Abstract
Background
Poor pulmonary function (PPF) is associated with increased risk of dementia yet it is unclear if PPF in early adulthood to midlife increases risk independent of smoking and subsequent vascular disease.
Objective
This study evaluates the association between multiple markers of PPF in early-adulthood to midlife and long-term risk of dementia.
Method
We evaluated 27,387 members of an integrated healthcare system with forced expiratory volume in 1 second (FEV1), 2 seconds (FEV2), and vital capacity (VC) collected from 1964-1973 (mean age=41.8±4.2 years). Associations of PPF with dementia diagnoses from 1/1/1996-9/30/2015 were evaluated with Cox proportional hazard models adjusted for demographics, height, body mass index, hypertension, smoking status, diabetes, stroke, and heart failure.
Results
7,519 individuals (27%) were diagnosed with dementia. In fully adjusted Cox proportional hazards models, for all PPF measures each liter decrease was associated with a 13-14% higher risk of dementia. Compared to the highest quintile, the 1st quintile of PPF measures were associated with a 24-28% increased risk of dementia; 2-4th quintiles showed strong dose dependent associations. Results were similar when stratified by smoking status.
Conclusion
In this large, diverse cohort multiple measures of PPF in early adulthood to midlife were associated with dementia risk independent of smoking and vascular comorbidities.
Keywords: lifecourse, longitudinal, dementia, pulmonary function, lung function
Although the human brain accounts for only 2.3% of our body weight[1], it utilizes one fourth of our oxygen intake[2]. The brain is sensitive to oxygen availability and respiratory conditions that result in reductions in oxygen to the brain can over time lead to decreased cognitive function [3–5]. Poor pulmonary function is an in vivo indicator of reduced oxygen uptake. Poor pulmonary function is associated with diminished cognitive function [6–8] and possibly dementia risk [6, 7, 9–11]. Recently a large body of evidence has demonstrated that risk factors for dementia need to be evaluated in early adulthood to midlife [11–17], long before neuropathological changes have commenced to establish temporality. To our knowledge no prior study has evaluated PPF in exclusively in adulthood to midlife and long-term dementia risk. We examine if numerous markers of pulmonary function in a large diverse sample of individuals ages 35-50 is associated with an elevated risk of dementia more than twenty years later taking into account smoking and vascular disease.
Methods
Study population
This study follows members of Kaiser Permanente Northern California (KPNC), an integrated health care system with over 3 million individual who are representative of the catchment area apart from the extremes of the income distribution [18–20]. We included individuals who were 35-50 years old when they participated in at least one check-up associated with the Multiphasic Health Checkups (MHC), a series of optional check-ups offered to members in San Francisco and Oakland, California in the 1960s and 1970s. During MHC visits, health questionnaires and clinical measurements collected information on demographics, lifestyle, pulmonary function, and cardiovascular health indicators.
We linked data from 33,045 members of KPNC who were 35-50 during a MHC visit between 1964 and 1973 to medical health records starting in 1996. We excluded 2,797 individuals without any measures of midlife pulmonary function, 2,736 individuals missing height measurements, 2 individuals missing information on sex, 110 individuals missing race/ethnicity and 13 missing weight. A total of 27,387 individuals were eligible and included in these analyses.
This study was approved by the Kaiser Internal Review and conducted in accordance to with the Helsinki Declaration of 1975.
Midlife pulmonary function
Midlife pulmonary function was assessed during MHC visits between 1964 and 1973 by measuring forced expiratory volume in 1 second (FEV1), forced expiratory volume in 2 seconds (FEV2), and vital capacity (VC). Forced expiratory volume is the volume (liters) of gas exhaled during the first second or two of expiration. VC is the total volume (liters) of air expelled after the deepest breath possible. Pulmonary function measurements were captured using a Vertek VR5000 Lung Function computer (Electro/Med. Instruments, Houston, TX) [21]. If individuals participated in more than one MHC visit, the first visit was used. Continuous measures of FEV1, FEV2, and VC were reverse coded ((maximum value + 1) - original value) so that higher values represented worse pulmonary function. To examine possible non-linear functional forms, each of the three measures of pulmonary function was divided into quintiles. Since sex is an important determinant of lung capacity, quintiles were created for each sex separately and then combined. Some individuals were missing one or two of the three pulmonary function tests: 26, 858 individuals had FEV1 values, 16,289 had FEV2 values, and 27,384 had VC values.
To better account for height and race, we also estimated percent predicted FEV1 and VC based off reference spirometeric values from a sample of Whites, African Americans, and Mexican Americans in the US.[22] Although we do not know the ancestry of the Hispanic population in our sample, the US Census reported that 78% of California’s Hispanic population in the 1970s were of Mexican descent.[23] The age, sex, and race specific equations incorporate information regarding height and age to calculate predicted FEV1 and VC. Percent predicted pulmonary function measures were then divided into quintiles.
Dementia diagnosis
Dementia diagnoses between January 1st, 1996 and Sept 30th, 2015 were ascertained from inpatient and outpatient electronic medical records. Consistent with previous studies in this population [24–27] the following International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes were used to define dementia: Alzheimer’s disease (331.0), vascular dementia (290.4x), and other/nonspecific dementia (290.0, 290.1x, 290.2x, 290.3, 294.1, 294.2x, and 294.8). A similar set of codes had a sensitivity of 77% and a specificity of 95% compared with a consensus dementia diagnosis [28].
Mortality
Death was obtained through KPNC electronic medical records, California State Mortality File, and Social Security Death records.
Covariates
Sex and educational attainment were captured in the 1964-1973 MHC questionnaires. Educational attainment was captured as the highest grade completed and was recoded as high school or less. Height, a well-established predictor of pulmonary function, weight, and blood pressure were measured during the 1964-1973 MHC. Height and weight were combined to calculate body mass index (BMI) and hypertension status was defined using blood pressure thresholds based off recommendations from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) [29]. Late-life heart failure, stroke, and diabetes were defined as ICD-9 diagnoses (Supplemental Table 1) identified in the electronic medical records between January 1st 1996 and January 1st, 1997 preceding any possible dementia diagnosis that may have also occurred during that time period. KPNC records provided information on self-reported race and ethnicity (White (reference), African American, Asian, Hispanic, and “other racial/ethnic identity”) and age. Self-reported smoking was captured by MHC questionnaires and categorized as current, past, or never smoker. Missing indicators were used for missing information on midlife smoking (n= 3,669).
Method of analysis
We examined the distribution of pulmonary function measures, demographics, height, smoking, and health conditions in mid- and late-life by dementia status at end of follow-up. For each of the pulmonary function measures, we ran a series of Cox proportional hazards models (age as time scale) examining the association of both continuous and quintile forms of pulmonary function with dementia. The highest quintile (i.e. best) was the reference group in analyses examining the association between quintiles of pulmonary function. We also examined the risk of dementia associated with being in the worst quintile of all pulmonary function measures (i.e. worst quintile of FEV1, FEV2, and VC) compared to not being in the worst quintile for any of the pulmonary measures.
Covariates were added to Cox proportional hazards models in two groups since mid- and late-life health indicators may behave as mediators. All models adjusted for age (as timescale), demographics, and height, a strong predictor of pulmonary function. Next, we further adjusted for midlife health indicators (BMI, hypertension, and smoking status) and late-life health indicators (diabetes, stroke, and heart failure). Although BMI is comprised of height and weight, BMI and height are not closely correlated (Pearson correlation coefficient =0.03) and it is common to concurrently adjust for both BMI and height when examining pulmonary function [7, 10, 30]. For all Cox models, individuals were censored at date of dementia diagnosis, death, the start of a membership gap lasting more than 90 days, or the end of the study period on September 30th, 2015.
We estimated and plotted the cumulative incidence of dementia associated with being in the worst versus best quintile of VC in 5-year increments from 10 to 35 years beginning at age 60. Estimates were conditional on survival free of dementia up to age 60. The Practical Incidence Estimator macro [31] was used to obtain these estimates, which incorporates information on death rates and assuming that individuals who die without a dementia diagnosis never develop dementia.
We examined possible effect modification of the relationship between pulmonary function and dementia by midlife smoking by contrasting estimated effects of quintiles of pulmonary function among midlife smokers versus midlife never smokers; individuals who reported past smoking at midlife (4,835 individuals) were excluded from these analyses. We also tested for possible effect modification of the relationship between being in the worst quintile for all three measures and dementia by smoking.
In sensitivity analyses, Cox proportional hazards models were implemented to examine the relationship between quintiles of percent predicted FEV1 and VC among Whites, African Americans, and Hispanics in our sample. The highest quintiles reflected the best pulmonary function and served as the reference group. All models adjusted for age (as timescale), demographics, and height and fully adjusted models also accounted for mid- and late-life health indicator.
Results
On average, pulmonary function measures were taken when individuals were in their early 40s (mean age=41.8 ±4.2 years) (Table 1). The mean FEV1, FEV2, and VC were 2.7 ± 0.8, 3.3±1.0, and 3.5 ±1.0 liters, respectively. Overall, the sample was 67% White, 17% African American, 6% Asian, 6% Hispanic, and 4% were categorized as “Other racial/ethnic identity”. At midlife, 35% reported being current smokers, 18% were past smokers, and 34% were never smokers. The mean age at the start of follow-up for dementia in 1996 was 69.8 ±5.4 years old. At the end of follow-up, 7,519 people (27%) received a dementia diagnosis, 8,346 people died (30%), 4,601 people (17%) were censored due membership lapse, and 6,921 people (25%) were alive, dementia-free, and still members of KPNC.
Table 1.
Sample characteristics by dementia status
| Dementia | No Dementia | Overall | |
|---|---|---|---|
| n (column %) | n (column %) | n (column %) | |
| N | 7519 (27.4)* | 19,868 (72.5)* | 27,387 (100) |
| FEV1 (L; mean (SD)) | 2.6 (0.8) | 2.7 (0.8) | 2.7 (0.8) |
| FEV2 (L; mean (SD)) | 3.1 (0.9) | 3.4 (1.0) | 3.3 (1.0) |
| VC (L; mean (SD)) | 3.4 (0.9) | 3.6 (1.0) | 3.5 (1.0) |
|
| |||
| Female | 4463 (59.4) | 10341 (52.1) | 14804 (54.1) |
| High school or less | 2712 (36.5) | 6314 (32.1) | 9026 (33.3) |
| Height (cm; mean, SD) | 166.9 (9.4) | 168.4 (9.7) | 168.0 (9.6) |
| Age (yrs; mean, SD) | |||
| At midlife MHC visit | 43.2 (4.1) | 41.3 (4.2) | 41.8 (4.2) |
| At start of dementia follow-up | 71.9 (4.8) | 69.0 (5.4) | 69.8 (5.4) |
| At dementia | 82.3 (6.1) | – | 82.3 (6.1) |
| Race/ethnicity | |||
| White | 4785 (63.6) | 13546 (68.2) | 18331 (66.9) |
| African American | 1480 (19.7) | 3140 (15.8) | 4620 (16.9) |
| Asian | 413 (5.5) | 1296 (6.5) | 1709 (6.2) |
| Hispanic | 483 (6.4) | 1250 (6.3) | 1733 (6.3) |
| Other racial/ethnic identity | 357 (4.8) | 634 (3.2) | 991 (3.6) |
| Midlife Health | |||
| Hypertension | 2255 (30.0) | 5796 (29.2) | 8051 (29.4) |
| BMI (mean, SD) | 25.1 (4.0) | 24.9 (4.2) | 24.9 (4.1) |
| Midlife smoking status | |||
| Current | 2474 (32.9) | 7149 (36.0) | 9623 (35.1) |
| Past | 1258 (16.7) | 3577 (18.0) | 4835 (17.7) |
| Never | 2645 (35.2) | 6615 (33.3) | 9260 (33.8) |
| Missing | 1142 (15.2) | 2527 (12.7) | 3669 (13.4) |
| Late-life Health | |||
| Stroke | 239 (3.2) | 391 (2.0) | 630 (2.3) |
| Diabetes | 913 (12.1) | 1989 (10.0) | 2902 (10.6) |
| Heart Failure | 201 (2.7) | 647 (3.3) | 848 (3.1) |
row percent; FEV=Forced expiratory volume; VC=vital capacity; L=liters; SD=standard deviation
For the three pulmonary function measures, each one liter difference below the reference value was associated with approximately 15-17% increase risk of dementia in models adjusting for demographics and height, and 13-14% in fully adjusted models (Table 2). For all three pulmonary function measures, in minimally and fully adjusted models, there were strong dose responses associations between quintiles of pulmonary function and dementia risk with the lowest (i.e. worst) quintile at the greatest risk of dementia. For example, in fully adjusted models, individuals in the 1st, 2nd, and 3rd quintile of VC were at 28% (HR=1.28; 95% CI: 1.17, 1.39), 20% (HR=1.20; 95% CI: 1.11, 1.30), and 13% (HR=1.13; 95% CI: 1.05, 1.22) greater risk of dementia than individuals in the best VC quintile. Individuals in the 4th quintile of VC were not at significantly greater risk of dementia (HR=1.07; 95% CI: 0.99, 1.16). In fully-adjusted models, compared to not being in the worst quintile for any of the three pulmonary measures, being in the worst quintile for all three measures was associated with an 28% increase in dementia risk compared to individuals who were in the best quintile for the three measures (HR=1.28; 95% CI: 1.16, 1.41).
Table 2.
Adjusted hazard ratios (aHR) for dementia by midlife pulmonary function modelled continuously and in quintiles
| FEV1 (n=26858; 7359 cases) |
FEV2 (n=16289; 3787 cases) |
VC (n=27384; 7518 cases) |
||||
|---|---|---|---|---|---|---|
| Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
|
| Continuous* | 1.15 (1.11, 1.20) | 1.13 (1.09, 1.18) | 1.17 (1.11, 1.23) | 1.13 (1.08, 1.19) | 1.16 (1.12, 1.21) | 1.14 (1.10, 1.18) |
|
| ||||||
| Quintile | ||||||
| 1- Worst | 1.29 (1.19, 1.39) | 1.24 (1.14, 1.34) | 1.37 (1.22, 1.54) | 1.27 (1.13, 1.43) | 1.34 (1.23, 1.45) | 1.28 (1.17, 1.39) |
| 2 | 1.19 (1.10, 1.29) | 1.16 (1.08, 1.26) | 1.29 (1.15, 1.44) | 1.24 (1.10, 1.39) | 1.23 (1.13, 1.34) | 1.20 (1.11, 1.30) |
| 3 | 1.18 (1.09, 1.28) | 1.16 (1.07, 1.26) | 1.15 (1.03, 1.29) | 1.11 (1.00, 1.25) | 1.15 (1.07, 1.24) | 1.13 (1.05, 1.22) |
| 4 | 1.04 (0.97, 1.13) | 1.04 (0.96, 1.12) | 1.15 (1.03, 1.28) | 1.12 (1.01, 1.25) | 1.08 (1.00, 1.16) | 1.07 (0.99, 1.16) |
| 5- Best | ref | ref | ref | ref | ref | ref |
Notes:
Continuous midlife pulmonary function was reverse coded such that high values reflect worse pulmonary function. Midlife pulmonary function quintiles estimated separately for men and women. Model 1 adjusts for demographics (age, race/ethnicity, education) and height. Model 2 adjusts demographic, height, midlife health indicator (hypertension, body mass index, smoking status), and late-life health indicator (stroke, diabetes, heart failure).
Estimates of the cumulative incidence of dementia were consistently higher for individuals in the worst quintile of VC compared to those in the best quintile (Table 3 and Figure 1). The 30-year incidence of dementia among individuals in the worst quintile of VC was 35.7% (95% CI: 33.7%, 37.3%) compared to 30.4% (95% CI: 28.3%, 32.0%) for individuals in the best VC quintile. Overall, there was a dose response association across quintiles of pulmonary function and cumulative incidence of dementia risk.
Table 3.
Estimates of cumulative incidence of dementia (95% confidence interval) for each quintile of vital capacity (VC)
| 1st quintile (Worst) Cumulative incidence (95% CI) |
2nd quintile Cumulative incidence (95% CI) |
3rd quintile Cumulative incidence (95% CI) |
4th quintile Cumulative incidence (95% CI) |
5th quintile (Best) Cumulative incidence (95% CI) |
|
|---|---|---|---|---|---|
| 10-year | 3.2 (2.2, 4.1) | 1.5 (0.8, 2.2) | 2.0 (1.1, 2.8) | 1.9 (1.2, 2.6) | 1.3 (0.8, 1.8) |
| 15-year | 8.3 (7.1, 9.5) | 5.6 (4.6, 6.6) | 5.5 (4.4, 6.5) | 5.5 (4.6, 6.4) | 4.1 (3.4, 4.8) |
| 20-year | 15.6 (14.2, 16.8) | 13.2 (11.9, 14.4) | 12.7 (11.4, 13.8) | 11.5 (10.3, 12.5) | 10.1 (9.1, 11.0) |
| 25-year | 25.7 (24.1, 27.1) | 23.4 (21.7, 24.8) | 22.0 (20.5, 23.4) | 21.3 (18.9, 22.6) | 19.6 (18.2, 20.8) |
| 30-year | 35.7 (33.7, 37.3) | 33.7 (31.4, 35.4) | 32.7 (30.8, 34.3) | 31.8 (29.9, 33.4) | 30.4 (28.3, 32.0) |
| 35-year | 42.2 (39.6, 44.0) | 41.3 (38.1, 43.3) | 40.5 (37.5, 42.3) | 40.6 (37.5, 42.5) | 38.2 (34.6, 40.3) |
Notes: Cumulative incidence estimates incorporate death rates and are conditional on survival free of dementia until age 60. The time period associated with the cumulative incidence estimates begins at age 60.
Figure 1.
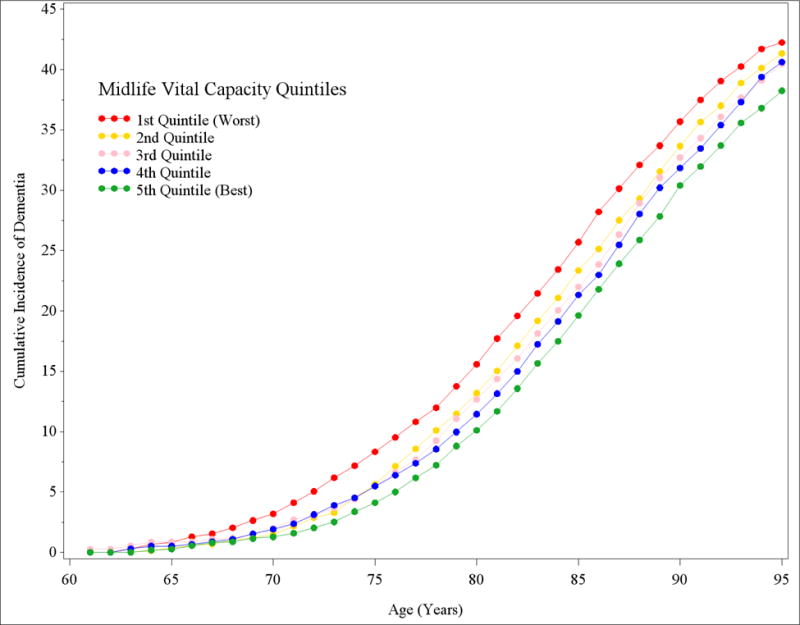
Cumulative incidence of dementia for individuals with worst and best quintile of midlife pulmonary vital capacity
While effect estimates of FEV1, FEV2, and VC quintiles tended to be slightly larger among midlife smokers than non-smokers, there was little evidence of effect modification by smoking: the confidence intervals overlapped and p-values for interaction terms were > 0.30; Table 4). The worst quintile of FEV2 was associated with a 28% increased risk of dementia for those who were never smokers in midlife (HR=1.28; 95% CI: 1.07-1.53) and 30% for those who were midlife smokers (HR=1.30; 95% CI: 1.07, 1.58). Overall, there was a dose-response relationship between pulmonary function and dementia for both midlife smokers and non-smokers. Compared to not being in the worst quintile for any of the three pulmonary measures, being in the worst quintile for all three was associated with a 31% increase in dementia risk for never smokers at midlife (HR=1.31; 95% CI: 1.12, 1.54) and a 20% increase for midlife smokers (HR=1.20; 95% CI: 1.02, 1.41).
Table 4.
Table 4 Adjusted hazard ratios (aHR) for dementia by midlife pulmonary function quintile stratified by midlife smoking, adjusted for demographics
| FEV1 | FEV2 | VC | ||||
|---|---|---|---|---|---|---|
| Quintile | Never smoker HR (95% CI) |
Current smoker HR (95% CI) |
Never smoker HR (95% CI) |
Current smoker HR (95% CI) |
Never smoker HR (95% CI) |
Current smoker HR (95% CI) |
| Observations | 9034 | 9433 | 5859 | 6143 | 9258 | 9622 |
| Cases | 2572 | 2424 | 1442 | 1393 | 2644 | 2474 |
|
| ||||||
| p-test for trend | 0.02 | 0.02 | 0.01 | 0.03 | 0.001 | 0.0004 |
| 1- Worst | 1.18 (1.03, 1.34) | 1.23 (1.06, 1.41) | 1.28 (1.07, 1.53) | 1.30 (1.07, 1.58) | 1.27 (1.11, 1.46) | 1.36 (1.18, 1.57) |
| 2 | 1.17 (1.03, 1.33) | 1.13 (0.99, 1.30) | 1.15 (0.95, 1.39) | 1.34 (1.10, 1.62) | 1.18 (1.03, 1.35) | 1.25 (1.08, 1.44) |
| 3 | 1.05 (0.91, 1.20) | 1.18 (1.02, 1.36) | 0.98 (0.82, 1.17) | 1.17 (0.97, 1.42) | 1.05 (0.93, 1.20) | 1.25 (1.09, 1.42) |
| 4 | 0.98 (0.87, 1.12) | 1.04 (0.90, 1.20) | 1.05 (0.89, 1.25) | 1.12 (0.92, 1.36) | 1.01 (0.89, 1.14) | 1.11 (0.96, 1.27) |
| 5- Best | ref | ref | ref | ref | ref | ref |
Notes: Individuals missing smoking information or self-reported as past smokers in midlife are excluded from these analyses. All models adjust for age, height, race/ethnicity, and education.
Among Whites, African Americans, and Hispanics, there were strong dose responses associations between quintiles of percent predicted FEV1 and dementia risk in minimally and fully adjusted models (Supplemental Table 2). For example, adjusting for demographics, individuals in the 1st, 2nd, and 3rd quintile of percent predicted FEV1 were at 20% (HR=1.20; 95% CI: 1.11, 1.29), 14% (HR=1.14; 95% CI: 1.05, 1.23), and 9% (HR=1.09; 95% CI: 1.01, 1.18) greater risk of dementia compared to those in the highest quintile of percent predicted FEV1. Individuals in the 4th quintile of percent predicted FEV1 were not at significantly greater risk of dementia (HR=1.00; 95% CI: 0.92, 1.08). Individuals in the 1st and 2nd quintile of percent predicted VC were at 24% (HR=1.24; 95% CI: 1.15, 1.34) and 14% (HR=1.14; 95% CI: 1.05, 1.23) increased risk of dementia compared to those in the highest quintile of percent predicted VC. Individuals in the 3rd and 4th quintile of percent predicted FEV1 were not at significantly greater risk of dementia (HR3rd quintile=1.00; 95% CI: 0.93, 1.08; HR4th quintile=1.01; 95% CI: 0.94, 1.09).
Discussion
In this large longitudinal study, several indicators of midlife pulmonary function were consistently associated with dementia risk. Each 1 L difference below the reference value of FEV1, FEV2, or VC was associated with 15% to 17% increase risk of dementia. There was a strong dose-response relationship with individuals with FEV1, FEV2, or VC measurements in the lowest (i.e. worst) quintile at the greatest risk of dementia. For example, individuals in the lowest quintile of VC were at 34% greater risk of dementia than individuals in the highest VC quintile. The 30-year cumulative incidence of dementia was 17% greater for individuals in the lowest VC quintile than those in the highest quintile. There was no evidence of effect modification by midlife smoking status. For FEV2, the worst quintile was associated with 28% greater risk of dementia for midlife non-smokers and 30% for midlife smokers. In analyses restricted to White, African Americans, and Hispanics, quintiles of percent predicted FEV1 and VC, which better account for height and race, were inversely associated with dementia risk. Those in the worst quintile of percent predicted FEV1 and VC were at 20% and 24% increased risk of dementia compared to those in the best quartiles in models accounting for demographics. To our knowledge, this is the first study to exclusively examine individuals 35 to 50 years old. Additionally, this is the largest and most diverse sample in the United States including 4,620 African Americans, 1,709 Asians, and 1,733 Hispanics.
Our study is consistent with a body of research examining the longitudinal association between midlife pulmonary function and dementia risk. Guo et al found that for every one standard deviation increase in any of the three measures of pulmonary function (FEV1, VC, and peak expiratory flow) there was a decrease in the risk of dementia by about 25% [10]. However, their sample was comprised of 1,135 women ages 44 + at the time of pulmonary function measurement, limiting generalizability to younger adults and men. Studies have since shown an association between pulmonary function and dementia incidence among men and women ages 45 and 64 [6, 7, 10], although one study did not find evidence of a relationship [11]. The largest study examining the association between pulmonary function and dementia risk is a meta-analysis involving 54,671 people ages 16 to 100 that found individuals in the worst quartile of pulmonary function were at double risk of dementia mortality than those in the best quartile of pulmonary function [32].
The mechanisms underlying the association between poor pulmonary function and long-term dementia risk remain unclear. Poor pulmonary function is associated with dementia risk factors such as a proinflammatory state [33–35] and white matter hyperintensities [36]. Poor pulmonary function may increase dementia risk due to hypoxia and hypoperfusion [10]. Cerebral hypoxia is associated with oxidative stress, a possible trigger for neuroinflammation resulting in neuronal apoptosis[37]. Hypoxia alters the ability of the brain to metabolize glucose and glucose hypometabolism may lead to a reduction in dendritic synaptic density and neuronal degeneration[38]. Hypoxia may also increase tau hyperphosphorylation and the level of amyloid precursor protein that is then converted to Aβ [38, 39] suggesting a direct role of hypoxia on neurodegenerative pathology. It is also possible that the association between pulmonary function and dementia is spurious and due to associations of cardiovascular disease, socioeconomic status[40], and physical activity[41] with both pulmonary function and dementia risk. In the current study the association between pulmonary function and dementia persisted even after controlling for a number of both mid- and late-life cardiovascular risk factors and comorbidities (e.g., blood pressure, heart failure, and stroke), suggesting that this is not a spurious association due to comorbid cardiovascular disease. Although we adjusted for educational attainment, residual confounding by socioeconomic status and other unmeasured confounders is possible. Smoking does not appear to explain the association between poor pulmonary function and dementia. Consistent with prior work, poor pulmonary function continued to have a strong dose response relationship with dementia risk among non-smokers in this sample [10].
Strengths of this study include a long follow-up and a well-characterized, large, diverse, sample starting in their mid 30s. The MHC and the electronic medical records provide prospectively collected information on a wide range of mid- and late-life health indicators associated with pulmonary function, dementia, and cardiovascular health. In sensitivity analyses we calculated percent predicted pulmonary function to more carefully adjust for difference by height and race/ethnicity. Unfortunately, the reference predictive equations we implemented did not provide reference values for racial and ethnic groups other than Whites, African Americans, and Mexican Americans.[22] Pulmonary function is associated with increased mortality risk and we were unable to assess if people who were censored due to death during follow-up would have otherwise developed dementia; this likely results in an underestimate of the true effect of pulmonary function on dementia risk. Lack of neuroimaging data restricted our ability to examine the structural cerebral differences associated with poor pulmonary function. Lastly, we were unable to examine possible biological pathways linking pulmonary function and dementia risk.
The results of this study suggest that pulmonary function is a strong predictor of dementia risk beginning in one’s mid 30s independent of smoking, mid-and late-life cardiovascular risk factors, and other comorbidities. A dose response relationship with dementia risk was present for all three measures of pulmonary function among both smokers and non-smokers. Further research is needed to delineate the neurobiological mechanisms through which poor pulmonary function elevates risk of dementia decades later.
Supplementary Material
Acknowledgments
Funding:
This study is support by the National Institutes on Aging (NIA RF1A6052132; Dr. Whitmer). Dr. Gilsanz is supported by the UCSF Training for Research on Aging and Chronic Disease (T32 AG049663). Dr. Mayeda is supported by the National Institute on Aging (5K99AG053410). Dr. Flatt is supported by the UCSF Center of Aging in Diverse Aging in Diverse Populations (P30AG015272) and the National Center for Advancing Translational Sciences (KL2TR001870).
References
- 1.Krompecher ST, Lipák J. A simple method for determining cerebralization. Brain weight and intelligence. The Journal of Comparative Neurology. 1966;127:113–120. doi: 10.1002/cne.901270108. [DOI] [PubMed] [Google Scholar]
- 2.Luo Q, Li LZ, Harrison DK, Shi H, Bruley DF, International Society on Oxygen Transport to Tissue. Annual Meeting Wuhan C . Oxygen transport to tissue XXXVIII. Springer; Switzerland: 2016. [Google Scholar]
- 3.Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev. 2018;38:39–49. doi: 10.1016/j.smrv.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Sanchez I, Rodriguez-Alzueta E, Cabrera-Martos I, Lopez-Torres I, Moreno-Ramirez MP, Valenza MC. Cognitive impairment in COPD: a systematic review. J Bras Pneumol. 2015;41:182–190. doi: 10.1590/S1806-37132015000004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathan SS, Gottesman RF, Mosley TH, Knopman DS, Sharrett AR, Alonso A. Association of lung function with cognitive decline and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Neurol. 2011;18:888–898. doi: 10.1111/j.1468-1331.2010.03340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giltay EJ, Nissinen A, Giampaoli S, Kromhout D. Apolipoprotein E genotype modifies the association between midlife lung function and cognitive function in old age. Dement Geriatr Cogn Disord. 2009;28:433–441. doi: 10.1159/000255600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chyou PH, White LR, Yano K, Sharp DS, Burchfiel CM, Chen R, Rodriguez BL, Curb JD. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. Am J Epidemiol. 1996;143:750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- 9.Vidal JS, Aspelund T, Jonsdottir MK, Jonsson PV, Harris TB, Lopez OL, Gudnason V, Launer LJ. Pulmonary function impairment may be an early risk factor for late-life cognitive impairment. J Am Geriatr Soc. 2013;61:79–83. doi: 10.1111/jgs.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo X, Waern M, Sjogren K, Lissner L, Bengtsson C, Bjorkelund C, Ostling S, Gustafson D, Skoog I. Midlife respiratory function and Incidence of Alzheimer’s disease: a 29-year longitudinal study in women. Neurobiol Aging. 2007;28:343–350. doi: 10.1016/j.neurobiolaging.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10:562–570. doi: 10.1016/j.jalz.2013.05.1772. [DOI] [PubMed] [Google Scholar]
- 12.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, Dean A, Whitmer RA. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89:1886–1893. doi: 10.1212/WNL.0000000000004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, Egan K. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 2017;8:165–178. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: Differential effects for alzheimer disease and vascular dementia. Archives of General Psychiatry. 2012;69:493–498. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;74(10):1246–1254. doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133:2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- 17.Rusanen M, Kivipelto M, Quesenberry CP, Jr, Zhou J, Whitmer RA. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med. 2011;171:333–339. doi: 10.1001/archinternmed.2010.393. [DOI] [PubMed] [Google Scholar]
- 18.Gordon NP. Kaiser Permanente. Northern California Division of Research; Oakland, CA: 2012. [Google Scholar]
- 19.Gordon NP, Kaplan GA. Some evidence refuting the HMO “favorable selection” hypothesis: the case of Kaiser Permanente. Adv Health Econ Health Serv Res. 1991;12:19–39. [PubMed] [Google Scholar]
- 20.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collen MF. Multiphasic Health Testing Services. John Wiley; New York: 1978. [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Bureau of the Census. Subject Reports: Persons of Spanish Origin. Table 3. Age of Persons of Spanish Origin by Sex and Urban and Rural Residence: 1970.
- 24.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 26.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 27.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Association Between Birth in a High Stroke Mortality State, Race, and Risk of Dementia. JAMA Neurol. 2017;74(9):1056–1062. doi: 10.1001/jamaneurol.2017.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katon WJ, Lin EH, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, Rutter C, Crane PK, Oliver M, Von Korff M. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010;25:423–429. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 30.Maiolo C, Mohamed EI, Carbonelli MG. Body composition and respiratoryfunction. Acta Diabetologica. 2003;40:s32–s38. doi: 10.1007/s00592-003-0023-0. [DOI] [PubMed] [Google Scholar]
- 31.Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Russ TC, Starr JM, Stamatakis E, Kivimäki M, Batty GD. Pulmonary function as a risk factor for dementia death: an individual participant meta-analysis of six UK general population cohort studies. Journal of Epidemiology and Community Health. 2015;69:550–556. doi: 10.1136/jech-2014-204959. [DOI] [PubMed] [Google Scholar]
- 33.Gan WQ, Man SFP, Sin DD. The Interactions Between Cigarette Smoking and Reduced Lung Function on Systemic Inflammation. Chest. 2005;127:558–564. doi: 10.1378/chest.127.2.558. [DOI] [PubMed] [Google Scholar]
- 34.Ahmadi-Abhari S, Kaptoge S, Luben RN, Wareham NJ, Khaw K-T. Longitudinal Association of C-Reactive Protein and Lung Function Over 13 Years: The EPIC-Norfolk Study. American Journal of Epidemiology. 2014;179:48–56. doi: 10.1093/aje/kwt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Guo X, Pantoni L, Simoni M, Gustafson D, Bengtsson C, Palmertz B, Skoog I. Midlife Respiratory Function Related to White Matter Lesions and Lacunar Infarcts in Late Life. The Prospective Population Study of Women in Gothenburg, Sweden. 2006;37:1658–1662. doi: 10.1161/01.STR.0000226403.00963.af. [DOI] [PubMed] [Google Scholar]
- 37.Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep. 2017;5(9):pii:e13258. doi: 10.14814/phy2.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daulatzai MA. Death by a thousand cuts in Alzheimer’s disease: hypoxia–the prodrome. Neurotox Res. 2013;24:216–243. doi: 10.1007/s12640-013-9379-2. [DOI] [PubMed] [Google Scholar]
- 39.Blass JP. Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia? J Neurosci Res. 2001;66:851–856. doi: 10.1002/jnr.10087. [DOI] [PubMed] [Google Scholar]
- 40.Gray LA, Leyland AH, Benzeval M, Watt GCM. Explaining the social patterning of lung function in adulthood at different ages: the roles of childhood precursors, health behaviours and environmental factors. Journal of Epidemiology and Community Health. 2013;67:905–911. doi: 10.1136/jech-2012-201704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nystad W, Samuelsen SO, Nafstad P, Langhammer A. Association between level of physical activity and lung function among Norwegian men and women: The HUNT Study. The International Journal of Tuberculosis and Lung Disease. 2006;10:1399–1405. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


