Abstract
Objective
Sedation and neuromuscular blockade (NMB) protocols in patients undergoing targeted temperature management (TTM) after cardiac arrest address patient discomfort and manage shivering. These protocols vary widely between centers and may affect outcomes.
Design
Consecutive patients admitted to 20 centers after resuscitation from cardiac arrest were prospectively entered into the International Cardiac Arrest Registry between 2006–2016. Additional data about each center’s sedation and shivering management practice was obtained via survey. Sedation and shivering practices (SP) were categorized as escalating doses of sedation and minimal or no NMB (SP1), sedation with continuous or scheduled NMB (SP2), or sedation with as-needed NMB (SP3). Good outcome was defined as cerebral performance category (CPC) score of 1 or 2. A logistic regression hierarchical model was created with two levels (patient-level data with standard confounders at level one and hospitals at level two) and sedation practice as a fixed effect at the hospital level. The primary outcome was dichotomized CPC at 6 months.
Setting
Cardiac arrest receiving centers in Europe and the United states from 2006–2016
Patients
Four thousand two hundred sixty seven cardiac arrest patients 18 years or older enrolled in the International Cardiac Arrest Registry.
Interventions
None
Measurements and Main Results
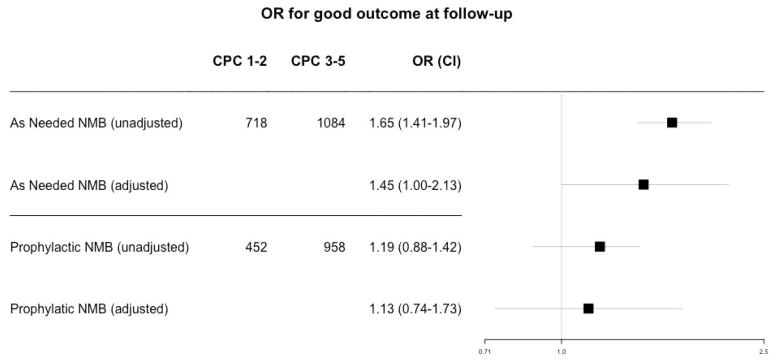
The mean age was 62±15 years, 36% were female, 77% out-of-hospital arrests, and mean ischemic time was 24(±18) minutes. Adjusted odds ratio (for age, return of spontaneous circulation, location of arrest, witnessed, initial rhythm, bystander CPR, and defibrillation, medical history country and size of hospital) was 1.13 (0.74–1.73, p=0.56) and 1.45 (1.00–2.13, p=0.046) for SP2 and SP3 respectively, referenced to SP1.
Discussion
Cardiac arrest patients treated at centers using as-needed NMB had increased odds of good outcomes compared to centers using escalating sedation doses and avoidance of NMB, after adjusting for potential confounders. These findings should be further investigated in prospective studies.
Keywords for Indexing: Cardiac Arrest, Sedation, Analgesia, Analagosedation, Shiver, Hypoxic Ischemic Encephalopathy
Introduction
Targeted temperature management (TTM) may improve functional outcomes of patients with hypoxic ischemic encephalopathy (HIE) after cardiac arrest, and is recommended for these patients after the return of spontaneous circulation (ROSC)(1). Sedative and analgesic infusions and neuromuscular blockade agents (NMB) are commonly used during TTM for comfort, suppression of shivering, and reduction of metabolic activity, but the optimal regimens are unknown, and dosing strategies vary widely(2–5). During TTM, shivering increases the systemic metabolic rate(6), reduces brain oxygen levels(7), and increases intracranial pressure(8), and cause variability in body temperature, each of which can worsen secondary neurologic injury. To counteract these effects, different strategies have been proposed, ranging from high dose of sedatives and analgesics without NMB, to much lower dose with intermittent or continuous NMB(2, 9, 10).
Deeper sedation is associated with worse outcomes in other medical and surgical ICU populations (8, 11–15). During TTM, observational studies suggest that sedatives and analgesics accumulate due to impaired metabolism, which can delay wakening, confound neurologic assessment, and potentially result in inappropriate withdrawal of life support(4, 9, 16–18). Deeper sedation also may induce more hypotension with or without lower cardiac index, which may also affect outcomes (19–22). The approach to NMB during TTM also varies widely, from recommendations to avoid its use, to observational data that continuous use may improve outcome after cardiac arrest(2, 4, 9, 10, 23–25). For these reasons, optimization of sedation is thought to be essential in the management of patients with critical illness(26), and the specific effects of sedation on cardiac arrest survivors undergoing TTM could be profound, but are unknown.
Current literature available to address this issue has used single-center data to associate NMB dosing to outcome, however has likely not appropriately adjusted for underlying severity of illness and is complicated by patients with very severe brain injury having less shivering. The only randomized trial comparing continuous versus as needed NMB dosing protcolized the as needed group to escalating doses of sedation as well, making the results difficult to interpret. To address these controversies and inconsistencies, we evaluated sedation and shivering management practices in the International Cardiac Arrest Registry (INTCAR), a multicenter registry of patients that have been successfully resuscitated after in-hospital and out-of-hospital cardiac arrests.
Materials and Methods
Centers and Patients
We included centers participating in the INTCAR registry between 2006 and 2017. The INTCAR registry consists of two iterations: a 1.0 dataset between the years of 2006–2011 and a 2.0 dataset between the years of 2011–2017. The core common variables of this retrospective data set were merged for this analysis. Centers enrolled consecutive adult patients admitted to an intensive care unit after in-hospital or out-of-hospital cardiac arrest. Only patients treated with TTM were included, and management varied according to local best practices. The database was maintained at Lund, Sweden on the Lytics© server. Centers participated in the registry on a volunteer basis, and there was no reimbursement for enrolling patients, and all had institutional review board approval at their center. The merging of the 1.0 and 2.0 iteration and the analysis below was competed in R software version 1.0.136(27).
Sedation Practices
Patient-level sedation data was not part of the INTCAR database. Center-specific sedation practices (SP) were assessed using a Research Electronic Data Capture (REDCap) based survey hosted at Tufts University(28). Surveys were sent by email to the investigators listed in the INTCAR system up to three times, on different days of the week. Centers that did not respond were then contacted directly by the administrators of INTCAR and asked to complete the survey. Two investigators independently assigned centers into one of the three categories based on their survey results: SP1 indicated escalating sedation dosing and avoidance of NMB, SP2 indicated sedation with either scheduled or continuous NMB to prevent shivering, and SP3 indicated sedation with as-needed NMB in response to shivering. Only centers that enrolled at least 20 patients and completed the survey were included in the analysis.
Outcome
The primary outcome was CPC at 6-month as a dichotomous variable, with good outcome defined as CPC of 1 or 2 and poor outcome as CPC of 3–5. Secondary outcome was CPC at ICU discharge. The 6-month CPC outcome was assessed with a review of medical records or telephone call to the patient or their proxy.
Predictors
Candidate variables included in both INTCAR iterations were age, sex, number of prior medical conditions (CAD, CHF, arrhythmia, COPD, hypertension, CKD, neurologic disease, liver disease, malignancy, obesity, IDDM and NIDDM), ischemic time, location of arrest (in hospital vs. out-of-hospital), rhythm (shockable, non-shockable, and unknown), bystander CPR, witnessed CPR, number of beds at each center and country of center (European versus United States). These were extracted by chart review at the individual centers and uploaded into the Lytics© server. Continuous variables were tested for linearity assumptions.
Missing data
Missing data was assessed and those with variables with greater than 10% missing data were estimated with multiple imputation. The imputation method was predictive mean metric, where 5 coefficients are assigned for each missing data point based on closest matches with complete data, then randomly samples of the donors are randomly selected and the observed value is returned. This was repeated 10 times and results pooled for the multivariate imputation by chained equations (mice) analysis. (29)
Statistical analysis methods
Predictors were separated into patient-level and center-level sets. To account for shared variance between center, sedation use, and patient use, a hierarchical model was used with two levels (patients at level one and centers at level two) with sedation practice as a fixed effect at center level. Explanatory variables were largely treated as dichotomous for yes/no variables and continuous variables were assessed for linearity with the logit of the outcome variables. Age was grouped by decade and ROSC was grouped by 5-minute intervals. Past medical history consisted of a sum of relevant pre-arrest diagnoses listed above. Candidate variables were assessed in a univariate manner using logistic regression and variables were retained in the model if the p-value was < 0.20. The decision was made a-priori to force certain selected variables into the model, regardless of significance based on clinical importance (ROSC, rhythm, location, bystander CPR).
Results
Survey
Thirty five eligible INTCAR centers were sent surveys, and 20 (57%) responded. Each center was assigned to one of the three categories based on their survey results without discrepancy between the assigners.
Patient population
A total of 4,267 patients at 20 centers were included in the dataset. The mean age was 62 (± 15) years, 34% (n= 1432) were female and 77% (n=3256) were out of hospital arrests. There were similar rates of shockable and non-shockable rhythms and mean ischemic time was 24 (± 18) minutes. Further characteristics, including characteristics from the complete INTCAR dataset, are described in Table 1. At 6 month follow-up 1,349 (32%) had a good outcome, with similar results at ICU discharge (1,313 (31%)).
Table 1.
Demographics and Clinical Characteristics of All Cardiac Arrest Patients and by Sedation and Shivering Practices
| Demographics and Clinical Factors | All INTCAR Centers | All Centers included in analysis | SP1 | SP2 | SP3 |
|---|---|---|---|---|---|
| Centers (n,%) | 35 | 20 | 6, 30 | 6, 30 | 8, 40 |
| Number of Patients (n,%) | 5450 | 4267 | 889, 21 | 1462, 34 | 1916, 45 |
| Age (mean, SD) | 62.0, 15 | 61.8, 15 | 65.4, 16 | 60.2, 15 | 61.2, 15 |
| Female (n,%) | 1792 (33) | 1432 (34) | 334, 38 | 509, 35 | 589, 30 |
| Medical History Diagnosis (n, IQR) | 2 1,3 | 2 2,2 | 2 2,2 | 2 2,2 | 2 2,2.25 |
| Out-of-hospital (n,%) | 4125, 76 | 3256, 77 | 634, 72 | 1183, 81 | 1439, 75 |
| Witnessed (n,%) | 4429, 82 | 3429, 81 | 710, 81 | 1161, 80 | 1558, 82 |
| Rhythm: PEA/Asystole (n,%) | 2478, 49 | 2050, 50 | 501, 60 | 675, 48 | 874, 47 |
| Shockable (n,%) | 2565, 49 | 1999, 49 | 320, 39 | 728, 51 | 951, 51 |
| Unknown (n,%) | 130, 2 | 64, 2 | 10, 1 | 15, 1 | 39, 2 |
| Time to ROSC (mean, SD) | 24, 18 | 24, 18 | 23, 18 | 26, 22 | 22, 15 |
| Bystander CPR (n,%) | 3718, 68 | 2008, 59 | 271, 57 | 715, 60 | 922, 60 |
| Defibrillation (n,%) | 3173, 59 | 2452, 59 | 422, 49 | 877, 61 | 1153, 61 |
| European (n,%) | 2449, 45 | 1365, 32 | 475, 53 | 245, 17 | 645, 34 |
| ICU CPC1–2 (n, %) | 1770, 32 | 1313, 32 | 225, 27 | 380, 28 | 780, 37 |
| 6 month CPC 1–2 | 1674, 31 | 1394, 35 | 224, 28 | 452, 32 | 718, 40 |
PEA: Pulseless electrical activity
VT/VF: ventricular fibrillation/ventricular tachycardia
ROSC: Return of spontaneous circulation
SP: Sedation and shivering practice
Missing data
At least one variable was missing from 23% of patients. 11% of patients had more than one variable of missing data. The most common variable with missing data was bystander CPR. Multiple imputation was performed with 5 imputations and pooled for the final analysis.
Model development
Variables were selected a priori from existing literature describing outcome prediction after cardiac arrest. The results of univariate analyses are shown in Supplement table 1–2. Age and ROSC were changed from continuous variables to intervals due to the nonlinear relationship of the raw variables with the outcome variables (Supplement figure 1,2). After model development, a priori interactions were tested and not found to be significant. All univariate variables were found to be significant and were retained in the model. First, the global p value for sedation level for each outcome was tested, followed by testing the three sedation practices referenced to sedation practice 1.
Model specification
The full model is shown in Supplementary Table 1. Variables retained in the model included age, sex, arrest location, medical history, ischemic time, witnessed arrest, bystander CPR, rhythm, country, defibrillation, and hospital size.
Outcome by sedation category
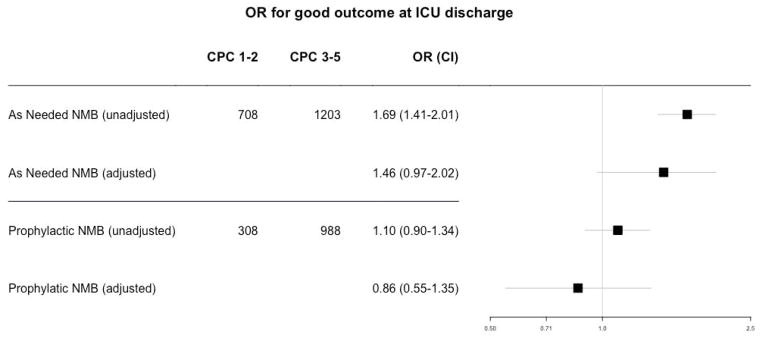
For the primary outcome of 6-month CPC referenced to SP1, the global p-value was significant at 0.003. The unadjusted odds ratios for good outcome were 1.19 (0.88–1.42, p=0.07) for SP2 and 1.65 (1.40–1.97, p<0.001) for SP3. The adjusted odds ratios were 1.13 (0.74–1.73, p=0.56) for SP2 and 1.45 (1.00–2.13, p=0.046) for SP3. For the secondary endpoint of dichotomized ICU discharge CPC, the global p-value was significant at 0.002. The unadjusted odds ratios for good outcome were 1.10 (0.90–1.34, p=0.34) for SP2 and 1.69 (1.41–2.01, p<0.001) for SP3, and the adjusted odds ratio was 0.86 (0.55–1.35, p=0.52) for SP2 and 1.46 (0.97–2.20, p=0.07) for SP3, all referenced to SP1.
Discussion
In patients receiving TTM after cardiac arrest, improved functional outcomes was associated with patients admitted to centers that used as-needed NMB with an adjunctive sedation regimen compared to centers using escalating sedation dosing and limiting NMB, including after adjustment for major confounders. This is the largest study to evaluate the impact of sedation and NMB on outcomes after cardiac arrest.
Current guidelines recommend “light” sedation (i.e. awake and responsive) in the general ICU population, which is associated with improved outcomes(13, 30), but applying this approach to cardiac arrest patients undergoing TTM is inappropriate. Monitoring the depth of sedation has not been validated in brain-injured patients, as mental status changes may be secondary to brain injury rather than sedation.
In addition to inconsistent sedation strategies during TTM, the use of NMB varies widely, and the benefits or risks are uncertain. A recent guideline for the use of NMB in the ICU made no recommendation regarding the use of NMB during TTM(31). Several observational TTM studies have concluded that NMB administration was associated with improved outcome (23–25) although these studies did not robustly adjust for underlying severity of illness. Some experts have recommended avoiding NMB during TTM(1, 10). Shivering itself is associated with good outcome after cardiac arrest,(33) possibly because the most severely injured brains do not mount a shivering response. Therefore requiring treatment for shivering with NMB may reflect a less severe brain injury and thus explain the association with better outcome. The most recent study was a single center study of 63 randomized continuous versus as needed NMB. This studies positive finding was fewer shivering episodes in the continuous NMB group. Secondary outcomes showed lower doses of sedatives and analgesics, decreased length of ICU stay and earlier awakening in the continuous NMB group. However, it is important to note that patients received escalating doses of sedatives and analgesics with each dose of rocuronioum per protocol(32). Therefore, it is unclear if the time to awakening and ICU stay is reflective of the NMB practice or the protocol of increasing sedation and analgesia in the as needed NMB group. Our analysis, where sedation and shivering strategies were defined at the hospital level, avoids this within-patient confounder and may be more generalizable to a center-treatment approach.
Worse outcomes associated with escalating sedation dosing sedation after cardiac arrest has several plausible explanations. The metabolism of many sedatives and analgesics decreases during hypothermia (34, 35),(16) likely resulting in accumulation of medication, which can confound neuroprognostication after cooling, resulting in inappropriate withdrawal of life support. Targeting a moderate depth of sedation and using NMB to treat shivering reduces the overall dose of sedatives, particularly for those who shiver vigorously, and appears to reduce the time to wakening after TTM. (4, 36) Higher-doses of sedation to control shivering without NNB may also reduce blood pressure, known to influence outcome in animal cardiac arrest models(37, 38) and retrospective human studies.(20, 39) In addition, escalating doses of sedation without NMB may incompletely control shivering, increase variability in body temperature or delay adequate suppression of shivering. Excess shivering has the potential to delay time to target temperature, elevate intracranial pressure, and lower brain oxygen levels. Sedation also has immunology effects,(40, 41) which may increase the rate of infection, time on the ventilator, and prolong ICU course.(2, 13). Lastly, improved outcome with as-needed doses of NMB may be a reflection of increased bedside monitoring of patients.
Although this is the largest study to evaluate the effects of sedation on outcomes after cardiac arrest, several limitations warrant discussion. The INTCAR data were collected prospectively, but sedation and NMB doses were not collected at the patient level. However, every center had a sedation protocol, and although between-patient variability occurs within each center, centers with established protocols may have less drastically divergent variations in treatment sufficient to confound these results. Also centers who choose to participate in INTCAR have a particular interest in post-resuscitation care and it is unclear how these results apply to other centers. Next, the effect of similar sedation approaches within each center may not be consistent across all patients. It is also possible that these effects on outcome may be due to variation in sedation protocols or from other unmeasured differences in practice, such as increased bedside attentiveness with as-needed NMB protocols. Also it should be noted that the groups were not comparable for several variables known to influence outcomes after cardiac arrest, however we used the preferred statistical method of hierarchal logistic regression to minimize bias between these groups. Also, it is possible that the sedation practice could have changed significantly over the course of the study. The survey attempted to address this, asking if and how practices changed after the year 2012 (start of INTCAR 2.0). Fifty percent of centers reported a significant change in their protocol however citing reasons would not have altered their SP category (ie changing from fentanyl to remifentanyl or inclusion of dexmedetomidine). There were two centers that responded that there was a significant change in their protocol but did not explain what that was. One of these centers included patients in both 1.0 and 2.0 datasets and 170 patients were in the 1.0 dataset, and could have been inappropriately assigned.
Protocols for sedation and shivering management of patients undergoing TTM are variable (TTM) and the only randomized trial to address this is difficult to interpret differences in NMB dosing or sedation dosing as an influence to outcome. Our data suggest that good outcome is associated with the use of a protocol that favors as needed NMB with basal sedation instead of increasing sedation to avoid NMB in response to shivering after cardiac arrest. Prospective study evaluating sedative and NMB use at the patient level, with outcomes adjusted for severity of illness, is warranted.
Supplementary Material
Figure 1.
Odds ratio for good outcome at s of SP2 (prophylactic NMB) and SP3 (as needed NMB) referenced to SP1 (avoiding NMB).
CPC: Cerebral Performance Category
Figure 2.
Odds ratio for good outcome at ICU discharge of SP2 (prophylactic NMB) and SP3 (as needed NMB) referenced to SP1 (avoiding NMB).
CPC: Cerebral Performance Category
Footnotes
Reprints: No reprints will be ordered
Disclosure: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number KL2TR001063. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Copyright form disclosure: Dr. May’s institution received funding from TUFTS CTSI Career development award (KL2) supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), Grant Number KL2TR0010063. Drs. May and Kent received support for article research from the NIH. Dr. Fraser disclosed off-label product use of all sedatives mentioned (with the exception of midazolam and dexmedetomidine). Dr. Nielsen received funding from BARD Medical (lecture fee) and BrainCool (advisory board). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S465–482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamorro C, Borrallo JM, Romera MA, et al. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesthesia and analgesia. 2010;110(5):1328–1335. doi: 10.1213/ANE.0b013e3181d8cacf. [DOI] [PubMed] [Google Scholar]
- 3.Choi HA, Ko SB, Presciutti M, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocritical care. 2011;14(3):389–394. doi: 10.1007/s12028-010-9474-7. [DOI] [PubMed] [Google Scholar]
- 4.May TL, Seder DB, Fraser GL, et al. Moderate-Dose Sedation and Analgesia During Targeted Temperature Management After Cardiac Arrest. Neurocritical care. 2014 doi: 10.1007/s12028-014-9998-3. [DOI] [PubMed] [Google Scholar]
- 5.Wong GC, van Diepen S, Ainsworth C, et al. Canadian Cardiovascular Society/Canadian Cardiovascular Critical Care Society/Canadian Association of Interventional Cardiology Position Statement on the Optimal Care of the Postarrest Patient. The Canadian journal of cardiology. 2017;33(1):1–16. doi: 10.1016/j.cjca.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Badjatia N, Strongilis E, Gordon E, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke; a journal of cerebral circulation. 2008;39(12):3242–3247. doi: 10.1161/STROKEAHA.108.523654. [DOI] [PubMed] [Google Scholar]
- 7.Oddo M, Frangos S, Maloney-Wilensky E, et al. Effect of shivering on brain tissue oxygenation during induced normothermia in patients with severe brain injury. Neurocritical care. 2010;12(1):10–16. doi: 10.1007/s12028-009-9280-2. [DOI] [PubMed] [Google Scholar]
- 8.Quenot JP, Ladoire S, Devoucoux F, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Critical care medicine. 2007;35(9):2031–2036. doi: 10.1097/01.ccm.0000282733.83089.4d. [DOI] [PubMed] [Google Scholar]
- 9.Riker RR, Gagnon DJ, May T, et al. Analgesia, sedation, and neuromuscular blockade during targeted temperature management after cardiac arrest. Best practice & research Clinical anaesthesiology. 2015;29(4):435–450. doi: 10.1016/j.bpa.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Critical care medicine. 2009;37(3):1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 11.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Critical care medicine. 1999;27(12):2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 12.De Jonghe B, Bastuji-Garin S, Fangio P, et al. Sedation algorithm in critically ill patients without acute brain injury. Critical care medicine. 2005;33(1):120–127. doi: 10.1097/01.ccm.0000150268.04228.68. [DOI] [PubMed] [Google Scholar]
- 13.Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. The New England journal of medicine. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 14.Treggiari MM, Romand JA, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Critical care medicine. 2009;37(9):2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 15.Kollef MH, Levy NT, Ahrens TS, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114(2):541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 16.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Critical care medicine. 2007;35(9):2196–2204. doi: 10.1097/01.ccm.0000281517.97507.6e. [DOI] [PubMed] [Google Scholar]
- 17.Perman SM, Kirkpatrick JN, Reitsma AM, et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia*. Critical care medicine. 2012;40(3):719–724. doi: 10.1097/CCM.0b013e3182372f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaniego EA, Mlynash M, Caulfield AF, et al. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocritical care. 2011;15(1):113–119. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YM, Youn CS, Kim SH, et al. Adverse events associated with poor neurological outcome during targeted temperature management and advanced critical care after out-of-hospital cardiac arrest. Crit Care. 2015;19:283. doi: 10.1186/s13054-015-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilgannon JH, Roberts BW, Jones AE, et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest*. Crit Care Med. 2014;42(9):2083–2091. doi: 10.1097/CCM.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 21.Kilgannon JH, Roberts BW, Reihl LR, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3):410–416. doi: 10.1016/j.resuscitation.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37(11):2895–2903. doi: 10.1097/ccm.0b013e3181b01d8c. quiz 2904. [DOI] [PubMed] [Google Scholar]
- 23.Salciccioli JD, Cocchi MN, Rittenberger JC, et al. Continuous neuromuscular blockade is associated with decreased mortality in post-cardiac arrest patients. Resuscitation. 2013;84(12):1728–1733. doi: 10.1016/j.resuscitation.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DH, Lee BK, Jeung KW, et al. Neuromuscular blockade requirement is associated with good neurologic outcome in cardiac arrest survivors treated with targeted temperature management. Journal of critical care. 2017;40:218–224. doi: 10.1016/j.jcrc.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Lascarrou JB, Le Gouge A, Dimet J, et al. Neuromuscular blockade during therapeutic hypothermia after cardiac arrest: observational study of neurological and infectious outcomes. Resuscitation. 2014;85(9):1257–1262. doi: 10.1016/j.resuscitation.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Kelly DF, Goodale DB, Williams J, et al. Propofol in the treatment of moderate and severe head injury: a randomized, prospective double-blinded pilot trial. Journal of neurosurgery. 1999;90(6):1042–1052. doi: 10.3171/jns.1999.90.6.1042. [DOI] [PubMed] [Google Scholar]
- 27.Debourgogne A, Goehringer F, Umhang G, et al. Primary cerebral alveolar echinococcosis: mycology to the rescue. Journal of clinical microbiology. 2014;52(2):692–694. doi: 10.1128/JCM.02843-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stef van Buuren KG-O. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011 [Google Scholar]
- 30.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the Intensive Care Unit: executive summary. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(1):53–58. doi: 10.1093/ajhp/70.1.53. [DOI] [PubMed] [Google Scholar]
- 31.Murray MJ, DeBlock H, Erstad B, et al. Clinical Practice Guidelines for Sustained Neuromuscular Blockade in the Adult Critically Ill Patient. Crit Care Med. 2016;44(11):2079–2103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 32.Stockl M, Testori C, Sterz F, et al. Continuous versus intermittent neuromuscular blockade in patients during targeted temperature management after resuscitation from cardiac arrest-A randomized, double blinded, double dummy, clinical trial. Resuscitation. 2017;120:14–19. doi: 10.1016/j.resuscitation.2017.08.238. [DOI] [PubMed] [Google Scholar]
- 33.Nair SU, Lundbye JB. The occurrence of shivering in cardiac arrest survivors undergoing therapeutic hypothermia is associated with a good neurologic outcome. Resuscitation. 2013;84(5):626–629. doi: 10.1016/j.resuscitation.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Bjelland TW, Klepstad P, Haugen BO, et al. Effects of hypothermia on the disposition of morphine, midazolam, fentanyl, and propofol in intensive care unit patients. Drug metabolism and disposition: the biological fate of chemicals. 2013;41(1):214–223. doi: 10.1124/dmd.112.045567. [DOI] [PubMed] [Google Scholar]
- 35.Fritz HG, Holzmayr M, Walter B, et al. The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesthesia and analgesia. 2005;100(4):996–1002. doi: 10.1213/01.ANE.0000146517.17910.54. [DOI] [PubMed] [Google Scholar]
- 36.Bjelland TW, Dale O, Kaisen K, et al. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: a randomised trial. Intensive care medicine. 2012;38(6):959–967. doi: 10.1007/s00134-012-2540-1. [DOI] [PubMed] [Google Scholar]
- 37.Sterz F, Leonov Y, Safar P, et al. Hypertension with or without hemodilution after cardiac arrest in dogs. Stroke; a journal of cerebral circulation. 1990;21(8):1178–1184. doi: 10.1161/01.str.21.8.1178. [DOI] [PubMed] [Google Scholar]
- 38.Leonov Y, Sterz F, Safar P, et al. Hypertension with hemodilution prevents multifocal cerebral hypoperfusion after cardiac arrest in dogs. Stroke; a journal of cerebral circulation. 1992;23(1):45–53. doi: 10.1161/01.str.23.1.45. [DOI] [PubMed] [Google Scholar]
- 39.Beylin ME, Perman SM, Abella BS, et al. Higher mean arterial pressure with or without vasoactive agents is associated with increased survival and better neurological outcomes in comatose survivors of cardiac arrest. Intensive care medicine. 2013;39(11):1981–1988. doi: 10.1007/s00134-013-3075-9. [DOI] [PubMed] [Google Scholar]
- 40.Corcoran TB, Engel A, Sakamoto H, et al. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. British journal of anaesthesia. 2006;97(6):825–831. doi: 10.1093/bja/ael270. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Barke RA, Charboneau R, et al. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. Journal of immunology (Baltimore, Md : 1950) 2005;174(1):426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.