Abstract
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF superfamily that can initiate the apoptosis pathway by binding to its associated death receptors DR4 and DR5. The activation of the TRAIL pathway in inducing tumor-selective apoptosis leads to the development of TRAIL-based cancer therapies, which include recombinant forms of TRAIL, TRAIL receptor agonists and other therapeutic agents. Importantly, TRAIL, DR4 and DR5 can all be induced by synthetic and natural agents that activate the TRAIL apoptosis pathway in cancer cells. Thus, understanding the regulation of the TRAIL apoptosis pathway can aid in the development of TRAIL-based therapies for the treatment of human cancer.
Keywords: TRAIL, apoptosis, resistance, cancer therapy
1 Introduction
Inducing apoptosis in tumor cells is a key anti-cancer treatment strategy [1]. There are two major signaling pathways that lead to apoptosis, namely the intrinsic (mitochondrial) pathway and the extrinsic (death receptor) pathway [2]. The intrinsic apoptosis pathway is controlled by the Bcl-2 family members, which can be initiated by the activation of the tumor suppressor p53 in response to DNA damage by chemotherapy and radiotherapy [2]. However, conventional chemotherapy often fails to activate the intrinsic apoptosis pathway due to the loss of p53-mediated apoptosis [3]. On the other hand, the extrinsic apoptosis pathway is initiated by death receptor activation by ligands such as TNF-Related Apoptosis-Inducing Ligand (TRAIL) [2,4]. TRAIL binds to the death receptors DR4 and DR5 and triggers the apoptotic cascade by recruiting Fas-associated protein with death domain (FADD) via death domain interactions and thereafter, by FADD binding to the death effector domain present on pro-caspases-8 and -10 to form a death-inducing signaling complex (DISC). TRAIL can thus lead to direct activation of the caspase cascade resulting in apoptotic cell death. However, the intrinsic and extrinsic apoptotic pathways can crosstalk through caspase-8-mediated cleavage of Bid, triggering the intrinsic apoptotic pathway.
The early observation that tumor cells exhibit exquisite sensitivity to TRAIL over normal cells highlighted its potential as a novel cancer agent [5]. Consequently, recombinant human TRAIL (RhTRAIL) proteins or agonistic antibodies against DR4 and DR5, also called PARAs (pro-apoptotic receptor agonists), have been developed and shown to be effective in inducing apoptosis in various tumor cell lines [6,7]. In addition, a number of studies have indicated that rhTRAIL and PARAs enhance tumor sensitivity to chemotherapy, targeted therapy and radiotherapy [8–10]. However, development of resistance to TRAIL-induced apoptosis can greatly diminish the clinical potential of TRAIL-based agents. Thus, identifying the mechanisms of TRAIL resistance and restoring the sensitivity of tumor cells to TRAIL-based therapies could help improve their therapeutic efficacies. In this review, we discuss the potential for targeting TRAIL signaling in human cancers and the diverse molecular mechanisms by which tumor cells develop TRAIL resistance. In addition, emphasis is placed on synthetic and natural agents that stimulate the expression of TRAIL and its death receptors to induce cancer cell death.
2 TRAIL signaling
TRAIL is a 281-amino acid type II transmembrane protein that belongs to the TNF superfamily [11]. TRAIL is expressed in a variety of human fetal and adult tissues including small intestine, colon, spleen, thymus, prostate and placenta, and in immune cells such as natural killer (NK) cells, B cells, monocytes and dendritic cells [12–16]. The extracellular domain of TRAIL can be shed from the cell surface and are active as self-assembling non-covalent trimers. Both the soluble and membrane-bounded TRAIL can bind to its five distinct receptors DR4, DR5 DcR1, DcR2, and osteoprotegrin (OPG) [17]. DR4 and DR5 are type I membrane proteins with 2–4 similar cysteine-rich domains in the extracellular portion and a ~70 amino acid “death domain” in the cytoplasmic portion. The death receptors induce apoptosis upon TRAIL binding. DcR1 and DcR2 are two membrane decoy receptors while OPG is a soluble decoy receptor. Those three decoy receptors compete with the death receptors for TRAIL binding and thus block apoptotic signals.
Activation of death receptors by TRAIL leads to the recruitment of caspase-8 and FADD to form the DISC [4]. This can result in the activation of downstream effector caspases-3, -6, and -7 and subsequently induce apoptosis. It has been shown that many other proteins can be recruited into the DISC, including Cul3, a member of the cullin family of E3 ligases and PP2AC, a catalytic subunit of protein phosphatase 2A [18–20] (Figure 1). In some cells, the death receptor-initiated signal is not sufficient to trigger the caspase cascade. Here, the death receptor-induced caspase-8 activation cleaves the pro-apoptotic BH3-only Bcl-2 family member, Bid, thereby generating active truncated Bid (tBid), which interacts with Bax and Bak at the mitochondrial membrane to promote the release of apoptotic factors [21]. These apoptotic factors bind to apoptotic peptidase activating factor 1 (Apaf-1) and pro-caspase-9 to form a functional apoptosome, which initiates the caspase cascade to induce apoptosis. TRAIL can also induce lysosomal translocation of Bim and Bax through recruitment of the multifunctional sorting protein phosphofurin acidic cluster sorting protein-2 (PACS-2) to DR5-positive endosomes [22]. PACS-2 forms a complex with Bim and Bax on lysosomal membranes and releases cathepsin B to induce apoptosis. The metabolic status of the cell significantly affects TRAIL-induced cell death. For instance, 2-deoxyglucose, an inhibitor of glycolysis enhances TRAIL-induced cell death [23]. Paradoxically, inhibition of glycolysis by means of glucose deprivation can inhibit apoptosis [23]. This varied responses to glycolytic inhibition is determined by the balance between the activation of AKT and AMPK (AMP-activated protein kinase) pathways. The consequence of the balance impacts protein translation and the levels of anti- and pro-apoptotic Bcl-2 family member proteins. This indicates that cellular metabolic status can regulate the mitochondrial apoptotic pathway and thereby sensitivity to antitumor agents such as TRAIL. Treatment with methylglyoxal, a side product of glycolysis, or inhibition of glyoxalase I (GLO1) can also sensitize cancer cells to TRAIL [24]. TRAIL signaling is also positively regulated by mitogen-activated protein kinase kinase (MEK)/extracellular-signal-regulated kinase (ERK) signaling as MEK inhibition decreases sensitivity of cancer cells to TRAIL treatment [25]. Mechanistically, MEK inhibition negatively regulates DR4 expression and cellular response to TRAIL-induced apoptosis [25]. Increasing evidence indicates that endoplasmic reticulum (ER) stress can stimulate the activation of TRAIL receptors [26,27]. In macrophages, ER stress is a potent inducer of TRAIL signaling, and specific inhibition of Jun N-terminal kinase (JNK) and transcription factor AP-1 can inhibit the expression of TRAIL [28]. Mechanistically, ER stress induces the expression of activating transcription factor 4 (ATF4), which in turn regulates ATF3 and CCAAT/enhancer-binding protein homologous protein (CHOP) expression. ATF3 physically interacts with CHOP forming a complex to regulate DR5 expression. Loss of ATF4, ATF3, or CHOP reduced the DR5 levels and decreased apoptosis [29]. Alternately, TRAIL can induce ER stress via caspase-8-mediated cleavage of B cell receptor-associated protein 31 (BAP31) [30]. Increased production of reactive oxygen species (ROS) can regulate TRAIL signaling by ROS-ERK-CHOP-mediated up-regulation of DR4 and DR5 expression [31]. ROS can also induce Bax phosphorylation at threonine-167, sensitizing cells to TRAIL-mediated apoptosis [32]. TRAIL signaling has been implicated in activating the NF-κB pathway via the TRAIL receptor death domain (DD), FADD, and caspase-8 [33]. Loss-of-function mutation in FADD halts the recruitment of caspase-8 and thus prevents NF-κB activation [33].
Figure 1.
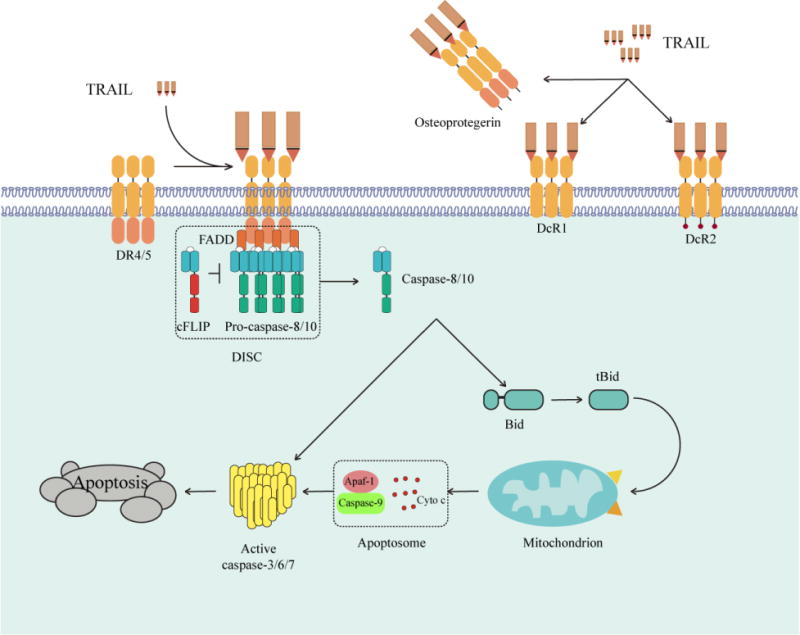
The TRAIL apoptosis pathway. In response to TRAIL stimulation, FADD, caspase-8/10 and c-FLIP are recruited to death receptors DR4 and DR5 to form the death-inducing signaling complex (DISC), which triggers apoptosis. DcR1, DcR2 and osteoprotegerin are three decoy receptors. DcR1 lacks intracellular domain while DcR2 contains truncated death domain. Osteoprotegerin is a soluble decoy receptor.
3 Physiological roles for TRAIL signaling
TRAIL signaling is known to regulate metabolism and differentiation and is also involved in some diseases. For example, in adipocytes TRAIL treatment results in a reduction of insulin-stimulated glucose uptake as well as de novo lipogenesis [34]. This is mediated by caspase-8/caspase-3 activation and cleavage of PPARgamma, which in turn down-regulates the expression of lipogenic genes, such as Glut-4 and FASN [34]. TRAIL also plays a role in spermatogenesis [35]. Specifically, Trail knockout (Trail−/−) mice exhibit significantly lower testis to body weight ratios and spermatid head counts, while displaying increased levels of basal germ cell apoptosis [35]. In addition, TRAIL is implicated in osteoclast differentiation through a TNF receptor-associated factor 6 (TRAF-6)-dependent signaling pathway [36]. TRAIL signaling is also involved in the pathogenesis of pulmonary arterial hypertension (PAH) [37]. TRAIL promotes microvascular hyperpermeability through caspase-3 cleavage of the endothelial adherens junctions, which is dependent on the phosphatidylinositol 3-kinase (PI3K) pathway [38]. Furthermore, data from multiple rodent models indicate that genetic deletion or antibody blockade of TRAIL can hinder the development of PAH [37]. In allergic airway inflammation, TRAIL regulates airway remodeling by up-regulating the E3 ubiquitin ligase Midline-1 (MID-1), which decreases the dephosphorylation of proinflammatory signaling molecules by protein phosphatase 2A [39]. Trail−/− mice lack airway remodeling, including peribronchial fibrosis, smooth muscle hypertrophy, and mucus hypersecretion [40]. Moreover, TRAIL signaling may be a new target for the treatment of some diseases, including liver fibrosis and influenza [41,42]. In addition, TRAIL signaling contributes to antiviral immunity by inducing apoptosis and promoting immune homeostasis during infection [43]. In a rat model of harmful focal ischemia, immunoneutralization of TRAIL significantly decreased tissue damage and exhibited functional recovery, underscoring a potential for the treatment of stroke [44]. Studies in the murine inner ear revealed a role for increased expression of TRAIL in triggering hair cell and neuronal degeneration, which can be suppressed with antibodies against DR5 [45].
4 Mechanisms of TRAIL resistance in cancer
TRAIL has been recognized as a promising target for cancer therapy because it can induce apoptosis in tumor cells but not normal cells. Although TRAIL shows high anti-tumor activity, resistance to TRAIL-induced apoptosis in tumor cells has been considered a clinical obstacle to its application. It is known that tumor cells with high nuclear localization of DR5 are resistant to TRAIL, whereas tumor cells without nuclear DR5 are highly sensitive to TRAIL [46]. The mutation of functional nuclear localization signals or knockdown of importin β1 can block the nuclear localization of DR5 and result in increased DR5 expression on the cell surface, and therefore, TRAIL sensitivity [46]. Low sensitivity to TRAIL also correlated with expression of anti-apoptotic members of the Bcl-2 family. For example, overexpression of Bcl-2 can inhibit TRAIL-induced apoptosis [47,48], and Bcl-xL inhibition significantly sensitized cells to TRAIL-induced apoptosis [49]. In addition, TRAIL resistance has been associated with lipid rafts, where the EGFR pathway is activated while TRAIL fails to induce effective death-inducing signaling complex formation [50]. Inhibition of epidermal growth factor receptor (EGFR) along with knockdown of casitas B-lineage lymphoma-b (Cbl-b) enhances TRAIL-induced apoptosis in these cells [50]. TRAIL-induced apoptosis is also altered by the multidrug transporter P-glycoprotein (Pgp) and the latter regulates endogenous TRAIL expression [51,52]. Blocking Pgp transport activity increases cell sensitivity to TRAIL [51,52]. It is also known that survivin and myeloid cell leukemia sequence 1 (Mcl-1) confer TRAIL resistance, and that inhibition of survivin and Mcl-1 sensitizes resistant tumor cells to TRAIL [53].
In breast cancer, cell lines of mesenchymal origin are susceptible to TRAIL while epithelial-like cell lines are TRAIL-resistant [54]. TRAIL sensitivity of breast cancer stem cells was inversely correlated with the cellular FLICE-like inhibitory protein (cFLIP), while overexpression of cFLIP in the cytosol relieved these cells from cytotoxicity [55]. The ERK1/2 pathway regulates cFLIP levels and thus impacts TRAIL sensitivity [56]. Caspase-8 is also involved in TRAIL sensitivity. Pro-caspase-8 mutations inhibit activation of the TRAIL pathway and confer resistance to death receptor activators [57]. Smad7, a negative regulator for the transforming growth factor-β (TGF-β) signaling pathway, binds to the caspase-8 promoter and enhances the recruitment of the interferon regulatory factor 1 (IRF1) transcription factor to the interferon-stimulated response element (ISRE) on the caspase-8 promoter [58]. Thus, Smad7 expression can restore the caspase cascade in apoptosis-resistant cells, rendering them susceptible to TRAIL-induced cell death. NF-κB signaling also plays a role in resistance against death receptor-induced apoptosis where TRAIL-resistant cells display a significant increase in TRAIL-inducible NF-κB activity, while TRAIL-sensitive cells display only a moderate level of NF-κB activity [59]. Activation of the NF-κB pathway upon TRAIL treatment is dependent on caspase-8-mediated cleavage of RIP1 [60]. Cleavage of RIP1 impairs IκB kinase (IKK) recruitment and thus NF-κB activation. In TRAIL-resistant cells, cFLIP restricts caspase-8 activity and RIP1 cleavage, which generates a cleaved fragment that can activate NF-κB but not apoptosis [60]. In vivo, the interaction of TRAIL, DR5 and NF-κB induces lung metastasis of melanoma in mice [61]. In cells with a defective mitochondrial apoptotic pathway, TRAIL induced phenotypic changes such as membrane blebbing and a transient loss of substrate adhesion properties while stimulating the migration potential of these cells [62]. Suppression of the apoptosis inhibitor cFLIP results in partial sensitization of TRAIL-resistant cancer cells to the pro-apoptotic effects of TRAIL, and the levels of cFLIP positively correlated with the survival of cancer patients [63,64].
The cytosolic translocation of the nuclear protein, HMGB1 (high mobility group box 1), plays a key role in TRAIL-mediated cancer cell death through autophagy [65]. TRAIL triggers PARP1 (poly [ADP-ribose] polymerase 1) activation and ADP-ribosylation of HMGB1 in cancer cells. PARP1 inhibition blocks HMGB1 cytoplasmic translocation and formation of the HMGB1-BECN1 complex, resulting in decreased autophagy, increased apoptosis, and increased sensitivity to TRAIL both in vitro and in vivo [65].
Promoter hypermethylation and/or inactivation of TRAIL decoy receptors are observed in a majority of cervical cancer patients. Such cervical cancer cell lines were able to effectively induce apoptosis upon treatment with TRAIL [66]. MicroRNAs (miRNAs) also play a role in the development of TRAIL resistance in different types of cancer. For example, miR-494 induces TRAIL resistance in non-small-cell lung cancer (NSCLC) by down-regulating the apoptosis regulator BIM [67]. In contrast, miR-212 inhibits the anti-apoptotic protein PED/PEA-15 and thereby overcomes TRAIL resistance [68].
5 Targeting the core machinery of the TRAIL pathway
Identification of TRAIL as an inducer of apoptosis that is selective towards cancer cells has been met with great enthusiasm, which has led to the development of TRAIL signaling agonists as anti-cancer agents, including TRAIL ligand and antibodies against DR4 and DR5 [69]. There are a number of TRAIL or TRAIL receptor-based clinical trials for cancer patients (Table 1). One such agent, recombinant human TRAIL (rhTRAIL), dulanermin, functions as a ligand to death receptors DR4 and DR5 [70]. In a clinical report, a patient with refractory chondrosarcoma who developed progressive metastatic chondrosarcoma to the lung showed a partial response to dulanermin treatment that was potentially mediated by DR4 present in the patient tumor cells [71]. However, resistance was observed after 62 months, which may have arisen due to the up-regulation of the pro-survival proteins, including NF-κB and Bcl-2 [71].
Table 1.
Completed clinical trials of agents that target the TRAIL core apoptotic pathway
| Therapeutic agents | In combination with | Trial type and tumor | Enrollment | Primary endpoint | Therapy effect | Reference |
|---|---|---|---|---|---|---|
| CPT | Thalidomide+ Dexamethasone |
Phase 2; myeloma | 71 | ORR | Benefit | ChiCTR-TRC-11001625 [135] |
| CPT | Thalidomide | Phase 2; myeloma | 43 | Safety | 2 CR, 7 PR | ChiCTR-ONC-1200206 [136] |
| Dulanermin | Rituximab | Phase 1b/2; lymphoma | 72 | Safety | No benefit | NCT00400764 [137] |
| Dulanermin | mFOLFOX6+ Bevacizumab |
Phase 1b; colorectal cancer | 23 | Safety | 13 PR, 7 SD | NCT00873756 [138] |
| Dulanermin | Phase 1a; cancer | 72 | Biomarkers | NR | [139] | |
| Dulanermin | Paclitaxel Carboplatin Bevacizumab |
Phase 2; NSCLC | 213 | ORR | 1 CR, 13 PR | NCT00508625 [140] |
| Dulanermin | Paclitaxel Carboplatin Bevacizumab |
Phase 1b; NSCLC | 24 | Safety | NR | NCT00508625 [141] |
| Dulanermin | Phase 1; cancer | 71 | Safety | NR | [142] | |
| Dulanermin | Camptosar/Erbitux Folfiri Bevacizumab |
Phase 1b; coloretal cancer | 42 | Safety | NR | NCT00671372 |
| Tigatuzumab | Phase 1; coloretal cancer | 19 | Distribution | 1 PR, 8 SD | NCT01220999 [143] | |
| Tigatuzumab | Sorafenib | Phase 2; liver cancer | 163 | Efficacy | No benefit | NCT01033240 [144] |
| Tigatuzumab | Paclitaxel | Phase 2; TNBC | 64 | ORR | 3 CR, 8 PR, 11SD | NCT01307891 [76] |
| Tigatuzumab | Gemcitabine | Phase 2; pancreatic cancer | 62 | Efficacy | Benefit | [145] |
| Tigatuzumab | Carboplatin/paclitaxel | Phase 2; NSCLC | 97 | Efficacy | No benefit | NCT00991796 [146] |
| Tigatuzumab | Phase 1; cancer | 17 | MTD | 7 SD | [147] | |
| Mapatumumab | Sorafenib | Phase 2; liver cancer | 101 | Efficacy | No benefit | NCT01258608 [148] |
| Mapatumumab | Paclitaxel Carboplatin |
Phase 2; NSCLC | 109 | Efficacy | No benefit | NCT00583830 [149] |
| Mapatumumab | Phase 1b/2; NHL | 40 | Efficacy | 2 CR, 1 PR | NCT00094848 [150] | |
| Mapatumumab | Phase 2; colorectal cancer | 38 | Efficacy | 12 SD | [151] | |
| Mapatumumab | Gemcitabine Cisplatin |
Phase 1; solid tumor | 49 | Safety | 12 PR, 25 SD | NCT01088347 [152] |
| Mapatumumab | Phase 1; solid tumor | 41 | MTD | 12 SD | [153] | |
| Mapatumumab | Paclitaxel Carboplatin |
Phase 1; solid tumor | 27 | Safety | 5 PR, 12 SD | [154] |
| Mapatumumab | Phase 2; NSCLC | 32 | Efficacy | 9 SD | NCT00092924 [155] | |
| Mapatumumab | Phase 1; solid tumor | 49 | Safety | 19 SD | [156] | |
| Mapatumumab | Bortezomib | Phase 2; Myeloma | 105 | Safety | NR | NCT00315757 |
| Mapatumumab | Sorafenib | Phase 1b; Liver cancer | 23 | Safety | NR | NCT00712855 |
| Mapatumumab | Cisplatin+ Radiotherapy |
Phase 1b/2; Cervical cancer | 9 | Safety | NR | NCT01088347 |
| Conatumumab | Paclitaxel Carboplatin |
Phase 2; NSCLC | 172 | PFS | No benefit | NCT00534027 [157] |
| Conatumumab/Ganitumab | FOLFIRI | Phase 2; colorectal cancer | 155 | PFS | Benefit | NCT00813605 [158] |
| Conatumumab | mFOLFOX6+ Bevacizumab |
Phase 1b/2; colorectal cancer | 202 | PFS | No benefit | NCT00625651 [159] |
| Conatumumab/Ganitumab | Gemcitabine | Phase 2; pancreatic cancer | 125 | OS | Benefit | NCT00630552 [160] |
| Conatumumab | Doxorubicin | Phase 1b/2; soft tissue sarcoma | 134 | PFS | No benefit | NCT00626704 [161] |
| Conatumumab | Phase 1; solid tumor | 18 | DLT | 2 SD | [162] | |
| Conatumumab | Phase 1; solid tumor | 37 | Safety | 1 PR, 15 SD | [163] | |
| Conatumumab | Panitumumab | Phase 1b/2; Colorectal cancer | 53 | Safety | NR | NCT00630786 |
| Conatumumab | Birinapant | Phase 1b; Ovarian cancer | 27 | Safety | NR | NCT01940172 |
| TAS266 | Phase 1; solid tumor | 4 | Safety | NR | [164] | |
| ONC201 | Phase 2; glioblastoma | 17 | Efficacy | Benefit | [130] | |
| ONC201 | Phase 1; solid tumor | 28 | Safety | Benefit | [129] |
Abbreviations: CPT, Circularly permuted TRAIL; NR, not reported; MTD, maximum tolerated dose; ORR, objective response rate; DLT, dose limiting toxicity; TNBC, triple-negative breast cancer; NHL, Non-Hodgkin’s lymphoma; NSCLC, non-small cell lung cancer; CR, complete response; PR, partial response; SD, stable disease.
While death receptor activation can be mediated through soluble human recombinant TRAIL, its short half-life as well as sequestration by decoy receptors limits its functionality. Generation of DR4 or DR5 agonist antibodies as therapeutic agents attempts to overcome the limitations of rhTRAIL [72]. Studies indicate that some monoclonal antibodies to DR4 or DR5 have been successful in mounting an anti-tumor response. One such example is LaDR5, which binds to DR5 and induces apoptosis in tumors [73]. Another DR5 agonist antibody, lexatumumab, induces apoptosis in a number of cancer cells [74]. Tigatuzumab (TIG), another anti-DR5 agonist antibody, resulted in apoptosis induction in basal-like breast cancer cells both in vitro and in vivo [75]. In a phase II trial in triple-negative breast cancer (TNBC) patients, use of TIG in combination with albumin-bound paclitaxel (nab-PAC) resulted in more patients with complete remission as well as prolonged progression free survival [76]. The efficacy of TRAIL receptor agonists also depends on antibody multimer formation that leads to receptor clustering on cancer cells. The agonist APG350 addresses this issue by incorporating six death receptor-binding sites per drug molecule and shows anti-tumor activity both in vitro and in vivo [77]. Secretory TRAIL-armed adenoviral (Ad.TRAIL) treatment has also exhibited enhanced apoptotic efficacy. In colorectal cancer xenograft models, the treatment with Ad.TRAIL blocked tumor growth and increased survival [78]. Ad.TRAIL in combination with mitomycin C and hyperthermia was shown to induce the JNK-Bak pathway, leading to apoptosis [79]. Strategies have also been developed for targeted delivery of TRAIL-based drugs. For example, TR3 is a TRAIL-based platform incorporating a genetically fused trimer that can be further modified to include tumor directed targeting moieties [80]. Meso-TR3 incorporates Mesothelin, which is known to interact with MUC16, a biomarker associated with several cancer types [81]. Meso-TR3 displayed binding selectivity and killing efficacy both in vitro and in a xenograft mouse model of MUC16-positive ovarian cancer [81]. In addition, other delivery systems, including nanoparticles and DNA vaccination were developed to target TRAIL-mediated tumor cell death [82,83]. For instance, Decarbazin (DTIC)-loaded polylactic acid (PLA) nanoparticles (DTIC-NPs), when conjugated to a highly specific targeting functional TRAIL-receptor 2 (DR5) monoclonal antibody, were able to specifically target DR5-overexpressing malignant melanoma cells and resulted in high cytotoxicity and increased apoptosis [84]. However, the current available data from these clinical trials are disappointed. The reasons of these disappointed results can be due to several aspects including resistance and patient selection. For example, in breast cancer, only TNBC cells but not other subtypes of breast cancer cells are susceptible to TRAIL. Therefore, it is conceivable that TRAIL-based therapy in the general breast cancer population is not expected to have a therapeutic benefit because TNBC only consists of 15–20 of all breast cancers.
6 Activation of the TRAIL pathway by anti-cancer agents
A major problem in clinical trials that use TRAIL-based therapeutics is that cancer cells are either intrinsically resistant or acquire resistance to TRAIL. Therefore, agents that can overcome TRAIL resistance have great therapeutic potential. Strategies have developed to increase the expression of TRAIL or its death receptors as novel cancer therapeutics.
6.1 Up-regulation of death receptors for activation of the TRAIL apoptosis pathway
Since DR5 was first shown to be induced by clinically used chemotherapeutic agents including doxorubicin and etoposide [85], a considerable interest has been generated to increase the expression of TRAIL death receptors as novel approaches for development of TRAIL-based cancer therapeutics. The idea behind this is that induced DR4 and DR5 can easily bind to TRAIL to induce apoptosis. Compounds that have abilities to increase death receptor expression can be categorized into three groups (Table 2): 1) clinically used anti-cancer drugs, 2) the agents that are currently being developed as anti-cancer agents, and 3) natural compounds that have anti-cancer activity.
Table 2.
The agents that increase the expression of TRAIL and its receptors DR4 and DR5 lead to the activation of the TRAIL apoptosis pathway.
| Therapeutic agents | Cancer type | Mechanism(s) | Reference |
|---|---|---|---|
| Chemotherapeutics | |||
| Doxorubicin | Ovarian terato-carcinoma | Inducing DR5 | [85] |
| Cisplatin | Glioblastoma | Inducing DR5 | [93] |
| Platinum(IV) LA-12 | Colon cancer | Activation of mitochondrial pathway | [94] |
| Carboplatin, pemetrexed and TRAIL(CPT) | Pleural mesothelioma | Inducing DR4 and DR5 | [95] |
| Trichostatin A(TSA) | Gastric cancer | Inducing DR5 | [96,97] |
| Natural products | |||
| Ginsenoside compound K (CK) | Colon cancer | Inducing DR5 | [107] |
| Magnolol and polyphenol mixture | NSCLC | Inducing DR5 | [108] |
| Chikusetsusaponin IVa butyl ester (CS-IVa-Be) | Breast cancer | Inducing DR5 | [109] |
| Zyflamend | Pancreatic cancer | Inducing ROS, CHOP and DR5 | [110] |
| Medicarpin(Med) | Myeloid leukemia cancer | Inducing ROS, CHOP and DR5 | [111] |
| Quercetin | Lung cancer | Induction of DR5 and inhibition of survivin | [112] |
| Triptolide | Leukemia | Decreasing XIAP | [113] |
| Wogonin | Solid tumors | Inducing DR5, decreasing c-FLIP | [114] |
| Other agents | |||
| Nutlin-3 | Colon cancer | Inducing DR5 | [98] |
| Edelfosine | Gastric cancer | Inducing DR5 | [99] |
| GW280264X | Glioblastoma | Inducing DR5 | [100] |
| NVP-AUY922 | Colorectal cancer | Suppressing the JAK2-STAT3-Mcl-1 pathway | [102] |
| Capsazepin | Colon cancer | Inducing DR4 and DR5 | [103] |
| Amurensin G | Leukemia | Inducing DR5 | [104] |
| Oligomycin A | Cervical | Inducing DR5 | [105] |
| ABC294640 | Lung cancer | Inducing DR4 and DR5 | [106] |
| ONC201 | Solid tumors | Inducing TRAIL and DR5 and ER stress | [122,125,126] |
6.1.1
Clinically used anti-cancer drugs include cisplatin, doxorubicin, etoposide, 5-FU, mitomycin c and mitoxantrone [86–92]. For example, cisplatin sensitized TRAIL-induced apoptosis by up-regulating DR5, leading to activation of caspases and apoptosis [93]. As a better alternative to cisplatin, Platinum(IV) complex LA-12 was tested to show higher sensitivity to TRAIL [94]. This was associated with Bax/Bak activation, decreased mitochondrial membrane potential, caspase-9 activation, and an increase in the expression of pro-apoptotic members of the Bcl-2 family of proteins. LA-12 was also a potent inducer of Noxa and BimL proteins. Treatment with Dulanermin (TRAIL) in combination with carboplatin and pemetrexed displayed increased sensitivity of malignant pleural mesothelioma cells compared to treatment with single agents [95]. The increased sensitivity to TRAIL was dependent on the increased expression of DR4 and DR5 in a p53-dependent manner mediated by carboplatin and pemetrexed [95]. Furthermore, it has been shown that the histone deacetylase (HDAC) inhibitors suberoylanilide hydroxamic acid and trichostatin A also enhanced TRAIL sensitivity in cancer cells through the up-regulation of DR4 and DR5 [96,97].
6.1.2
The second group of compounds is those agents that are being developed as cancer therapeutics with ability to increase DR4 and DR5 expression. For example, Nutlin-3, a small molecule inhibitor of MDM2, can increase DR5 expression and sensitize cancer cells to TRAIL-induced cell death [98]. This induces TRAIL-mediated apoptosis in cancer cells expressing wild-type p53. The ether phospholipid edelfosine, an asynthetic anti-tumor alkyllysophospholipid, can up-regulate DR5 to enhance rhTRAIL-induced apoptosis [99]. It has been shown that DR5 expression can be regulated by the Notch1 signaling pathway through Sp1-dependent activation of DR5 transcription [100]. Therefore, modulation of this pathway impacts TRAIL-induced apoptosis. Indeed, GW280264X, an inhibitor for a metalloproteinase needed for activation of the Notch1 pathway by cleavage, was able to increase DR5 expression and subsequently sensitize glioblastoma cells to TRAIL-induced apoptosis [100]. Suppression of heat shock protein 70 (HSP70) has also been shown to induce TRAIL-mediated apoptosis by increasing DR4 and DR5 expression [101]. Similar to HSP70 inhibition, the HSP90 inhibitor NVP-AUY922 was shown to overcome TRAIL resistance through dephosphorylating JAK2 and STAT3 and decreasing Mcl-1, which triggers the release of cytochrome c [102]. Another agent, Capsazepin, a capsaicin antagonist, was shown to sensitize colon cancer cells to TRAIL by inducing DR4 and DR5 via the ROS/JNK/CHOP pathway [103]. The SIRT1 inhibitor Amurensin G also induced DR5 expression, resulting in enhanced TRAIL sensitivity in TRAIL-resistant human leukemic K562 cells [104]. The small molecule ATP synthase inhibitor Oligomycin A (OMA) triggers the ER stress signaling pathway [105]. OMA induces the inositol-requiring enzyme 1 (IRE1) signaling pathway, resulting in the splicing of X-binding protein 1 (XBP1) and increasing expression of CHOP, where CHOP can bind to the DR5 promoter, thus enhancing TRAIL-mediated apoptosis [105]. In addition, the sphingosine kinase 2 inhibitor ABC294640 has been shown to induce DR4 and DR5 expression to enhance apoptosis by TRAIL in lung cancer cells [106].
6.1.3
The third group of compounds is some natural products that can activate the TRAIL pathway. There are many natural compounds that have been shown to activate the TRAIL pathway, particularly those that can induce DR5 expression. For example, ginsenoside compound K, an active ingredient of ginseng, sensitizes human colon cancer cells to TRAIL-induced apoptosis by up-regulation of DR5 through both autophagy-dependent and -independent mechanisms [107]. Magnolol and polyphenol mixture (PM), a natural product derived from Magnolia officinalis, inhibits class I HDACs, leading to epigenetic activation of DR5 and thus significant enhancement of TRAIL-mediated apoptosis in non-small lung cancer (NSCLC) cells [108]. Chikusetsusaponin IVa butyl ester (CS-IVa-Be), a triterpenoid saponin extracted from Acanthopanas gracilistylus W.W.Smith that acts as an IL6R antagonist, can sensitize the breast cancer MDA-MB-231 cells to TRAIL-induced apoptosis via up-regulation of DR5 [109]. Zyflamend®, a polyherbal preparation, can sensitize tumor cells to TRAIL through ROS-CHOP-mediated up-regulation of TRAIL death receptors [110]. Medicarpin, a naturally occurring phytoalexin l, can activate the ROS-JNK-CHOP pathway and induce DR5 expression, thus sensitizing myeloid leukemia cells to TRAIL-induced apoptosis [111]. Quercetin, a natural flavonoid, sensitizes lung cancer cells to TRAIL by increasing DR5 expression and inhibiting survivin expression [112]. In addition, the diterpene triepoxide, triptolide, sensitizes acute myeloid leukemia cells to TRAIL-induced apoptosis by increasing p53-dependent DR5 expression [113]. It has also shown that Wogonin and the structurally related natural flavones apigenin can overcome TRAIL resistance by down-regulation of c-FLIP and up-regulation of DR5 [114].
6.2 Up-regulation of TRAIL for inducing cancer cell death
In addition to induce death receptors as approaches to promote apoptosis in cancer cells, strategies have also developed to induce TRAIL expression as an approach for cancer therapies. TRAIL can be regulated by both transcriptional and post-translational mechanisms [115]. It has been shown that TRAIL is induced by retinoids and HDACis in leukemia cells, and that induction of TRAIL is the underlying mechanism by which retinoids or HDACIs cause apoptosis [116,117]. In breast cancer cells, TRAIL is induced by several anti-cancer agents including TNFα, the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine, and the HDAC inhibitor MS275 [118–120]. In addition, TRAIL can be positively regulated by p53 [121]. Induction of TRAIL by the different agents sensitizes cancer cells to clinically used chemotherapeutic agents. Therefore, it becomes clear that identifying small molecule compounds that are capable of increasing TRAIL expression can be a strategy for development of novel cancer therapy. In this regard, screening the NIH small molecule compound library led to the identification of the TRAIL-inducible small molecule ONC201 (TIC10) [122]. ONC201 is a member of the imipridone small molecule family [123]. Mechanistically, ONC201 induces apoptosis by inactivating AKT and ERK-mediated Foxo3a phosphorylation, resulting in Foxo3a translocation into the nucleus, where Fox3a activates TRAIL transcription by directly binding to the TRAIL promoter [122,124]. ONC201 has also been shown to induce the unfolded protein response (UPR) and integrated stress response (ISR) pathways [125–127]. This leads to increased transcription factor ATF4 levels, which promotes apoptosis. Further elucidation of this mechanism showed that ONC201 induces TRAIL via an ISR pathway involved the transcription factor ATF4, the transactivator CHOP, and DR5, where ATF4 or CHOP knockdown diminished ONC201-induced DR5 expression and apoptosis in cancer cells [125]. A recent study indicated that ONC201 is a selective inhibitor for the dopamine D2-like receptors [128]. These studies collectively suggest that ONC201 can inhibit cancer cell growth through multiple mechanisms.
Through extensive pre-clinical studies, ONC201 was approved by the FDA for phase I clinical trials for the treatments of several cancers in 2014. In 2016, the phase I dose-escalation study was completed and the safety profile of ONC201 was established [129]. Based on the safety profile, ONC201 is now in phase II clinical trials for patients with different malignancies including glioblastoma, lymphoma, multiple myeloma, and endometrial cancer. Importantly, a recent study showed that ONC201 is very effective against glioblastoma with a H3.3K27M mutation as two patients with this mutation exhibited significant clinical response to ONC201 [130]. Based on these encouraging preclinical and phase I studies, 12 phase II clinical trials have been approved by the FDA for evaluating ONC201’s anti-cancer activity in patients with various tumors (Table 3).
Table 3.
Ongoing clinical trials of TRAIL-based therapies and ONC201
| Therapeutic agents | In combination with | Trial type and tumor | Reference |
|---|---|---|---|
| ABBV-621 | Solid tumor/Hematologic malignancies | NCT03082209 | |
| DS8273 | Solid tumor | NCT02076451 | |
| ONC201 | Glioma | NCT03134131 | |
| ONC201 | Glioblastoma | NCT02525692 | |
| ONC201 | Glioma | NCT03295396 | |
| ONC201 | NHL | NCT02420795 | |
| ONC201 | Leukemias | NCT02392572 | |
| ONC201 | Myeloma | NCT02863991 | |
| ONC201 | Solid tumor/Myeloma | NCT02609230 | |
| ONC201 | Solid tumor | NCT02324621 | |
| ONC201 | Solid tumor | NCT02250781 | |
| ONC201 | Neuroendocrine tumor | NCT03034200 | |
| ONC201 | Breast cancer/Endometrial carcinoma | NCT03394027 | |
| ONC201 | Endometrial cancer | NCT03099499 |
Abbreviations: CPT, Circularly permuted TRAIL; NR, not reported; MTD, maximum tolerated dose; ORR, objective response rate; DLT, dose limiting toxicity; TNBC, triple-negative breast cancer; NHL, non-Hodgkin’s lymphoma; NSCLC, non-small cell lung cancer; CR, complete response; PR, partial response; SD, stable disease.
7 Conclusions
Accumulating evidence indicates that TRAIL and TRAIL receptor agonists offer new approaches for targeted therapy, as exampled by ONC201. Acquired resistance has limited the effectiveness of TRAIL-based therapy. More work needs to be done to evaluate the mechanisms of TRAIL resistance, which will open up new therapeutic approaches that restore TRAIL sensitivity. Novel treatment has been proposed to restore TRAIL-induced apoptosis through TRAIL and DR5 up-regulation. Significant progress has been made and more efficient natural and synthetic agents will be exploited in combination with TRAIL. However, several challenges remain in the TRAIL field. For example, TRAIL can selectively induce apoptosis of transformed or tumor cells but the mechanisms of TRAIL insensitivity in normal cells are still not fully understood. Therefore, understanding the mechanism of TRAIL resistance is still a primary focus in the field. Furthermore, it is known that TRAIL can induce tumor metastasis and activate survival pathways [131–134], but the detailed function of the TRAIL pathway in tumor metastasis and resistance is not fully understood. Thus, it is conceivable that understanding these issues will help develop TRAIL-based therapy as a novel anti-cancer agent for the treatment of human cancer.
Acknowledgments
This work was supported by NIH grant R01 CA174949 to G.S.W., and Natural Science Foundation of China #81572608 to K.W., and Wuhan Science and Technology Bureau #2017060201010170 to K.W.
Footnotes
Conflict of interest: The authors declare that they have no competing interest.
References
- 1.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 2.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 3.Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25(1):104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu GS. TRAIL as a target in anti-cancer therapy. Cancer Letters. 2009;285(1):1–5. doi: 10.1016/j.canlet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104(2):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuntharapai A, Dodge K, Grimmer K, Schroeder K, Marsters SA, Koeppen H, et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J Immunol. 2001;166(8):4891–4898. doi: 10.4049/jimmunol.166.8.4891. [DOI] [PubMed] [Google Scholar]
- 8.Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, et al. Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res. 2008;14(23):7733–7740. doi: 10.1158/1078-0432.CCR-08-0670. doi:14/23/7733 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Luster TA, Carrell JA, McCormick K, Sun D, Humphreys R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol Cancer Ther. 2009;8(2):292–302. doi: 10.1158/1535-7163.MCT-08-0918. [DOI] [PubMed] [Google Scholar]
- 10.Marini P, Junginger D, Stickl S, Budach W, Niyazi M, Belka C. Combined treatment with lexatumumab and irradiation leads to strongly increased long term tumour control under normoxic and hypoxic conditions. Radiat Oncol. 2009;4:49. doi: 10.1186/1748-717X-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14(3-4):337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 12.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 13.Kemp TJ, Moore JM, Griffith TS. Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation. J Immunol. 2004;173(2):892–899. doi: 10.4049/jimmunol.173.2.892. [DOI] [PubMed] [Google Scholar]
- 14.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188(12):2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Secchiero P, Rimondi E, di Iasio MG, Agnoletto C, Melloni E, Volpi I, et al. C-Reactive protein downregulates TRAIL expression in human peripheral monocytes via an Egr-1-dependent pathway. Clin Cancer Res. 2013;19(8):1949–1959. doi: 10.1158/1078-0432.CCR-12-3027. [DOI] [PubMed] [Google Scholar]
- 16.Gandini M, Gras C, Azeredo EL, Pinto LM, Smith N, Despres P, et al. Dengue virus activates membrane TRAIL relocalization and IFN-alpha production by human plasmacytoid dendritic cells in vitro and in vivo. PLoS Negl Trop Dis. 2013;7(6):e2257. doi: 10.1371/journal.pntd.0002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22(53):8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 18.Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137(4):721–735. doi: 10.1016/j.cell.2009.03.015. doi:S0092-8674(09)00275-X [pii] [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Xu Z, Zhou JY, Zhuang Z, Wang E, Boerner J, et al. Regulation of the Src-PP2A interaction in tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2013;288(46):33263–33271. doi: 10.1074/jbc.M113.508093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Zhou JY, Xu Z, Kho DH, Zhuang Z, Raz A, et al. The role of Cullin3-mediated ubiquitination of the catalytic subunit of PP2A in TRAIL signaling. Cell Cycle. 2014;13(23):3750–3758. doi: 10.4161/15384101.2014.965068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang K, Zhang J, O’Neill KL, Gurumurthy CB, Quadros RM, Tu Y, et al. Cleavage by Caspase 8 and Mitochondrial Membrane Association Activate the BH3-only Protein Bid during TRAIL-induced Apoptosis. J Biol Chem. 2016;291(22):11843–11851. doi: 10.1074/jbc.M115.711051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werneburg NW, Bronk SF, Guicciardi ME, Thomas L, Dikeakos JD, Thomas G, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein-induced lysosomal translocation of proapoptotic effectors is mediated by phosphofurin acidic cluster sorting protein-2 (PACS-2) J Biol Chem. 2012;287(29):24427–24437. doi: 10.1074/jbc.M112.342238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacFarlane M, Robinson GL, Cain K. Glucose–a sweet way to die: metabolic switching modulates tumor cell death. Cell Cycle. 2012;11(21):3919–3925. doi: 10.4161/cc.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi H, Horinaka M, Yoshida T, Yano K, Goda AE, Yasuda S, et al. Targeting the glyoxalase pathway enhances TRAIL efficacy in cancer cells by downregulating the expression of antiapoptotic molecules. Mol Cancer Ther. 2012;11(10):2294–2300. doi: 10.1158/1535-7163.MCT-12-0031. [DOI] [PubMed] [Google Scholar]
- 25.Yao W, Oh YT, Deng J, Yue P, Deng L, Huang H, et al. Expression of Death Receptor 4 Is Positively Regulated by MEK/ERK/AP-1 Signaling and Suppressed upon MEK Inhibition. J Biol Chem. 2016;291(41):21694–21702. doi: 10.1074/jbc.M116.738302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279(44):45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 27.Iurlaro R, Munoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283(14):2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Wang Y, Li X, Chen Z, Li X, Wang H, et al. Molecular mechanism of ER stress-induced gene expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in macrophages. FEBS J. 2015;282(12):2361–2378. doi: 10.1111/febs.13284. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Su L, Liu X. PKCdelta regulates death receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis in human lung cancer cells. Mol Cancer Ther. 2012;11(10):2174–2182. doi: 10.1158/1535-7163.MCT-12-0602. [DOI] [PubMed] [Google Scholar]
- 30.Lee DH, Sung KS, Guo ZS, Kwon WT, Bartlett DL, Oh SC, et al. TRAIL-Induced Caspase Activation Is a Prerequisite for Activation of the Endoplasmic Reticulum Stress-Induced Signal Transduction Pathways. J Cell Biochem. 2016;117(5):1078–1091. doi: 10.1002/jcb.25289. [DOI] [PubMed] [Google Scholar]
- 31.Gupta SC, Francis SK, Nair MS, Mo YY, Aggarwal BB. Azadirone, a limonoid tetranortriterpene, induces death receptors and sensitizes human cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through a p53 protein-independent mechanism: evidence for the role of the ROS-ERK-CHOP-death receptor pathway. J Biol Chem. 2013;288(45):32343–32356. doi: 10.1074/jbc.M113.455188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quast SA, Berger A, Eberle J. ROS-dependent phosphorylation of Bax by wortmannin sensitizes melanoma cells for TRAIL-induced apoptosis. Cell Death Dis. 2013;4:e839. doi: 10.1038/cddis.2013.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunert M, Gottschalk K, Kapahnke J, Gundisch S, Kieser A, Jeremias I. The adaptor protein FADD and the initiator caspase-8 mediate activation of NF-kappaB by TRAIL. Cell Death Dis. 2012;3:e414. doi: 10.1038/cddis.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keuper M, Wernstedt Asterholm I, Scherer PE, Westhoff MA, Moller P, Debatin KM, et al. TRAIL (TNF-related apoptosis-inducing ligand) regulates adipocyte metabolism by caspase-mediated cleavage of PPARgamma. Cell Death Dis. 2013;4:e474. doi: 10.1038/cddis.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin YC, Richburg JH. Characterization of the role of tumor necrosis factor apoptosis inducing ligand (TRAIL) in spermatogenesis through the evaluation of trail gene-deficient mice. PLoS One. 2014;9(4):e93926. doi: 10.1371/journal.pone.0093926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen ML, Hsu PN, Liao HJ, Lee BH, Tsai HF. TRAF-6 dependent signaling pathway is essential for TNF-related apoptosis-inducing ligand (TRAIL) induces osteoclast differentiation. PLoS One. 2012;7(6):e38048. doi: 10.1371/journal.pone.0038048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hameed AG, Arnold ND, Chamberlain J, Pickworth JA, Paiva C, Dawson S, et al. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J Exp Med. 2012;209(11):1919–1935. doi: 10.1084/jem.20112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stagg HW, Bowen KA, Sawant DA, Rodriguez M, Tharakan B, Childs EW. Tumor necrosis factor-related apoptosis-inducing ligand promotes microvascular endothelial cell hyperpermeability through phosphatidylinositol 3-kinase pathway. Am J Surg. 2013;205(4):419–425. doi: 10.1016/j.amjsurg.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Sokulsky LA, Collison AM, Nightingale S, Fevre AL, Percival E, Starkey MR, et al. TRAIL deficiency and PP2A activation with salmeterol ameliorates egg allergen-driven eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2016;311(6):G998–G1008. doi: 10.1152/ajpgi.00151.2016. [DOI] [PubMed] [Google Scholar]
- 40.Collison A, Li J, Pereira de Siqueira A, Zhang J, Toop HD, Morris JC, et al. Tumor necrosis factor-related apoptosis-inducing ligand regulates hallmark features of airways remodeling in allergic airways disease. Am J Respir Cell Mol Biol. 2014;51(1):86–93. doi: 10.1165/rcmb.2013-0490OC. [DOI] [PubMed] [Google Scholar]
- 41.Arabpour M, Poelstra K, Helfrich W, Bremer E, Haisma HJ. Targeted elimination of activated hepatic stellate cells by an anti-epidermal growth factor-receptor single chain fragment variable antibody-tumor necrosis factor-related apoptosis-inducing ligand (scFv425-sTRAIL) J Gene Med. 2014;16(9-10):281–290. doi: 10.1002/jgm.2776. [DOI] [PubMed] [Google Scholar]
- 42.Peteranderl C, Morales-Nebreda L, Selvakumar B, Lecuona E, Vadasz I, Morty RE, et al. Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest. 2016;126(4):1566–1580. doi: 10.1172/JCI83931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith W, Tomasec P, Aicheler R, Loewendorf A, Nemcovicova I, Wang EC, et al. Human cytomegalovirus glycoprotein UL141 targets the TRAIL death receptors to thwart host innate antiviral defenses. Cell Host Microbe. 2013;13(3):324–335. doi: 10.1016/j.chom.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantarella G, Pignataro G, Di Benedetto G, Anzilotti S, Vinciguerra A, Cuomo O, et al. Ischemic tolerance modulates TRAIL expression and its receptors and generates a neuroprotected phenotype. Cell Death Dis. 2014;5:e1331. doi: 10.1038/cddis.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kao SY, Soares VY, Kristiansen AG, Stankovic KM. Activation of TRAIL-DR5 pathway promotes sensorineural degeneration in the inner ear. Aging Cell. 2016;15(2):301–308. doi: 10.1111/acel.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kojima Y, Nakayama M, Nishina T, Nakano H, Koyanagi M, Takeda K, et al. Importin beta1 protein-mediated nuclear localization of death receptor 5 (DR5) limits DR5/tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced cell death of human tumor cells. J Biol Chem. 2011;286(50):43383–43393. doi: 10.1074/jbc.M111.309377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun SY, Yue P, Zhou JY, Wang Y, Kim HC, Lotan R, et al. Overexpression of bcl2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. BBRC. 2001;280:788–797. doi: 10.1006/bbrc.2000.4218. [DOI] [PubMed] [Google Scholar]
- 48.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21(15):2283–2294. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 49.Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95-and TRAIL-receptor-mediated apoptosis. Oncogene. 2000;19(48):5477–5486. doi: 10.1038/sj.onc.1203936. [DOI] [PubMed] [Google Scholar]
- 50.Xu L, Zhang Y, Liu J, Qu J, Hu X, Zhang F, et al. TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in lipid rafts antagonises TRAIL-induced apoptosis in gastric cancer cells. Eur J Cancer. 2012;48(17):3288–3299. doi: 10.1016/j.ejca.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Galski H, Oved-Gelber T, Simanovsky M, Lazarovici P, Gottesman MM, Nagler A. P-glycoprotein-dependent resistance of cancer cells toward the extrinsic TRAIL apoptosis signaling pathway. Biochem Pharmacol. 2013;86(5):584–596. doi: 10.1016/j.bcp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souza PS, Madigan JP, Gillet JP, Kapoor K, Ambudkar SV, Maia RC, et al. Expression of the multidrug transporter P-glycoprotein is inversely related to that of apoptosis-associated endogenous TRAIL. Exp Cell Res. 2015;336(2):318–328. doi: 10.1016/j.yexcr.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemke J, von Karstedt S, Abd El Hay M, Conti A, Arce F, Montinaro A, et al. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ. 2014;21(3):491–502. doi: 10.1038/cdd.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahman M, Davis SR, Pumphrey JG, Bao J, Nau MM, Meltzer PS, et al. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2009;113(2):217–230. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.French R, Hayward O, Jones S, Yang W, Clarkson R. Cytoplasmic levels of cFLIP determine a broad susceptibility of breast cancer stem/progenitor-like cells to TRAIL. Mol Cancer. 2015;14:209. doi: 10.1186/s12943-015-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yerbes R, Lopez-Rivas A, Reginato MJ, Palacios C. Control of FLIP(L) expression and TRAIL resistance by the extracellular signal-regulated kinase1/2 pathway in breast epithelial cells. Cell Death Differ. 2012;19(12):1908–1916. doi: 10.1038/cdd.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C, Egloff AM, Sen M, Grandis JR, Johnson DE. Caspase-8 mutations in head and neck cancer confer resistance to death receptor-mediated apoptosis and enhance migration, invasion, and tumor growth. Mol Oncol. 2014;8(7):1220–1230. doi: 10.1016/j.molonc.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong S, Kim HY, Kim J, Ha HT, Kim YM, Bae E, et al. Smad7 protein induces interferon regulatory factor 1-dependent transcriptional activation of caspase 8 to restore tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. J Biol Chem. 2013;288(5):3560–3570. doi: 10.1074/jbc.M112.400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M. Constitutive activation of nuclear factor-kappaB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene. 2001;20(29):3888–3896. doi: 10.1038/sj.onc.1204525. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Blackwell K, Workman LM, Chen S, Pope MR, Janz S, et al. RIP1 Cleavage in the Kinase Domain Regulates TRAIL-Induced NF-kappaB Activation and Lymphoma Survival. Mol Cell Biol. 2015;35(19):3324–3338. doi: 10.1128/MCB.00692-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi K, Takeda K, Saiki I, Irimura T, Hayakawa Y. Functional roles of tumor necrosis factor-related apoptosis-inducing ligand-DR5 interaction in B16F10 cells by activating the nuclear factor-kappaB pathway to induce metastatic potential. Cancer Sci. 2013;104(5):558–562. doi: 10.1111/cas.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somasekharan SP, Koc M, Morizot A, Micheau O, Sorensen PH, Gaide O, et al. TRAIL promotes membrane blebbing, detachment and migration of cells displaying a dysfunctional intrinsic pathway of apoptosis. Apoptosis. 2013;18(3):324–336. doi: 10.1007/s10495-012-0782-6. [DOI] [PubMed] [Google Scholar]
- 63.Piggott L, Omidvar N, Marti Perez S, French R, Eberl M, Clarkson RW. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Res. 2011;13(5):R88. doi: 10.1186/bcr2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ullenhag GJ, Al-Attar A, Mukherjee A, Green AR, Ellis IO, Durrant LG. The TRAIL system is over-expressed in breast cancer and FLIP a marker of good prognosis. J Cancer Res Clin Oncol. 2015;141(3):505–514. doi: 10.1007/s00432-014-1822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M, Liu L, Xie M, Sun X, Yu Y, Kang R, et al. Poly-ADP-ribosylation of HMGB1 regulates TNFSF10/TRAIL resistance through autophagy. Autophagy. 2015;11(2):214–224. doi: 10.4161/15548627.2014.994400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narayan G, Xie D, Ishdorj G, Scotto L, Mansukhani M, Pothuri B, et al. Epigenetic inactivation of TRAIL decoy receptors at 8p12-21.3 commonly deleted region confers sensitivity to Apo2L/trail-Cisplatin combination therapy in cervical cancer. Genes Chromosomes Cancer. 2016;55(2):177–189. doi: 10.1002/gcc.22325. [DOI] [PubMed] [Google Scholar]
- 67.Romano G, Acunzo M, Garofalo M, Di Leva G, Cascione L, Zanca C, et al. MiR-494 is regulated by ERK1/2 and modulates TRAIL-induced apoptosis in non-small-cell lung cancer through BIM down-regulation. Proc Natl Acad Sci U S A. 2012;109(41):16570–16575. doi: 10.1073/pnas.1207917109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iaboni M, Russo V, Fontanella R, Roscigno G, Fiore D, Donnarumma E, et al. Aptamer-miRNA-212 Conjugate Sensitizes NSCLC Cells to TRAIL. Mol Ther Nucleic Acids. 2016;5:e289. doi: 10.1038/mtna.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26(21):3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 70.Soria J, Smit EF, Khayat D, Besse B, Burton J, Yang X, et al. Phase Ib study of recombinant human (rh) Apo2L/TRAIL in combination with paclitaxel, carboplatin, and bevacizumab (PCB) in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2008;26(15S (May 20 Supplement)):3539. [Google Scholar]
- 71.Subbiah V, Brown RE, Buryanek J, Trent J, Ashkenazi A, Herbst R, et al. Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol Cancer Ther. 2012;11(11):2541–2546. doi: 10.1158/1535-7163.MCT-12-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17(6):352–366. doi: 10.1038/nrc.2017.28. [DOI] [PubMed] [Google Scholar]
- 73.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7(8):954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Zhang X, Barrisford GW, Olumi AF. Lexatumumab (TRAIL-receptor 2 mAb) induces expression of DR5 and promotes apoptosis in primary and metastatic renal cell carcinoma in a mouse orthotopic model. Cancer Lett. 2007;251(1):146–157. doi: 10.1016/j.canlet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 75.Malin D, Chen F, Schiller C, Koblinski J, Cryns VL. Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer. [Research Support, Non-U.S. Gov’t] Clin Cancer Res. 2011;17(15):5005–5015. doi: 10.1158/1078-0432.CCR-11-0099. [DOI] [PubMed] [Google Scholar]
- 76.Forero-Torres A, Varley KE, Abramson VG, Li Y, Vaklavas C, Lin NU, et al. TBCRC 019: A Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer. Clin Cancer Res. 2015;21(12):2722–2729. doi: 10.1158/1078-0432.CCR-14-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gieffers C, Kluge M, Merz C, Sykora J, Thiemann M, Schaal R, et al. APG350 induces superior clustering of TRAIL receptors and shows therapeutic antitumor efficacy independent of cross-linking via Fcgamma receptors. Mol Cancer Ther. 2013;12(12):2735–2747. doi: 10.1158/1535-7163.MCT-13-0323. [DOI] [PubMed] [Google Scholar]
- 78.Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61(8):3330–3338. [PubMed] [Google Scholar]
- 79.Kim SY, Lee DH, Song X, Bartlett DL, Kwon YT, Lee YJ. Role of Bcl-xL/Beclin-1 in synergistic apoptotic effects of secretory TRAIL-armed adenovirus in combination with mitomycin C and hyperthermia on colon cancer cells. Apoptosis. 2014;19(11):1603–1615. doi: 10.1007/s10495-014-1028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spitzer D, McDunn JE, Plambeck-Suess S, Goedegebuure PS, Hotchkiss RS, Hawkins WG. A genetically encoded multifunctional TRAIL trimer facilitates cell-specific targeting and tumor cell killing. Mol Cancer Ther. 2010;9(7):2142–2151. doi: 10.1158/1535-7163.MCT-10-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garg G, Gibbs J, Belt B, Powell MA, Mutch DG, Goedegebuure P, et al. Novel treatment option for MUC16-positive malignancies with the targeted TRAIL-based fusion protein Meso-TR3. BMC Cancer. 2014;14:35. doi: 10.1186/1471-2407-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perlstein B, Finniss SA, Miller C, Okhrimenko H, Kazimirsky G, Cazacu S, et al. TRAIL conjugated to nanoparticles exhibits increased anti-tumor activities in glioma cells and glioma stem cells in vitro and in vivo. Neuro Oncol. 2013;15(1):29–40. doi: 10.1093/neuonc/nos248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piechocki MP, Wu GS, Jones RF, Jacob JB, Gibson H, Ethier SP, et al. Induction of proapoptotic antibodies to triple-negative breast cancer by vaccination with TRAIL death receptor DR5 DNA. Int J Cancer. 2012 doi: 10.1002/ijc.27534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding B, Wu X, Fan W, Wu Z, Gao J, Zhang W, et al. Anti-DR5 monoclonal antibody-mediated DTIC-loaded nanoparticles combining chemotherapy and immunotherapy for malignant melanoma: target formulation development and in vitro anticancer activity. Int J Nanomedicine. 2011;6:1991–2005. doi: 10.2147/IJN.S24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu GS, Burns TF, McDonald ER, Jiang W, Meng R, Krantz ID, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 86.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60(4):847–853. [PubMed] [Google Scholar]
- 87.Wu XX, Kakehi Y, Mizutani Y, Nishiyama H, Kamoto T, Megumi Y, et al. Enhancement of TRAIL/Apo2L-mediated apoptosis by adriamycin through inducing DR4 and DR5 in renal cell carcinoma cells. Int J Cancer. 2003;104(4):409–417. doi: 10.1002/ijc.10948. [DOI] [PubMed] [Google Scholar]
- 88.Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL. Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol. 2000;20(1):205–212. doi: 10.1128/mcb.20.1.205-212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients’ colon tumors grown in SCID mice. Cancer Res. 2002;62(20):5800–5806. [PubMed] [Google Scholar]
- 90.Wang S, El-Deiry WS. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004;64(18):6666–6672. doi: 10.1158/0008-5472.CAN-04-1734. [DOI] [PubMed] [Google Scholar]
- 91.Cheng H, Hong B, Zhou L, Allen JE, Tai G, Humphreys R, et al. Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: evidence for the role of c-Jun N-terminal kinase activation. Cell Cycle. 2012;11(17):3312–3323. doi: 10.4161/cc.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Senbabaoglu F, Cingoz A, Kaya E, Kazancioglu S, Lack NA, Acilan C, et al. Identification of Mitoxantrone as a TRAIL-sensitizing agent for Glioblastoma Multiforme. Cancer Biol Ther. 2016;17(5):546–557. doi: 10.1080/15384047.2016.1167292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding L, Yuan C, Wei F, Wang G, Zhang J, Bellail AC, et al. Cisplatin restores TRAIL apoptotic pathway in glioblastoma-derived stem cells through up-regulation of DR5 and down-regulation of c-FLIP. Cancer Invest. 2011;29(8):511–520. doi: 10.3109/07357907.2011.605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jelinkova I, Safarikova B, Vondalova Blanarova O, Skender B, Hofmanova J, Sova P, et al. Platinum(IV) complex LA-12 exerts higher ability than cisplatin to enhance TRAIL-induced cancer cell apoptosis via stimulation of mitochondrial pathway. Biochem Pharmacol. 2014;92(3):415–424. doi: 10.1016/j.bcp.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 95.Pasello G, Urso L, Silic-Benussi M, Schiavon M, Cavallari I, Marulli G, et al. Synergistic antitumor activity of recombinant human Apo2L/tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in combination with carboplatin and pemetrexed in malignant pleural mesothelioma. J Thorac Oncol. 2014;9(7):1008–1017. doi: 10.1097/JTO.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 96.Sung ES, Kim A, Park JS, Chung J, Kwon MH, Kim YS. Histone deacetylase inhibitors synergistically potentiate death receptor 4-mediated apoptotic cell death of human T-cell acute lymphoblastic leukemia cells. Apoptosis. 2010;15(10):1256–1269. doi: 10.1007/s10495-010-0521-9. [DOI] [PubMed] [Google Scholar]
- 97.Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene. 2004;23(37):6261–6271. doi: 10.1038/sj.onc.1207830. [DOI] [PubMed] [Google Scholar]
- 98.Hori T, Kondo T, Kanamori M, Tabuchi Y, Ogawa R, Zhao QL, et al. Nutlin-3 enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through up-regulation of death receptor 5 (DR5) in human sarcoma HOS cells and human colon cancer HCT116 cells. Cancer Lett. 2010;287(1):98–108. doi: 10.1016/j.canlet.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 99.Lim SC, Parajuli KR, Han SI. The alkyllysophospholipid edelfosine enhances TRAIL-mediated apoptosis in gastric cancer cells through death receptor 5 and the mitochondrial pathway. Tumour Biol. 2016;37(5):6205–6216. doi: 10.1007/s13277-015-4485-9. [DOI] [PubMed] [Google Scholar]
- 100.Fassl A, Tagscherer KE, Richter J, De-Castro Arce J, Savini C, Rosl F, et al. Inhibition of Notch1 signaling overcomes resistance to the death ligand Trail by specificity protein 1-dependent upregulation of death receptor 5. Cell Death Dis. 2015;6:e1921. doi: 10.1038/cddis.2015.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhuang H, Jiang W, Zhang X, Qiu F, Gan Z, Cheng W, et al. Suppression of HSP70 expression sensitizes NSCLC cell lines to TRAIL-induced apoptosis by upregulating DR4 and DR5 and downregulating c-FLIP-L expressions. J Mol Med (Berl) 2013;91(2):219–235. doi: 10.1007/s00109-012-0947-3. [DOI] [PubMed] [Google Scholar]
- 102.Lee DH, Sung KS, Bartlett DL, Kwon YT, Lee YJ. HSP90 inhibitor NVP-AUY922 enhances TRAIL-induced apoptosis by suppressing the JAK2-STAT3-Mcl-1 signal transduction pathway in colorectal cancer cells. Cell Signal. 2015;27(2):293–305. doi: 10.1016/j.cellsig.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sung B, Prasad S, Ravindran J, Yadav VR, Aggarwal BB. Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic Biol Med. 2012;53(10):1977–1987. doi: 10.1016/j.freeradbiomed.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim HB, Kim MJ, Lee SH, Lee JW, Bae JH, Kim DW, et al. Amurensin G, a novel SIRT1 inhibitor, sensitizes TRAIL-resistant human leukemic K562 cells to TRAIL-induced apoptosis. Biochem Pharmacol. 2012;84(3):402–410. doi: 10.1016/j.bcp.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 105.He L, Jang JH, Choi HG, Lee SM, Nan MH, Jeong SJ, et al. Oligomycin A enhances apoptotic effect of TRAIL through CHOP-mediated death receptor 5 expression. Mol Carcinog. 2013;52(2):85–93. doi: 10.1002/mc.21831. [DOI] [PubMed] [Google Scholar]
- 106.Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, et al. ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biol Ther. 2015;16(8):1194–1204. doi: 10.1080/15384047.2015.1056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L, Meng Y, Sun Q, Zhang Z, Guo X, Sheng X, et al. Ginsenoside compound K sensitizes human colon cancer cells to TRAIL-induced apoptosis via autophagy-dependent and -independent DR5 upregulation. Cell Death Dis. 2016;7(8):e2334. doi: 10.1038/cddis.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Y, Tong Y, Yang X, Li F, Zheng L, Liu W, et al. Novel histone deacetylase inhibitors derived from Magnolia officinalis significantly enhance TRAIL-induced apoptosis in non-small cell lung cancer. Pharmacol Res. 2016;111:113–125. doi: 10.1016/j.phrs.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 109.Yang J, Qian S, Cai X, Lu W, Hu C, Sun X, et al. Chikusetsusaponin IVa Butyl Ester (CS-IVa-Be), a Novel IL6R Antagonist, Inhibits IL6/STAT3 Signaling Pathway and Induces Cancer Cell Apoptosis. Mol Cancer Ther. 2016;15(6):1190–1200. doi: 10.1158/1535-7163.MCT-15-0551. [DOI] [PubMed] [Google Scholar]
- 110.Kim JH, Park B, Gupta SC, Kannappan R, Sung B, Aggarwal BB. Zyflamend sensitizes tumor cells to TRAIL-induced apoptosis through up-regulation of death receptors and down-regulation of survival proteins: role of ROS-dependent CCAAT/enhancer-binding protein-homologous protein pathway. Antioxid Redox Signal. 2012;16(5):413–427. doi: 10.1089/ars.2011.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trivedi R, Maurya R, Mishra DP. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014;5:e1465. doi: 10.1038/cddis.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen W, Wang X, Zhuang J, Zhang L, Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis. 2007;28(10):2114–2121. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- 113.Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108(2):630–637. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding J, Polier G, Kohler R, Giaisi M, Krammer PH, Li-Weber M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J Biol Chem. 2012;287(1):641–649. doi: 10.1074/jbc.M111.286526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allen JE, El-Deiry WS. Regulation of the human TRAIL gene. Cancer Biol Ther. 2012;13(12):1143–1151. doi: 10.4161/cbt.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7(6):680–686. doi: 10.1038/89050. [DOI] [PubMed] [Google Scholar]
- 117.Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 118.Xu J, Zhou JY, Wu GS. Tumor necrosis factor-telated apoptosis-inducing ligand is required for tumor necrosis factor {alpha}-mediated sensitization of human breast cancer cells to chemotherapy. Cancer Res. 2006;66(20):10092–10099. doi: 10.1158/0008-5472.CAN-06-1633. [DOI] [PubMed] [Google Scholar]
- 119.Xu J, Zhou JY, Tainsky MA, Wu GS. Evidence that tumor necrosis factor-related apoptosis-inducing ligand induction by 5-aza-2′-deoxycytidine sensitizes human breast cancer cells to adriamycin. Cancer Res. 2007;67(3):1203–1211. doi: 10.1158/0008-5472.CAN-06-2310. [DOI] [PubMed] [Google Scholar]
- 120.Xu J, Zhou JY, Wei WZ, Philipsen S, Wu GS. Sp1-mediated TRAIL induction in chemosensitization. Cancer Res. 2008;68(16):6718–6726. doi: 10.1158/0008-5472.CAN-08-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuribayashi K, Krigsfeld G, Wang W, Xu J, Mayes PA, Dicker DT, et al. TNFSF10 (TRAIL), a p53 target gene that mediates p53-dependent cell death. Cancer Biol Ther. 2008;7(12) doi: 10.4161/cbt.7.12.7460. [DOI] [PubMed] [Google Scholar]
- 122.Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5(171):171ra117. doi: 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allen JE, Kline CL, Prabhu VV, Wagner J, Ishizawa J, Madhukar N, et al. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget. 2016;7(45):74380–74392. doi: 10.18632/oncotarget.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prabhu VV, Allen JE, Dicker DT, El-Deiry WS. Small-Molecule ONC201/TIC10 Targets Chemotherapy-Resistant Colorectal Cancer Stem-like Cells in an Akt/Foxo3a/TRAIL-Dependent Manner. Cancer Res. 2015;75(7):1423–1432. doi: 10.1158/0008-5472.CAN-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kline CL, Van den Heuvel AP, Allen JE, Prabhu VV, Dicker DT, El-Deiry WS. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2alpha kinases. Sci Signal. 2016;9(415):ra18. doi: 10.1126/scisignal.aac4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ishizawa J, Kojima K, Chachad D, Ruvolo P, Ruvolo V, Jacamo RO, et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal. 2016;9(415):ra17. doi: 10.1126/scisignal.aac4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan X, Kho D, Xu J, Gajan A, Wu K, Wu GS. ONC201 activates ER stress to inhibit the growth of triple-negative breast cancer cells. Oncotarget. 2017;8(13):21626–21638. doi: 10.18632/oncotarget.15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kline CLB, Ralff MD, Lulla AR, Wagner JM, Abbosh PH, Dicker DT, et al. Role of Dopamine Receptors in the Anticancer Activity of ONC201. Neoplasia. 2017;20(1):80–91. doi: 10.1016/j.neo.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stein MN, Bertino JR, Kaufman HL, Mayer T, Moss R, Silk A, et al. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin Cancer Res. 2017;23(15):4163–4169. doi: 10.1158/1078-0432.CCR-16-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8(45):79298–79304. doi: 10.18632/oncotarget.17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trauzold A, Wermann H, Arlt A, Schutze S, Schafer H, Oestern S, et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001;20(31):4258–4269. doi: 10.1038/sj.onc.1204559. [DOI] [PubMed] [Google Scholar]
- 132.Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, et al. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25(56):7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- 133.Xu J, Zhou JY, Wei WZ, Wu GS. Activation of the Akt survival pathway contributes to TRAIL resistance in cancer cells. PLoS One. 2010;5(4):e10226. doi: 10.1371/journal.pone.0010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hartwig T, Montinaro A, von Karstedt S, Sevko A, Surinova S, Chakravarthy A, et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Mol Cell. 2017;65(4):730–742 e735. doi: 10.1016/j.molcel.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leng Y, Hou J, Jin J, Zhang M, Ke X, Jiang B, et al. Circularly permuted TRAIL plus thalidomide and dexamethasone versus thalidomide and dexamethasone for relapsed/refractory multiple myeloma: a phase 2 study. Cancer Chemother Pharmacol. 2017;79(6):1141–1149. doi: 10.1007/s00280-017-3310-0. [DOI] [PubMed] [Google Scholar]
- 136.Geng C, Hou J, Zhao Y, Ke X, Wang Z, Qiu L, et al. A multicenter, open-label phase II study of recombinant CPT (Circularly Permuted TRAIL) plus thalidomide in patients with relapsed and refractory multiple myeloma. Am J Hematol. 2014;89(11):1037–1042. doi: 10.1002/ajh.23822. [DOI] [PubMed] [Google Scholar]
- 137.Cheah CY, Belada D, Fanale MA, Janikova A, Czucman MS, Flinn IW, et al. Dulanermin with rituximab in patients with relapsed indolent B-cell lymphoma: an open-label phase 1b/2 randomised study. Lancet Haematol. 2015;2(4):e166–174. doi: 10.1016/S2352-3026(15)00026-5. [DOI] [PubMed] [Google Scholar]
- 138.Wainberg ZA, Messersmith WA, Peddi PF, Kapp AV, Ashkenazi A, Royer-Joo S, et al. A phase 1B study of dulanermin in combination with modified FOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2013;12(4):248–254. doi: 10.1016/j.clcc.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 139.Pan Y, Xu R, Peach M, Huang CP, Branstetter D, Novotny W, et al. Evaluation of pharmacodynamic biomarkers in a Phase 1a trial of dulanermin (rhApo2L/TRAIL) in patients with advanced tumours. Br J Cancer. 2011;105(12):1830–1838. doi: 10.1038/bjc.2011.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(33):4442–4451. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- 141.Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol. 2010;28(9):1527–1533. doi: 10.1200/JCO.2009.25.4847. [DOI] [PubMed] [Google Scholar]
- 142.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28(17):2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 143.Ciprotti M, Tebbutt NC, Lee FT, Lee ST, Gan HK, McKee DC, et al. Phase I Imaging and Pharmacodynamic Trial of CS-1008 in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2015;33(24):2609–2616. doi: 10.1200/JCO.2014.60.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng AL, Kang YK, He AR, Lim HY, Ryoo BY, Hung CH, et al. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: A phase 2 randomized study. J Hepatol. 2015;63(4):896–904. doi: 10.1016/j.jhep.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Forero-Torres A, Infante JR, Waterhouse D, Wong L, Vickers S, Arrowsmith E, et al. Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013;2(6):925–932. doi: 10.1002/cam4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reck M, Krzakowski M, Chmielowska E, Sebastian M, Hadler D, Fox T, et al. A randomized, double-blind, placebo-controlled phase 2 study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naive metastatic/unresectable non-small cell lung cancer. Lung Cancer. 2013;82(3):441–448. doi: 10.1016/j.lungcan.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 147.Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer Biother Radiopharm. 2010;25(1):13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ciuleanu T, Bazin I, Lungulescu D, Miron L, Bondarenko I, Deptala A, et al. A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma. Ann Oncol. 2016;27(4):680–687. doi: 10.1093/annonc/mdw004. [DOI] [PubMed] [Google Scholar]
- 149.von Pawel J, Harvey JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, et al. Phase II trial of mapatumumab, a fully human agonist monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2014;15(3):188–196 e182. doi: 10.1016/j.cllc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 150.Younes A, Vose JM, Zelenetz AD, Smith MR, Burris HA, Ansell SM, et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Br J Cancer. 2010;103(12):1783–1787. doi: 10.1038/sj.bjc.6605987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C, et al. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer. 2010;102(3):506–512. doi: 10.1038/sj.bjc.6605507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mom CH, Verweij J, Oldenhuis CN, Gietema JA, Fox NL, Miceli R, et al. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study. Clin Cancer Res. 2009;15(17):5584–5590. doi: 10.1158/1078-0432.CCR-09-0996. [DOI] [PubMed] [Google Scholar]
- 153.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14(11):3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 154.Leong S, Cohen RB, Gustafson DL, Langer CJ, Camidge DR, Padavic K, et al. Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study. J Clin Oncol. 2009;27(26):4413–4421. doi: 10.1200/JCO.2008.21.7422. [DOI] [PubMed] [Google Scholar]
- 155.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008;61(1):82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 156.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25(11):1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 157.Paz-Ares L, Balint B, de Boer RH, van Meerbeeck JP, Wierzbicki R, De Souza P, et al. A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol. 2013;8(3):329–337. doi: 10.1097/JTO.0b013e31827ce554. [DOI] [PubMed] [Google Scholar]
- 158.Cohn AL, Tabernero J, Maurel J, Nowara E, Sastre J, Chuah BY, et al. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol. 2013;24(7):1777–1785. doi: 10.1093/annonc/mdt057. [DOI] [PubMed] [Google Scholar]
- 159.Fuchs CS, Fakih M, Schwartzberg L, Cohn AL, Yee L, Dreisbach L, et al. TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: A randomized phase 1b/2 trial. Cancer. 2013;119(24):4290–4298. doi: 10.1002/cncr.28353. [DOI] [PubMed] [Google Scholar]
- 160.Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Jr, Rocha-Lima CM, et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23(11):2834–2842. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 161.Demetri GD, Le Cesne A, Chawla SP, Brodowicz T, Maki RG, Bach BA, et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study. Eur J Cancer. 2012;48(4):547–563. doi: 10.1016/j.ejca.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 162.Doi T, Murakami H, Ohtsu A, Fuse N, Yoshino T, Yamamoto N, et al. Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68(3):733–741. doi: 10.1007/s00280-010-1544-1. [DOI] [PubMed] [Google Scholar]
- 163.Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu CP, Goyal L, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010;16(23):5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 164.Papadopoulos KP, Isaacs R, Bilic S, Kentsch K, Huet HA, Hofmann M, et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody(R) targeting the DR5 receptor. Cancer Chemother Pharmacol. 2015;75(5):887–895. doi: 10.1007/s00280-015-2712-0. [DOI] [PubMed] [Google Scholar]


