33 (63.5) | 18 (43.9) | 12 (28.6) | 0.003 |
| Bone with or without pulmonary metastases | 34 (25.2) | 12 (23.1) | 6(14.6) | 16(38.1) | 0.042 |
| Additional metastatic foci (brain, pleura, skin) | 31 (23.0) | 5 (9.6) | 14 (34.1) | 12 (28.6) | 0.011 |
| Gross extrathyroidal extension | 40 (29.6) | 16 (30.8) | 12 (29.3) | 12 (28.6) | 0.875 |
| Cumulative RAI Dose (median, IQR) mCi | 419 (245–755) | 406 (220–625) | 934.6 (400–1200) | 335 (178–489.5) | < 0.0001 |
| Number of repeated RAI dosages (median, IQR) | 2 (1–3) | 2 (1–5) | 4 (1–8) | 1 (1–3) | <0.0001 |
| Duration of follow up (median, IQR) years | 5.1 (3.0–8.1) | 5.7 (3.5–9.5) | 4.5 (2.3–9.7) | 4.5 (2.0–6.3) | 0.138 |
P value refers to a difference between THW, THW+Li and rhTSH groups; IQR – 25–75 percentiles
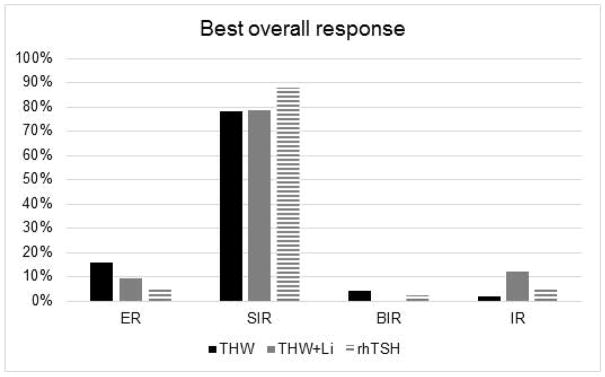
There was no difference in the best overall response to treatment between the study groups (p=0.11), (Figure 1). Minority of patients in each group had excellent response to treatment - THW 9/52 (17.3%) vs THW+Li 3/41 (7.3%) vs rhTSH 2/42 (4.8%).
Figure 1.
No difference in best overall response between the study groups. ER – excellent response, SIR – structural incomplete response, BIR- biochemical incomplete response, IR – indeterminate response.
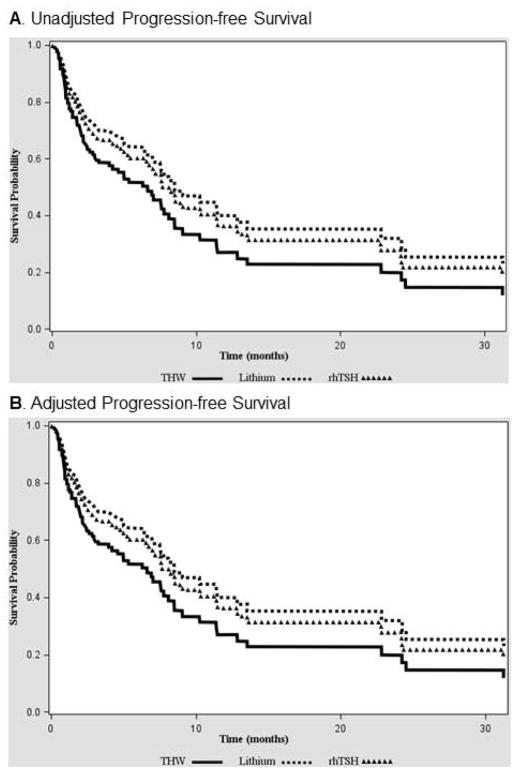
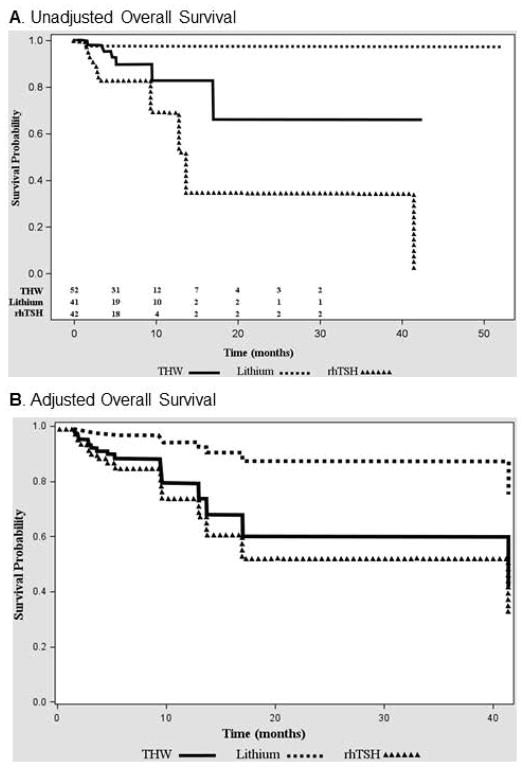
During the follow up of 5.1 (IQR=3.0–8.1 years), 52% of patients (70/135) had disease progression and 12.6% of patients (n=17/135) died. There was no difference in PFS between the study groups (p=0.47) (Figure 2A). The stepwise Cox proportional model, utilizing baseline characteristics and RAI dose as covariates, revealed that the factor independently associated with better PFS is presence of metastatic disease confined only to pulmonary metastases (HR 0.35 (0.20– 0.60), p=0.0002), (Table 2, Figure 2B). THW+Li group was characterized by the longest OS (p=0.007) (Figure 3A). However, after adjusting by the known factors affecting the OS, only age (HR 1.05, CI 1.01 – 1.09, p=0.01) and disease burden (HR 3.83, CI 1.24–11.78, p=0.02) remained statistically significant, while the method of preparation showed a trend towards improved OS in THW+Li vs rhTSH group (HR 0.16, CI 0.02–1.3, p= 0.08) (Table 2, Figure 3B).
Figure 2.
No difference in progression free survival between the study groups. A) Unadjusted Kaplan-Meier survival curves, B) Survival curves adjusted by presence of solely pulmonary metastases - the factor selected in logistic regression model as affecting PFS.
Table 2.
Stepwise variable selection for PFS and OS. Age and presence of widespread disease with metastases to the brain, spine, kidney and skin are independent prognostic factors associated with decreased overall survival. Disease confined to the pulmonary metastases is associated with better progression free survival
| Characteristics | Progression-Free Survival Adjusted HR (95% CI) |
P-value | Overall Survival Adjusted HR (95% CI) |
P-value |
|---|---|---|---|---|
| Group | ||||
| Lithium vs. THW | 0.66 (0.36 – 1.21) | 0.18 | 0.21 (0.03 – 1.82) | 0.16 |
| Lithium vs rhTSH | 0.88 (0.48 – 1.61) | 0.68 | 0.16 (0.02 – 1.30) | 0.08 |
| THW vs rhTSH | 1.34 (0.73 – 2.47) | 0.34 | 0.74 (0.25 – 2.19) | 0.58 |
|
| ||||
| Age at Diagnosis | NA | NA | 1.05 (1.01 – 1.09) | 0.01 |
|
| ||||
| Lung metastasis only | 0.35 (0.20– 0.60) | 0.0002 | NA | NA |
|
| ||||
| Widespread disease (lung, bone and others) | NA | NA | 3.83 (1.24–11.78) | 0.02 |
Figure 3.
A) Significantly longer OS in THW-Li group compared with rhTSH group (p=0.007). B) No significant difference in OS THW+Li group compared with rhTSH and THW group after adjustment for factors significantly affecting OS – age and presence of widespread disease with metastases affecting multiple organs, not only the lungs and the bones.
Discussion
We performed a first cohort study aiming at evaluation of long-term outcomes in patients with metastatic DTC prepared for dosimetry based RAI therapy with thyroid hormone withdrawal with an adjunct of 7 days therapy with lithium carbonate. We hypothesized that the addition of Li, which is associated with increased RAI residence time in metastatic lesions 11, may result in improved outcomes. To test this hypothesis, we compared the results of this cohort study to the long-term outcomes of two retrospective cohorts of patients treated in another tertiary referral center and prepared for dosimetry-based RAI therapy either with THW or rhTSH. Interestingly, patients from THW+Li group were treated with the highest cumulative RAI activity. This observation is most likely related to the fact that THW+Li group received the largest number of repeated RAI dosages - median 4, (range 1–8) compared with median 2 (range 1–5) in THW group and median 1 (range 1–3) in rhTSH group. The prospective design of THW+Li cohort could have let to more systematic administration of repeated dosimetry-based RAI dosages for persistent RAI-avid disease, while follow up and repeated therapy scheme could have been less stringently controlled in two retrospective control cohorts of patients, despite the similar duration of follow up in all study groups.
Consistently with previously published data, we found no difference in best overall response to treatment, PFS or OS in patients prepared for RAI therapy either with rhTSH or THW 17,18. This observation is concordant with the results of our previous retrospective study involving 56 patients with metastatic DTC, among whom 41 were prepared for RAI therapy with THW and 15 with rhTSH, showing that the method of TSH stimulation did not affect the best overall response to treatment nor PFS. In fact, the only independent factor associated with shorter PFS in that study was patients’ older age at diagnosis 17. The current study found that addition of lithium carbonate to THW is associated with similar best overall response to treatment and PFS as preparation with THW or rhTSH alone. There is only one small retrospective case series study involving 12 patients serving as their own historical controls showing similar results. In that study, the patients who failed previous RAI treatments underwent lithium-aided subsequent RAI therapy, without a significant improvement in disease control 19. Our study documented that the only factor significantly associated with better PFS is presence of metastatic disease confined only to pulmonary metastases. Similarly, Haq et al. documented that metastatic disease confined to the lungs is associated with improved disease specific survival (HR 0.37, CI 0.19–0.72),20. In contrast to lack of superiority of Li-aided RAI therapy in metastatic thyroid cancer patients, Yamazaki et al. documented that addition of Li improved the rate of remnant ablation with 30 mCi of RAI in low risk thyroid cancer patients 21.
Tala et al. in a retrospective study involving 175 metastatic DTC patients, among whom 35 patients were treated with THW-aided RAI therapy, 58 patients with rhTSH-aided RAI and 82 patients with both methods of TSH stimulation, showed that the method of TSH stimulation does not affect the 5- year survival rate 18. This study concluded that in patients with metastatic RAI-avid DTC, age, but not the method of TSH stimulation, was an independent and strong predictor of OS. There are no previous studies focused on the association between preparation for RAI therapy with TSH stimulation combined with lithium carbonate with OS. Our current study shows that endogenous TSH stimulation with adjunct pretreatment with Li is associated with improved OS in patients with metastatic RAI-avid thyroid cancer. However, this observation needs to be interpreted with caution, as the study groups were disbalanced in terms of baseline characteristics, with rhTSH group characterized by the oldest age and both rhTSH and THW+Li group characterized by the most widespread disease. After adjusting by the factors affecting OS, which were found to be significant in a stepwise logistic regression model, only age and disease burden were found to be strong independent predictors of OS. Of note, the group of patients prepared for RAI with THW and Lithium was characterized by the youngest age, most likely contributing to the best OS.
Consistent with our findings, the ATA guidelines also underscore that morbidity and mortality in metastatic thyroid cancer are dependent on distribution and number of sites of metastasis, tumor burden, and age at diagnosis of metastases 1,22–28.
Interestingly, recent large epidemiological data document that melanoma patients exposed to Li therapy were characterized by lower disease-specific mortality than Li non-exposed melanoma patients (4.68/1,000 person-years vs 7.21/1,000 person-years, respectively) 29. These data suggest that Li carbonate may exert some pleiotropic effects in cancer patients. It is worthwhile to speculate that these effects might be related to the molecular signature of certain types of cancer. Of note, both thyroid cancer and melanoma are characterized by a high prevalence of BRAFV600E mutation, leading to activation of Raf/MEK/ERK signaling pathway. Lithium is a well-known inhibitor of glycogen synthase kinase-3β, a downstream target of the Raf/MEK/ERK pathway 30. Recent in vitro studies in leukemia documented that the antineoplastic effects of Li are MEK/ERK-dependent 31. In addition, O’Donovan et al. showed that Li modulates autophagy in esophageal and colorectal cancer leading to improved disease control in vivo when combined with chemotherapeutic agent with more than 50% of the animals achieving long term cure without re-occurrence (> 1-year tumor free) 32. However, relatively short exposure to lithium, of only 7 days preceding each repeated RAI dosage in our cohort, may preclude translation of these observations to our cohort of patients with metastatic thyroid cancer.
Our study has several limitations associated with its design as a cohort study. Moreover, the outcomes of prospective cohort study conducted at the NIH were compared to two retrospective cohorts from another tertiary referral center. Therefore, since there was no randomization, potential confounders are not equally distributed across the study groups. In fact, we do have an evidence that the groups were disbalanced in terms of age and disease burden. To reduce this bias, we included all known confounders such as age, gender, histology, TNM status, localization of distant metastases, RAI dosage in a stepwise logistic regression model, finding that the age and disease burden are key factors affecting OS. Since the number of RAI therapies and cumulative dosage of RAI were significantly higher in THW+Li group, it is difficult to separate potential effects of Li preparation with the intensity of RAI therapy. However, our multivariate model documented that neither RAI activity nor method of preparation for RAI was an independent factor affecting the outcomes. Although our study was the largest to date addressing potential adjuvant role of Lithium therapy in preparation for RAI treatment, it may be underpowered in terms of detection of differences in clinical outcomes. The other limitation is a lack of additional group of patients prepared for RAI with rhTSH combined with lithium.
Conclusion
The older age at diagnosis is associated with shorter OS, while disease burden affects both OS and PFS in patients with metastatic differentiated thyroid cancer. The method of preparation for RAI therapy either by exogenous or endogenous TSH stimulation with or without addition of Lithium does not significanly affect PFS and OS.
Acknowledgments
This work was supported by the NIH intramural grant DK047053. We thank Mathhew Breymayer and Frank Velez for database manegement. Special thanks to Dr Lee Weinstein and Dr Douglas Van Nostrand for critical review of the paper. We thank all our patients for particpation in this study.
References
- 1.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klubo-Gwiezdzinska J, Van Nostrand D, Atkins F, et al. Efficacy of dosimetric versus empiric prescribed activity of 131I for therapy of differentiated thyroid cancer. The Journal of clinical endocrinology and metabolism. 2011;96(10):3217–3225. doi: 10.1210/jc.2011-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deandreis D, Rubino C, Tala H, et al. Comparison of Empiric Versus Whole-Body/-Blood Clearance Dosimetry-Based Approach to Radioactive Iodine Treatment in Patients with Metastases from Differentiated Thyroid Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58(5):717–722. doi: 10.2967/jnumed.116.179606. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni K, Van Nostrand D, Atkins F, Aiken M, Burman K, Wartofsky L. The relative frequency in which empiric dosages of radioiodine would potentially overtreat or undertreat patients who have metastatic well-differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2006;16(10):1019–1023. doi: 10.1089/thy.2006.16.1019. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle RM, Leboeuf R, Robbins RJ, et al. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47(10):1587–1591. [PubMed] [Google Scholar]
- 6.Luster M, Sherman SI, Skarulis MC, et al. Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinoma. European journal of nuclear medicine and molecular imaging. 2003;30(10):1371–1377. doi: 10.1007/s00259-003-1230-1. [DOI] [PubMed] [Google Scholar]
- 7.Taieb D, Jacob T, Zotian E, Mundler O. Lack of efficacy of recombinant human thyrotropin versus thyroid hormone withdrawal for radioiodine therapy imaging in a patient with differentiated thyroid carcinoma lung metastases. Thyroid : official journal of the American Thyroid Association. 2004;14(6):465–467. doi: 10.1089/105072504323150804. [DOI] [PubMed] [Google Scholar]
- 8.Driedger AA, Kotowycz N. Two cases of thyroid carcinoma that were not stimulated by recombinant human thyrotropin. The Journal of clinical endocrinology and metabolism. 2004;89(2):585–590. doi: 10.1210/jc.2003-031650. [DOI] [PubMed] [Google Scholar]
- 9.Van Nostrand D, Khorjekar GR, O’Neil J, et al. Recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal in the identification of metastasis in differentiated thyroid cancer with 131I planar whole-body imaging and 124I PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53(3):359–362. doi: 10.2967/jnumed.111.096016. [DOI] [PubMed] [Google Scholar]
- 10.Plyku D, Hobbs RF, Huang K, et al. Recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal in 124I-PET/CT based dosimetry for 131I therapy of metastatic differentiated thyroid cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017 doi: 10.2967/jnumed.116.179366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koong SS, Reynolds JC, Movius EG, et al. Lithium as a potential adjuvant to 131I therapy of metastatic, well differentiated thyroid carcinoma. The Journal of clinical endocrinology and metabolism. 1999;84(3):912–916. doi: 10.1210/jcem.84.3.5527. [DOI] [PubMed] [Google Scholar]
- 12.Van Nostrand D, Atkins F, Yeganeh F, Acio E, Bursaw R, Wartofsky L. Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2002;12(2):121–134. doi: 10.1089/105072502753522356. [DOI] [PubMed] [Google Scholar]
- 13.Benua RS, Cicale NR, Sonenberg M, Rawson RW. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. The American journal of roentgenology, radium therapy, and nuclear medicine. 1962;87:171–182. [PubMed] [Google Scholar]
- 14.Robbins RJ, Larson SM, Sinha N, et al. A retrospective review of the effectiveness of recombinant human TSH as a preparation for radioiodine thyroid remnant ablation. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2002;43(11):1482–1488. [PubMed] [Google Scholar]
- 15.Goebel J, Hoischen J, Gramsch C, et al. Tumor response assessment: comparison between unstructured free text reporting in routine clinical workflow and computer-aided evaluation based on RECIST 1.1 criteria. Journal of cancer research and clinical oncology. 2017 doi: 10.1007/s00432-017-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer (Oxford, England : 1990) 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Mete M, Jonklaas J, Wartofsky L. Radioiodine treatment of metastatic thyroid cancer: relative efficacy and side effect profile of preparation by thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid : official journal of the American Thyroid Association. 2012;22(3):310–317. doi: 10.1089/thy.2011.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tala H, Robbins R, Fagin JA, Larson SM, Tuttle RM. Five-year survival is similar in thyroid cancer patients with distant metastases prepared for radioactive iodine therapy with either thyroid hormone withdrawal or recombinant human TSH. The Journal of clinical endocrinology and metabolism. 2011;96(7):2105–2111. doi: 10.1210/jc.2011-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YY, van der Pluijm G, Karperien M, et al. Lithium as adjuvant to radioiodine therapy in differentiated thyroid carcinoma: clinical and in vitro studies. Clinical endocrinology. 2006;64(6):617–624. doi: 10.1111/j.1365-2265.2006.02515.x. [DOI] [PubMed] [Google Scholar]
- 20.Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clinical endocrinology. 2005;63(1):87–93. doi: 10.1111/j.1365-2265.2005.02304.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki CA, Padovani RP, Biscolla RP, et al. Lithium as an adjuvant in the postoperative ablation of remnant tissue in low-risk thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2012;22(10):1002–1006. doi: 10.1089/thy.2011.0372. [DOI] [PubMed] [Google Scholar]
- 22.Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. The Journal of clinical endocrinology and metabolism. 1997;82(11):3637–3642. doi: 10.1210/jcem.82.11.4386. [DOI] [PubMed] [Google Scholar]
- 23.Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So. 2004;48(1):12–19. [PubMed] [Google Scholar]
- 24.Shoup M, Stojadinovic A, Nissan A, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. Journal of the American College of Surgeons. 2003;197(2):191–197. doi: 10.1016/S1072-7515(03)00332-6. [DOI] [PubMed] [Google Scholar]
- 25.Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma -- surgery or conventional therapy? Clinical endocrinology. 2002;56(3):377–382. doi: 10.1046/j.1365-2265.2002.01482.x. [DOI] [PubMed] [Google Scholar]
- 26.Pittas AG, Adler M, Fazzari M, et al. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid : official journal of the American Thyroid Association. 2000;10(3):261–268. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 27.Schlumberger M, Challeton C, De Vathaire F, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1996;37(4):598–605. [PubMed] [Google Scholar]
- 28.Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. The Journal of clinical endocrinology and metabolism. 1995;80(7):2041–2045. doi: 10.1210/jcem.80.7.7608252. [DOI] [PubMed] [Google Scholar]
- 29.Asgari MM, Chien AJ, Tsai AL, Fireman B, Quesenberry CP., Jr Association between Lithium Use and Melanoma Risk and Mortality: A Population-Based Study. The Journal of investigative dermatology. 2017;137(10):2087–2091. doi: 10.1016/j.jid.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Molecular cancer therapeutics. 2007;6(3):1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 31.Zassadowski F, Pokorna K, Ferre N, et al. Lithium chloride antileukemic activity in acute promyelocytic leukemia is GSK-3 and MEK/ERK dependent. Leukemia. 2015;29(12):2277–2284. doi: 10.1038/leu.2015.159. [DOI] [PubMed] [Google Scholar]
- 32.O’Donovan TR, Rajendran S, O’Reilly S, O’Sullivan GC, McKenna SL. Lithium Modulates Autophagy in Esophageal and Colorectal Cancer Cells and Enhances the Efficacy of Therapeutic Agents In Vitro and In Vivo. PloS one. 2015;10(8):e0134676. doi: 10.1371/journal.pone.0134676. [DOI] [PMC free article] [PubMed] [Google Scholar]