Abstract
Sodium–glucose cotransporter 2 (SGLT2) inhibitors offer attractive metabolic and cardiorenal benefits for patients with type 2 diabetes, but are associated with a number of safety issues. A recent study documented that SGTL2 inhibition indirectly triggers the FGF23/1,25-dihydroxyvitamin D/parathyroid hormone axis, which may contribute to adverse effects on bone health.
Sodium glucose cotransporter-2 (SGLT2) inhibitors promote urinary glucose excretion, thereby decreasing plasma glucose1. This class of therapeutics provides additional benefits by decreasing body weight, blood pressure, risk of major adverse cardiovascular events, hospitalization for heart failure, and progression of diabetic kidney disease. Health authorities including the U. S. Food and Drug Administration have warned about multiple safety concerns, including genitourinary infections, urosepsis, acute kidney injury, ketoacidosis, lower limb amputations, and bone fractures.
An excess of bone fractures was first reported in clinical studies with dapagliflozin. In a study of diabetic patients with moderate renal impairment, 9.4% of patients treated with dapagliflozin (10 mg/d) experienced bone fractures2. No fractures were observed in placebo-treated patients2. Canagliflozin (300 mg/d) accelerated loss of total hip bone mineral density compared to placebo (−2.1% vs. −0.9%) during a 104 week trial in diabetic patients 55–80 years of age3. In the Canagliflozin Cardiovascular Assessment Study, canagliflozin was associated with an increased incidence of fractures: 4.0% of canagliflozin-treated vs. 2.6% of placebo-treated patients 4. Thus far, health authorities have warned about increased risk of bone fractures with canagliflozin, but have not announced a firm conclusion whether this is a class effect.
Mechanisms for increased fracture risk
Bone health is a complex biological problem. Patients receiving SGLT2 inhibitors often have concomitant conditions affecting bone health: post-menopausal osteopenia/osteoporosis, co-existing chronic kidney disease (CKD), as well as type 2 diabetes itself. Several mechanisms have previously been suggested to mediate adverse effects of SGLT2 inhibitors on bone. SGLT2 inhibitors promote weight loss, and the magnitude of weight loss was correlated with a biomarker for bone resorption (collagen type 1 β-carboxy-telopeptide). However, weight loss explained only a small percentage (~3%) of the variance of bone resorption biomarkers3. Furthermore, SGLT2 inhibitors can induce postural hypotension, which may increase the risk of falls4. It is plausible that falling could increase fracture risk, but unlikely that this accounts for loss in bone mineral density. Finally, women receiving canagliflozin (300 mg) experienced a 9.2% decrease in estradiol levels3. While this could increase fracture risk, the statistical analysis did not establish whether loss of bone mineral density was indeed correlated with decreased estradiol levels. In summary, mechanisms mediating canagliflozin-induced fractures have not been fully elucidated.
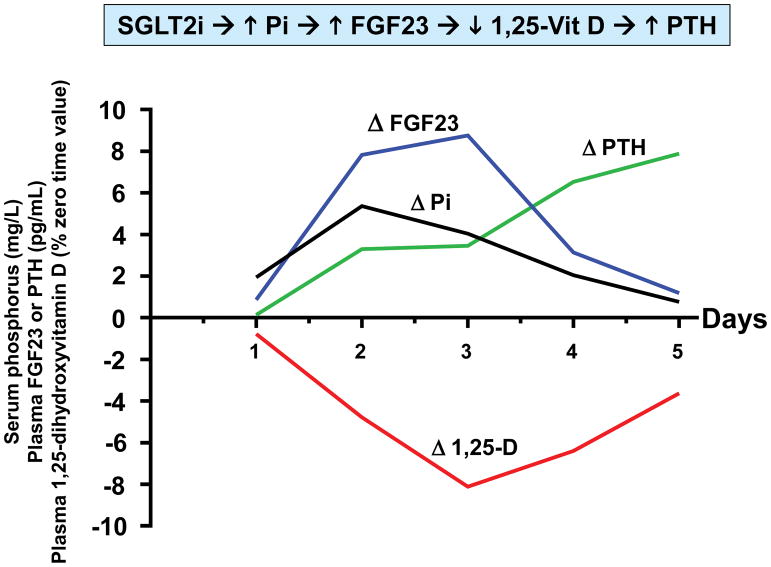
In a recent study of canagliflozin in healthy volunteers, we identified drug-induced endocrine changes that may mediate the drug’s adverse effect on bone health5. Canagliflozin (300 mg/d) increased mean serum phosphorus levels – with a peak increase of 0.61 mg/dL observed after 36 hours of drug administration. The increase in serum phosphorus was correlated with increased transtubular reabsorption of phosphate. This increase in serum phosphorus is reminiscent of electrolyte changes characterizing CKD – albeit changes are smaller in SGLT2 inhibitor-treated patients. Whereas increased serum phosphorus is driven by decreased glomerular filtration in CKD, SGLT2 inhibitors increase proximal tubular reabsorption of phosphate. Nevertheless, both CKD and SGLT2 inhibitors trigger the FGF23/1,25-dihydroxyvitamin D/PTH axis. Plasma FGF23 was increased by ~20% within 24 hours after initiation of canagliflozin (Fig. 1). This was followed by a decrease in plasma levels of 1,25-dihydroxyvitamin D (-10%) and increased plasma PTH (+25%). The decrease in 1,25-dihydroxyvitamin D levels was likely mediated by FGF23-induced decrease in expression of the CYP27B1 gene. We hypothesize that decreased levels of 1,25-dihydroxyvitamin D decrease gastrointestinal absorption of dietary calcium, which in turn promotes PTH secretion. Consistent with this hypothesis, we observed a decrease in 24 hour urinary calcium excretion on days 4 and 5 of our study.
Figure 1. Time course of pharmacodynamic responses to canagliflozin.
Healthy volunteers (N=25) were admitted to the NIH Clinical Center to participate in a randomized crossover trial 5. Research subjects participated in two admissions during which they received either placebo or canagliflozin (300 mg/d) for five days. Each research subject was randomized between placebo or canagliflozin for the first admission, and then crossed over to the other treatment for the second admission. Four blood samples were obtained on each day (8 AM, 10 AM, 12 noon, and 8 PM). The details of the study design are described in Blau et al. 5 We calculated averages of the timed blood samples on each of the five days of the study. This figure presents drug-induced changes of those averages (i.e., placebo-subtracted values) for each day. In order to display all four parameters on the same axes, we expressed the data in the following units: serum phosphorus (mg/L); plasma FGF23 (pg/mL); plasma PTH (pg/mL); and 1,25-dihydroxyvitamin D (% of time zero value).
Clinical implications
The combination of decreased 1,25-dihydroxyvitamin D and increased PTH levels would be expected to be detrimental to bone health, and may contribute to SGLT2 inhibitor-associated fracture risk. It is important to note that increased levels of serum phosphorus have been observed with all drugs in the SGLT2 inhibitor class. In 1944, Pitts & Alexander reported that phlorizin (a non-selective inhibitor of SGLT-family cotransporters) promoted tubular phosphate reabsorption6. They postulated that glucose transporters and phosphate transporters compete for “an element common to the two mechanisms.” We now know that both glucose and phosphate are reabsorbed by Na+-dependent cotransporters: SGLT1 (SLC5A1) and SGLT2 (SLC5A2) for glucose; NaPi-IIa (SLC34A1) and NaPi-IIc (SLC34A3) for phosphate. The electrochemical gradient for Na+ represents the common element for which these Na+-dependent cotransporters compete. Based on these considerations, it is likely that triggering the FGF23/1,25-dihydroxyvitamin D/PTH axis represents a mechanism-based class effect shared by all SGLT2 inhibitors. Nevertheless, it is possible that this adverse effect might vary in magnitude among different members of the SGLT2 inhibitor class. Such differences could be caused by differences in selectivity (SGLT1 vs. SGLT2) or differences in the efficacy of approved doses for different drugs.
The drug-induced increase in serum phosphorus was transient in our healthy volunteer study. The increase in serum phosphorus was followed by induction of two phosphaturic hormones (FGF23 and PTH), which function in tandem to restore serum phosphorus levels toward normal. Levels of FGF23 and 1,25-dihydroxyvitamin D were also restored toward baseline, but PTH levels remained elevated at the end of our five day study. In contrast to our data with healthy volunteers, the mean increase in serum phosphorus (≈ 0.15 mg/dL) was sustained for at least 26 weeks in type 2 diabetic patients treated with canagliflozin. The mean increase was greater (≈ 0.35 mg/dL) in diabetic patients with impaired renal function (mean eGFR = 39 mL/min/m2). Just as the increase in serum phosphorus was sustained in diabetic patients, we hypothesize that canagliflozin-induced changes in FGF23, 1,25-dihydroxyvitamin D, and PTH are also sustained in diabetic patients. Additional studies will be required to assess the magnitude of changes in bone-related hormones in response to chronic therapy with SGLT2 inhibitors in diabetic patients. These studies are challenging for a variety of reasons, including diurnal variation in levels of serum phosphorus, FGF23, 1,25-dihydroxyvitamin D, and PTH. Further, the amplitude of the diurnal variation is similar in magnitude to drug-induced changes. For example, in placebo-treated individuals, peak serum phosphorus levels (observed at 8 PM) averaged ~3.8 mmol/L while trough levels (observed at 12 noon) averaged ~3.3 mmol/L. Because the amplitude of diurnal variation (~0.5 mmol/L) is similar in magnitude to the mean drug effect (~0.6 mmol/L), it is critical to pay strict attention to timing of blood tests to minimize the risk that diurnal variation would hide the drug effect.
Most SGLT2 inhibitor-treated patients do not experience fractures. How can physicians identify the minority of patients who are at greatest risk to develop fractures? There is considerable inter-individual variation with respect to the magnitude of canagliflozin-induced changes in serum phosphorus and bone-related hormones. We hypothesize that patients with the largest changes in 1,25-dihydroxyvitamin D and/or PTH may be at greatest risk. Further, we speculate that calcitriol treatment might be beneficial for patients in whom an SGLT2 inhibitor induces the greatest decrease in 1,25-dihydroxyvitamin D levels. Additional research will be required to test these hypotheses.
Conclusions
In designing drugs, scientists focus primarily on selecting molecules that will provide clinically meaningful therapeutic benefits. Nevertheless, most drugs also exert unintended pharmacological activities leading to undesirable side effects. Some side effects are mediated by off-target activities. In theory, chemists can modify the drug’s structure to eliminate these off-target activities. In the case of selective SGLT2 inhibitors, side effects appear to be mediated by on-target activity. Systems biology teaches that a perturbation of the function of a single protein can lead to numerous physiologically important alterations in downstream pathways. The nephron provides a perfect example. The electrochemical gradient for Na+ drives active transport of many ions and solutes, resulting in cross-talk among the various Na+-dependent transport mechanisms. Alterations in renal tubular function can change circulating concentrations of key ions, metabolites and hormones. Some changes may be beneficial while others may cause adverse effects. By understanding mechanisms of these complex downstream changes, physicians will be better able to manage and possibly mitigate adverse effects of these drugs.
Acknowledgments
SIT acknowledges funding from NIDDK (grant number P30DK072488). JEB is supported by funding from the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosures
SIT is a consultant for Ionis Pharmaceuticals, has research support provided to the University of Maryland School of Medicine by Regeneron Pharmaceuticals, and has ownership of stock in Celgene, Amgen, and Abbott Laboratories.
References
- 1.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 2.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilezikian JP, et al. Evaluation of Bone Mineral Density and Bone Biomarkers in Patients With Type 2 Diabetes Treated With Canagliflozin. J Clin Endocrinol Metab. 2016;101:44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts NB, et al. Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2016;101:157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blau JE, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3 doi: 10.1172/jci.insight.99123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitts RF, Alexander RS. The renal reabsorptive mechanism for inorganic phosphate in normal and acidotic dogs. Am J Physiol. 1944;142:648–662. [Google Scholar]