Abstract
Objectives
The association of diet with inflammation is well documented. Yet, no evidence exists on the relationship between inflammatory potential of the diet and low-grade inflammation (LGI) as measured by a composite score of plasma and cellular biomarkers. The aim of the present study is to assess the association between Dietary Inflammatory Index (DII®) and LGI in a large population-based cohort.
Methods
Cross-sectional analyses were conducted on data from 20,823 subjects (age≥35 years, 48% male) without acute inflammation, recruited within the general population of the Moli-sani study from 2005 to 2010. LGI was measured by using a composite score (INFLA-score) including platelet and leukocyte counts, the granulocyte to lymphocyte ratio and C-reactive protein. DII® scores were computed based on dietary intake assessed by the EPIC food frequency questionnaire. Multivariable linear regression models were fit to produce adjusted regression coefficients and 95% confidence intervals.
Results
Higher DII scores were associated with increased LGI (β=0.131; 95%CI 0.089 to 0.174 for the highest vs lowest quintile of DII) after adjusting for age, sex, lifestyle, prevalence of chronic diseases and health conditions. A higher DII score also was positively associated with each single biomarker of inflammation included in the INFLA-score, unhealthy behaviours (smoking, sedentary behaviour) and insulin.
Conclusions
Higher DII scores, indicating greater inflammatory potential of the diet, were directly associated with LGI, as measured by a composite score of plasma and cellular biomarkers of inflammation. These findings are consistent with the contributing role of diet-mediated inflammation in increasing risk of inflammation-related chronic diseases.
Keywords: dietary inflammatory index, low grade inflammation, Italy, FFQ, Moli-sani study
Graphical Abstract
INTRODUCTION
Dietary components have been shown to be associated with a variety of chronic conditions ranging from cardiovascular diseases to cancer and mental disorders [1–4]. Acute inflammatory response is the process of body’s natural reaction to injury or infection in order to heal wounds and promote tissue regeneration [5,6]. Chronic, low-grade (or subclinical) systemic inflammation is associated with numerous chronic conditions [7,8] and the ironic inability to mount a competent immune response to injury or infection [9]. A number of biomarkers, both circulating (i.e., C-reactive protein (CRP)) and cellular (i.e., leukocyte count) have been associated with the onset of major chronic diseases, such as cardiovascular diseases [10,11]and cancer ( 8, 9) and, more recently, also with higher risk of death [12].
The Mediterranean dietary pattern, which is high in fruits, vegetables, olive oil, whole grains, and fish, and low in red meat and butter, with moderate alcohol intake, has been associated with lower levels of inflammation [13]. By contrast, the Western-type diet, which is high in red meat, high-f at dairy products, and refined grains, has been associated with higher levels of CRP, IL-6 and fibrinogen [14]. Specific nutrients also have consistently been associated with lower levels of inflammation. These include n-3 poly-unsaturated fatty acids (PUFA) [15], fibre [16], vitamin E [17]and β-carotene[18].
The dietary inflammatory index (DII®) was developed by researchers at the University of South Carolina to estimate the overall inflammatory potential of the diet [19]. The DII is based upon an extensive literature search incorporating cell culture, laboratory animal, and epidemiologic studies of the effect of diet on inflammation. Previously, the DII has been shown to be associated with single markers of inflammation; specifically CRP, IL-6, TNF-α levels [20–28]. Specifically, in Italy, the DII has been shown to be associated with several cancers and myocardial infarction [29–36]. To date, no work has been conducted to validate the DII with low-grade inflammation as measured by a composite score of plasma and cellular biomarkers [12]. The objective of the current study is to conduct a cross-sectional analysis to examine the association between DII and low-grade inflammation in the general adult population of the Moli-sani study.
MATERIALS and METHODS
Study population
Data presented here are from the Moli-sani study, a large population-based cohort study that recruited 24,325 men and women aged ≥35 years from the general population of the Molise region, in central-southern Italy in 2005–2010[37].
For the purpose of the present analyses, we excluded individuals with implausible energy intakes (<800 kcal/day in men and <500 kcal/day in women or >4,000 kcal/day in men and >3,500 kcal/day in women; 3.2%), missing data on dietary habits (0.4%) or on markers of low-grade inflammation (3.3%), C-reactive protein levels ≥10 mg/L (4%), unreliable medical or dietary questionnaires (1% and 3.9%, respectively), missing data on main covariates (4.5%). The final sample consisted of 20,823 individuals. The Moli-sani study complies with the Declaration of Helsinki and was approved by the ethical committee of the Catholic University in Rome, Italy. All participants provided written informed consent.
Inflammatory biomarkers and INFLA-score
Blood samples were obtained from participants who had fasted overnight and had refrained from smoking for at least 6 h. A full description of biomarkers measurement is described elsewhere [38].
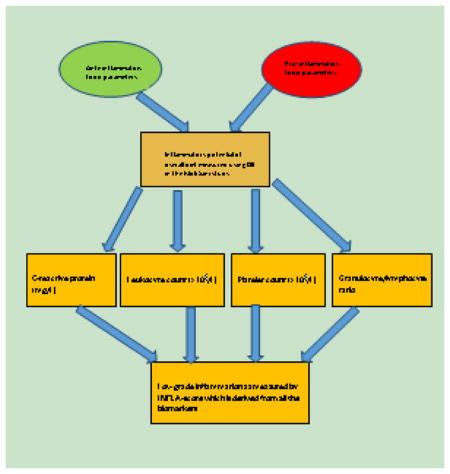
Low-grade inflammation was assessed by an INFLA-score, which has already been defined and used within the Moli-sani cohort [39] and includes C-reactive protein (CRP, mg/l), leukocyte (WBC, ×109/L) and platelet counts (×109/L) and the granulocyte to lymphocyte ratio (G/L ratio). For all four components, being in the highest deciles (7 to 10) was scored from +1 to +4; while being in the lowest deciles (1 to 4) was negatively scored from −4 to −1. The mid-deciles (5 or 6) were assigned a score of zero (0). The INFLA-score, which is sum of the four components, ranged between −16 and +16. Higher scores represented greater low-grade inflammation. For analytic purposes, the INFLA-score was rescaled to have a mean of zero and a standard deviation of one.
Assessment of risk factors
History of CVD included documented angina, myocardial infarction, revascularization procedures and cerebrovascular events. History of cancer included self-reported diagnosis of cancer. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or treatment for hypertension. Hypercholesterolemia was defined if total cholesterol ≥240 mg/dl or by use of specific medication. Diabetes was defined as fasting blood glucose ≥126 mg/dl, or on the basis of specific pharmacological treatment
Socioeconomic variables
Education was based on the highest qualification attained and was categorized as up to lower secondary school (≤8 years of study), up to higher secondary school (8–13 years of study) and post-graduate (>13 years of study). Household income, expressed as earned Euros per year, was a 5-level variable (<10,000; 10,000–25,000; 25,000–40,000; >40,000 with missing values collapsed into a non-respondent category). Occupational social class was based on the Registrar General’s occupation based classification scheme but, differently from the original UK classification, the social class for women was obtained as done for men [40].
Dietary information
Food intake during the year before enrolment was assessed by the validated Italian EPIC food frequency questionnaire [41]. Adherence to the Mediterranean diet (MD) was defined according to the Mediterranean Diet Score [42], with scores ranging from 0 to 9.
Food antioxidant content (FAC) was appraised by a score determining the content in antioxidant vitamins and phytochemicals of each food group and ranged from −99 to 99 with higher values indicating increased consumption of foods rich in antioxidants [43]. The polyphenol content of diet was measured by a polyphenol antioxidant content (PAC)-score calculated as in Pounis et al [43].
Variety of fruit and vegetable intake was assessed by a Diet Diversity Score [44] derived from the total number of individual vegetable and fruit items eaten at least once in two weeks (range 0–37). FFQ-derived dietary information was used to calculate DII scores for all subjects, as described in detail elsewhere [19]. Briefly, the dietary data for each study participant were first linked to the regionally representative global database that provided a robust estimate of a mean and standard deviation for each of the food parameters (i.e., foods, nutrients, and other food components such as flavonoids) considered. A z-score was derived by subtracting the “standard global mean” from the amount reported and then dividing this value by the standard deviation. To minimize the effect of “right skewing” (a common occurrence with dietary data), this value was then converted to a centered proportion, which was then multiplied by the respective food parameter inflammatory effect score (derived from a literature review and scoring of 1943 “qualified” articles) to obtain the subject’s food parameter-specific DII score. All of the food parameter-specific DII scores were then summed to create the overall DII score for every subject in the study. For the current study, data were available for a total of 34 food parameters (carbohydrate, protein, total fat, alcohol, fibre, cholesterol, saturated fat, monounsaturated fat, polyunsaturated fat, omega-3, omega-6 fatty acid, niacin, thiamin, riboflavin, vitamin B12, vitamin B6, iron, magnesium, zinc, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, anthocyanidins, flavan3ol, flavones, flavonols, flavonones, isoflavones, garlic, onion, tea). A description of validation work of the DII score, based on both dietary recalls and the 7-day dietary recall, a structured questionnaire similar in terms of its layout to an FFQ, is available elsewhere [23].
Risk factor assessment
Leisure-time physical activity (LTPA) was expressed as daily energy expenditure in metabolic equivalent task-hours (MET-h/d). Body mass index (BMI) was calculated as weight(kg)/height(m)2 and then grouped into three categories as normal (≤25kg/m2), overweight (>25 to <30 kg/m2) or obese (≥30kg/m2). Abdominal obesity was defined as waist-to-hip ratio ≥0.85 or ≥0.90 for women and men, respectively [45]. Subjects were classified as never-smokers, current smokers or ex-smokers (quitting from at least 1 year).
Statistical analysis
Characteristics of the study population were presented as numbers and percentages, or mean values and standard deviation for continuous variables. Differences in Tables 1 and 2 were calculated by using the analysis of variance (ANOVA) adjusted for age and sex (PROC GENMOD and PROC GLM in SAS for categorical and continuous variables, respectively). Beta-coefficients (±SE) with 95% confidence intervals (95%CI) from multivariable linear regression analysis were used to estimate the association of the INFLA-score or single inflammatory biomarkers (used as dependent variable) with DII, wither as quintiles or as a continuous variable (Tables 4,5). When quintiles are considered, beta-coefficients represent the change in INFLA-score (or single inflammatory biomarkers) for each quintile (2 to 5) in comparison with the lowest quintile (1). The regression models included age, sex, smoking, leisure-time PA, BMI (categorical), abdominal obesity, education, income, occupational class, CVD, cancer, diabetes, hypercholesterolemia and hypertension. Association with platelet count was further adjusted for haematocrit. A two-sided α=0.05 was phenomenal cutpoint for statistical significance. The data analysis was generated using SAS/STAT software, Version 9.4 of the SAS System for Windows©2009. SAS Institute Inc. and SAS are registered trademarks of SAS Institute Inc., Cary, NC, USA.
Table 1.
Characteristics of the study population by quintiles of dietary inflammation index, Moli-sani study, 2005–2010.
| Quintiles of dietary inflammation index (DII®) Score | P value | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1st | 2nd | 3rd | 4th | 5th | ||
| Means, SD | −0.99 (0.81) | 0.59 (0.31) | 1.58 (0.27)- | 2.53 (0.27)- | 3.67 (0.60) | |
|
| ||||||
| N, % | 4164 (20.0) | 4165 (20.0) | 4165 (20.0) | 4165 (20.0) | 4164 (20.0) | - |
| Age | 58.1 (11.6) | 57.0 (11.7) | 55.8 (11.7) | 54.5 (11.5) | 51.2 (10.8) | <.0001 |
| Sex (men, %) | 36.6 | 44.6 | 51.5 | 51.4 | 52.6 | <.0001 |
| Education (%) | 0.0002 | |||||
| Up to lower secondary school | 52.7 | 54.1 | 52.9 | 52.8 | 47.3 | |
| Up to higher secondary school | 33.8 | 32.9 | 33.9 | 35.1 | 38.8 | |
| Postgraduate | 13.5 | 13.0 | 13.2 | 12.1 | 13.9 | |
| Household income (%) | 0.006 | |||||
| <10,000 | 6.8 | 5.3 | 5.9 | 5.5 | 4.0 | |
| 10,000–25,000 | 27.9 | 29.6 | 30.3 | 33.5 | 30.1 | |
| 25,000–40,000 | 19.8 | 19.7 | 20.6 | 20.7 | 22.5 | |
| >40,000 | 11.9 | 12.1 | 11.8 | 10.9 | 12.7 | |
| Non-respondents | 33.5 | 33.3 | 31.4 | 29.5 | 30.7 | |
| Occupational class (%) | <.0001 | |||||
| Professional/managerial | 22.3 | 20.4 | 21.0 | 19.8 | 20.1 | |
| Skilled non-manual | 36.5 | 36.2 | 34.4 | 35.3 | 39.3 | |
| Skilled manual | 14.6 | 17.2 | 19.9 | 19.2 | 18.6 | |
| Semi-skilled/unskilled | 18.6 | 19.5 | 18.7 | 20.6 | 16.7 | |
| Unclassified | 7.8 | 6.8 | 5.9 | 5.0 | 5.3 | |
| Leisure-time PA (met-h/d) | 4.0 (4.1) | 3.7 (4.1) | 3.6 (4.1) | 3.3 (3.8) | 3.0 (3.7) | <.0001 |
| Smokers (%) | 17.6 | 19.8 | 20.7 | 24.3 | 30.5 | <.0001 |
| BMI | 28.1 (4.7) | 28.1 (4.7) | 27.7 (4.5) | 27.8 (4.5) | 27.7 (4.6) | <.0001 |
| Abdominal obesity (%) | 75.1 | 74.7 | 73.2 | 73.1 | 69.4 | 0.005 |
| Cardiovascular disease (%) | 6.8 | 5.5 | 5.4 | 4.6 | 2.8 | 0.002 |
| History of cancer (%) | 4.0 | 3.3 | 3.2 | 2.9 | 2.5 | 0.73 |
| Diabetes (%) | 11.7 | 9.9 | 9.2 | 8.2 | 5.7 | <.0001 |
| Blood glucose (mg/dL) | 103 (26) | 101 (25) | 101 (24) | 100 (22) | 100 (19) | 0.005 |
| Hypertension (%) | 59.7 | 60.6 | 56.5 | 54.0 | 45.9 | 0.002 |
| Systolic BP (mm Hg) | 141 (22) | 141 (20) | 140 (20) | 141 (21) | 140 (19) | 0.005 |
| Diastolic BP (mm Hg) | 82 (9) | 83 (10) | 82 (9) | 82 (10) | 82 (10) | 0.41 |
| Hypercholesterolemia (%) | 35.6 | 33.0 | 29.9 | 29.6 | 26.7 | <.0001 |
| Total cholesterol (mg/dL) | 213 (42) | 213 (42) | 212 (41) | 213 (41) | 213 (41) | 0.13 |
| HDL-cholesterol (mg/dL) | 57 (15) | 58 (15) | 58 (15) | 58 (15) | 58 (15) | 0.09 |
| LDL-cholesterol (mg/dL) | 131 (36) | 130 (36) | 129 (35) | 131 (35) | 131 (34) | 0.34 |
| Triglycerides (mg/dL) | 125 (62) | 125 (63) | 124 (66) | 124 (65) | 123 (65) | 0.11 |
Continuous variables are presented as means and standard deviation (SD).
Means and p values adjusted for age and sex.
Table 2.
Dietary inflammation index and biomarkers of inflammation, Moli-sani study, 2005–2010.
| Quintiles of dietary inflammation index (DII®) Score | P value | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1st | 2nd | 3rd | 4th | 5th | ||
| N, % | 4164 (20.0) | 4165 (20.0) | 4165 (20.0) | 4165 (20.0) | 4164 (20.0) | - |
| INFLA-score | −0.07 (1.02) | −0.03 (0.99) | 0.002 (1.00) | 0.02 (0.98) | 0.07 (1.00) | <.0001 |
| C-reactive protein (mg/L) | 2.03 (1.95) | 2.01 (1.89) | 2.05 (1.93) | 2.02 (1.80) | 2.08 (1.85) | 0.43 |
| Leukocyte count (×109/L) | 6.11 (1.97) | 6.11 (1.55) | 6.15 (1.62) | 6.18 (1.57) | 6.26 (1.65) | 0.0002 |
| Platelet count (×109/L) | 245 (66) | 247 (64) | 249 (63) | 249 (63) | 250 (62) | 0.0052 |
| Granulocyte/lymphocyte ratio | 1.93 (0.74) | 1.97 (0.75) | 1.98 (0.78) | 1.97 (0.73) | 2.04 (1.29) | <.0001 |
Values are reported as means with standard deviation in brackets.
Mean and p values are adjusted for age and sex. Means for platelet count were further adjusted for haematocrit.
Table 4.
Association of dietary inflammation index with the INFLA-score and biomarkers of inflammation, Moli-sani study, 2005–2010.
| Quintiles of dietary inflammation index (DII®) Score | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1st | 2nd | 3rd | 4th | 5th | Continuous | |
|
| ||||||
| Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
||
| INFLA-score | Ref | 0.034 (−0.008 to 0.075) | 0.086 (0.044 to 0.128) | 0.092 (0.049 to 0.134) | 0.131 (0.089 to 0.174) | 0.029 (0.021 to 0.037) |
| C-reactive protein (mg/L) | Ref | −0.012 (−0.051 to 0.027) | 0.028 (−0.010 to 0.068) | 0.020 (−0.010 to 0.068) | 0.054 (0.014 to 0.094) | 0.012 (0.004 to 0.019) |
| Leukocyte count (×109/L) | Ref | 0.012 (−0.028 to 0.052) | 0.045 (0.005 to 0.085) | 0.054 (0.013 to 0.094) | 0.078 (0.037 to 0.120) | 0.016 (0.009 to 0.024) |
| Platelet count (×109/L) | Ref | 0.024 (−0.017 to 0.065) | 0.065 (0.023 to 0.106) | 0.067 (0.026 to 0.109) | 0.074 (0.032 to 0.117) | 0.016 (0.008 to 0.024) |
| Granulocyte/lymphocyte ratio | Ref | 0.054 (0.011 to 0.096) | 0.064 (0.021 to 0.107) | 0.049 (0.006 to 0.092) | 0.098 (0.054 to 0.142) | 0.020 (0.012 to 0.028) |
Regression coefficients (with 95% CI) obtained from a multivariable model adjusted for age, sex, smoking, leisure-time PA, BMI, abdominal obesity, education, income, occupational class, CVD, cancer, diabetes, hypercholesterolemia and hypertension. Association with platelet count was further adjusted for haematocrit.
Table 5.
Sub-group analyses for the association of dietary inflammation index with the INFLA-score and biomarkers of inflammation, Moli-sani study, 2005–2010.
|
|
||||||
|---|---|---|---|---|---|---|
| Men (n=9,861) | Women (n=10,962) | Aged<50 y (n=7,870) | Aged 50–64 y (n=8,296) | Aged ≥ 65 y (n=4,657) | Healthy sample (n=6,385)1 |
|
|
| ||||||
| Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
Regression coefficient (95%CI) |
|
| INFLA-score | 0.027 (0.015 to 0.039) | 0.031 (0.021 to 0.042) | 0.025 (0.013 to 0.038) | 0.034 (0.022 to 0.047) | 0.014 (−0.003 to 0.032) | 0.031 (0.017 to 0.045) |
| C-reactive protein (mg/L) | 0.016 (0.004 to 0.027) | 0.009 (−0.0007 to 0.019) | 0.007 (−0.005 to 0.019) | 0.022 (0.011 to 0.034) | −0.002 (−0.019 to 0.013) | 0.012 (−0.001 to 0.026) |
| Leukocyte count (×109/L) | 0.015 (0.004 to 0.027) | 0.018 (0.008 to 0.028) | 0.019 (0.007 to 0.031) | 0.014 (0.002 to 0.026) | 0.011 (−0.007 to 0.028) | 0.014 (0.0004 to 0.027) |
| Platelet count (×109/L) | 0.025 (0.014 to 0.037) | 0.010 (−0.0002 to 0.021) | 0.018 (0.006 to 0.031) | 0.015 (0.002 to 0.027) | 0.014 (−0.003 to 0.031) | 0.023 (0.009 to 0.038) |
| Granulocyte/lymphocyte ratio | 0.006 (−0.007 to 0.018) | 0.032 (0.021 to 0.043) | 0.017 (0.004 to 0.029) | 0.021 (0.008 to 0.034) | 0.012 (−0.007 to 0.031) | 0.023 (0.009 to 0.037) |
Regression coefficients (with 95% CI) obtained from a multivariable model adjusted for age, sex, smoking, leisure-time PA, BMI, abdominal obesity, education, income, occupational class, CVD, cancer, diabetes, hypercholesterolemia and hypertension. Association with platelet count was further adjusted for haematocrit.
Subjects free from CVD, cancer, diabetes, hypercholesterolemia and hypertension.
RESULTS
Table 1 shows the characteristics of the participants across quintiles of DII. Changes were observed across quintiles for several characteristics. Participants in quintile 5 were younger, more likely to be males, smokers, have postgraduate education and income, be in a skilled occupation, have low physical activity, lower BMI, higher abdominal obesity, and lower prevalence of CVD, cancer, hypertension, diabetes and hypercholesterolemia. Table 2 shows distribution of biomarkers of inflammation across quintiles of DII. Participants in quintile 5 had higher levels of most markers (INFLA-score, leukocyte count, platelet count, and granulocyte/lymphocyte ratio) compared to participants in quintile 1.
Table 3 shows the distribution of dietary characteristics across DII quintiles, with participants in quintile 5 having higher energy intake and lower Mediterranean diet score, polyphenol content, antioxidant content and fruits and vegetables, compared to subjects in the lowest quintile.
Table 3.
Dietary inflammation index and dietary characteristics of the population, Moli-sani study, 2005–2010.
| Quintiles of dietary inflammation index (DII®) Score | P value | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1st | 2nd | 3rd | 4th | 5th | ||
| N, % | 4164 (20.0) | 4165 (20.0) | 4165 (20.0) | 4165 (20.0) | 4164 (20.0) | - |
| Energy intake (Kcal/d) | 1883 (519) | 2025 (519) | 2105 (547) | 2153 (567) | 2256 (576) | <.0001 |
| Mediterranean diet score | 5.3 (1.4) | 4.8 (1.5) | 4.4 (1.5) | 4.0 (1.5) | 3.3 (1.5) | <.0001 |
| Polyphenol content (PAC-score) | 11.8 (10.8) | 6.1 (10.7) | 1.2 (10.8) | −4.4 (10.6) | −12.2 (9.9) | <.0001 |
| Antioxidant content (FAC-score) | 38 (42) | 18 (42) | 3 (41) | −14 (41) | −37 (39) | <.0001 |
| Fruit and vegetable variety | 16 (4) | 15 (3) | 14 (3) | 13 (4) | 11 (4) | <.0001 |
Continuous variables are presented as means and standard deviation (SD).
Means and p values adjusted for age and sex.
Analysis of inflammatory markers
Analyses adjusting for covariates showed positive associations between DII and INFLA-score (β DIIq5 vs 1=0.13; 95%CI 0.09 to 0.17), CRP (βDIIq5 vs 1=0.05; 95%CI 0.01 to 0.09), leukocyte count (β DIIq5 vs 1=0.08; 95%CI 0.04 to 0.12), platelet count ( βDIIq5 vs 1=0.07; 95%CI 0.03 to 0.12), granulocyte/lymphocyte ratio (βDIIq5 vs 1=0.10; 95%CI 0.05 to 0.14), (Table 4).
Subgroup analyses, as shown in Table 5, revealed a positive association between DII as continuous and: 1. INFLA-score among both men and women, those aged 50–65 years and among participants without CVD, cancer, diabetes, hypercholesterolemia and hypertension.; 2. CRP among men, those aged 50–64 years; 3. Leukocyte count among both men and women, those aged <50, 50–64 years, and healthy sample; 4. Platelet count among men, those aged <50, 50–64 years, and healthy sample; 5. Granulocyte/lymphocyte ration among women, those aged <50, 50–64 years, and healthy sample.
DISCUSSION
This is the first study in Italy to observe an association between inflammatory potential of diet, as measured by DII score, and low-grade inflammation assessed by a composite score including cellular and circulating biomarkers that have previously been found to be associated with increased risk of death [12]. The results also revealed a positive association between DII score and individual biomarkers of low-grade inflammation. These findings reinforce the idea that a diet rich in pro-inflammatory food parameters (sweets, butter and other animal fats, cholesterol, saturated fat), and relatively poor in anti-inflammatory food parameters (vegetables and fruits) contribute to low-grade, systemic inflammation among adults in the general Italian population. Overall, our study results are consistent with the hypothesis that diet modulates inflammation. The inference is that by modulating low-grade inflammation diet may exert an effect on chronic diseases such as several cancers and cardiovascular diseases. Higher scores also could result from low consumption of food items considered to be anti-inflammatory such as fruits and vegetables. Adherence to an anti-inflammatory dietary pattern, such as the Mediterranean diet, has been shown to reduce the risk of cardiovascular and cerebrovascular diseases [46,47]. All of these chronic health conditions are related to inflammation [48–50]. In subgroup analyses, the DII was associated with low-grade inflammation among most subgroups except those aged ≥65 years. The reason for this is not clear; therefore, further analyses should be carried out to investigate the possibility of other factors such as infections or normal cellular senescence may play a major role in modulating inflammation among elderly [51].
We also observed that participants in DII quintile 5 had lower prevalence of CVD, cancer, hypertension, diabetes and hypercholesterolemia despite that these participants were more likely to smoke, be male, and have low physical activity. This can be explained by the fact that they were significantly younger than individuals in other quintiles (e.g., nearly 7 years younger than those in quintile 1). Another explanation is that older individuals, who are more likely to have had a diagnosed chronic disease are more likely to have adopted a healthier, anti-inflammatory diet. Of course, these explanations are not mutually exclusive.
In a previous study within the Moli-sani cohort, three dietary patterns were identified by principal factor analysis, the “Olive Oil and Vegetables” pattern, was associated with relatively lower values of CRP, the “Pasta and Meat” pattern, was positively associated with CRP and the “Eggs and Sweets” pattern, was associated with high values of CRP [52]. We also have reported previously an inverse association between INFLA-score and adherence to MD (56); while in another report polyphenol content of diet was negatively associated with low-grade inflammation as determined through INFLA-score [39]. As shown from the current analyses, higher DII scores are associated with lower Mediterranean diet score. The polyphenol and antioxidant content and lower fruit and vegetable consumption of the MD also are associated with lower DII scores.
The strengths of our study include extensive information on diet, lifestyle and cardiovascular risk profile, the large size of the study population, and the sampling from the general population, with a high response rate (70%) [37]. As previously mentioned, this is the first study to investigate the association of biomarkers included in the INFLA-score with DII in a Mediterranean population. Previously, the DII has been shown to be associated with a variety of low grade inflammation-related outcomes in Italy both in prospective and case-control studies [29–35,53–56]. Another strength includes the large number of inflammation markers, measured in plasma and analysed centrally in the same laboratory and the large battery of lifestyle and anthropometric variables assessed according to standardized procedures is an important advantage of this study as it allows adjustment for several possibly confounding factors.
Several methodological limitations of the present study should be noted. The cross-sectional design of the study provided a picture of eating habits and other characteristics at a point in time, thus the possibility of inverse causation bias arises. Another limitation is the fact that DII scores were computed from self-reports via a food frequency questionnaire, which carried an inherent degree of recall bias and can lead to a potential misclassification of the exposure. Third, the DII was calculated using data on only 34 nutrients and food components derived from FFQ. In the DII validation study, sensitivity analysis was conducted comparing DII calculated from multiple (up to 15/person) 24HR (which provided data on 44 nutrients and food components) with DII calculated from 7-day dietary recalls (which provided data on 28 nutrients and food components) [23]. We found that the association with CRP was not attenuated when using the more limited list available with the 7-day dietary recalls. Still, the attenuation in the food parameter list could explain the absence of an association between DII and CRP in this study. Despite the reduction in the number of parameters, we still could successfully observe associations with some of the inflammatory markers [23].
Conclusion
Results from this study suggest that consuming a pro-inflammatory diet high in food components such as sugar and saturated fat leads to a persistent state of low grade inflammation, which may increase the risk of a range of chronic diseases like cancers and cardiovascular diseases throughout life.
Supplementary Material
Highlights.
Dietary Inflammatory Index (DII) measures dietary inflammation potential.
This study assessed the association between DII and low-grade inflammation.
This is the first study to examine this association in an Italian population.
Results showed that proinflammatory diet was associated with higher level of LGI.
Acknowledgments
The Moli-sani research group thanks the Associazione Cuore-Sano Onlus (Campobasso, Italy) for its cultural and financial support.
Funding: The enrolment phase of the Moli-sani Study was supported by research grants from Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy)–Programma Triennale di Ricerca, Decreto no.1588 and Instrumentation Laboratory, Milan, Italy. Funders had no role in study design, collection, analysis, and interpretation of data; in the writing of the manuscript and in the decision to submit the article for publication. Marialaura Bonaccio was supported by a Fondazione Umberto Veronesi Fellowship. Simona Costanzo is the recipient of a Fondazione Umberto Veronesi Travel Grant.
All Authors were and are independent from funders. The present analyses were partially supported by the Italian Ministry of Health 2013 (Young investigator grant to MB, number: GR-2013-02356060) and by the Italian Association for Cancer Research (A.I.R.C.) with grant AIRC “5×1000” Ref. n. 12237. Drs. Shivappa, Hébert, were supported by the United States National Institute for Diabetes, Digestive and Kidney Diseases (grant no. R44DK103377). This research received no specific grant from any funding agency, commercial or not-for-profit sectors. EC and NCI had no role in the design, analysis or writing of this article.
Footnotes
Conflict of interest: All authors declare no conflict of interest.
Disclosure: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 2.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 3.Akbaraly T, Kerlau C, Wyart M, Chevallier N, Ndiaye L, Shivappa N, Hebert JR, Kivimaki M. Dietary inflammatory index and recurrence of depressive symptoms: Results from the Whitehall II Study. Clinical psychological science: a journal of the Association for Psychological Science. 2016;4(6):1125–1134. doi: 10.1177/2167702616645777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tse G, Eslick GD. Soy and isoflavone consumption and risk of gastrointestinal cancer: a systematic review and meta-analysis. Eur J Nutr. 2016;55(1):63–73. doi: 10.1007/s00394-014-0824-7. [DOI] [PubMed] [Google Scholar]
- 5.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15(17):1949–1955. doi: 10.2174/138161209788453167. [DOI] [PubMed] [Google Scholar]
- 6.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009;57(11):4467–4477. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- 7.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients. 2018;10(2) doi: 10.3390/nu10020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients. 2017;9(9) doi: 10.3390/nu9091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-Reactive Protein and Low-Density Lipoprotein Cholesterol Levels in the Prediction of First Cardiovascular Events. New England Journal of Medicine. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 12.Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L Moli-sani Study I. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani study. Haematologica. 2016;101(11):1434–1441. doi: 10.3324/haematol.2016.144055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Johansson-Persson A, Ulmius M, Cloetens L, Karhu T, Herzig KH, Onning G. A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur J Nutr. 2013;7:7. doi: 10.1007/s00394-013-0496-8. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers.[see comment] Journal of Clinical Endocrinology & Metabolism. 2006;91(2):439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ, 3rd, Li W, Pagoto SL, Hafner AR, Ockene IS. Association between dietary fiber and serum C-reactive protein. American Journal of Clinical Nutrition. 2006;83(4):760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertran N, Camps J, Fernandez-Ballart J, Arija V, Ferre N, Tous M, Simo D, Murphy MM, Vilella E, Joven J. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J Lab Clin Med. 2005;145(1):41–46. doi: 10.1016/j.lab.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Erlinger TP, Guallar E, Miller ER, 3rd, Stolzenberg-Solomon R, Appel LJ. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch Intern Med. 2001;161(15):1903–1908. doi: 10.1001/archinte.161.15.1903. [DOI] [PubMed] [Google Scholar]
- 19.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2015;45(1):177–183. doi: 10.1111/cea.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, Martin LW, Millen AE, Park HL, Rosal MC, Shikany JM, Shivappa N, Ockene JK, Hebert JR. Construct validation of the dietary inflammatory index among postmenopausal women. Annals of epidemiology. 2015;25(6):398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hebert JR. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2014;56(9):986–989. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutr. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivappa N, Hebert JR, Marcos A, Diaz LE, Gomez S, Nova E, Michels N, Arouca A, Gonzalez-Gil E, Frederic G, Gonzalez-Gross M, Castillo MJ, Manios Y, Kersting M, Gunter MJ, De Henauw S, Antonios K, Widhalm K, Molnar D, Moreno L, Huybrechts I. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Molecular nutrition & food research. 2017;61(6) doi: 10.1002/mnfr.201600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. The British journal of nutrition. 2015;113(4):665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vahid F, Shivappa N, Hekmatdoost A, Hebert JR, Davoodi SH, Sadeghi M. Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: case-control study. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2017;42(5):511–516. doi: 10.1139/apnm-2016-0274. [DOI] [PubMed] [Google Scholar]
- 27.Shivappa N, Wirth MD, Hurley TG, Hebert JR. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Molecular nutrition & food research. 2017;61(4) doi: 10.1002/mnfr.201600630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boden S, Wennberg M, Van Guelpen B, Johansson I, Lindahl B, Andersson J, Shivappa N, Hebert JR, Nilsson LM. Dietary inflammatory index and risk of first myocardial infarction; a prospective population-based study. Nutrition journal. 2017;16(1):21. doi: 10.1186/s12937-017-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zucchetto A, Serraino D, Shivappa N, Hebert JR, Stocco C, Puppo A, Falcini F, Panato C, Dal Maso L, Polesel J. Dietary inflammatory index before diagnosis and survival in an Italian cohort of women with breast cancer. The British journal of nutrition. 2017;117(10):1456–1462. doi: 10.1017/S0007114517001258. [DOI] [PubMed] [Google Scholar]
- 30.Shivappa N, Hebert JR, Taborelli M, Montella M, Libra M, Zucchetto A, Crispo A, Grimaldi M, La Vecchia C, Serraino D, Polesel J. Dietary inflammatory index and non-Hodgkin lymphoma risk in an Italian case-control study. Cancer causes & control: CCC. 2017;28(7):791–799. doi: 10.1007/s10552-017-0905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivappa N, Hebert JR, Rosato V, Garavello W, Serraino D, La Vecchia C. Inflammatory potential of diet and risk of oral and pharyngeal cancer in a large case-control study from Italy. International journal of cancer. 2017;141(3):471–479. doi: 10.1002/ijc.30711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shivappa N, Hebert JR, Ferraroni M, La Vecchia C, Rossi M. Association between Dietary Inflammatory Index and Gastric Cancer Risk in an Italian Case-Control Study. Nutrition and cancer. 2016;68(8):1262–1268. doi: 10.1080/01635581.2016.1224367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shivappa N, Hebert JR, Rosato V, Montella M, Serraino D, La Vecchia C. Association between the dietary inflammatory index and breast cancer in a large Italian case-control study. Molecular nutrition & food research. 2017;(61):3. doi: 10.1002/mnfr.201600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivappa N, Hebert JR, Rosato V, Rossi M, Libra M, Montella M, Serraino D, La Vecchia C. Dietary Inflammatory Index and Risk of Bladder Cancer in a Large Italian Case-control Study. Urology. 2017;100:84–89. doi: 10.1016/j.urology.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shivappa N, Hebert JR, Rosato V, Serraino D, La Vecchia C. Inflammatory potential of diet and risk of laryngeal cancer in a case-control study from Italy. Cancer causes & control: CCC. 2016;27(8):1027–1034. doi: 10.1007/s10552-016-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivappa N, Tavani A, Hebert JR, Rosato V, La Vecchia C. Dietary inflammatory index and acute myocardial infarction in a large Italian case-control study. European journal of public health. 2017 doi: 10.1093/eurpub/ckx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Castelnuovo A, Costanzo S, Persichillo M, Olivieri M, de Curtis A, Zito F, Donati MB, de Gaetano G, Iacoviello L, Investigators M-SP. Distribution of short and lifetime risks for cardiovascular disease in Italians. Eur J Prev Cardiol. 2012;19(4):723–730. doi: 10.1177/1741826711410820. [DOI] [PubMed] [Google Scholar]
- 38.Santimone I, Di Castelnuovo A, De Curtis A, Spinelli M, Cugino D, Gianfagna F, Zito F, Donati MB, Cerletti C, de Gaetano G, Iacoviello L, Investigators M-SP. White blood cell count, sex and age are major determinants of heterogeneity of platelet indices in an adult general population: results from the MOLI-SANI project. Haematologica. 2011;96(8):1180–1188. doi: 10.3324/haematol.2011.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pounis G, Bonaccio M, Di Castelnuovo A, Costanzo S, de Curtis A, Persichillo M, Sieri S, Donati MB, Cerletti C, de Gaetano G, Iacoviello L. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thrombosis and Haemostasis. 2016;115(2):344–352. doi: 10.1160/TH15-06-0487. [DOI] [PubMed] [Google Scholar]
- 40.Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L, Investigators M-SS. Relative contribution of health-related behaviours and chronic diseases to the socioeconomic patterning of low-grade inflammation. Int J Public Health. 2017;62(5):551–562. doi: 10.1007/s00038-016-0939-0. [DOI] [PubMed] [Google Scholar]
- 41.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26:S152–S160. doi: 10.1093/ije/26.suppl_1.S152. [DOI] [PubMed] [Google Scholar]
- 42.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. New England Journal of Medicine. 2003;348(26):2599–2608. doi: 10.1056/Nejmoa025039. [DOI] [PubMed] [Google Scholar]
- 43.Pounis G, Costanzo S, di Giuseppe R, de Lucia F, Santimone I, Sciarretta A, Barisciano P, Persichillo M, de Curtis A, Zito F, Di Castelnuovo AF, Sieri S, Donati MB, de Gaetano G, Iacoviello L Investigators M-sP. Consumption of healthy foods at different content of antioxidant vitamins and phytochemicals and metabolic risk factors for cardiovascular disease in men and women of the Moli-sani study. European Journal of Clinical Nutrition. 2013;67(2):207–213. doi: 10.1038/ejcn.2012.201. [DOI] [PubMed] [Google Scholar]
- 44.Jeurnink SM, Buchner FL, Bueno-de-Mesquita HB, Siersema PD, Boshuizen HC, Numans ME, Dahm CC, Overvad K, Tjonneland A, Roswall N, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Kaaks R, Teucher B, Boeing H, Buijsse B, Trichopoulou A, Benetou V, Zylis D, Palli D, Sieri S, Vineis P, Tumino R, Panico S, Ocke MC, Peeters PHM, Skeie G, Brustad M, Lund E, Sanchez-Cantalejo E, Navarro C, Amiano P, Ardanaz E, Quiros JR, Hallmans G, Johansson I, Lindkvist B, Regner S, Khaw KT, Wareham N, Key TJ, Slimani N, Norat T, Vergnaud AC, Romaguera D, Gonzalez CA. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European prospective investigation into cancer and nutrition. International journal of cancer. 2012;131(6):E963–E973. doi: 10.1002/ijc.27517. [DOI] [PubMed] [Google Scholar]
- 45.Waist Circumference and Waist-Hip Ratio; Geneva. 8–11 December (2008); [Accessed June 2016]. [Google Scholar]
- 46.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA, Investigators PS. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. New England Journal of Medicine. 2013;368(14):1279–1290. doi: 10.1056/Nejmoa1200303. [DOI] [PubMed] [Google Scholar]
- 47.Tuttolomondo A, Casuccio A, Butta C, Pecoraro R, Di Raimondo D, Della Corte V, Arnao V, Clemente G, Maida C, Simonetta I, Miceli G, Lucifora B, Cirrincione A, Di Bona D, Corpora F, Maugeri R, Iacopino DG, Pinto A. Mediterranean Diet in patients with acute ischemic stroke: Relationships between Mediterranean Diet score, diagnostic subtype, and stroke severity index. Atherosclerosis. 2015;243(1):260–267. doi: 10.1016/j.atherosclerosis.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Bluher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clinical science. 2016;130(18):1603–1614. doi: 10.1042/CS20160005. [DOI] [PubMed] [Google Scholar]
- 49.Moreno LA, Bel-Serrat S, Santaliestra-Pasias A, Bueno G. Dairy products, yogurt consumption, and cardiometabolic risk in children and adolescents. Nutrition reviews. 2015;73(Suppl 1):8–14. doi: 10.1093/nutrit/nuv014. [DOI] [PubMed] [Google Scholar]
- 50.Sleiman D, Al-Badri MR, Azar ST. Effect of mediterranean diet in diabetes control and cardiovascular risk modification: a systematic review. Frontiers in public health. 2015;3:69. doi: 10.3389/fpubh.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machado MC, Coelho AM, Carneiro D’Albuquerque LA, Jancar S. Effect of ageing on systemic inflammatory response in acute pancreatitis. International journal of inflammation. 2012;2012:270319. doi: 10.1155/2012/270319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centritto F, Iacoviello L, di Giuseppe R, De Curtis A, Costanzo S, Zito F, Grioni S, Sieri S, Donati MB, de Gaetano G, Di Castelnuovo A, Moli-sani I. Dietary patterns, cardiovascular risk factors and C-reactive protein in a healthy Italian population. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2009;19(10):697–706. doi: 10.1016/j.numecd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Shivappa N, Hebert JR, Rosato V, Rossi M, Montella M, Serraino D, La Vecchia C. Dietary inflammatory index and ovarian cancer risk in a large Italian case-control study. Cancer causes & control: CCC. 2016;27(7):897–906. doi: 10.1007/s10552-016-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zucchetto A, Gini A, Shivappa N, Hebert JR, Stocco C, Dal Maso L, Birri S, Serraino D, Polesel J. Dietary inflammatory index and prostate cancer survival. International journal of cancer. 2016;139(11):2398–2404. doi: 10.1002/ijc.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivappa N, Hebert JR, Polesel J, Zucchetto A, Crispo A, Montella M, Franceschi S, Rossi M, La Vecchia C, Serraino D. Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. The British journal of nutrition. 2016;115(2):324–331. doi: 10.1017/S0007114515004419. [DOI] [PubMed] [Google Scholar]
- 56.Shivappa N, Hebert JR, Taborelli M, Zucchetto A, Montella M, Libra M, La Vecchia C, Serraino D, Polesel J. Association between dietary inflammatory index and Hodgkin’s lymphoma in an Italian case-control study. Nutrition. 2018;53:43–48. doi: 10.1016/j.nut.2018.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


