Abstract
MHC class II-restricted antigen presentation by dendritic cells is necessary for activation of naïve CD4 T cells, whereas class II-restricted antigen presentation by B lymphocytes and macrophages is important for the recruitment of CD4+ helper and regulatory T cells. Antigen presentation by B cells is also important for induction of T cell tolerance. B cells are unique among these three types of MHC class II-expressing antigen presenting cells (APC) as they constitutively express high levels of cell surface class II molecules and express a clonally restricted antigen specific receptor, the B cell receptor (BCR). Here, I review our current understanding of three major steps that underlie the processing and presentation of BCR-bound cognate antigen: (1) endocytosis of antigen-BCR (Ag-BCR) complexes, (2) Ag-BCR trafficking to intracellular antigen processing compartments and (3) generation of antigenic peptide-MHC class II complexes, with a particular focus on the role of BCR ubiquitination in each. I will highlight potential topics for future research and briefly discuss the impact of the cell biology of BCR-mediated antigen processing on the response of the B cell and T cell to the cell-cell interactions mediated by B cell-expressed peptide-class II complexes.
Keywords: B cell receptor (BCR), ubiquitin, antigen processing, MHC class II, antigen presentation
1 -. Introduction
While dendritic cells could be considered the most altruistic antigen presenting cell (APC), focusing their efforts on the activation of naïve T cells that are unprepared to provide T cell help, B cells and macrophages (MØ) are greedy APCs, primarily focusing their efforts on the recruitment of effector T cells that are able to provide T cell help. B cells are also highly specialized APCs. While B cells can process and present non-cognate (e.g., self-) antigens internalized via fluid-phase (F-P) endocytosis (likely involved in T cell tolerance, see below and [1, 2]), it is BCR-mediated processing and presentation of cognate foreign antigen that is central to the recruitment of T cell help, which supports the induction and control of an antigen-specific humoral immune response. In addition to driving final maturation of effector CD4 T cells, BCR-mediated antigen processing drives germinal center (GC) formation, which allows GC B cells that have undergone somatic hyper-mutation and immunoglobulin class switching to test the antigen binding capacity and functionality their mutated BCR using antigen captured on the surface of follicular dendritic cells. These B cells then process and present this antigen to follicular helper T cells (TFH) to elicit survival signals and ultimately drive production of high affinity IgG antibodies.
Pioneering studies by Chesnut and Grey [3] as well as Lanzavecchia and colleagues [4] demonstrates that BCR-mediated processing of cognate antigen is orders of magnitude more efficient than the F-P processing of non-cognate antigen, allowing the processing and presentation of BCR-bound cognate antigen to occur at immunologically-relevant concentrations. However, these studies raised the question of whether the function of the BCR is simply to allow enhanced antigen uptake at low antigen concentrations (along with driving B cell signaling) or if the role of the BCR in antigen processing/presentation is more elaborate. Subsequent studies have revealed a much more intricate story. Here, I will review our current understanding of three major steps in the BCR-mediated processing of cognate antigen: (1) endocytosis of cell surface antigen-BCR (Ag-BCR) complexes, (2) trafficking of Ag-BCR complexes to the MIIC antigen processing compartment and (3) formation of antigenic peptide-MHC class II complexes, and will highlight the role of BCR ubiquitination in each. I will also call attention to some of the remaining questions that need to be addressed by future study.
2 -. Mutually Exclusive BCR Functions
Upon binding of cognate antigen, the BCR simultaneously performs two distinct functions; lymphocyte signaling and the endocytosis of BCR bound antigen. However, if we look at a molecular level we find that each cell surface BCR molecule can only carryout one of these two mutually exclusive functions (Figure 1 and [5]). BCR endocytosis occurs via clathrin coated pits (CCP) and is restricted to non-signaling BCR molecules. In contrast, BCR signaling (which is important for BCR ubiquitination, see below) is mediated by lipid raft resident BCR molecules which cannot be readily internalized via CCP [6, 7]. Moreover, evidence suggests that the fraction of BCR molecules that enter each of these pathways is determined, at least in part, by the crosslinking potential of the bound antigen.
Figure 1. Mutually Exclusive BCR Functions.
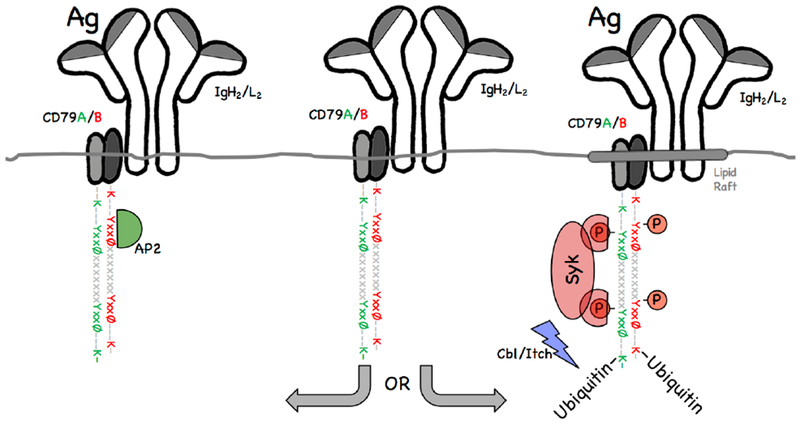
B cell receptor (BCR) molecules are composed of an immunoglobulin heavy and light chain (IgH2/L2) antigen-binding subunit as well as a ITAM-bearing CD79A/B signaling subunit. Upon antigen binding, BCR molecules enter one of two distinct functional pathways. A fraction of Ag-BCR complexes remain ITAM non-phosphorylated and interacts with the AP2 endocytic adaptor via the membrane proximal ITAM-embedded YxxØ motif of CD79B. These molecules are rapidly cleared from the cell surface via clathrin coated pits (CCP). A distinct fraction of BCR molecules enters lipid rafts and undergoes ITAM phosphorylation. These ITAM phosphorylated BCR molecules then recruit Syk and Cbl/Itch ubiquitin ligases, resulting in ubiquitination of CD79 lysine (K) residues. Each CD79 subunit possesses two putative ubiquitination sites (i.e., K residues). However, the exact site(s) of CD79 ubiquitination are unclear. Ubiquitination is arbitrarily illustrated on the CD79 C-terminal sites.
2.1 -. BCR Endocytosis
The role of CCP in BCR endocytosis was initially reported by Salisbury and colleagues in 1980 [8]. However, it took another 20-plus years before the molecular mechanisms underlying CCP-mediated BCR endocytosis were uncovered. In general, CCP-mediated endocytosis is driven by endocytosis motifs present in a receptor’s cytosolic domain or tail, with the YxxØ (Ø = large hydrophobic R group) motif being the most well characterized [9]. The YxxØ motif binds clathrin adaptor protein-2 (AP-2) and the tyrosine (Y) and Ø R groups plug into two snug binding pockets on one face of the AP-2 protein.
Identification of the BCR endocytosis motif was not a simple task and there are still some unanswered questions. The BCR comes in a variety of isotypes from IgM to IgA, and the cytoplasmic tail of each BCR heavy chain is different (Table I), with some, but not all, possessing a YxxØ motif that might contribute to BCR endocytosis (discussed below). In addition, all BCR molecules possess a CD79 heterodimeric signaling module, which could also support BCR internalization (Figure 1). The cytoplasmic tails of CD79A and CD79B (Table I) each possess an immune-receptor tyrosine-based activation motif (ITAM) with a consensus sequence of [D/E]x7[D/E]xxYxxLx6-8 YxxL/I. Upon antigen binding, the two ITAM tyrosine residues are phosphorylated by a Src family kinase and these dually phosphorylated ITAMs act as docking sites for Syk, which then activates multiple downstream signaling pathways. However, both ITAM tyrosine residues are also part of a YxxØ potential endocytosis motif, and there is a third C-terminal non-ITAM YxxØ motif in CD79A. Thus, each BCR molecule possesses between 5 and 7 YxxØ putative AP-2 binding motifs (5 motifs in CD79 and 2 in the IgG or IgE paired heavy chain cytoplasmic tails), any one of which might be involved in Ag-BCR endocytosis via CCP. Interestingly, it turns out that not all of these motifs are active.
TABLE I. Primary Structure of BCR Cytoplasmic Domains.
(the YxxØ putative endocytosis motifs in each tail are highlighted)
| Chain | Cytoplasmic Domain |
|---|---|
| muCD79A1 | RKRWQNEKFGVDMPDDYEDENLYEGLNLDDCSMYEDISRGLQGTYQDVGNLHIGDAQLEKP |
| huCD79A2 | RKRWQNEKLGLDAGDEYEDENLYEGLNLDDCSMYEDISRGLQGTYQDVGSLNIGDVQLEKP |
| muCD79B3 | DKDDGKAGMEEDHTYEGLNIDQTATYEDIVTLRTGEVKWSVGEHPGQE |
| huCD79B4 | DKDDSKAGMEEDHTYEGLDIDQTATYEDIVTLRTGEVKWSVGEHPGQE |
| muIgM | KVK |
| muIgD | KVK |
| muIgG15,6 | KVKWIFSSVVELKQTLVPEYKNMIGQAP |
| muIgG2a6 | KVKWIFSSVVELKQTISPDYRNMIGQGA |
| muIgG2b6 | KVKWIFSSVVELKQKISPDYRNMIGQGA |
| muIgG36 | KVKWIFSSVVQVKQTAIPDYRNMIGQGA |
| huIgG36 | KVKWIFSSVVDLKQTIIPDYRNMIGQGA |
| muIgE6 | KVKWVLSTPMQDTPQTFQDYANILQTRA |
| muIgA6 | TVRGPFGSKEVPQY |
| huIgA16 | SVRGPSGNREGPQY |
While literature from the 1990’s suggests that the longer cytoplasmic tails of the IgG and IgE BCR heavy chains possess active endocytosis motifs [10, 11], it appears that the shared CD79 heterodimer is the primary driver of CCP-mediated BCR endocytosis. Focusing on CD79, work from the Matsuuchi lab and our group has revealed a complex interplay between the two CD79 cytoplasmic domains that controls YxxØ AP-2 binding motif activity and identified the membrane proximal ITAM-embedded YxxØ motif of CD79B as the only endocytosis motif that is active within the complete BCR molecule (i.e., IgH2/L2–CD79A/B, Figure 1, [12, 13]). Currently, the mechanism(s) that control the ability of each CD79 YxxØ endocytosis motif to interact with AP-2 are unknown, but are likely to be structural in nature [12, 14, 15] and these structural considerations could also impact BCR ubiquitination (see below).
As mentioned above, some BCR functions such as signaling and endocytosis are mutually exclusive events when considered at a molecular level ([5] and Figure 1). However, it was initially unclear how this division of labor was controlled. Landmark work on another immune receptor, CTLA-4, established that phosphorylation of the tyrosine residue of an immune-receptor tyrosine-based inhibition motif (ITIM)-embedded YxxØ endocytosis motif prevents AP-2 binding and receptor endocytosis (the phospho-tyrosine side chain is too large to fit into the appropriate AP-2 binding pocket [16]). Building on these studies, Clark and colleagues reported in 2006 that upon antigen binding, only a subset of BCR molecules become ITAM phosphorylated to drive B cell signaling. Moreover, phosphorylation of the ITAM tyrosines of these signaling active BCR molecules prevents the AP-2 binding of the ITAM-embedded YxxØ motifs, blocking CCP-mediated uptake of these actively signaling BCR molecules. At the same time, the ITAMs of a distinct subset of BCR molecules remain unphosphorylated, allowing interaction of ITAM-embedded YxxØ motifs with AP-2, driving CCP-mediated BCR endocytosis [6, 7, 12]. Interestingly, like BCR ITAM phosphorylation, ubiquitination also seems to be restricted to a subset of BCR molecules (see bellow).
2.2 -. BCR Ubiquitination
Numerous published studies have revealed a central role for cytoplasmic domain ubiquitination in controlling the intracellular trafficking of the epidermal growth factor receptor (EGFR) and other cell surface signaling molecules, and have identified multiple ubiquitin ligases that can mediate this receptor modification (reviewed in [17]). In the case of the BCR, three distinct ubiquitin ligases have been implicated in ubiquitination of the receptor’s cytoplasmic tail; Itch [18], c-Cbl [19] and Cbl-b [20]. Interestingly, the Cbl-family of ubiquitin ligases has also been implicated in the regulation of BCR signaling, mediating Syk ubiquitination as a key step in the down-regulation of receptor signaling (reviewed in [21]). Syk activation involves its binding to dually phosphorylated CD79 ITAM motifs (see above) and Syk ubiquitination appears to be the result of a direct Cbl-Syk interaction [21]. This suggests that Syk could also be acting as an adaptor or bridge to mediate Cbl–BCR interactions and hints that Cbl-driven BCR ubiquitination might be restricted to the subset of signaling-involved BCR molecules. Consistent with this notion, we have reported that BCR ubiquitination is restricted to the subset of lipid raft-resident Ag-BCR complexes (Figure 1 and [22]), which is in line with the known role of lipid rafts as BCR signaling platforms. However, this scenario would focus ubiquitination onto BCR molecules bearing phosphorylated ITAMs, raising the question of how ubiquitnated Ag-BCR complexes are internalized? This is unlikely to occur via AP-2/CCP, as the YxxØ endocytosis motifs embedded in the CD79 ITAMs are likely phosphorylated and thus endocytically-inactive (see above and additional discussion below). Instead, internalization of ubiquitinated BCR molecules could be driven by CCP adaptors that directly recognize ubiquitin (reviewed in [9]). Alternatively, work from the Pure lab has shown that BCR internalization can occur via a Cbl-dependent mechanism that involves the actin cytoskeleton [23] and which is likely distinct from CCP.
Another ligase implicated in BCR ubiquitination is Itch [18]. Clark and colleagues demonstrated that ItchΔ B cells exhibit a marked but incomplete deficit in ligand-induced BCR ubiquitination as well as altered intracellular Ag-BCR trafficking (see below). Here, the authors noted that BCR ubiquitination is not required for BCR internalization, but did not specifically investigate the role of signaling in Itch-dependent BCR ubiquitination. However, as previously discussed [19], c-Cbl is known to be a target of Itch-mediated ubiquitination, which would suggest that Syk molecules bound to ITAM phosphorylated BCR molecules may act as an adaptor to recruit both c-Cbl and Itch to drive robust and dynamic BCR ubiquitination. The work of Clark and colleagues also identified CD79B as the major site of early Itch-dependent BCR ubiquitination, suggesting a possible complex interplay between CD79B-driven BCR endocytosis (above) and BCR signaling/ubiquitination.
While B cells can readily respond to soluble antigens, a role for antigen captured on the surface of cells such as follicular dendritic cells in B cell activation has recently received a lot of attention [24-26]. To date, no one has investigated BCR ubiqutination subsequent to B cell-FDC interactions, but it is possible to make some prognostications. Depending on conditions, B cells can extract antigen from the FDC surface by two distinct mechanisms, force-dependent extraction or enzymatic liberation [24, 25]. Force-dependent extraction is an active event that involves the acto-myosin cytoskeleton and BCR signaling. Relatedly, we have shown that highly cross-linking soluble BCR ligands such as polyclonal anti-BCR antibodies drive a greater fraction of BCR molecules into lipid rafts, resulting in an internalization mechanism that is now partially BCR signaling dependent [6]. Since we have shown that lipid raft-resident Ag-BCR complexes are the unique targets of signaling-dependent ubiquitionation (above and [22]), this suggests that BCR recognition of antigen on FDCs might result in extensive BCR lipid raft partitioning/signaling and enhanced BCR ubiquitination. This could have profound effects on the ability of GC B cells that recognize minute amounts of antigen on the surface of FDCs to efficiently process and present that antigen to follicular helper T cells. However, this intriguing possibility requires experimental investigation.
Overall, it is now clear that while cell surface BCR molecules can carry-out multiple simultaneous functions some of these functions are mutually-exclusive events when viewed at a molecular level. Ag-BCR complexes not directly involved in BCR signaling (thus having non-phosphorylated CD79 ITAMs) can interact with the endocytic adaptor AP-2 via the membrane proximal YxxØ motif in CD79B and undergo rapid endocytosis via AP-2/CCP. In contrast, Ag-BCR complexes that are directly involved in BCR signaling are localized to src family kinase-enriched lipid rafts and have phosphorylated CD79 ITAMs. These signaling involved BCR molecules become ubiquitined and are then internalized via poorly defined mechanism, possibly via a cytoskeleton-dependent mechanism or a mechanism involving clathrin and a ubiquitin-recognizing endocytosis adaptor [9]. Because there is differential ubiquitination of these two BCR populations, their trafficking within the endocytic pathway is likely different (discussed below).
Questions of future investigation:
What is the mechanism of uptake of lipid raft-resident, ubiquitnated Ag-BCR complexes? If it is CCP, what endocytic adaptor protein is involved? If it is not CCP, what is the underlying molecular mechanism?
How are the three distinct ubiquitin ligases recruited to the BCR? Can one BCR associate with more than one ubiquitin ligase? Do they work in parallel or sequentially? Do CD79A and CD79B bind different ligases to result in different patterns of BCR modification? What are the target residues of each ubiquitin ligase?
How does the physical state of the antigen (e.g., soluble vs. FDC-associated) impact the level of BCR ubiquitination and subsequent antigen processing/presentation?
3 -. Ubiquitin and the Intracellular Trafficking of Antigen-BCR Complexes
Seminal studies on the intracellular trafficking of the EGFR and other signaling receptors has resulted in development of a paradigm of receptor trafficking to and within multi-vesicular bodies (MVB, Figure 2), which are a central element of the late endocytic pathway (reviewed in [17]). Proceeding or upon delivery to the limiting membrane (LM) of MVB, the EGFR cytoplasmic tail is multi-ubiquitined (multiple sites of mono-ubiquitination as opposed to addition of a single poly-ubiquitination chain) by a member of the Cbl family of ubiquitin ligases. The EGFR-Ubi is then recognized by a series of proteins of the Hrs/ESCRT (hepatocyte growth factor-regulated tyrosine kinase substrate/endosomal sorting complexes required for transport) complex and the receptor selectively incorporated into inward budding nascent intraluminal vesicles (ILV). Finally, the receptor is de-ubiquitinated and the nascent ILV released from the LM into the lumen of the MVB. The EGFR in the ILV is ultimately targeted to lysosomes for degradation. Interestingly, the lipid raft resident protein flotillin has recently been shown to have a role in regulating the transfer of ubiquitinated EGFR between modules of the ESCRT complex [27], suggesting that lipid raft-resident Ag-BCR complexes may also exhibit more efficient trafficking through this MVB sorting pathway (see above).
Figure 2. Working Model of the Pathway of Ubiquitination-Dependent BCR-Mediated Antigen Processing and Presentation.
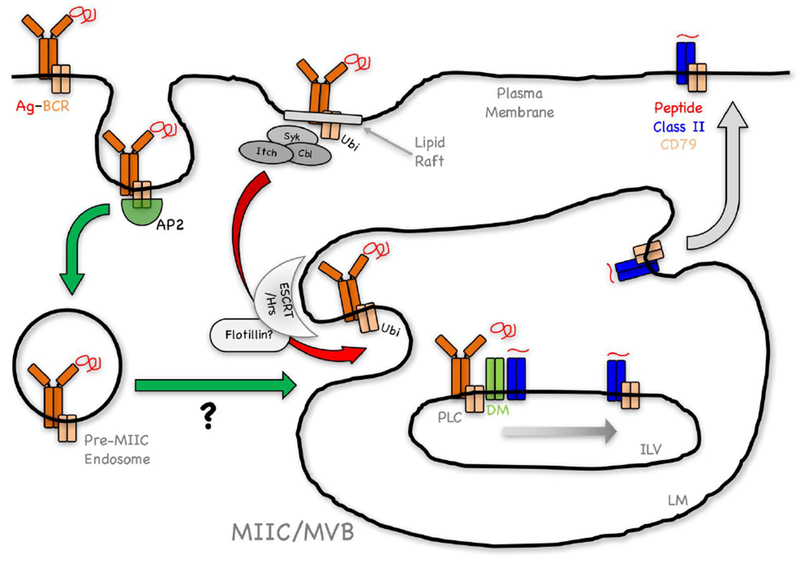
Antigen binding drives two concurrent events; signaling and Ag-BCR internalization. A fraction of Ag-BCR complexes not involved in signaling are internalized via an AP-2/CCP-dependent pathway (green arrow), mediated by the membrane proximal ITAM YxxØ motif of CD79B (see text). The ultimate intracellular fate of these complexes (delayed delivery to MIIC?) is currently unclear. A second population of Ag-BCR complexes within lipid rafts undergoes signaling-dependent ubiquitination (Ag-BCR-ubi) by either c-Cbl, Cbl-b and/or Itch (Figure 1, see text). Endocytosis of these Ag-BCR-ubi complexes via a poorly defined mechanism that may not be CCP (see text), results in their rapid delivery to the limiting membrane (LM) of multi-vesicular body (MVB)-like MIIC (red arrow). Recognition of these Ag-BCR-ubi complexes by the ESCRT/Hrs complex (possibly via a flotillin-dependent mechanism, see text) results in Ag-BCR de-ubiquitination and delivery to nascent intra-lumenal vesicles (ILV). Within ILV, Ag-BCR complexes associate with M1 paired MHC class II molecules (see text), associated with the class II chaperone HLA-DM/H-2M (DM). Within this MHC class II peptide loading complex (PLC), DM catalyzes Ag release from the BCR and the binding of antigen-derived peptides to the class II molecule. The PLC may also facilitate the transfer of the BCR CD79 signaling module to these nascent peptide-class II complexes. The resulting ILV peptide-MHC class II-CD79 complexes are then delivered to the MIIC LM by “back-fusion” and then to the cell surface for recognition by CD4 T cells.
Multi-vesicular bodies are very similar to the MHC class II-enriched multi-vesicular MIIC that are the site for antigen processing and formation of peptide-class II complexes [28-30]. Thus, it was not surprising to find that ubiquitination is important for BCR-mediated MHC class II-restricted antigen processing and presentation (Figure 2). However, the details of this process remain unclear.
3.1 -. Trafficking of Ag-BCR Complexes
In the context of BCR biology, ubiquitination is most widely studied as a mechanism to regulate receptor signaling [21]. However, similar to the EGFR, BCR ubiquitination is also important in the trafficking of internalized Ag-BCR complexes and delivery of these complexes to the cell’s antigen processing compartment (i.e., MIIC). This was first suggested by our 2006 report on the effect of treatment of B cells with proteasome inhibitors on antigen presentation [31]. Proteasome blockade results in accumulation of ubiquitinated cellular proteins, a corresponding depletion of the cellular pool of free ubiquitin and ultimately the inhibition of other ubiquitin-dependent cellular events. While proteasome inhibition does not alter the kinetics of Ag-BCR internalization, it does elicit a notable delay in the disassembly/degradation of internalized Ag-BCR complexes and a remarkable change in the intracellular distribution of these complexes. Moreover, this treatment completely blocks formation of peptide-class II complexes derived from BCR internalized antigen, without having any effect on the level of complexes formed by fluid-phase endocytosis.
As noted above, three different ubiquitin ligases have been implicated in BCR ubiquitination that controls the receptor’s intracellular trafficking; Itch [18], c-Cbl (aka, Cbl) [19] and Cbl-b [20]. In 2007, Zhang and colleagues reported that Itch is critical to BCR-mediated antigen processing as Itch deficient (ItchΔ) B cells exhibit altered Ag-BCR trafficking and a profound defect in BCR-mediated antigen presentation [18]. Here, Itch-dependent BCR ubiquitination seems to be focused on CD79B and to be highest immediately upon receptor engagement (This finding is consistent with our unpublished results showing that constructs bearing a CD79B tail [alone or paired with CD79A] can be robustly ubiquitinated, while those possessing only a CD79A tail cannot, Katkere and Drake). However, Zhang and colleagues found that CD79A ubiquitintion becomes more pronounced at later time points (i.e., at 30 min.) when CD79B ubiquitination is waning, suggesting a dynamic nature to BCR ubiquitination. “Late” CD79A ubiquitination would be consistent with our observation that CIIV (a type of MIIC isolated from B cells [32]) are enriched in a ~50 kDa protein recognized by an anti-CD79A antibody [33]. Initially, we termed this protein p50Igα (because of its molecular mass and immunological properties) and proposed that it was encoded by a CD79A-like gene, but in hindsight it is most likely di-ubiquitinated CD79A. Interestingly, BCR ubiquitination is not completely abolished in ItchΔ B cells, leaving open the possibility of other parallel mechanisms of receptor ubiquitination such as one of the Cbl family of ubiquitin ligases.
In 2012, my lab reported a role for c-Cbl (aka, Cbl) in BCR-mediated antigen processing [19]. We found that shRNA-driven depletion of c-Cbl (but not Cbl-b) results in a marked selective decrease in BCR-mediated antigen processing/presentation (F-P processing was unaffected). Keeping in mind the observation that c-Cbl can bind to and ubiquitinate Syk upon initiation of BCR signaling [21], we also investigated the role of Syk signaling in BCR-mediated antigen processing and found that blocking Syk signaling selectively blocks BCR-mediated antigen processing without inhibiting receptor endocytosis. This observation is consistent with the earlier findings of Le Roux and colleagues who demonstrated that Syk is critical for BCR-mediated antigen processing/presentation [34]. Moreover, these findings are consistent with our earlier observation that ubiquitinated Ag-BCR complexes are restricted to lipid rafts [22], which are known BCR signaling platforms and suggest that only a subset of BCR molecules undergo modification.
In 2014, Veselits and colleagues reported that Cbl-b, but not c-Cbl, is recruited to clusters of BCR molecules and that Cbl-b recruitment is necessary for trafficking of the BCR to late endocytic compartments [20]. Interestingly, it seems that the E3 ubiquitin ligase activity of Cbl-b is not necessary for proper BCR trafficking (In this report, the authors did not report the effect of Cbl-b knockdown on BCR-mediated antigen processing.). Instead, it appears that the protein’s ubiquitin associated UBA domain (which can bind ubiquitin) is acting as a “scaffold” to coordinate proper BCR trafficking. In the report, the authors raise two interesting possibilities. First, Cbl-b is known to act with the motor protein dynein, which they suggest could be involved in intracellular BCR trafficking. Alternatively (or in addition), Cbl-b (and c-Cbl) are known to interact with the Hrs protein (aka, ESCRT-0), which is involved in the early steps of MVB delivery/sorting, which could mean that Cbl-b (and/or c-Cbl) is controlling Ag-BCR entry into the ESCRT complex. These findings bring to attention the idea that ubiquitin ligases like Cbl-b have multiple functions (some of which are independent of ubiquitin ligase activity) and may function in a highly complex manner to assure correct trafficking of Ag-BCR complexes to and within MVB-like MIIC. Hence, the ubiquitin moiety attached to the BCR may not be able on its own to mediate all BCR trafficking, but rather it may need the help of “accessory” proteins such as Cbl-b.
Finally, in 2015 Satpathy and colleagues reported the BCR “ubiquitome” [35]. While the primary focus of this report was BCR signaling, the authors did observe ubiquitination of both the CD79A and CD79B subunits of the BCR. Interestingly, c-Cbl and Cbl-b were found to be targets of both BCR-induced phosphorylation and ubiquitination, consistent with a possible role of Syk in the recruitment of these ubiquitin ligases to the BCR ([21, 36] and discussed above). In contrast, Itch was found not to be a target of either BCR-induced phosphorylation or ubiquitination. The authors also reported that while ubiquitinated CD79A is also phosphorylated (presumably on one or both ITAM tyrosine residues), ubiquitinated CD79B is not phosphorylated. This is especially intriguing as the membrane proximal ITAM tyrosine of CD79B is responsible for mediating BCR endocytosis ([12, 13] and above) and the lack of phosphorylation of this residue would leave open the possibility of AP-2/CCP-mediated internalization of ubiquitinated Ag-BCR complexes (see above). Finally, the authors note that “ubiquitylation is dynamically regulated at hundreds of [molecular] sites within minutes after BCR stimulation”, suggesting that we still have much to learn about the roles of ubiquitination in BCR function and B cell immunobiology.
The EGFR paradigm discussed above would suggest that BCR ubiquitination is important for trafficking of Ag-BCR complexes to MVB-like MIIC and ultimately for sorting of these complexes into MIIC ILV. This framework would suggest a role for the Hrs/ESCRT complex in the BCR-mediated processing of cognate antigen (Figure 2). While this idea has yet to be directly tested, Nagata and colleagues have reported the development of mice selectively deficient for expression of the Hrs protein in B cells [37]. While the authors did not investigate the cell biology of BCR-mediated antigen processing in this system, they did report a reduction in the in vivo levels of T cell-dependent antibody production in response to immunization with a haptenated carrier protein, suggesting a possible alteration in BCR-mediated antigen processing/presentation. However, this intriguing possibility requires direct investigation.
Together, the results discussed above reveal that BCR ubiquitination is a dynamic process that is central to the intracellular trafficking of Ag-BCR complexes and delivery of these complexes to the intracellular compartment where antigen processing leads to the formation of derivative antigenic peptide-MHC class II complexes (Figure 2).
3.2 -. Trafficking of MHC Class II Molecules
MHC class II molecules bind peptides derived from the processing of BCR-bound cognate antigen and present these peptides to CD4 T cells. Interestingly, class II molecules are also ubiquitinated and this modification impacts their intracellular trafficking (recently reviewed in [38-40]). However, unlike the BCR, MHC class II ubiquitination is catalyzed by MARCH family ubiquitin ligases and is generally thought to drive class II down-regulation. However, the story might not be that simple. A 2013 report from the Ishido lab revealed that while DCs engineered to express class II molecules refractory to ubiquitination (a class II β chain cytoplasmic tail K>R mutant) express higher levels of class II molecules (as expected), these DCs exhibit an unexpected defect in CD4 T cell activation, suggesting a potential positive role for class II ubiquitination in antigen presentation ([41], and reviewed in [40]).
HLA-DM/H-2M (DM) is a molecular chaperone that associates with MHC class II molecules and facilitates class II peptide loading [42]. Within the ILV of MIIC, MHC class II and DM molecules are in close physical proximity [43], implicating MIIC ILV as a site of MHC class II peptide loading (see below and Figure 2). This idea is further supported by the observation that HLA-DO/H-2O molecules, which are an inhibitor of DM action, are excluded from MIIC ILV and restricted to the limiting membrane of this compartment [43]. In fact, DO can actually block DM delivery to ILV [44], suggesting that DO may regulate DM activity by regulating its intra-vesicular distribution.
Currently, the mechanism controlling class II trafficking to MIIC ILV is unclear. While MARCH1-mediated ubiquitination of the class II β chain is critical for class II down-regulation, class II incorporation into exosomes (a form of ILV released from the cell upon MVB/MIIC fusion with the plasma membrane) appears to occur via a ubiquitination-independent mechanism [39, 45, 46]. Here, it is possible that class II ILV/exosome sorting involves the CD9 tetraspan protein and lipid rafts [46], which could mean that only lipid raft-resident class II molecules are targeted to ILV (see below). It is unlikely that this sorting pathway involves ubiquitination of class II associated invariant chain (Ii), as Ii contains a sorting signal that shunts nascent class II-Ii complexes away from MARCH1 bearing endosomes (at least in DCs) [47].
To add another layer of complexity and interest to the story, we recently reported that MHC class II molecules exist in two distinct conformational states, based on alternative pairing of transmembrane (TM) domain GxxxG dimerization motifs ([48, 49] and reviewed in [50]). These two distinct conformers (termed “M1” and “M2” paired class II, for the use of the class II α chain GxxxG motif 1 or motif 2) are linked to different signaling pathways, exhibit differential localization to membrane lipid domains and possess disparate abilities to drive T cell activation. In addition, these two class II conformers have differential access to peptides derived from BCR-bound antigen (below), suggesting their trafficking to and within MIIC might be different.
In all, these findings highlight the previously unappreciated complexity of protein sorting to and within MIIC/MVB [51] and suggest that there are multiple mechanisms controlling the delivery of Ag-BCR, MHC class II and DM to the ILV of MIIC for antigen processing and peptide-class II complex formation.
Questions of future investigation:
What are the roles of c-Cbl, Cbl-b and Itch in BCR trafficking? Which functions are dependent on BCR ubiquitination and which are not? Are the roles dependent on the state of B cell maturation or expressed BCR isotype?
What are the functions of CD79A vs. CD79B ubiquitination, which exhibit different kinetics? Is the BCR heavy chain also a target for ubiqutination?
What is the relationship between BCR ubiquitination and other BCR modifications and/or functions, such as ITAM phosphorylation/signaling, AP-2 binding/endocytosis or BCR cytoplasmic tail arginine methylation [52]? Which modifications can occur on the same receptor molecule and which are mutually exclusive?
What are the immunological implications of ubiquitin-dependent Ag-BCR trafficking to MIIC (see below)?
What is the mechanism of trafficking of class II and/or DM into the ILV of MIIC for antigen processing?
4 -. Formation of Peptide–Class II Complexes: An MHC Class II Peptide Loading Complex?
4.1 -. Two Pathways of Antigen Endocytosis and Processing
Unlike dendritic cells and macrophages, B cells constitutively express robust levels of MHC class II molecules, and these molecules have two very distinct immunological functions. The first well appreciated function is to present foreign antigen-derived peptides to effector CD4 T cells to elicit T cell help. The second less well appreciated function is presentation of self-peptides to CD4 T cells to drive peripheral (and possibly central) T cell tolerance [1, 2, 53]. This is relevant to this discussion, as the presentation of non-self/foreign-peptides is generally dependent on ubiquitin-dependent Ag-BCR trafficking, whereas presentation of self-peptides by non-autoreactive B cells would be the result of the ubiquitination-independent fluid-phase (F-P) uptake and processing of self-proteins [31].
In addition to allowing for antigen uptake at immunologically-relevant concentrations, BCR-mediated antigen processing results in the formation of antigenic peptide-class II complexes with unique biological and immunological properties. Evidence for this phenomenon can be found as far back as the 1970’s, when Mitchison investigated the in vivo response of in vitro antigen-pulsed B cells. Mitchison found that when B cells were pulsed only with a low dose of haptenated carrier protein such as NP-ovalbumin (which would be processed via the BCR of hapten-specific B cells) and transferred into a carrier primed mouse (bearing ovalbumin-specific helper T cells), the B cells supported a readily detectable anti-hapten antibody response. In contrast, if the B cells were instead pulsed with a low dose of haptenated carrier plus a high dose of a non-haptenated form of the same carrier (e.g., ovalbumin, which would be internalized and processed in parallel via a F-P pathway), the B cells exhibited a blunted response [54, 55]. Thus, while T cell recognition of peptide–class II complexes formed via BCR-mediated processing of hapten-carrier conjugates (p-MHCIIBCR) support B cell activation, T cell recognition of additional peptide-class II complexes formed via parallel F-P processing of a high-dose of the same carrier (p-MHCIIF-P) has an inhibitory effect.
Using a more highly-defined model system based on B cells expressing a transgenic HEL specific IgM BCR and I-Ak class II, we reported that while either mAb or TCR engagement of p-MHCIIBCR results in B cell activation in vitro, mAb/TCR engagement of p-MHCIIF-P blocks this response [56]. These finding are also in line with the observations of Cyster and colleagues who reported that in vivo B cell-T cell interactions occurring in the absence of foreign peptide (presumably mediated by self-peptide-class II complexes formed via F-P processing of self-antigens) are short-lived (lasting less than 10 min.) and non-activating, whereas in vivo B-T interactions mediated by BCR-generated foreign peptide-class II complexes are prolonged (lasting for 60 minutes or longer) and ultimately result in the B cell leading the acquiescent T cell to forming germinal centers (GC) [57]. As discussed below, consideration of the unique aspects of the ubiquitin-dependent trafficking of Ag-BCR complexes to and within MIIC provides insight into the molecular mechanisms that likely underlies the unique properties of p-MHCIIBCR vs. p-MHCIIF-P.
4.2 -. An MHC Class II Peptide Loading Complex
Numerous studies have established the MIIC as a major subcellular compartment for class II peptide loading in B cells and other antigen presenting cells. However, two important observations suggest that MHC class II peptide loading does not occur by simple class II capture of peptides “floating around” in the lumen of the MIIC (a subcellular compartment that can be accessed by both BCR-mediated and F-P endocytosis). In a 1995 paper, Mitchell and colleagues report that peptides derived from the processing of two different BCR-bound antigens can compete for binding to a subset of class II molecules that cannot be accessed by peptides derived from the processing of antigen internalized by F-P endocytosis [58]. This observation suggests that peptides derived from the processing of BCR-bound foreign antigen are loaded onto a select subset of MHC class II molecules. In the same paper, the authors analyzed the intracellular itinerary of a mutant BCR previously shown to exhibit a selective defect in antigen presentation (i.e., an immunoglobulin heavy chain transmembrane [TM] domain Y587 to F mutation, [59]). They demonstrate that while the wild type (WT) and Y>F mutant BCRs are internalized and delivered to intracellular antigen processing compartments (e.g., MIIC) in similar fashion, there is a striking difference in what happens next. Whereas antigen bound to the WT BCR is processed to peptides that readily bind MIIC resident MHC class II molecules, antigen bound to the mutant BCR is processed to peptides but these peptides fail to bind to the MIIC resident class II molecules. This observation suggests that class II molecules do not simply capture random peptides from the MIIC lumen, but rather that there is a mechanism or complex that orchestrates loading of a subset of class II molecules with peptides derived from the processing of BCR bound cognate antigen and that the IgH TM domain Y>F mutation may prevent the BCR from entering this complex.
Consistent with this notion of an MHC class II peptide loading complex (PLC), we recently reported the identification and characterization of intracellular Ag-BCR–class II complexes that are involved in the formation of derivative peptide-class II complexes (i.e., p-MHCIIBCR, [60]). Using both biochemical and imaging/FRET approaches, we demonstrated the physical association of internalized Ag-BCR complexes with intracellular MHC class II molecules. Interestingly, the class II molecules in these molecular assemblies are a conformational subset of class II molecules termed M1 paired class II (which represent about 10% of all cellular class II molecules and which have unique biochemical and immunological properties [48, 49, 61], see above). Consistent with the idea that this molecular assembly of Ag-BCR and class II represents an MHC class II peptide loading complex (PLC), we found that peptides derived from the processing of BCR-bound cognate antigen are selectively loaded onto M1 paired class II. Moreover, these class II molecules possess associated CD79 signaling molecules [62], possibly obtained from the BCR molecule, likely explaining unique signaling properties of these p-MHCIIBCR complexes (discussed above and Figure 2). In contrast, peptides derived from the processing of F-P internalized protein are non-selectively loaded onto both M1 and M2 paired class II molecules.
The observation of intracellular complexes of Ag-BCR and MHC class II is also in line with the 2014 report of Hauser and Lindner, who demonstrated that under some conditions, cell surface Ag-BCR complexes and Ii-class II complexes can coalesce in the same membrane micro-domains [63, 64]. This coalescence requires both antigen-driven BCR cross-linking as well as Ii-MHC crosslinking by molecules such as MIF (migration inhibition factor, an Ii ligand [63, 64]). While it is currently unclear how frequently the required level of Ii-class II crosslinking would occur in vivo, the finding does demonstrate the propensity of emergent MHC class II molecules to associate with at least one source of antigenic peptides (i.e., Ag-BCR complexes) in a putative MHC class II PLC. In addition, the idea of a role for Ii-class II complexes in the intracellular trafficking of Ag-BCR complexes is consistent with findings from the Schröder lab, who documented a molecular-level association between the BCR and Ii [65] and found that B cells with a deficiency in signal peptide peptidase-like 2a (SPPL2a), which is responsible for the endosomal degradation of N-terminal Ii fragments, exhibit a defect in BCR endosomal trafficking [66]. Whether formation of an MHC class II PLC happens in other APCs expressing other antigen receptors, such as Fc receptors in MØ, is currently unclear.
While the exact sub-compartment within the MIIC where this putative MHC class II PLC forms is still unclear, ILVs are a likely candidate (Figure 2). As noted above, MIIC ILV are rich in DM-class II complexes but relatively devoid of the DO, meaning that ILV class II molecules are ripe for peptide loading. Moreover, delivery of Ag-BCR complexes to ILV likely requires BCR ubiquitination, which has been shown to be critical for BCR-mediated antigen processing/presentation (discussed above). Under this scenario, DM molecules could be a central regulator of PLC activity within the ILV. In addition to stabilizing empty MHC class II molecules, DM has recently been shown to stimulate dissociation of Ag-BCR complexes [67]. Thus, upon entry into MIIC ILV, Ag-BCR complexes could intercept class II-DM complexes, resulting in a DM-driven Ag-BCR dissociation. This could facilitate the processing of the released BCR internalized antigen and allow for the subsequent directed loading of resultant antigen-derived peptides onto the associated class II molecules. The PLC could also coordinate the transfer of the CD79 signaling module from the BCR to the resulting peptide class II complexes [62], potentially explaining the unique signaling properties of p-MHCIIBCR (discussed above). Moreover, an inability of the Y587 BCR mutant to access this class II PLC might explain the observed selective defect in BCR-mediated antigen processing and presentation exhibited by this BCR [58].
Together, these results reveal the existence of a coordinated mechanism that controls the loading of antigen-derived peptides onto MHC class II molecules, analogous to the MHC class I PLC that resides in the endoplasmic reticulum of the cell. This model represents a more sophisticated picture of the immunobiology of exogenous antigen processing and presentation and provides a framework for future investigation.
Questions of future investigation:
What is the subcellular location of formation of the putative MHC class II PLC (i.e., Ag-BCR–class II complexes)? If it is the ILV of MIIC, what is the role of BCR ubiquitination in controlling PLC assembly? What is the fate of non-ubiquitinated Ag-BCR complexes (persistence and delayed processing [68])?
What controls class II entry into the PLC, restricting entry to M1 paired class II molecules? Does this involve class II ubiquitination and is this impacted by the TM domain pairing status of the molecule (the MARCH1 ubiquitin ligase is a TM protein, which possesses a GxxxG motif)?
Are the Ii and DM chaperone molecules involved in assembly and/or function of the PLC? If so, what is their precise function? The DM molecule possesses TM domain GxxxG protein-protein interaction motifs identical to those that drive MHC class II conformer formation [49]; are they involved in DM-class II interactions within the PLC?
Does the IgM TM domain Y587 mutation block BCR entry into the class II PLC? If so, what critical molecular interactions are being blocked by this mutation?
5 -. Summary and Future Directions
Our understanding of the immunobiology of the BCR-mediated processing of cognate antigen has increased greatly since the initial observations of Chesnut, Grey and Lanzavecchia. Building from the EGFR/MVB trafficking paradigm, we have gained a greater understanding of the molecular mechanism of BCR ubiquitination and the role of this pathway in the formation of derivative peptide-class II complexes (Figure 2).
Upon antigen binding the BCR simultaneously carries out two main functions, signaling and antigen internalization. However, multiple studies have revealed that at a molecular level these two distinct functions are mutually exclusive events. For example, BCR signaling appears to be focused on lipid raft-resident Ag-BCR complexes that, in addition to undergoing ITAM phosphorylation, also undergo signaling-dependent ubiquitination. In contrast, CCP-mediated endocytosis appears to be primarily based on non-signaling BCR molecules where non-phosphorylated ITAM-embedded YxxØ motifs can mediate receptor interaction with the AP-2 endocytosis adaptor and result in internalization. However, it is currently unclear what specific molecular events are mutually exclusive and which are not (e.g., can all signaling BCR molecules drive all types of BCR signaling?). How is this distribution of functions is controlled (e.g., raft vs. non-raft localization) and how does this molecular division of labor impact B cell antigen processing and presentation? Gaining a better understanding of these issues at a molecular level will be one of the next major steps forward in our understanding of BCR function.
Within the B cell, BCR ubiquitination appears to be the passport that allows Ag-BCR complexes to enter MVB-like MIIC and an MHC class II peptide loading complex (PLC), where a subset of class II molecules accepts delivery of both antigen-derived peptide and the CD79 signaling molecule. While these findings provide a likely explanation of the unique biological and immunological properties of BCR-generated peptide-class II complexes, the full immunological impact of these properties remains to be determined. In addition, this viewpoint raises the question of the function of the non-ubiquitinated Ag-BCR complexes (do they undergo slower processing and presentation to allow for prolonged expression of peptide-class II complexes [68]?) as well as the impact of how B cell recognition of antigen on the surface of FDCs (which is central to the process of affinity maturation) impacts ubiquitin-dependent BCR functions.
While we have come a long way in our understanding of the immunobiology of the BCR-mediated processing and presentation of cognate antigen, there is still much to learn and the results of future investigations promise to be exciting and enlightening
Highlights:
The B cell receptor (BCR) mediates the processing and presentation of cognate foreign antigen
Ubiquitination of the BCR controls its trafficking to and within intracellular antigen processing compartments
BCR-mediated processing of cognate antigen results in the formation of peptide-MHC class II complexes with unique biological and immunological properties
Acknowledgements:
The author would like to thank Lisa Drake and Dr. Michelle Lennartz for critical reading of the manuscript. This work was supported in part by N.I.H. grants AI-097673 to JRD and AI-056320 to the Department of Immunology and Microbial Disease. The N.I.H had no direct role in the writing or submission of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no competing interests to declare
References:
- 1. Frommer F Heinen TJ Wunderlich FT Yogev N Buch T Roers A Bettelli E Muller W Anderton SM Waisman A Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion J Immunol 2008. 181 8 5748–59 [DOI] [PubMed] [Google Scholar]
- 2. Yamano T Nedjic J Hinterberger M Steinert M Koser S Pinto S Gerdes N Lutgens E Ishimaru N Busslinger M Brors B Kyewski B Klein L Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction Immunity 2015. 42 6 1048–61 [DOI] [PubMed] [Google Scholar]
- 3. Chesnut RW Grey HM Studies on the capacity of B cells to serve as antigen-presenting cells J Immunol 1981. 126 3 1075–9 [PubMed] [Google Scholar]
- 4. Lanzavecchia A Antigen-specific interaction between T and B cells Nature 1985. 314 6011 537–9 [DOI] [PubMed] [Google Scholar]
- 5.Hou P, Araujo E, Zhao T, Zhang M, Massenburg D, Veselits M, Doyle C, Dinner AR, Clark MR. B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 2006;4(7):e200. doi: 10.1371/journal.pbio.0040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caballero A Katkere B Wen XY Drake L Nashar TO Drake JR Functional and structural requirements for the internalization of distinct BCR-ligand complexes Eur J Immunol 2006. 36 12 3131–45 [DOI] [PubMed] [Google Scholar]
- 7. Putnam MA Moquin AE Merrihew M Outcalt C Sorge E Caballero A Gondre-Lewis TA Drake JR Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking J Immunol 2003. 170 2 905–12 [DOI] [PubMed] [Google Scholar]
- 8. Salisbury JL Condeelis JS Satir P Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells J Cell Biol 1980. 87 1 132–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traub LM, Bonifacino JS. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb Perspect Biol. 2013;5(11):a016790. doi: 10.1101/cshperspect.a016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Achatz G Nitschke L Lamers MC Effect of transmembrane and cytoplasmic domains of IgE on the IgE response Science 1997. 276 5311 409–11 [DOI] [PubMed] [Google Scholar]
- 11. Kaisho T Schwenk F Rajewsky K The roles of gamma 1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses Science 1997. 276 5311 412–5 [DOI] [PubMed] [Google Scholar]
- 12.Busman-Sahay K, Drake L, Sitaram A, Marks M, Drake JR. Cis and trans regulatory mechanisms control AP2-mediated B cell receptor endocytosis via select tyrosine-based motifs. PLoS One. 2013;8(1):e54938. doi: 10.1371/journal.pone.0054938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jang C Machtaler S Matsuuchi L The role of Ig-alpha/beta in B cell antigen receptor internalization Immunol Lett 2010. 134 1 75–82 [DOI] [PubMed] [Google Scholar]
- 14.Rosenlow J, Isaksson L, Mayzel M, Lengqvist J, Orekhov VY. Tyrosine phosphorylation within the intrinsically disordered cytosolic domains of the B-cell receptor: an NMR-based structural analysis. PLoS One. 2014;9:e96199. doi: 10.1371/journal.pone.0096199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sigalov A Aivazian D Stern L Homooligomerization of the cytoplasmic domain of the T cell receptor zeta chain and of other proteins containing the immunoreceptor tyrosine-based activation motif Biochemistry 2004. 43 7 2049–61 [DOI] [PubMed] [Google Scholar]
- 16. Shiratori T Miyatake S Ohno H Nakaseko C Isono K Bonifacino JS Saito T Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2 Immunity 1997. 6 5 583–9 [DOI] [PubMed] [Google Scholar]
- 17. Haglund K Dikic I The role of ubiquitylation in receptor endocytosis and endosomal sorting J Cell Sci 2012. 125 Pt 2 265–75 [DOI] [PubMed] [Google Scholar]
- 18. Zhang M Veselits M O’Neill S Hou P Reddi AL Berlin I Ikeda M Nash PD Longnecker R Band H Clark MR Ubiquitinylation of Ig beta dictates the endocytic fate of the B cell antigen receptor J Immunol 2007. 179 7 4435–43 [DOI] [PubMed] [Google Scholar]
- 19. Katkere B Rosa S Drake JR The Syk-binding ubiquitin ligase c-Cbl mediates signaling-dependent B cell receptor ubiquitination and B cell receptor-mediated antigen processing and presentation J Biol Chem 2012. 287 20 16636–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veselits M, Tanaka A, Lipkowitz S, O’Neill S, Sciammas R, Finnegan A, Zhang J, Clark MR. Recruitment of Cbl-b to B cell antigen receptor couples antigen recognition to Toll-like receptor 9 activation in late endosomes. PLoS One. 2014;9(3):e89792. doi: 10.1371/journal.pone.0089792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao N Dodge I Band H The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system J Leukoc Biol 2002. 71 5 753–63 [PubMed] [Google Scholar]
- 22. Katkere B Rosa S Caballero A Repasky EA Drake JR Physiological-range temperature changes modulate cognate antigen processing and presentation mediated by lipid raft-restricted ubiquitinated B cell receptor molecules J Immunol 2010. 185 9 5032–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacob M Todd L Sampson MF Pure E Dual role of Cbl links critical events in BCR endocytosis Int Immunol 2008. 20 4 485–97 [DOI] [PubMed] [Google Scholar]
- 24. Pierobon P Lennon-Dumenil AM To use or not to use the force: How B lymphocytes extract surface-tethered antigens J Cell Biol 2017. 216 1 17–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tolar P Spillane KM Force generation in B-cell synapses: mechanisms coupling B-cell receptor binding to antigen internalization and affinity discrimination Adv Immunol 2014. 123 69–100 [DOI] [PubMed] [Google Scholar]
- 26.Yuseff MI, Lennon-Dumenil AM. B Cells use Conserved Polarity Cues to Regulate Their Antigen Processing and Presentation Functions. Front Immunol. 2015;6:251. doi: 10.3389/fimmu.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meister M, Banfer S, Gartner U, Koskimies J, Amaddii M, Jacob R, Tikkanen R. Regulation of cargo transfer between ESCRT-0 and ESCRT-I complexes by flotillin-1 during endosomal sorting of ubiquitinated cargo. Oncogenesis. 2017;6(6):e344. doi: 10.1038/oncsis.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kleijmeer MJ Morkowski S Griffith JM Rudensky AY Geuze HJ Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments J Cell Biol 1997. 139 3 639–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleijmeer MJ Oorschot VM Geuze HJ Human resident langerhans cells display a lysosomal compartment enriched in MHC class II J Invest Dermatol 1994. 103 4 516–23 [DOI] [PubMed] [Google Scholar]
- 30. Kleijmeer MJ Ossevoort MA van Veen CJ van Hellemond JJ Neefjes JJ Kast WM Melief CJ Geuze HJ MHC class II compartments and the kinetics of antigen presentation in activated mouse spleen dendritic cells J Immunol 1995. 154 11 5715–24 [PubMed] [Google Scholar]
- 31. Drake L McGovern-Brindisi EM Drake JR BCR ubiquitination controls BCR-mediated antigen processing and presentation Blood 2006. 108 13 4086–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amigorena S Drake JR Webster P Mellman I Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes Nature 1994. 369 6476 113–20 [DOI] [PubMed] [Google Scholar]
- 33. Drake JR Webster P Cambier JC Mellman I Delivery of B cell receptor-internalized antigen to endosomes and class II vesicles J Exp Med 1997. 186 8 1299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Roux D Lankar D Yuseff MI Vascotto F Yokozeki T Faure-Andre G Mougneau E Glaichenhaus N Manoury B Bonnerot C Lennon-Dumenil AM Syk-dependent actin dynamics regulate endocytic trafficking and processing of antigens internalized through the B-cell receptor Mol Biol Cell 2007. 18 9 3451–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satpathy S, Wagner SA, Beli P, Gupta R, Kristiansen TA, Malinova D, Francavilla C, Tolar P, Bishop GA, Hostager BS, Choudhary C. Systems-wide analysis of BCR signalosomes and downstream phosphorylation and ubiquitylation. Mol Syst Biol. 2015;11(6):810. doi: 10.15252/msb.20145880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sohn HW Gu H Pierce SK Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk J Exp Med 2003. 197 11 1511–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagata T Murata K Murata R Sun SL Saito Y Yamaga S Tanaka N Tamai K Moriya K Kasai N Sugamura K Ishii N Hepatocyte growth factor regulated tyrosine kinase substrate in the peripheral development and function of B-cells Biochem Biophys Res Commun 2014. 443 2 351–6 [DOI] [PubMed] [Google Scholar]
- 38.Cho KJ, Roche PA. Regulation of MHC Class II-Peptide Complex Expression by Ubiquitination. Front Immunol. 2013;4:369. doi: 10.3389/fimmu.2013.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garstka MA Neefjes J How to target MHC class II into the MIIC compartment Mol Immunol 2013. 55 2 162–5 [DOI] [PubMed] [Google Scholar]
- 40. Oh J Shin JS Molecular mechanism and cellular function of MHCII ubiquitination Immunol Rev 2015. 266 1 134–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishikawa R Kajikawa M Ishido S Loss of MHC II ubiquitination inhibits the activation and differentiation of CD4 T cells Int Immunol 2014. 26 5 283–9 [DOI] [PubMed] [Google Scholar]
- 42.Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, Hung SC, Mellins ED. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front Immunol. 2017;8:319. doi: 10.3389/fimmu.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zwart W Griekspoor A Kuijl C Marsman M van Rheenen J Janssen H Calafat J van Ham M Janssen L van Lith M Jalink K Neefjes J Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape Immunity 2005. 22 2 221–33 [DOI] [PubMed] [Google Scholar]
- 44. Xiu F Cote MH Bourgeois-Daigneault MC Brunet A Gauvreau ME Shaw A Thibodeau J Cutting edge: HLA-DO impairs the incorporation of HLA-DM into exosomes J Immunol 2011. 187 4 1547–51 [DOI] [PubMed] [Google Scholar]
- 45. Gauvreau ME Cote MH Bourgeois-Daigneault MC Rivard LD Xiu F Brunet A Shaw A Steimle V Thibodeau J Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway Traffic 2009. 10 10 1518–27 [DOI] [PubMed] [Google Scholar]
- 46. Buschow SI Nolte-’t Hoen EN van Niel G Pols MS ten Broeke T Lauwen M Ossendorp F Melief CJ Raposo G Wubbolts R Wauben MH Stoorvogel W MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways Traffic 2009. 10 10 1528–42 [DOI] [PubMed] [Google Scholar]
- 47. Furuta K Walseng E Roche PA Internalizing MHC class II-peptide complexes are ubiquitinated in early endosomes and targeted for lysosomal degradation Proc Natl Acad Sci U S A 2013. 110 50 20188–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Busman-Sahay K Sargent E Harton JA Drake JR The Ia.2 epitope defines a subset of lipid raft-resident MHC class II molecules crucial to effective antigen presentation J Immunol 2011. 186 12 6710–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dixon AM Drake L Hughes KT Sargent E Hunt D Harton JA Drake JR Differential transmembrane domain GXXXG motif pairing impacts major histocompatibility complex (MHC) class II structure J Biol Chem 2014. 289 17 11695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harton J, Jin L, Hahn A, Drake J. Immunological Functions of the Membrane Proximal Region of MHC Class II Molecules. F1000Res. 2016;5 doi: 10.12688/f1000research.7610.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roche PA Furuta K The ins and outs of MHC class II-mediated antigen processing and presentation Nat Rev Immunol 2015. 15 4 203–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Infantino S Benz B Waldmann T Jung M Schneider R Reth M Arginine methylation of the B cell antigen receptor promotes differentiation J Exp Med 2010. 207 4 711–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamano T, Steinert M, Klein L. Thymic B Cells and Central T Cell Tolerance. Front Immunol. 2015;6:376. doi: 10.3389/fimmu.2015.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mitchison NA Antigen Recognition Responsible for the Induction In Vitro of the Secondary Response Cold Spring Harb Symp Quant Biol 1967. 32 431–439 [Google Scholar]
- 55. Mitchison NA The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis Eur J Immunol 1971. 1 1 10–7 [DOI] [PubMed] [Google Scholar]
- 56. Nashar TO Drake JR The pathway of antigen uptake and processing dictates MHC class II-mediated B cell survival and activation J Immunol 2005. 174 3 1306–16 [DOI] [PubMed] [Google Scholar]
- 57.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3(6):e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mitchell RN Barnes KA Grupp SA Sanchez M Misulovin Z Nussenzweig MC Abbas AK Intracellular targeting of antigens internalized by membrane immunoglobulin in B lymphocytes J Exp Med 1995. 181 5 1705–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shaw AC Mitchell RN Weaver YK Campos-Torres J Abbas AK Leder P Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation Cell 1990. 63 2 381–92 [DOI] [PubMed] [Google Scholar]
- 60. Barroso M Tucker H Drake L Nichol K Drake JR Antigen-B Cell Receptor Complexes Associate with Intracellular major histocompatibility complex (MHC) Class II Molecules J Biol Chem 2015. 290 45 27101–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Drake LA Drake JR A triad of molecular regions contribute to the formation of two distinct MHC class II conformers Mol Immunol 2016. 74 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lang P Stolpa JC Freiberg BA Crawford F Kappler J Kupfer A Cambier JC TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-alpha/beta dimers Science 2001. 291 5508 1537–40 [DOI] [PubMed] [Google Scholar]
- 63. Hauser JT Lindner R Coalescence of B cell receptor and invariant chain MHC II in a raft-like membrane domain J Leukoc Biol 2014. 96 5 843–55 [DOI] [PubMed] [Google Scholar]
- 64.Lindner R. Invariant Chain Complexes and Clusters as Platforms for MIF Signaling. Cells. 2017;6(1) doi: 10.3390/cells6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huttl S Klasener K Schweizer M Schneppenheim J Oberg HH Kabelitz D Reth M Saftig P Schroder B Processing of CD74 by the Intramembrane Protease SPPL2a Is Critical for B Cell Receptor Signaling in Transitional B Cells J Immunol 2015. 195 4 1548–63 [DOI] [PubMed] [Google Scholar]
- 66. Schneppenheim J Loock AC Huttl S Schweizer M Lullmann-Rauch R Oberg HH Arnold P Lehmann CHK Dudziak D Kabelitz D Lucius R Lennon-Dumenil AM Saftig P Schroder B The Influence of MHC Class II on B Cell Defects Induced by Invariant Chain/CD74 N-Terminal Fragments J Immunol 2017. 199 1 172–185 [DOI] [PubMed] [Google Scholar]
- 67. Macmillan H Strohman MJ Ayyangar S Jiang W Rajasekaran N Spura A Hessell AJ Madec AM Mellins ED The MHC class II cofactor HLA-DM interacts with Ig in B cells J Immunol 2014. 193 6 2641–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gondre-Lewis TA Moquin AE Drake JR Prolonged antigen persistence within nonterminal late endocytic compartments of antigen-specific B lymphocytes J Immunol 2001. 166 11 6657–64 [DOI] [PubMed] [Google Scholar]


