Abstract
Background
Oral pre-exposure prophylaxis (PrEP) prevents HIV infection in men who have sex with men (MSM); however, adherence is an ongoing concern. Long-acting injectable PrEP (LAI-PrEP) is being tested in phase III trials and may address challenges associated with adherence. We examined the potential effectiveness of LAI-PrEP compared to oral PrEP among MSM.
Methods
We used an agent-based model to simulate HIV transmission in a dynamic network of 11,245 MSM in Atlanta. We used raw data from macaque studies and pharmacokinetic data from safety trials to estimate the time-varying efficacy of LAI-PrEP. The impact of LAI-PrEP on the cumulative number of HIV infections over 10 years was compared to oral PrEP across a range of coverage levels. Sensitivity analyses were conducted with varying maximum efficacy and drug half-life values.
Findings
In the absence of PrEP, the model predicts 2,374 new infections over a decade. The cumulative number of new HIV infections was reduced in all scenarios in which MSM received LAI-PrEP compared to oral PrEP. At a coverage level of 35%, compared to no PrEP, LAI-PrEP led to a 44.0% reduction in new HIV infections versus 33.4% for oral PrEP. The relative benefit of LAI-PrEP was sensitive to the assumed efficacy of injections received every 8 weeks, discontinuation rates, and terminal drug half-life.
Interpretation
LAI-PrEP has the potential to produce larger reductions in HIV transmission among MSM than oral PrEP. However, the real-world, population-level impact of LAI-PrEP will depend on uptake of this prevention modality, its efficacy, as well as retention in clinical care.
Keywords: agent-based modeling, pre-exposure prophylaxis, men who have sex with men, cabotegravir, HIV, microsimulation
INTRODUCTION
Men who have sex with men (MSM) are at substantial risk for HIV infection in nearly all contexts studied globally (1). The prevalence of HIV infection among MSM is elevated relative to the prevalence among the general adult male population in all regions (1). Despite declines in select settings, incidence rates remain high and show little sign of decline in many contexts (1).
Pre-exposure prophylaxis (PrEP) represents one promising approach to decreasing the incidence of HIV infection among at-risk groups, including MSM (2). The efficacy of oral PrEP among at-risk MSM has been demonstrated in several clinical trials and has since been shown to be effective in real-world settings (2–4). In the United States (US), PrEP is currently recommended for adult, HIV-uninfected MSM who report engagement in condomless anal intercourse with more than one HIV-uninfected partner, have been recently diagnosed with a bacterial sexually transmitted infection, and/or are in an ongoing partnership with an HIV-positive partner (5).
PrEP use among MSM in the US has increased, but its uptake among this population has been slow (6). In addition, an individual must take at least 4 doses per week to achieve intracellular drug concentrations consistent with 96% reduction in risk of HIV infection (7). To address challenges associated with adherence to a daily pill-based regimen, injectable formulations of antiretroviral drugs are being tested as candidates for use as long-acting injectable PrEP (LAI-PrEP) (8). Recent studies have shown high interest in LAI-PrEP among MSM (9, 10).
Long-acting injectable cabotegravir (LAI-CAB), an integrase inhibitor formulated as an injectable nanoparticle suspension, has been demonstrated to exert a strong protective effect against intravenous simian immunodeficiency virus (SIV) challenge in a macaque model (11). The safety and tolerability of LAI-CAB among healthy men has been demonstrated by two phase IIa trials (12, 13). In these trials, LAI-CAB was well tolerated, with an acceptable safety profile despite high incidence of transient, mild-to-moderate injection-site reactions (12, 13). An assessment of pharmacokinetic data has suggested that LAI-CAB may confer consistent high levels of protection when given every eight weeks (13). The efficacy of LAI-CAB administered in this regimen is currently being evaluated in comparison to oral PrEP among MSM at high risk of HIV infection at ten sites in eight countries as part of a phase III trial (14).
Although LAI-PrEP is a promising HIV prevention approach, it is currently unknown what the population-level impact will be on the incidence of HIV infection among MSM. Employing an agent-based model (ABM), we aimed to quantify the potential impact of LAI-PrEP on HIV incidence in a population of MSM in Atlanta, Georgia, an area with high HIV incidence among MSM (15). The primary objective of this study was to measure the impact of LAI-PrEP, in comparison to oral PrEP, on cumulative HIV incidence among MSM in Atlanta over 10 years.
METHODS
Model Setting
The ABM simulated HIV transmission over a 10-year period beginning in 2015 within a population of MSM in the city of Atlanta, Georgia. MSM are estimated represent 5·4% of the adult male population of the broader Atlanta-Sandy Springs-Roswell metropolitan statistical area (16). Demographic, behavioral, and clinical characteristics, including race (i.e. black/African American or white), were assigned to each agent in the model, with distributions for these characteristics informed by local data and existing literature. The ABM modeled a dynamic population in steady state, in which agents left the population at death or due to aging out at 65 years. Demographic, behavioral, and clinical characteristics for incoming agents were randomly drawn from the same distributions used to create the base population (Table 1). The model was calibrated to reproduce the trajectories in observed HIV diagnoses among MSM in Georgia from 2007 to 2015 (17). Further details regarding model calibration are available in the Supplemental File.
Table 1.
Key model processes and parameters
| Domain | Description |
|---|---|
| Demographics | |
| Population size | n = 11,245 |
| Age | General male population distribution |
| Mortality | Varies by HIV/AIDS status |
| HIV risk behaviors | |
| Per-partner sex frequency | Number of sex acts per partner per year |
| Per-act probability of condomless anal intercourse | Varies by number of prior contacts with partner |
| Per-act transmission risk (condomless anal intercourse) | Varies by sexual role, PrEP, HIV diagnosis, ART |
| Receptive Partner Base Risk: 1.38% per-act | |
| Insertive Partner Base Risk: 0.11% per-act | |
| Sexual Networks | |
| Partner Number (Annual) | Number of anal sex partners per year |
| Duration of Relationships | Duration of relationship duration in month |
| Daily Oral Pre-Exposure Prophylaxis | |
| Population-Level Coverage | Percentage of agents using daily oral PrEP |
| Probability Retention in Clinical Care | 3 months post-initiation: 72.5% |
| 6 months post-initiation: 59.6% | |
| Probability of Full Adherence (≥4 pills per week) | 92.3% |
| Percent Reduction in Per-Act Transmission Risk | 96.0% (≥4 pills), 76.0% (2–3 pills) |
| Long-Acting Injectable Pre-Exposure Prophylaxis | |
| Population-Level Coverage | Percentage of agents using LAI-PrEP |
| Probability of Retention in Clinical Care | 2 months post-initiation: 84.8% |
| Percent Reduction in Per-Act Transmission Risk | Varies as a function of time since last injection |
| HIV Testing | |
| Probability of having ever tested | 92.8% |
| Annual probability of obtaining HIV testing | 69.0% |
| HIV Treatment | |
| Proportion of PLWH aware of their HIV infection status | 84.4% (white MSM), 74.7% (black MSM) |
| Proportion of PLWH on ART | 46.7% (white MSM), 26.2% (black MSM) |
| Proportion of PLWH with viral load suppression | 40.5% (white MSM), 21.0% (black MSM) |
| Percent Reduction in Per-Act Transmission with ART | 96.0%, among PLWH with viral load suppression |
Abbreviations: PLWH, people living with HIV; ART, antiretroviral therapy
Note: full list of parameter values, sources, and discussion are provided in the Supplemental File.
Sexual Networking
As the model progressed in monthly time-steps over a period of ten years, agents formed and dissolved sexual partners, engaged in sexual acts, and acquired HIV infection within serodiscordant partnerships. Target annual partner numbers and numbers of sex acts per partner were assigned to agents in a stochastic fashion each year. Upon formation, each partnership was assigned a set duration. Population sexual networks were generated within the model through the formation and dissolution of relationships as the model progressed.
Sexual Behavior and Transmission of HIV Infection
Partner-level sexual role classes were assigned to each agent to determine their behavior within a sexual partnership. An exclusively insertive agent would engage as the insertive partner only in anal intercourse and a receptive agent would engage as the receptive partner only, while versatile agents could engage in either role. At each time-step, agents within sexual partnerships engaged in a specified number of sex acts with their respective partners; their probability of condom use decreased as a function of the number of prior contacts with that partner (18). Each condomless sex act within a serodiscordant dyad carried a probability of HIV transmission. The base initial per-act probabilities for condomless receptive anal intercourse and condomless insertive anal intercourse were set as 1·38% and 0·11%, respectively (19). The probabilities of transmission were then scaled based on whether the HIV-uninfected agent used PrEP, and in the oral PrEP scenarios, their adherence to the therapy; and whether the HIV-infected agent was diagnosed with HIV infection, and if so, had achieved viral load suppression based on adherence to antiretroviral treatment. At model initialization, the proportion of HIV-infected MSM who were virally suppressed was set at 24·7% (20). Condom-protected acts were assumed to carry no risk of HIV transmission.
Deriving Efficacy of LAI-PrEP
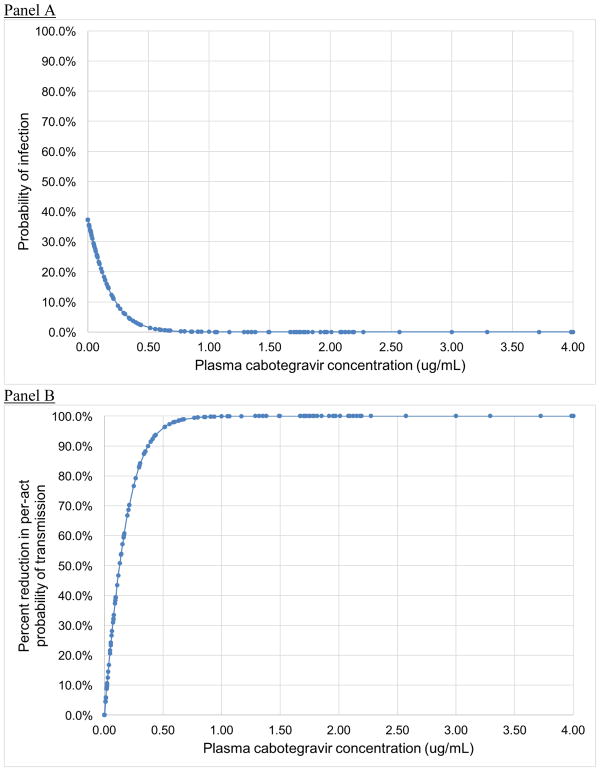
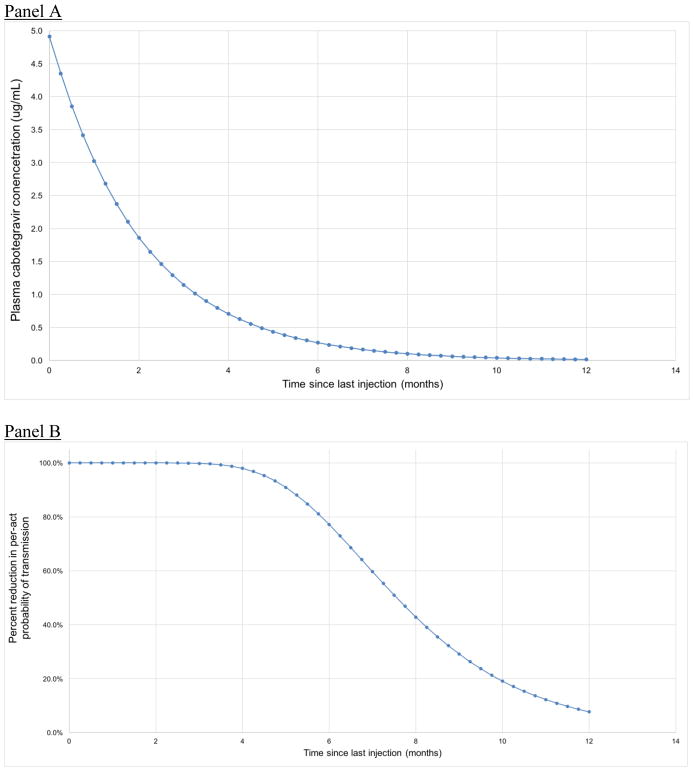
We used raw data from a study examining the efficacy of LAI-CAB in reducing risk for SIV acquisition in a macaque model to estimate the percent reduction in risk for HIV acquisition conferred as a function of serum CAB concentration (Figure 1) (11). We obtained raw data from the study authors, and used logistic regression to model the probability of seroconversion as a function of serum CAB concentration. From this curve, we estimated the percent reduction in the probability of infection at each serum CAB concentration compared to controls. We then modeled serum CAB concentrations from each LAI-PrEP injection over time (Figure 2, Panel A), with a half-life based on data from Phase II trials in humans (12). Based on these curves, agents in the model who were assigned to LAI-PrEP maintained serum CAB concentrations for up to 12 months following their last injection, where each value corresponds to an estimated percent reduction in the risk of HIV acquisition (Figure 2, Panel B).
Figure 1.
Estimated (A) probability of infection from rectal SIV exposure in a macaque model (11), and (B) reduction in per-act probability of SIV transmission in macaques, by plasma cabotegravir concentration.
Figure 2.
Estimated (A) plasma cabotegravir concentration and (B) reduction in per-act probability of HIV transmission by time since last injection
Note: Percent reduction in per-act HIV transmission after a final LA-CAB injection was estimated based on half-life values reported in Markowitz et al., 2017 (12)
Model Scenarios
The main analyses evaluated three scenarios: 1) oral PrEP exclusively, 2) LAI-PrEP exclusively, and 3) no PrEP availability. Ten-year cumulative HIV incidence was calculated for each of these three scenarios across coverage targets ranging from 5% to 35% in 5% increments. Each scenario was initialized in January 2015 with a set coverage level of each intervention that was maintained throughout the 10-year simulation. In all cases, agents who were not retained in PrEP care were eligible to re-initiate daily oral PrEP or LAI-PrEP twelve months after discontinuation, with the same probability as all other HIV-uninfected agents.
For scenarios involving daily oral PrEP, agents’ engagement in care was based on data regarding adherence and retention from MSM in three real-world PrEP clinics in the US (21). Parameters (see Table 1) pertaining to PrEP use were based on the assumption that a patient will receive a 90-day prescription at each clinical care visit and that a patient is considered retained in care if they had a clinical care visit in the past 6 months. Patients were categorized as not retained in care—and therefore no longer users of PrEP—if they had not had a clinical care visit within 6 months. This definition of retention in care does not allow for intermittent clinic attendance but does allow for variation in the length of time an individual uses PrEP. Active oral PrEP users were also categorized as optimally adherent or sub-optimally adherent to capture heterogeneity in daily pill-taking. For scenarios involving LAI-PrEP, agents’ engagement in care was based on observed retention rates in phase II trials of CAB as LAI-PrEP (13). In these cases, a patient was considered retained if they returned for a follow-up injection, occurring 2 months after their previous injection. For LAI-PrEP, retention in care and adherence are interchangeable, given the need for an individual to attend a clinic visit and for a clinician to administer the injections.
Outcome Measures
The primary outcome was the cumulative number of new HIV infections over 10 years (2015–2024) with either oral PrEP or LAI-PrEP at each coverage level compared to the base model where neither modality was available. Outcomes were also expressed as a percent reduction in the cumulative number of new HIV infections from the base model to the given PrEP coverage scenario. In addition, the percent reduction in the cumulative number of new HIV infections in scenarios with LAI-PrEP from scenarios with oral PrEP at the same coverage level is presented as a measure of the performance of LAI-PrEP in comparison to oral PrEP. All scenarios were run 500 times. Estimates are presented with 95% simulation intervals (SIs) to represent the overall stochasticity of the model.
Sensitivity Analysis
Sensitivity analyses were conducted to examine the robustness of the main analysis to uncertain model parameters likely to affect the overall impacts of LAI-PrEP, including retention in care, its maximum efficacy, terminal half-life, and the duration of a period of waning protection following a final injection. In addition, we modeled a scenario where individuals who seroconvert with waning serum CAB concentrations following their last injection have a 1% probability of a drug-resistant infection, as a means of measuring the potential cumulative number of HIV infections with resistance to CAB. Although existing data from macaques suggest this resistance will be rare (22), the Q148R mutation of the HIV integrase is associated in humans with emerging resistance to CAB, raltegravir, and elvitegravir (23).
RESULTS
The ABM simulated a total of 11,245 MSM in the city of Atlanta, Georgia. In a model without either of the two PrEP modalities, there were an estimated 2,374 new infections (95% SI: 2,345–2,412) from 2015 to 2024, producing an average 10-year incidence rate of 3·57 (95% SI: 3·42–3·70) per 100 person-years.
The estimated percentage reduction in infection per exposure as a function of plasma CAB concentration is shown in Figure 1. These calculations yielded a theoretical efficacy for the two-month period following an injection of >99·9% (this level of protection was assumed to be maintained as long as an individual is retained in care). In the period following a final injection (i.e., when an agent discontinues therapy), the level of protection diminished in accordance with the published half-life of LA-CAB (see Figure 2).
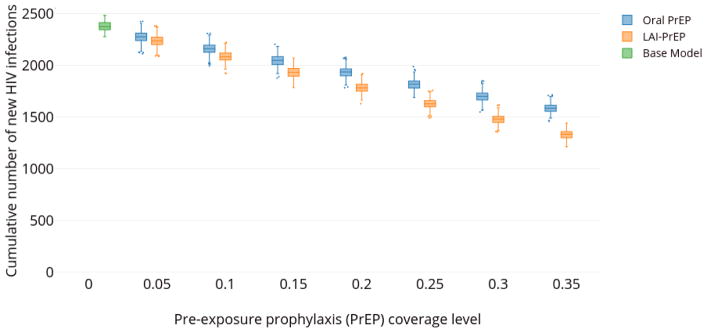
At all coverage levels, increases in oral PrEP and LAI-PrEP coverage resulted in decreases in the cumulative number of new HIV infections (Figure 3). As coverage with either of the two PrEP modalities increased, the cumulative number of new infections in the ten-year period decreased. For example, at 15% population-level coverage, there were 2,048 (95% SI: 2,008–2,084) and 1,932 (95% SI: 1,894–1,970) new infections with daily oral PrEP and LAI-PrEP, respectively.
Figure 3.
Estimated cumulative number of new HIV infections estimated among adult MSM in Atlanta, Georgia (2015–2024), at different coverage levels of oral PrEP (blue), LAI-PrEP (orange), and a base case assuming no PrEP (green).
Note: For each prevention method and coverage level, a box and whisker plot is shown, indicating the median cumulative number of new HIV infections, along with the first and third quartiles associated with this distribution. Whisker lengths represent the range of values larger than the third quartile plus 1.5× the interquartile range, and the range of values smaller than the first quartile minus 1.5× the interquartile range. Dots indicate outlying extreme values outside of this range.
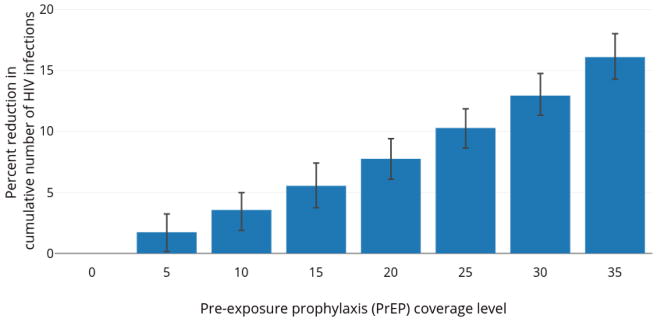
LAI-PrEP use at a given coverage level outperformed daily oral PrEP at the same level of coverage in reducing the cumulative number of new HIV infection between 2015 and 2024 (Figure 4). For example, at a population-level coverage of 35%, daily oral PrEP averted 792 infections (95% SI: 763–821), whereas with LAI-PrEP, 1,044 new infections were averted (95% SI: 1,018–1,077), corresponding to 16·1% relative reduction in cumulative incidence with LAI-PrEP compared to a scenario with oral PrEP.
Figure 4.
Percent reduction in number of new HIV infections with LAI-PrEP relative to daily oral PrEP among MSM in Atlanta, Georgia (2015–2024).
The average percent reduction is plotted comparing the number of new HIV infections with LAI-PrEP relative to daily oral PrEP. Whiskers indicate 95% simulation intervals.
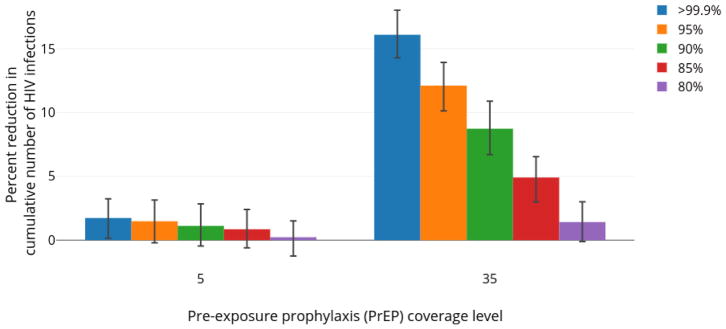
Sensitivity Analyses
The impact of LAI-PrEP on the cumulative number of new HIV infections was sensitive to the efficacy of LAI-PrEP in preventing HIV acquisition, the half-life of CAB, and the duration of protection afforded by CAB following a final injection. As the potential efficacy of LAI-PrEP was decreased from >99% to 80%, LAI-PrEP performed more similarly to daily oral PrEP (see Figure 5). A similar trend was observed as the half-life of CAB decreased from 40·0 to 18·4 days (Supplemental Figure S3). The impact of LAI-PrEP decreased as well in scenarios in which agents did not have a period of waning protection following their final injection (Supplemental Figure S4). As the probability of retention on LAI-PrEP decreased, the relative impact of LAI-PrEP increased, since, for a fixed population-level coverage, poorer retention resulted in a greater number of agents in the population with partial LAI-PrEP protection (Supplemental Figure S5). In scenarios where agents with waning serum CAB concentrations following a final injection had a 1% probability of developing a drug-resistant infection, on average, less than 1 drug-resistant infection occurred in the simulation period with 5% coverage of LAI-PrEP and less than 2 drug-resistant infections occurred with 35% coverage of LAI-PrEP.
Figure 5.
Percent reduction in cumulative number of new HIV infections with LAI-PrEP among MSM in Atlanta, Georgia (2015–2024) relative to equivalent coverage of daily oral PrEP, with varying maximum efficacy of LAI-PrEP.
The average percent reduction is plotted comparing the number of new HIV infections with LAI-PrEP relative to daily oral PrEP. Whiskers indicate 95% simulation intervals.
DISCUSSION
This study is the first to evaluate the potential population-level impact of LAI-PrEP on HIV incidence among MSM. Our model predicts that, with an estimated theoretical efficacy of greater than 99% protection from HIV acquisition with bimonthly CAB injections and waning protection for up to 12 months following a final injection, LAI-PrEP prevents more HIV infections than oral PrEP at the same coverage level. Should its efficacy in humans be equivalent to its efficacy in reducing risk of SIV infection in macaques (11). LAI-PrEP will be a highly promising HIV prevention approach.
Two studies have investigated the effectiveness of LAI-PrEP on HIV incidence among heterosexual couples in South Africa. Walensky and colleagues used a state-transition model to compare scenarios in which daily oral PrEP was 62% effective and LAI-PrEP was 75% effective to a scenario where no PrEP modalities were available. In scenarios where PrEP was available, the risk of lifetime infection declined to 540 cases per 1,000 with daily oral PrEP and 510 cases per 1,000 with LAI-PrEP (24). These estimates are consistent with our finding that LAI-PrEP outperformed daily oral PrEP at all levels of coverage in reducing HIV transmission in our target population (i.e., MSM). In a dynamic compartmental model representing heterosexual adults in KwaZulu-Natal, South Africa, Glaubius et al. found that 9·1% of new HIV infections were prevented over a ten-year period in a scenario where LAI-PrEP by 15% of all heterosexual adults (25). The results from our model are broadly similar (LAI-PrEP use by 15% of MSM prevented 12·4% of new infections).
These two previous modeling studies used different, arbitrary estimates for the efficacy of LAI-PrEP, ranging from 70% to 90% (24, 25). Our model, based on raw data from a macaque challenge study, was parameterized with a >99% estimate for LAI-PrEP efficacy. Of note, LAI-PrEP did not significantly outperform oral PrEP when maximum efficacy of the bimonthly injection was reduced to 80%. Furthermore, our analysis incorporated a 12-month period of waning partial protection from HIV infection after a final injection based the half-life reported in previous studies; however, there is variation in the length of the pharmacokinetic tail of CAB in humans (12). In scenarios with a shorter half-life or without this waning period of protection, the number of new HIV infections averted by LAI-PrEP also decreased. These two features of LAI-PrEP (maximum efficacy and longevity of protection after a final injection) will be key features in determining its real-world impact. The efficacy and longevity of protection conferred by LAI-PrEP is currently being determined in phase III trials, which will assess key factors that determine whether LAI-PrEP should be recommended as a viable HIV prevention tool, and how it should be implemented (e.g., how often injections should be given) (14).
A significant concern in the implementation of LAI-PrEP in clinical settings is that individuals who discontinue LAI-PrEP would have sub-therapeutic serum CAB concentrations for up to 12 months, leading to the potential development of integrase inhibitor resistance. This concern is of particular relevance because these agents are recommended as first-line treatment options. Despite increasing use of integrase inhibitors in HAART, resistance to these drugs remains low (26). Although further research is required, our results suggest that emergence of resistance to CAB will be rare, even in the setting where a significant proportion of individuals drop out of care is high, as has been seen with oral PrEP.
The population-level impact of LAI-PrEP will also be affected by retention in care. In our model, we estimated that 85% of individuals who receive their first injection for LAI-PrEP would receive their next injection (13). In phase IIa trials of CAB as LAI-PrEP among MSM, there has been variation in the severity and duration of the main side effect, injection-site pain (12, 13). Retention in care will likely be impacted by the willingness of LAI-PrEP users to repeatedly tolerate injection-site pain and other adverse events over the course of the injection schedule. Therefore, retention in care could be lower during real world implementation of LAI-PrEP than in clinical trials. This could adversely impact population effectiveness, particularly if new patients do not initiate LAI-PrEP at a sufficient rate to maintain high coverage in the at-risk population. In observational studies of real-world daily oral PrEP use, retention in clinical care has been identified as a key feature in sustaining the population-level impact of daily oral PrEP (21). However, we found that—given the waning period of protection from HIV infection following a final injection—decreasing retention in care on LAI-PrEP while maintaining population-level coverage improves its population-level impact, as individuals who discontinue LAI-PrEP maintain some level of protection against HIV infection for up to twelve months after their last injection. This counterintuitive finding is the result of necessary artificialities in our model. Constant population-level coverage is needed to isolate the effects of retention in care from other factors (e.g., initiation rates, efficacy) on the benefits of LAI-PrEP relative to oral PrEP. This predicted benefit of transitory usage (assuming a fixed number of patients in care) must be counter-balanced with consideration of the predictable increased potential for the evolution of resistance in the many consequent individuals with sub-preventative levels of injected CAB. Real-world LAI PrEP programs may not have a population-level impact if high discontinuation rates cannot be offset to some extent by efforts to attract new users or re-engage those who discontinue. Future studies should assess the impact of varying coverage and retention patterns on the effectiveness of LAI-PrEP among MSM.
Limitations
Several limitations to the current study demand further investigation to ensure the validity of predictions made by our analysis. First, in all scenarios, daily oral PrEP and LAI-PrEP were not targeted to agents based on specific demographic or behavioral characteristics. Currently there are clinical recommendations for daily oral PrEP initiation among MSM,5 but clinical recommendations do not exist for LAI-PrEP as these formulations are currently being tested. Targeting of daily oral PrEP to individuals who engage in high-risk behaviors has been shown to increase the efficiency and impact of the intervention compared to scenarios where uptake is non-targeted (27), so our analysis likely underestimates the possible impact of both prevention modalities. Second, our models simulate scenarios in which all agents who use PrEP use daily oral PrEP or LAI-PrEP exclusively. Should LAI-PrEP be deemed efficacious in clinical trials and approved for real-world use, it will be implemented alongside other efficacious prevention strategies and it is likely that individuals will be able to choose a strategy that fits their behaviors and preferences. Future modeling studies should identify optimal combinations of daily oral PrEP and LAI-PrEP coverage, matching preferences across behavioral stratifications, to understand how to best implement these strategies together. Moreover, we assumed that the coverage and effectiveness of other interventions in the model (e.g., HIV testing, access to treatment) remained stable over the course of the simulation, which may not reflect real-world improvements in HIV prevention and treatment over the next decade. However, this limitation is likely to impact each PrEP scenario in a similar manner, and therefore does not affect the relative comparative benefits of LAI-PrEP versus oral PrEP. A final limitation to our study is that, although local data was used to parameterize the model wherever possible, several potentially important parameters were estimated using data from other localities or geographic scales, including annual distributions of numbers of sexual partners, sex acts, and frequency and concurrency of partnerships of longer duration, which can affect the overall impact of both strategies. These differences could affect specific predictions for Atlanta. Furthermore, the specific predictions for Atlanta could be unrepresentative of other locales.
Conclusions
Based on current human CAB pharmacokinetic data from phase IIa trials and efficacy observed in macaque models, LAI-PrEP is predicted to be significantly more effective than daily oral PrEP for the prevention of HIV transmission among MSM. However, the real-world population impact and relative benefits of LAI-PrEP compared to oral PrEP will depend on its efficacy in humans, as assessed in ongoing phase III trials, as well as on discontinuation rates and the duration of protection conferred.
Supplementary Material
Research in Context.
Evidence before this study
Daily oral pre-exposure prophylaxis (PrEP), formulated as a single tablet containing tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), has been shown to be an effective HIV prevention approach for gay, bisexual, and other men who have sex with men (MSM) and other at-risk populations. Previous modeling studies have shown that increased uptake of daily oral PrEP, in combination with other effective prevention and treatment strategies, could significantly reduce HIV incidence in MSM. However, inconvenience and other concerns about taking a daily pill—including being assumed by others to be HIV-positive or being labeled as promiscuous—can make adherence a challenge for many people. Long-acting injectable formulations of antiretroviral drugs have been developed as potential solutions for these challenges. Long-acting injectable cabotegravir (LAI-CAB), an integrase strand inhibitor formulated as an injectable nanoparticle suspension, was shown to have a strong protective effect against intravenous simian immunodeficiency virus (SIV) challenge in a macaque model. The safety and tolerability of long-acting injectable PrEP (LAI-PrEP) among healthy men has been demonstrated in two phase IIa randomized, double-blind, placebo-controlled studies. Its efficacy in comparison to daily oral PrEP among MSM is currently being assessed in a global phase III trial.
Added value of this study
Our agent-based model (ABM) simulated HIV transmission among a virtual population of MSM in Atlanta, Georgia with scenarios of varying coverage of daily oral PrEP and LAI-PrEP over a ten-year period (2015–2024). The efficacy of LAI-PrEP in preventing HIV infection was parameterized based on observed efficacy in preventing SIV infection in rhesus macaques—similar to that of daily oral PrEP in humans. We compared the cumulative number of HIV infections and the number of HIV infections averted across these scenarios to a base case without PrEP. Over the ten-year simulation period, the cumulative number of new HIV infections was reduced in scenarios in which agents received LAI-PrEP compared to scenarios in which all received oral PrEP, at every target coverage level. The relative benefit of LAI-PrEP was sensitive to the assumed efficacy of bimonthly CAB injections and the terminal half-life of CAB after a final injection.
Implications of all the available evidence
LAI-PrEP may be more effective than oral PrEP for preventing HIV acquisition in MSM due to the longer period of protection from HIV infection conferred by LAI PrEP that is independent of individual behavior (e.g., daily pill-taking). However, the population-level impact and relative benefit of LAI-PrEP will depend on its efficacy as observed in ongoing phase III trials, as well as the extent and duration of protection among persons who are not retained in care.
Acknowledgments
Funding: This work was supported by the National Institute on Drug Abuse [DP2DA040236] and by the National Institute of Mental Health [R21MH109360]. The funders had no role in the study analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
We would like to thank Chastity D. Andrews and David D. Ho for sharing a dataset originally described in Andrews et al., Science, 2014. We would also like to thank Jesse Yedinak for her research and administrative assistance.
Abbreviations
- ABM
Agent-based model
- CAB
Cabotegravir
- FTC
Emtricitabine
- HIV
Human immunodeficiency virus
- LAI-PrEP
Long-acting injectable pre-exposure prophylaxis
- MSM
Men who have sex with men
- PrEP
Pre-exposure prophylaxis
- SI
Simulation interval
- SIV
Simian immunodeficiency virus
- TDF
Tenofovir disoproxil fumarate
- US
United States
Footnotes
Data Availability: Model code and output are available upon request.
Presentation: Portions of these data have been presented in poster form at the 2018 Conference on Retroviruses and Opportunistic Infections in Boston, Massachusetts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beyrer C, Baral SD, Van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61(10):1601–3. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Public Health Service. Pre-Exposure Prophylaxis for the Prevention of HIV Infection in the United States: A Clinical Guideline. Atlanta, Georgia: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 6.Elion R, Coleman M. The PrEP revolution: From clinical trials to routine practice: Implementation view from the US. Curr Opin HIV AIDS. 2016;11(1):67–73. doi: 10.1097/COH.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra25–ra25. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8(6):565. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene GJ, Swann G, Fought AJ, Carballo-Diéguez A, Hope TJ, Kiser PF, et al. Preferences for long-acting pre-exposure prophylaxis (PrEP), daily oral PrEP, or condoms for HIV prevention among US men who have sex with men. AIDS Behav. 2017;21(5):1336–49. doi: 10.1007/s10461-016-1565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biello KB, Mimiaga MJ, Santostefano CM, Novak DS, Mayer KH. MSM at highest risk for HIV acquisition express greatest interest and preference for injectable antiretroviral PrEP compared to daily, oral medication. AIDS Behav. 2018;22(4):1158–64. doi: 10.1007/s10461-017-1972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343(6175):1151–4. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz M, Frank I, Grant RM, Mayer KH, Elion R, Goldstein D, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): A multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4(8):e331–e40. doi: 10.1016/S2352-3018(17)30068-1. [DOI] [PubMed] [Google Scholar]
- 13.Landovitz R, Li S, Grinsztejn B, Dawood H, Liu A, Magnus M, et al. Safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected women and men: HPTN 077. IAS Conference on HIV Science; Paris. 2017. [Google Scholar]
- 14.HIV Prevention Trials Network. HPTN 083: Give PrEP a Shot. 2017 [Available from: https://giveprepashot.org/]
- 15.Sullivan PS, Rosenberg ES, Sanchez TH, Kelley CF, Luisi N, Cooper HL, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: A prospective observational cohort study. Ann Epidemiol. 2015;25(6):445–54. doi: 10.1016/j.annepidem.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grey JA, Bernstein KT, Sullivan PS, Purcell DW, Chesson HW, Gift TL, et al. Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health Surveill. 2016;2(1):e14. doi: 10.2196/publichealth.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgia Department of Public Health. HIV Surveillance Summary, Georgia, 2015. Atlanta, Georgia: Georgia Department of Public Health; 2017. [Google Scholar]
- 18.Rosenberger JG, Reece M, Schick V, Herbenick D, Novak DS, Van Der Pol B, et al. Condom use during most recent anal intercourse event among a US sample of men who have sex with men. J Sex Med. 2012;9(4):1037–47. doi: 10.1111/j.1743-6109.2012.02650.x. [DOI] [PubMed] [Google Scholar]
- 19.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: A systematic review. AIDS. 2014;28(10):1509–19. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg ES, Millett GA, Sullivan PS, del Rio C, Curran JW. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: A modelling study. Lancet HIV. 2014;1(3):e112–e8. doi: 10.1016/S2352-3018(14)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan PA, Mena L, Patel R, Oldenburg CE, Beauchamps L, Perez-Brumer AG, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. doi: 10.7448/IAS.19.1.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews C, Mohri H, Bernard LS, Poon A, Spreen W, Gettie A, et al. Evaluation of resistance to cabotegravir long-acting (CAB LA) in SIVmac251-infected macaques. AIDS Res Hum Retroviruses. 2016;32(Suppl 1):43. [Google Scholar]
- 23.Hassounah SA, Mesplède T, Quashie PK, Oliveira M, Sandstrom PA, Wainberg MA. Effect of HIV-1 integrase resistance mutations when introduced into SIVmac239 on susceptibility to integrase strand transfer inhibitors. J Virol. 2014;88(17):9683–92. doi: 10.1128/JVI.00947-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walensky RP, Jacobsen MM, Bekker L-G, Parker RA, Wood R, Resch SC, et al. Potential clinical and economic value of long-acting preexposure prophylaxis for South African women at high-risk for HIV infection. J Infect Dis. 2015;213(10):1523–31. doi: 10.1093/infdis/jiv523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaubius RL, Parikh UM, Hood G, Penrose KJ, Bendavid E, Mellors JW, et al. Deciphering the effects of injectable pre-exposure prophylaxis for combination human immunodeficiency virus prevention. Open Forum Infect Dis. 2016;3(3):ofw125. doi: 10.1093/ofid/ofw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geretti AM, Armenia D, Ceccherini-Silberstein F. Emerging patterns and implications of HIV-1 integrase inhibitor resistance. Current opinion in infectious diseases. 2012;25(6):677–86. doi: 10.1097/QCO.0b013e32835a1de7. [DOI] [PubMed] [Google Scholar]
- 27.Jenness SM, Goodreau SM, Rosenberg E, Beylerian EN, Hoover KW, Smith DK, et al. Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis. 2016;214(12):1800–7. doi: 10.1093/infdis/jiw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.