Abstract
Gene therapy has become a promising approach for neurodegenerative disease treatment, however there is an urgent need to develop an efficient gene carrier to transport gene across the blood brain barrier (BBB). In this study, we strategically designed dual functionalized liposomes for efficient neuronal transfection by combining transferrin (Tf) receptor targeting and enhanced cell penetration utilizing penetratin (Pen). A triple cell co-culture model of BBB confirmed the ability of the liposomes to cross the barrier layer and transfect primary neuronal cells. In vivo quantification of PenTf-liposomes demonstrated expressive accumulation in the brain (12%), without any detectable cellular damage or morphological change. The efficacy of these nanoparticles containing plasmid β-galactosidase in modulating transfection was assessed by β-galactosidase expression in vivo. As a consequence of accumulation in the brain, PenTf-liposomes significantly induced gene expression in mice. Immunofluorescence studies of brain sections of mice after tail vein injection of liposomes encapsulating pDNA encoding GFP (pGFP) illustrate the superior ability of dual- functionalized liposomes to accumulate in the brain and transfect neurons. Taken together, the multifunctional liposomes provide an excellent gene delivery platform for neurodegenerative diseases.
Keywords: dual-functional liposomes, blood brain barrier, gene delivery, transferrin, penetratin
Graphical abstract

1. Introduction
With the increase in life expectancy, there has been an upsurge in neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease [1] These diseases currently affect more than 5.5 million. Americans resulting an estimated health care cost above $200 billion [2]. The blood brain barrier (BBB) creates major hurdle in the effective treatment of brain disorders by blocking access of most therapeutic molecules to central nervous system (CNS) [3]. In parallel, the current available treatments for these diseases only provide symptomatic relief and are unable to halt the progression of the disease and restore normal neurological function [4].
Gene therapy has emerged as an innovative treatment for different gene related disorders, especially for CNS diseases, offering long-term effect in a single administration. However, the progression of gene therapy largely depends on the development of safe and effective vectors to improve membrane permeability and half-life of therapeutic genes [5,6]. To this end, the vector should overcome extracellular and intracellular barriers, ensure that DNA is delivered to the nucleus, where it can be transcribed and translated into a therapeutic protein [7]. Several researchers have focused on the development of effective non-invasive delivery systems with targeting properties that are able to reach brain parenchyma at therapeutic doses [8–12]. Non-viral vectors, such as liposomes, have attracted much attention due to their favorable characteristics over viral vectors such as safety, ease of preparation, low immunogenicity, low cost and ability to deliver a wide range of plasmid sizes [7,13]. Furthermore, liposomes have efficiently protected plasmid DNA (pDNA) from DNase degradation [14,15].
Some transport mechanisms are present on BBB that enable nanoparticle transport into the brain, amongst which receptor-mediated transcytosis is most common and used in many platforms for brain delivery [16]. The high endocytotic potential of Transferrin receptor (TfR) makes it an interesting route for targeted delivery to the brain [17,18]. TfR are expressed in different types of tissues in the body [19] and the brain possesses high densities of TfR localized in the brain endothelium and neurons [20,21]. Liposome surface modification with 80 kDa Tf protein have been used as targeting ligand for BBB, leading to their sequential uptake and presentation of therapeutic molecule to the neurons [22]. Receptor-mediated transcytosis is normally a saturable process and dual targeting has become a strategy to overcome receptor saturation and provide efficient carrier delivery [23,24]. For this purpose, protein-transduction domains, also known as cell-penetrating peptides (CPP) that are small sequences of peptides, have demonstrated ability to transport cargo into cells in a non-invasive manner. These peptides have been widely used in the delivery of a variety of molecules across the cell membrane [25,26]. Cationic CPP, such as penetratin (Pen), have demonstrated other biological activity besides efficient cellular uptake, including facilitating intracellular delivery and endosomal escape [26,27]. Different studies have reported successful delivery of therapeutic molecules into the brain using receptor-targeted liposomes conjugated to CPP [28,29]. This dual targeting design of liposomes is intended to achieve a high degree of internalization and accumulation in the target site for therapeutic function.
However, there are limited investigation on multi-functional gene vehicles for CNS diseases treatment [30]. In the present study, liposomes were designed to achieve high neuronal transfection through dual functionalization. Tf was used for BBB targeting and transcytosis, while Pen aided in overcoming receptor saturation and enhancing liposome internalization. Hydrophilic plasmid for β-galactosidase (pβgal) was encapsulated in the liposomes as a gene model. The pDNA was complexed with chitosan in an attempt to utilize chitosan’s superior ability to protect DNA from enzymatic degradation and enhance transfection [31,32]. An in vitro triple cell co-culture BBB model was developed to study the transport of dual functionalized Pen-Tf liposomes across BBB and transfection of loaded genes in primary neuronal cells. Thereafter, the transfection efficiency of PenTf-liposomes containing pDNA was evaluated in vivo. These designed liposomes represent a promising delivery system to overcome BBB, reach brain parenchyma and target neuronal cells, enabling gene therapy as a viable treatment option for CNS diseases.
2. Material and Methods
2.1. Material
Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), Dioleoyl-3-trimethylammonium- propane chloride (DOTAP), l,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy (PEG)2000](DSPE-PEG-COOH) and Lissamine Rhodamine B were obtained from Avanti Polar Lipids (Birmingham, AL, USA). Cholesterol, 4-(2-hydroxyethyl)-l- piperazineethanesulfonic acid (HEPES), Ethylenediaminetetraacetic acid (EDTA), Hoechst 33342 and Triton™ X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Chitosan (MW 30 kDa) was purchased from Glentham Life Sciences (Corsham, UK). Plasmid DNA encoding beta-galactosidase (gWiz-βGal) and plasmid DNA encoding Green Fluorescent Protein (gWiz-GFP) were purchased from Aldevron LLC (Fargo, ND, USA). Dulbecco’s Modified Eagle Medium (DMEM), and phosphate buffered saline (PBS) were purchased from Coming Incorporated (Corning, NY, USA). Fetal bovine serum (FBS) was purchased from Omega Scientific (Tarzana, CA, USA). Beta-galactosidase enzyme assay kit with reporter lysis buffer was supplied by Promega (Madison, WI, USA). l,l’-Dioctadecyl- 3,3,3’,3’-tetramethylindotricarbocyanine iodide (DiR) was obtained from Marker Gene Technologies (Eugene, OR, USA). Rabbit anti-GFP antibody and rabbit Alexa Fluor 488 were purchased from Invitrogen (Carlsbad, CA, USA). Mouse anti-NeuN antibody was obtained from EMD Millipore Co. (Burlington, MA, USA)
2.2. Preparation and characterization of liposomes
Liposomes were prepared using thin lipid film hydration method while dual-functionalized liposomes were prepared by incorporating Tf-micelles into Pen-liposomes by post-insertion technique as previously reported [29]. Briefly, Pen and DSPE-PEG-COO- (1:5 molar ratio) were dissolved in dimethylformamide and the pH was adjusted to 8 by triethylamine. The reaction was kept under stirring at room temperature. After 5 days of reaction, the mixture was dialyzed (MWCO 3,500 Da) against deionized water and lyophilized. Tf was conjugated to DSPE-PEG-COO- (125μg Tf/μmol DSPE-PEG-COO-) and stirred for 24 h at room temperature. Uncoupled Tf was removed by dialysis (MWCO 3,500). Coupling efficiency for both reactions was determined using micro BCA assay. DOPE/DOTAP/Cholesterol/Pen- DSPE-PEG (45:45:2:4 mol %) were combined in chloroform:methanol (2:1, v/v) and dried to form a thin lipid film. For preparation of rhodamine-labeled liposomes or DiR-labeled liposomes, 0.5 mol % of the dyes were added to the lipid mixture. The film was hydrated using HEPES buffer (pH 7.4) to form Pen-liposomes. Tf-micelles were stirred overnight with Pen-liposomes to form PenTf-liposomes. The free Tf-micelles were separated from PenTf- liposomes by passing through Sephadex G-100 column and the percentage of Tf incorporation in PenTf-liposome surface was quantitated using micro BCA assay. The liposomes were characterized for hydrodynamic size and zeta potential by dynamic light scattering (DLS) using Zetasizer Nano ZS 90 (Malvern Instruments, Malvern, UK) at 25°C.
2.3. Blood compatibility study
Blood compatibility of liposomal formulations was evaluated in vitro by analyzing the hemolytic activity of these nanoparticles in blood freshly collected from Sprague-Dawley rats. Blood was centrifuged (1,500 rpm for 10 min) and washed three times with PBS 10 mM CaCl2. Erythrocytes solution 2% (v/v) was incubated with negative control (PBS), positive control (Triton X-100 1% v/v) and liposomal formulations (31.25–1000 μΜ) for 1 h at 37 °C, 5% CO2. Thereafter, cell suspension was centrifuged (1,500 rpm for 10 min), supernatant was removed, and the absorbance of released hemoglobin was measured (Spectramax M5 microplate spectrophotometer, Molecular Devices, Sunnyvale, CA) at 540 nm. Percent hemolysis was calculated by considering the absorbance in presence of Triton X-100 as 100%.
2.4. Cell lines and cell culture
Primary rat astrocytes and primary rat neuronal cells were isolated from 1-day old rats [33,34]. After dissecting the brains and removing the meninges and blood vessels, the brain tissue was minced into small pieces and incubated with DMEM containing 0.25% trypsin and DNase I (8 μg/mL) at 37 °C. The cells were isolated and cultured in DMEM with 10% v/v fetal bovine serum (FBS) and 1% v/v penicillin streptomycin fungizone (Psf). The same procedure was followed to obtain primary neuronal cells, except for the use of 10% horse serum in the procedure. Additionally, on day 3, the culture was treated with 10 μM cytosine arabinoside. After 2 days, the media was replaced with DMEM containing 10% v/v horse serum and after 7 days these cells were used in experiments. The purity of astrocytes and primary neuronal cells were tested by immunostaining for glial fibrillary acidic protein (GFAP) and anti-MAP2 antibody and were considered ideal when the culture consisted of >80% astrocytes or primary neuronal cells, respectively. bEnd.3 cells (obtained from ATCC) were cultured in DMEM 10% v/v FBS and 1% v/v Psf. Cells were incubated at 37 °C and 5% of CO2 in a humidified incubator.
2.5. Design of in vitro BBB model
The in vitro BBB model was constructed using a combination of bEnd.3 and primary rat astrocyte cell cultures [35,36]. Astrocytes (1.5 × 104 cells/cm2) were seeded on the bottom side of collagen-coated polyethylene terephthalate (PET) membrane (0.4 μm pore size, effective growth area 0.33 cm2) of transwell inserts (BD BioCoat™; BD Biosciences, NC) in DMEM with 20% v/v FBS. The cells were allowed to adhere to the membrane overnight. Following this, bEnd.3 cells (1.5 × 104 cells/cm2 per culture insert) were seeded on the inside of culture inserts that were placed in 24-well plates and cultured in DMEM with 20% v/v FBS and 1% v/v Psf. The cell culture inserts were kept in the 24-well plates in the culture medium for 6–7 days to allow the formation of tight junctions as assessed from their high transendothelial electrical resistance (TEER) values (measured by EVOM2; World Precision Instruments, Sarasota, FL, USA). As a control, models with only bEnd.3 cells on the upper side of the culture inserts, and models with only astrocytes on the underside of the membrane, were also constructed and maintained similarly. The cells were regularly checked for confluency under the microscope, and the cell culture medium was replaced when deemed necessary.
2.6. Transport across in vitro BBB model
The transport of the lissamine rhodamine B-liposomal formulations were measured across the in vitro BBB model according to a previous report with a minor modification [37]. To mimic the in vivo environment, flux of these liposomes was evaluated in sterile PBS (pH 7.4) containing 10% v/v FBS. The inserts were transferred to 24-well plates containing 0.5 mL PBS in the lower compartment and the culture medium inside was replaced with the liposomal suspensions (100 nM) in fresh serum-containing buffer. The inserts were then transferred to new wells with serum-PBS at 0.25, 0.5, 1, 2, 4 and 8 h. The concentration of liposomes in upper and lower compartments was determined by measuring the fluorescence intensity of the dye molecule in the samples using spectrophotometer (excitation/emission wavelengths: 568/583 nm, respectively). Apparent permeability coefficient (Papp) for each liposomal formulation was calculated using the following equation:
where dQ/dt is the amount of liposomes transported per min (μg/min), A is the surface area of the transwell membrane (cm2), C0 is the initial concentration of liposomes (μg/mL) and 60 is the conversion factor from min to sec. Papp for liposomes was also evaluated across cell free inserts. This apparent permeability was termed as Pt for the permeability coefficient of the total system (in vitro model) and Pf for the permeability coefficient across cell free inserts. The permeability of the endothelial barrier (Pe) was then calculated using the following equation:
The % transport, for all liposomal formulations were calculated over a period of 8 h.
In order to verify the intactness of the barrier layer and if the liposomal formulations do not cause membrane disruption, the TEER was calculated before and after (8 h) liposome transport across the in vitro BBB using EVOM2.
2.7. Evaluation of liposome transfection efficiency in the triple cell culture BBB model
Primary rat neuronal cells were seeded in 24-well plates. The culture inserts seeded with bEnd.3 cells and primary rat astrocytes were placed in the same well containing primary rat neuronal cells [38]. Liposomal suspensions (100 nM) encapsulating pGFP (1 μg) were added to the upper compartment of the inserts and incubated for 8 h. Thereafter, the inserts were removed, and the media replaced with fresh media. The primary rat neuronal cells were incubated for 48 h. GFP expression was analyzed through flow cytometry using FACS analysis- Accuri C6 flow cytometer (Ann Arbor, MI, USA) with laser excitation wavelength 488 nm, emission wavelength using optical filter FL1 533/30 nm and fluorescence microscopy.
2.8. In vivo study
C57BL/6J mice were maintained under standard housing conditions with free access to food and water and exposed to 12 h light-dark cycle. The studies were conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at North Dakota State University. Biodistribution, biocompatibility and transfection efficiency studies were conducted after a 7-day acclimation period.
2.8.1. Biodistribution and biocompatibility
Six C57BL/6J mice were injected with DiR-labeled liposomes (excitation: 748 nm and emission: 782 nm) via tail vein at a dose of ~15.2 μmoles phospholipids/kg body weight. After 24 h, ex vivo fluorescent images were taken by near infrared (NIR) imaging. Different organs such as brain, heart, liver, spleen, lungs, kidneys and blood were collected, weighed, homogenized with PBS (200 μl), and the fluorescent dye extracted in chloroform:methanol (2:1, v/v). The samples were then centrifuged at 4,000 rpm for 10 min and fluorescence intensity of the supernatant (100 μl) measured using spectrophotometric analysis. The fluorescence signals were normalized for sample weight and dilution factor. For biocompatibility, the organs were embedded in Tissue-Tek OCT compound and snap frozen in liquid nitrogen. The frozen tissues were sectioned using cryostat, mounted on poly lysine coated slides, fixed appropriately and evaluated for morphological alterations using hematoxylin-eosin (H&E) staining.
2.8.2. In vivo gene transfection
Six C57BL/6J mice were injected with single dose of liposomal formulations (~15.2 μmoles phospholipids/kg body weight) encapsulating pβgal (30 μg/mouse), or pβgal in buffer or buffer alone. After 5 days, different organs (brain, liver, kidneys, heart, lungs, spleen and blood) were removed, weighed, transferred to tissue lysis/protein extraction buffer (200 μl), homogenized and centrifuged at 4,000 rpm, 4 °C for 15 min. The supernatant was extracted with an equal volume of pβgal assay buffer (β-gal assay kit - Promega, Madison, WI) containing the substrate, o-nitrophenyl-β-D-galactopyranoside (ONPG) and incubated at 37 °C for 60 min. Addition of sodium carbonate stopped the reaction and the absorbance was measured at 420 nm. Tissue samples from control mice (PBS administration) were similarly processed to quantify the endogenous βgal activity of individual organs.
2.8.3. Immunofluorescence studies of brain sections
Two C57BL/6J mice were injected with single dose of liposomal formulations (~15.2 μmoles phospholipids/kg body weight) encapsulating pGFP (30 μg/mouse). A control group of two mice did not receive the treatment. After 7 days, the brains were embedded in OCT compound and snap frozen in liquid nitrogen. Tissue sections (30 μm thick) were cut using cryostat, fixed in acetone and methanol and incubated with primary antibody (rabbit anti-GFP antibody 1:100, Invitrogen, Carlsbad, CA) at 4 °C overnight. Thereafter, sections were incubated with secondary antibody (rabbit Alexa Fluor 488, 1:200, Invitrogen) or mouse anti- NeuN antibody (1:200, Millipore, Burlington, MA) and imaged using confocal microscope.
2.9. Statistical analysis
GraphPad 5.0 Prism software was used for all statistical analyses by one-way analysis of variance (ANOVA) followed by Tukey multiple comparison post-hoc test. Minimum of four independent in vitro experiments were performed and the data was expressed as mean ± SD. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Characterization of liposomes
Pen-DSPE-PEG coupling efficiency was found to be 89.1±1.1%, while that for Tf-DSPEPEG was 90.8±6.5%. Incorporation efficiency of Tf in Pen-liposomes surface to form PenTf-liposomes was 72.9±3.7%. Dialysis process removed all uncoupled Pen and Tf from the mixture. CMC of Tf-phospholipids was not determined since all free Tf was removed from Tf- and PenTf-liposomes by passing the liposomes through Sephadex G-100 column. However, since the CMC of DSPE-PEG-2000 micelles is reported to be 1 μM [39], we believe that the CMC of Tf-DSPE-PEG-2000 micelles would be around that value. This was followed by extrusion of liposomes that helps in the production of smaller and homogeneous particles, which can facilitate the translocation of the particles across BBB [10,40,41]. In this study, extrusion of liposomes through 100-nm and 200-nm polycarbonate filters was performed for all liposomal formulations. Liposome size less than 100 nm could significantly decrease the amount of chitosan-DNA complex in the formulation and therefore we did not process them through smaller sized filters. The size of formulations was optimized to obtain stable delivery systems with efficient cargo loading and targeting properties. The liposomal formulations with chitosan-pDNA complex loaded in the hydrophilic core, were spherical in shape with homogeneous particle size distribution. Details of liposome composition, hydrodynamic size distribution, zeta potential and polydispersity index (PDI) are shown in Table 1. DLS analysis demonstrated that all nanoparticles had an average size of 147–167 nm with no significant difference between the sizes and zeta potential between 15–34 mV. The encapsulation efficiencies of Plain-, Tf-, Pen- and PenTf-liposomes loaded with pGFP (Figure 1 A) were 90.4±3.3%, 88.5±2.4%, 86.1±3.6% and 93.6±2.8%, respectively while that for Plain-, Tf-, Pen- and PenTf-liposomes loaded with pβgal (Figure 1 B) were 84.2±5.2%, 92.9±2.9%, 87.3±2.6% and 91.6±3.7%, respectively. The surface modifications on liposomes did not significantly affect the loading efficiency of pDNA (p<0.05).
Table 1:
Liposome-pDNA: particle size, zeta potential and PDI
| Liposomes | Particle size (nm) | Zeta potential (mV) | PDIa |
|---|---|---|---|
| Plain-liposome | |||
| pGFP | 147.9±1.12 | 21.9±0.48 | 0.166±0,04 |
| Pβgal | 155.7±0.85 | 33.9±1.20 | 0.075±0,03 |
| Tf-liposome | |||
| pGFP | 157.7±1.93 | 15.9±1.32 | 0.183±0.04 |
| Pβgal | 167.2±2.43 | 23.3±0.83 | 0.142±0.04 |
| Pen-liposome | |||
| pGFP | 167.3±2.21 | 28.3±1.73 | 0.194±0.03 |
| Pβgal | 154.2±1.98 | 21.7±2.56 | 0.179±0.04 |
| PenTf-liposome | |||
| pGFP | 152.1±2.36 | 18.9±0.63 | 0.171±0.03 |
| Pβgal | 150.4±3.75 | 22.1±3.11 | 0.198±0.03 |
Data are presented as mean ± SD from four different preparations.
PDI: polydispersity index
Figure 1:

A) Encapsulation efficiency of Plain-, Tf-, Pen- and PenTf-liposomes containing pGFP. B) Encapsulation efficiency of Plain-, Tf-, Pen- and PenTf-liposomes containing pβgal. All data are expressed as mean± SD (n=4).
3.2. Blood compatibility
Under the experimental conditions, the hemolytic activity of liposomal formulations demonstrated no significant negative impact on blood. As shown in Figure 2, the hemoglobin release due to erythrocyte lysis was concentration dependent and incubation with the highest phospholipid concentration (1,000 nM) of Plain-, Tf-, Pen- and PenTf-liposomes resulted in 7.6±0.6%, 8.1±0.3%, 9.7±0.6% and 8.1±0.2% hemolysis, respectively. According to ISO/TR 7406 [42], materials exhibiting hemolysis less than 5% are considered to have good biocompatibility. At 500 nM phospholipid concentration, Plain-, Tf-, Pen- and PenTf- liposomes caused 3.3±0.2%, 3.6±0.5%, 4.8±0.2% and 3.8±0.4% hemolysis, respectively and were therefore within the critical safe hemolysis threshold. At 500 and 1000 nM phospholipid concentration, Pen-liposomes exhibited significantly higher hemolysis percentage than the other liposomal formulations.
Figure 2:

Hemolytic activity of Plain-, Tf-, Pen- and PenTf-liposomes at different phospholipid concentration (31.25–1000 μΜ). Hemolytic activity of 1% (v/v) Triton X-100 was considered as 100%. Data expressed as mean± SD (n=4). ***p<0.001, **p<0.005
3.3. Transport across in vitro BBB
The in vitro BBB model was evaluated by microscopic observations as well as by measuring TEER. In the developed in vitro BBB, TEER of cell culture inserts containing either bEnd.3 cells in the upper side of inserts or primary astrocytes on underside of inserts showed TEER of about 92.6±1.8 and 73.9±1.8 Ωcm−2, respectively as shown in Figure 3A. As expected, TEER of co-cultured insert was significantly (p<0.0001) higher (154.1±3.7 Ωcm−2) than the monolayer models, and there was no evident change in resistance after the co-culture was confluent (Figure 3A). Astrocytes are postulated to increase the tightness of endothelial monolayers as reflected by the TEER values of the co-culture. TEER was also used to check membrane integrity before and after the transport study. As depicted in Figure 3B, the liposomal formulations did not cause membrane rupture during transport study and no significant difference was observed in TEER before (144.8±9.9, 142.7±13.5, 139.3±7.1, 147.9±15.4 Ωcm−2 Plain-, Tf-, Pen- and PenTf-liposomes, respectively) or after (140.1±9.8, 140.1±7.1, 136.9±6.7, 146.3±8.9 Ωcm−2 Plain-, Tf-, Pen- and PenTf-liposomes, respectively) transport, thus indicating the low cytotoxicity of the liposomal formulations.
Figure 3:

A) Transendothelial electrical resistance (TEER, expressed as Ωcm−2) of different BBB models constmcted using astrocytes and bEnd.3 monolayers and co-culture of bEnd.3 and astrocytes. A significantly higher statistical difference in the TEER values between the groups was noted (***p<0.0005). B) TEER of cocultured BBB model before and after 8 h of transport study upon incubation with Plain-, Tf-, Pen- and PenTf- liposomes. All data are expressed as mean± SD (n=4).
Liposome transport across the barrier layer gradually increased with time showing 15.2% transport for PenTf-liposome after 8 h, which was significantly higher (p<0.001) than Plain- (10.9%), Tf- (11.1%) and Pen-liposomes (11.5%), shown in Figure 4A. The permeability of liposome formulations for the in vitro barrier was also monitored, and the Pe value for PenTf-liposomes (3.2×10−6cm/sec) was found to be significantly higher than Plain- (2.3×10−6cm/sec), Tf- (2.3×10−6cm/sec) and Pen-liposomes (2.4×10−6cm/sec), as shown in Figure 4B.
Figure 4:
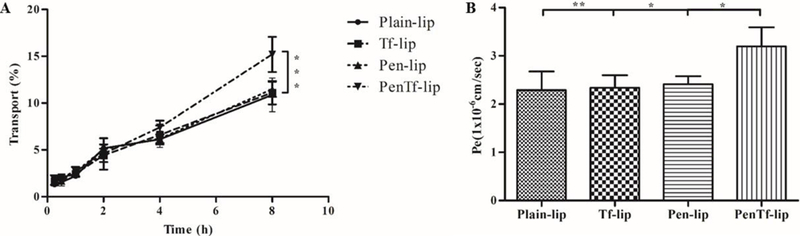
A) Liposomal transport through BBB model over a period of 8 h corresponding to Plain-, Tf-, Pen- and PenTf-liposomes. Data are expressed as mean ± SD (n=4), ***p<0.0001. B) Endothelial cell permeability (Pe, expressed in lxlO−6 cm/s) coefficient for Plain-, Tf-, Pen- and PenTf-liposomes. Data are expressed as mean ± SD (n=4). * p<0.05, **p<0.005.
3.4. Transfection after transport
To get information about the ability of liposomes to transfect cells, liposomes were challenged to transfect primary neuronal cells after transport through in vitro BBB. GFP expression of transfected cells was quantified by flow cytometry. PenTf-liposome containing pGFP (7.1%) increased GFP expression significantly (p<0.0001) compared to Pen- (2.6%), Tf- (3.5%) and Plain-liposomes (2.5%) (Figures 5 A and B). Fluorescence images of transfected cells confirmed the flow cytometry results. Higher number of cells were transfected by PenTf-liposomes and expressed the green fluorescence, as represented in Figure 5C.
Figure 5:

A) Transfection efficiency of Plain-, Tf-, Pen- and PenTf-liposomes containing pGFP in primary neuronal cells after transport through the barrier layer as determined by flow cytometry. B) Histogram plot Plain-, Tf-, Pen- and PenTf-liposomes in primary neuronal cells at the end of transport study. C) Fluorescence images of primary neuronal cells transfected with Plain-, Tf-, Pen- and PenTf-liposomes containing pGFP after transport study (10x magnification). Data are expressed as mean ± SD (n=5). ***p<0.005.
3.5. Biodistribution and biocompatibility
The biodistribution of DiR-liposomal formulations was tracked using NIR imaging, as shown in Figure 6. Quantitative estimation of liposome distribution showed accumulation mainly in the brain and to a lower extent in the liver and lungs. The fluorescence intensity found in brain treated with PenTf-liposomes was 11.9±1.3%, which indirectly indicated that 12% of PenTf-liposomes reached the brain (Figure 7). Significantly lower (p<0.001) fluorescence was detected for Plain- (8.3±1.1%), Tf- (7.7±2.3%) and Pen-liposomes (8.1±1.7%).
Figure 6:

Biodistribution of DiR-liposomes in C57BL/6J mice. Plain-, Tf-, Pen-, PenTf-liposomes (15.2 μΜ of phospholipids/kg body weight) were intravenously administered to mice. NIR images were acquired 24 h after administration.
Figure 7:

Biodistribution of DiR-liposomes in C57BL/6J mice after 24 h. Quantification was performed in different organs (brain, liver, kidneys, heart, lungs, spleen and blood) collected from mice that were intravenously administered with Plain-, Tf-, Pen-, PenTf-liposomes (15.2 μΜ of phospholipids/kg body weight). Six mice were used in each group. Data are expressed as mean ± SD of injected dose percentage (%ID) per gram of tissue. **p<0.001.
Considering the fact that liposomal formulations were preferentially accumulating in the brain, the low elimination rate of brain might have interfered with liposome elimination resulting in their accumulation. Each organ has a specific elimination rate and no significant fluorescence signals was observed in the other organs analyzed.
Histological examination of organs was performed to investigate biocompatibility of the liposomal formulations. The tissue samples from animals administered with PBS were used as control. H&E staining of the organs revealed no alterations in morphological appearance of the tissues, as shown in Figure 8. Additionally, no signs of necrosis, inflammation or cytotoxicity were observed, even in liver, lungs and brain, which demonstrated high accumulation of liposomes.
Figure 8:

Histological analysis through H&E staining of organ sections of C57B1/6J mice subjected to various liposomal formulations treatment. Representative sections (20 X magnification) of brain, liver, kidneys, heart, lungs and spleen of mice treated with Plain-, Tf-, Pen- and PenTf-liposomes. The tissue sections from mice administered with PBS were used as controls.
3.6. In vivo transfection efficiency
The capacity of liposomes to transfect cells was evaluated using liposomes containing plasmid βgal and quantified using βgal kit. The transfection of tissues was similar to the biodistribution of liposomal formulations. As shown in Figure 9, βgalactosidase activity was negligible in the heart, lungs, spleen and blood and no significant difference was found between the endogenous levels of βgal and treatment with naked pDNA. Brain, liver and kidneys demonstrated significantly higher (p<0.05) βgalactosidase activity with Plain-, Tf-, Pen- and PenTf-liposomes compared to the endogenous levels. Furthermore, PenTf- liposomes demonstrated better transfection capacity in the brain (15.7 milliunits βgal activity), that was significantly higher (p<0.05) than Plain-liposomes (11.7 milliunits). For liver and kidneys, no significant difference (p<0.05) was found between the liposomal formulations.
Figure 9:

(βgal expression in C57BL/6J mice treated with liposomal fonnulations containing pβgal after 5 days of liposome administration. βgalactosidasc activity was quantified in different organs (brain, liver, kidneys, heart, lungs, spleen and blood) harvested from mice treated with Plain-. Tf-, Pen-, PenTf-liposomes (15.2 μΜ of phospholipids/kg body weight) encapsulating 30μg pβgal. Six mice were used per group. Data are expressed as mean± SD. *p<0.05, **p<0.005, ***p<0.0005.
3.7. Immunofluorescence of brain sections
We confirmed the expression of GFP protein in hippocampus as well as in cortex sections of brain (Figure 10). Liposomes were able to cross BBB and transfect the brain cells. Slightly increased fluorescence was observed in cortex and hippocampus of mouse treated with PenTf-liposomes compared to Plain-liposomes. These observations correlate well with the biodistribution and transfection efficiency profile of these liposomal formulations. Furthermore, the specificity of PenTf-liposomes to transfect neurons was demonstrated by GFP that was mainly expressed in neurons co-localized with the neuronal marker NeuN (Figure 10B).
Figure 10:

Immunofluorescence analysis of mouse brain sections. A) Cortex and hippocampus sections from non-treated mouse (control) and mouse treated with Plain- and Pentf-liposomes containing 30 μg of pGFP stained with anti-GFP antibody. B) Cortex and hippocampus sections of mouse treated with PenTf-liposomes containing 30 μg of pGFP. Sections were stained with anti-GFP antibody (green) and anti-NeuN antibody (red) (40x magnification).
4. Discussion
Numerous treatment approaches for neurodegenerative diseases are currently under investigation at clinical stages with the focus mostly on small molecules and immunotherapy [43]. Liposomes have been widely investigated for drug delivery and many products are either commercially available or are currently being investigated in clinical trials [44,45]. However, a lot progress is still to be made toward the development of consistent carriers, especially for gene delivery since the current liposomal formulations in clinical trials are primarily for cancer treatment [46]. To this end, we designed liposomal nanoparticles to improve transport across BBB and deliver encapsulated genes to neuronal cells after systemic administration. Multiple strategies as explained below were applied to improve the ability of liposome to target BBB, translocate across the barrier and subsequently transfect the brain cells.
-
a)
Cationic liposomes were used to favor electrostatic interactions with the negatively charged cell membrane and facilitate gene encapsulation [47].
-
b)
PEG incorporation in phospholipid was intended to enhance systemic circulation of liposomes [48] and increase nanoparticle accumulation in the target site.
-
c)
Active uptake by brain through liposome surface modification by transferrin provided liposomes the ability to reach the target site by binding specifically to brain transferrin receptors while decreasing the accumulation in non-targeted organs [49].
-
d)
Enhanced carrier internalization and overcoming receptor saturation, was achieved through additional surface modification of liposomes with penetratin (Pen), a cell penetrating peptide (CPP). CPPs have the capability to translocate a variety of cargo into the cell in a non- invasive manner, without the need for receptors [50], but generally without any specialized targeting properties. However, Pen is known to have the ability to penetrate neurons and accumulate in the nucleus [51]. Additionally, superior effectiveness of Pen in delivering therapeutic macromolecules, compared to various types of CPPs has been demonstrated by different studies [52,53].
-
e)
Complexing plasmid DNA to chitosan enabled chitosan to condense DNA to sizes compatible for cellular uptake [54], protect DNA molecules against enzymatic degradation [55] and promote transfection [56].
These aforementioned strategies when individually applied to the liposomes offer limited advantage but when combined, are believed to synergistically improve efficiency of the brain-targeted gene nanocarrier. This notion was tested by studying the ability of the liposomes to cross an in vitro BBB model, followed by in vivo biodistribution and transfection efficiency studies.
Preparation of Pen-liposomes through thin lipid film rehydration method allows accurate control of the amount of CPP used and to produce stable nanoparticles [57,58]. On the other hand, post-insertion technique is a suitable method for preparation of Tf ligand based stealth liposomes [59]. The insertion of PEG-derivative phospholipids into pre-formed liposomes is a spontaneous process, which is driven mainly by hydrophobic interactions of membrane lipids with the hydrophobic parts of PEG-derivative phospholipids [60]. With a smaller hydrophobic domain of Pen [61] compared to Tf, [62] we believe the association of Pen with the liposomes would be higher when incorporated through film rehydration method compared to spontaneous post insertion method. This technique has been used by other researchers and commonly the ligands were coupled to PEG-phospholipids, which produced a variety of lipid-based nanoparticles [60].
The control of liposome characteristics such as particle size, surface charge and surface modification are of major importance since they closely influence liposome properties, which likely determine their biological fate [63]. The presence of a targeting ligand and a cell-penetrating peptide might synergistically facilitate the transport across BBB via receptor-mediated transcytosis and adsorptive transcytosis, respectively [64]. Considering these cases, liposome surface modification with specific ligands play an important role in CNS delivery. Initially, the physico-chemical characteristics of the liposomal formulations were optimized (particle size ~150 nm, carrying slightly positively charge and high pDNA encapsulation efficiency) to obtain a suitable long circulating delivery system. Plasmid incorporation and Tf and CPP conjugation did not significantly affect the size distribution and morphology of liposomes.
Although cationic liposomes afford high transfection activity, their use in intravenously administered formulations require major attention since these nanoparticles can interact electrostatically with various negatively charged surfaces such as red blood cells and plasma proteins. The interaction can promote erythrocyte lysis and release of hemoglobin [65]. For this, in vitro evaluation of formulation-induced erythrocyte hemolysis is considered a simple and reliable measure for estimating blood compatibility of biomaterials. The behavior of liposomes can be predicted by investigating the in vitro degree of hemolysis [42]. Liposomal formulations exhibited a concentration dependent hemolytic activity and the hemolysis levels were within the range suggestive of good biocompatibility. The cationic character that Pen added to formulations could explain the hemolytic properties of Pen-liposomes and their higher degree of hemolysis.
Modeling BBB indeed has become a useful tool for screening formulations developed for brain delivery [66,67]. The liposomal formulations demonstrated capability of crossing in vitro BBB without disrupting the membrane integrity, as noted through TEER measurements. The significant transport of PenTf-liposomes through the barrier compared to the other liposomes suggests the importance of both ligands, Tf and Pen, on liposome-cell interaction leading to additive/synergistic enhancement in transcytosis. Tf ligand is reported to facilitate liposome transport across BBB via receptor-mediated transcytosis, while cationic CPP such as Pen aids transport via adsorption-mediated transcytosis. Evaluation of transport across BBB in vitro using Tf- and Pen-liposomes indicated that each ligand individually contributed to liposome transport in a similar manner. Interestingly, we did not find that Tf- and Pen- liposomes significantly improved transport across BBB in vitro compared to Plain-liposomes. This outcome may be attributed to the incubation period that was used in the experiment, which may have allowed time for non-specific interaction and uptake of Plain-liposomes. Furthermore, transwell co-culture systems do not replicate the sink conditions in vivo. Despite this drawback, primary cells based in vitro BBB model is considered a reliable model for investigating transcytosis [68]. Additional investigation is necessary to elucidate the mechanisms and the contribution of each surface modification involved in liposome uptake. On the other hand, Pen-Tf liposomes demonstrated better efficiency to cross the barrier in vitro, in conjunction with better ability to transfect primary neuronal cells compared to the other liposomal formulations. It is believed that liposome surface modifications through Tf receptor mediated uptake of formulations, Pen based enhanced internalization through cell membrane interaction and chitosan-pDNA complexation to assist endosomal escape may have contributed to the strong increase in GFP expression in primary neuronal cells.
Tf receptors are likely expressed at low levels in most normal human tissues [69]. For instance, hepatocytes express an average of 20,000 surface Tf receptors per cell, while nonparenchymal liver cells express around 5,000 surface receptors per cell [70]. On the other hand, high expression of Tf receptors has been reported in BBB endothelium [71], with an average of 7×104 Tf receptors/cell in astrocytes [72] and 3xl07 receptors/cell in brain endothelial cells [73]. Considering the higher expression of Tf receptors in brain endothelium compared to rest of the body, we expected pronounced accumulation of Tf-modified liposomes in the brain and low accumulation in other different tissues. In addition, difference in clearance pathways of organs may influence liposomal accumulation, especially in the brain, which present lower elimination rate due to the complex structure of BBB and its transport systems [74,75]. Conversely, high perfusion rates of organs such as liver, kidneys and heart could disfavor liposome accumulation in these organs. Several studies have explored the efficacy of Tf-liposomes mediated drug/gene delivery and have attributed the low accumulation of liposomes in these organs to rapid blood clearance [49,76–82]. In this study, we observed low liposome fluorescence intensity in liver, kidneys, lungs, heart and spleen and significantly high liposomal accumulation in the brain through NIR imaging and DiR-liposome quantification after 24 h of administration which could be related to the differential blood clearance from these organs. Similar observation was also reported in a study which characterized the biodistribution kinetics profile of a dual-functionalized liposome, however, the analysis was performed over a period of 12 h [78]. We observed that despite liposome clearance, the dual-functional nanoparticles efficiently translocated across BBB and accumulated in the brain.
The compartmentalized distribution of each liposomal formulation in the brain could not be explained by simply analyzing the NIR imaging of brain. Additional investigation is necessary to elucidate the mechanisms involved in uptake, transport and distribution of liposomes in the brain. Immunofluorescence images of brain sections transfected with GFP stained with neuronal specific antibody was performed only in PenTf-liposomes due to significantly higher accumulation and transfection observed in the brain. However, based on the images of GFP expression exhibited by brain cells, we could only infer that cell transfection was a result of liposome ability to target neuronal cells and deliver the gene to cell nucleus. Further studies are needed to determine the exact nature of liposome distribution within specific regions of the brain, which are being planned for further characterization of the formulation.
The fluorescence levels of DiR observed upon treatment with Plain-, Tf- and Pen-liposomes in the brain were similar, but significantly lower than the levels of PenTf-liposomes. Other researchers working with brain targeted-liposomes have observed initial significantly higher brain accumulation compared to non-targeted liposome (between 1 to 4 h) [83,84]. However, after 24 h, a continuous decrease in liposome brain accumulation led to presence of similar levels of targeted and non-targeted liposomes. Since, quantification of liposomal biodistribution in our study was only one time point based (at 24 h after liposome administration), the results obtained may have undervalued the targeting ability, hence accumulation of PenTf-liposomes in the brain at this time point. Additionally, we are unable to provide evidence whether Plain-liposomes accumulated in brain capillaries or crossed BBB and accumulated in brain parenchyma. Likewise, if there is any facilitated preferential accumulation of other formulations (Tf- and Pen-liposomes) in capillaries and transcytosis thereafter into the brain, it needs to be investigated. In our future studies, brain capillary depletion study will be performed to address these questions [47,64,85]. Different scattering and absorption properties of the various organs studied may have been a factor involved in the difference observed between the qualitative and quantitative biodistribution data in this study [86]. As a consequence, the fluorescence intensity detected by fluorescence imaging may not necessarily be proportional to the number of molecules present in the sample, providing a less accurate but informative way to assess the biodistribution.
Administration of PenTf-liposomes led to higher uptake in the brain after 24 h compared to the other liposomal formulations. This is the first report that shows significant liposome targeting to the brain as compared to the percentage of injected dose (%ID) reported in other studies using targeted nanoparticles [12,29,81,82], that emphasizes the role of dualmodification (PenTf) of liposomes for brain targeting and accumulation. The observed 12% ID for PenTf-liposomes and approximately 8% ID for Plain-, Tf- and Pen-liposomes in mice brain are amongst the highest levels that we have seen in the literature so far. Different studies that similarly quantified multi-functionalized liposomes in the brain reported values lower than 1% [10,40,41], which highlight the magnitude of our results.
The in vivo biocompatibility of formulations administered systemically is fundamental to its safety. Low doses of cationic liposomes have shown good biocompatibility, biodegradability and low cytotoxicity [9,87]. Histological examination of brain, liver, kidneys, heart, lungs and spleen tissues demonstrated the non-cytotoxic profile of liposomal formulation, showing no cellular damage or morphological alterations. The H&E analysis of organs and hemocompatibility study confirmed the low toxicity and good biocompatibility of liposomal formulations. Additional detailed investigation is needed to evaluate in vivo liposomal toxicity including assessment of genotoxicity and inflammation markers, which are not obtained by histology and hematological analyses [87]. The use of organ specific biomarkers may be a more accurate way to investigate toxicity in drug development. For example, standard biomarkers of drug-induced liver injury including alanine aminotransferase and aspartate aminotransferase can be studied [88,89]. Also, evaluation of oxidative stress and quantification of blood biomarkers such as Tau and GFPA can potentially indicate brain injury [90,91]. Investigation of these different toxicity biomarkers will be included in our future studies. Based on H&E staining and blood compatibility studies, the dual- functionalized liposomes can be considered a suitable formulation for systemic administration. Analysis of brain histological sections together with immunofluorescence images of cortex and hippocampus did not indicate any disruption of the BBB. Therefore, liposome accumulation in the brain suggests their ability to cross the BBB without damaging the barrier cells. During the experimental study, no sign of toxicity or change in animal behavior was observed. The pharmacological effect as a result of improved delivery of PenTf-liposomes to the brain was shown through quantification of βgal expression after 5 days of liposome administration.
Quantification of transfection efficiency of liposomal formulation containing the reporter gene βgal in different organs was a strategy used to determine pharmacological consequence of differential biodistribution of the liposomes. Additionally, quantification of βgal activity enable determination of gene delivery specifically to cell nucleus. Upon single administration, dual-functionalized liposomal formulations (PenTf-liposomes) were not only able to reach brain parenchyma in significant amount (~12%), but also transfect brain cells with βgal gene. However, it must be noted that PEG based formulations can be subjected to accelerated blood clearance. This phenomenon usually happens due to multiple administration of PEG-modified formulations, which is characterized by rapid clearance of circulating PEG-liposomes after repeated administration [92]. A single liposome administration can avoid this undesired effect and most likely, this phenomenon did not affect liposomal biodistribution in our study since it was based on a single administration of the formulation. Transfection efficiency of liposomal formulations followed the pattern observed in biodistribution study. The results indicate that the formulation accumulated at high levels mainly in the brain, liver, kidneys and to a lesser extent in the heart. The targeting properties along with protection from enzymatic degradation of PenTf-liposomes might have contributed to the higher protein expression observed in the brain. Immunofluorescence images of GFP expression in the brain induced by liposomes confirmed the ability of these nanoparticles to reach brain parenchyma and specially transfect neurons. The dual liposome surface modification with Tf ligand and Pen may have exerted an additive/synergistic effect on BBB targeting as well as neuronal transfection. Overall, these dual-functionalized liposomes have the potential to accurately and efficiently deliver pDNA into brain and should be considered for further development.
5. Conclusions
Dual-functionalized liposomes were developed to cross BBB and deliver gene to brain cells. The system could reach brain parenchyma, release pDNA in the cytoplasm of neurons, induce β-galactosidase production in vivo and elicit a better effect than non-modified liposomes or naked pDNA. The study illustrates the superior ability of dual-functionalized liposomes to accumulate in the brain and transfect neurons. These results suggest that PenTf liposomal formulation might be an efficient non-viral gene carrier and a promising approach for gene therapy of CNS diseases. Additional studies are needed to more clearly understand nanoparticle transcytosis across BBB and into the brain.
Highlights.
Dual-functionalized liposomes were designed to cross BBB and deliver gene to brain cells
The designed liposomes, surface modified with transferrin (Tf) and cell penetrating peptide penetratin (Pen), were preferentially accumulated in the brain compared to liposomes with single or no modification.
Dual-functionalized liposomes carrying gene complexed with chitosan demonstrated significantly higher gene transfection and protein expression in the brain
The study suggests that the PenTf liposomal formulation is a promising gene delivery platform for treatment of neurodegenerative diseases
Acknowledgments
This research was supported by National Institutes of Health Grant R01AG051574. B.S.R. is supported by a fellowship from Sciences without Borders Program (Conselho Nacional de Desenvolvimento Científico e Technologico - CNPq, Brazil).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP, The global prevalence of dementia: A systematic review and metaanalysis, Alzheimer’s Dement. 9 (2013) 63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- [2].Alzheimer’s association, 2017 AL ZHE IME R ‘ S DISE A SE FAC TS AND FIGURES Includes a, (2017) 88. [Google Scholar]
- [3].Jeffrey P, Summer S, Neurobiology of Disease Assessment of the blood - brain barrier in CNS drug discovery, 37 (2010) 33–37. doi: 10.1016/j.nbd.2009.07.033. [DOI] [PubMed] [Google Scholar]
- [4].Saraiva C, Pra??a C, Ferreira R, Santos T, Ferreira L, Bernardino L, Nanoparticle- mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases, J. Control. Release. 235 (2016) 34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- [5].Naldini L, Gene therapy returns to centre stage, Nature. 526 (2015) 351–360. doi: 10.1038/naturel5818. [DOI] [PubMed] [Google Scholar]
- [6].Pardo J, Morel GR, Astiz M, Schwerdt JI, León ML, Rodríguez SS, Hereñú CB, Goya RG, Gene Therapy and Cell Reprogramming for the Aging Brain : Achieve- ments and Promise, (2014) 24–34. [DOI] [PubMed] [Google Scholar]
- [7].Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, Non- viral vectors for gene-based therapy, Nat Rev Genet. 15 (2014) 541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- [8].Liu Y, An S, Li J, Kuang Y, He X, Guo Y, Ma H, Biomaterials Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer ‘ s disease mice, 80 (2016)33–45. doi: 10.1016/j.biomaterials.2015.11.060. [DOI] [PubMed] [Google Scholar]
- [9].Zheng X, Zhang C, Guo Q, Wan X, Shao X, Liu Q, Zhang Q, Dual-functional nanoparticles for precise drug delivery to Alzheimer’s disease lesions: Targeting mechanisms, pharmacodynamics and safety, Int. J. Pharm. 525 (2017) 237–248. doi: 10.1016/j.ijpharm.2017.04.033. [DOI] [PubMed] [Google Scholar]
- [10].Ordóñez-Gutiérrez L, Posado-Fernández A, Ahmadvand D, Lettiero B, Wu L, Antón M, Flores O, Moghimi SM, Wandosell F, ImmunoPEGliposome-mediated reduction of blood and brain amyloid levels in a mouse model of Alzheimer’s disease is restricted to aged animals, Biomaterials. 112 (2016). doi: 10.1016/j.biomaterials.2016.07.027. [DOI] [PubMed] [Google Scholar]
- [11].Gregori M, Taylor M, Salvati E, Re F, Mancini S, Balducci C, Forloni G, Zambelli V, Sesana S, Michael M, Michail C, Tinker-Mill C, Kolosov O, Scherer M, Harris S, Fullwood NJ, Masserini M, Allsop D, Retro-inverso peptide inhibitor nanoparticles as potent inhibitors of aggregation of the Alzheimer’s Αβ peptide, Nanomedicine Nanotechnology, Biol. Med. (2016) 1–10. doi: 10.1016/j.nano.2016.10.006. [DOI] [PubMed] [Google Scholar]
- [12].Sánchez-López E, Ettcheto M, Egea MA, Espina M, Calpena AC, Folch J, Camins A, García ML, New Potential Strategies for Alzheimer’s Disease Prevention: Pegylated Biodegradable Dexibuprofen Nanospheres Administration to APPswe/PSldE9, Nanomedicine Nanotechnology, Biol. Med. (2016). doi: 10.1016/j.nano.2016.12.003. [DOI] [PubMed] [Google Scholar]
- [13].Tapeinos C, Battaglini M, Ciofani G, Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases, J. Control. Release. 264 (2017) 306–332. doi: 10.1016/j.jconrel.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Obata Y, Saito S, Takeda N, Takeoka S, Plasmid DNA-encapsulating liposomes: Effect of a spacer between the cationic head group and hydrophobic moieties of the lipids on gene expression efficiency, Biochim. Biophys. Acta - Biomembr. 1788 (2009) 1148–1158. doi: 10.1016/j.bbamem.2009.02.014. [DOI] [PubMed] [Google Scholar]
- [15].Yang Y, Yang Y, Xie X, Cai X, Zhang H, Gong W, Wang Z, Mei X, Biomaterials PEGylated liposomes with NGR ligand and heat-activable cell- penetrating peptide e doxorubicin conjugate for tumor-specific therapy, Biomaterials. 35 (2014) 4368–4381. doi: 10.1016/j.biomaterials.2014.01.076. [DOI] [PubMed] [Google Scholar]
- [16].Vieira DB, Gamarra LF, Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier, Int. J. Nanomedicine. 11 (2016) 5381–5414. doi: 10.2147/UN.S117210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Paterson J, Webster CI, Exploiting transferrin receptor for delivering drugs across the blood-brain barrier, Drug Discov. Today Technol. 20 (2016) 49–52. doi: 10.1016/j.ddtec.2016.07.009. [DOI] [PubMed] [Google Scholar]
- [18].Agrawal M, Ajazuddin, Tripathi DK, Saraf S, Saraf S, Antimisiaris SG, Mourtas S, Hammarlund-Udenaes M, Alexander A, Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease, J. Control. Release. 260 (2017) 61–77. doi: 10.1016/j.jconrel.2017.05.019. [DOI] [PubMed] [Google Scholar]
- [19].Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY, Transferrin receptors in human tissues: their distribution and possible clinical relevance., J. Clin. Pathol. 36 (1983) 539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hersom M, Helms HC, Pretzer N, Goldeman C, Jensen AI, Severin G, Nielsen MS, Holm R, Brodin B, Transferrin receptor expression and role in transendothelial transport of transferrin in cultured brain endothelial monolayers, (2016). doi: 10.1016/j.men.2016.08.009. [DOI] [PubMed] [Google Scholar]
- [21].Jefferies WA, Brandon MR, V Hunt S, Williams AF, Gatter KC, Mason DY, Transferrin receptor on endothelium of brain capillaries., Nature. 312 (n.d.) 162–3. http://www.ncbi.nlm.nih.gov/pubmed/6095085 (accessed December 6, 2017). [DOI] [PubMed] [Google Scholar]
- [22].Johnsen KB, Moos T, Revisiting nanoparticle technology for blood-brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor- targeted liposomes, J. Control. Release. 222 (2016) 32–46. doi: 10.1016/j.jconrel.2015.11.032. [DOI] [PubMed] [Google Scholar]
- [23].Xiao G, Gan L-S, Receptor-mediated endocytosis and brain delivery of therapeutic biologies., Int. J. Cell Biol 2013 (2013) 703545. doi: 10.1155/2013/703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Srinivasarao M, Low PS, Ligand-Targeted Drug Delivery, Chem. Rev. 117 (2017) 12133–12164. doi: 10.1021/acs.chemrev.7b00013. [DOI] [PubMed] [Google Scholar]
- [25].Zhang D, Wang J, Xu D, Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems, J. Control. Release. 229 (2016) 130–139. doi: 10.1016/j.jconrel.2016.03.020. [DOI] [PubMed] [Google Scholar]
- [26].Bashyal S, Noh G, Keum T, Choi YW, Lee S, Cell penetrating peptides as an innovative approach for drug delivery; then, present and the future, J. Pharm. Investig. 46 (2016) 205–220. doi: 10.1007/s40005-016-0253-0. [DOI] [Google Scholar]
- [27].Brugnano J, Ward BC, Panitch A, Cell penetrating peptides can exert biological activity: A review, Biomol. Concepts. 1 (2010) 109–116. doi: 10.1515/bmc.2010.016. [DOI] [PubMed] [Google Scholar]
- [28].Yang Z, Li J, Wang Z, Dong D, Qi X, Biomaterials Tumor-targeting dual peptides- modi fi ed cationic liposomes for delivery of siRNA and docetaxel to gliomas, Biomaterials. 35 (2014) 5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- [29].Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, Singh J, Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection, J. Control. Release. 167 (2013) 1–10. doi: 10.1016/j.jconrel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- [30].Pankevich DE, Altevogt BM, Dunlop J, Gage FH, Hyman SE, Improving and accelerating drug development for nervous system disorders, Neuron. 84 (2014) 546–553. doi: 10.1016/j.neuron.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cifani N, Chronopoulou L, Pompili B, Di A, @bullet M, Bordi F, Sennato S, Enea @bullet, Di G, @bulle D, Palocci C, Ascenzioni F, Improved stability and efficacy of chitosan/pDNA complexes for gene delivery, (2014). doi: 10.1007/sl0529-014-1727-7. [DOI] [PubMed] [Google Scholar]
- [32].Nimesh S, Thibault MM, Lavertu M, Buschmann MD, Enhanced Gene Delivery Mediated by Low Molecular Weight Chitosan/DNA Complexes: Effect of pH and Serum, (2010). doi: 10.1007/s12033-010-9286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sumners C, Fregly MJ, Modulation of angiotensin II binding sites in neuronal cultures by mineralocorticoids, Am. J. Physiol. 256 (1989) C121–C129. [DOI] [PubMed] [Google Scholar]
- [34].Sumners C, Tang WEI, Raizada MK, Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain, Proc. Natl. Acad. Sci. U. S. A. 88 (1991) 7567–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel Á, Tanaka K, Niwa M, A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes, Neurochem. Int. 54 (2009) 253–263. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- [36].Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud P-O, Deli MA, Förster C, Galla HJ, Romero IA, Shusta EV, Stebbins MJ, Vandenhaute E, Weksler B, Brodin B, In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use, J. Cereb. Blood FlowMetab. 36 (2016) 862–890. doi: 10.1177/0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sharma G, Modgil A, Sun C, Singh J, Grafting of cell-penetrating peptide to receptor-targeted liposomes improves their transfection efficiency and transport across blood-brain barrier model., J. Pharm. Sci. 101 (2012)2468–78. doi: 10.1002/jps.23152. [DOI] [PubMed] [Google Scholar]
- [38].Xue Q, Liu Y, Qi H, Ma Q, Xu L, Chen W, Chen G, Xu X, A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat, Int. J. Biol. Sci. 9 (2013) 174–189. doi: 10.7150/ijbs.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ashok B, Arleth L, Hjelm RP, Rubinstein I, Önyüksel H, In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: Effects of PEG chain length and PC incorporation, J. Pharm. Sci. (2004). doi: 10.1002/jps.20150. [DOI] [PubMed] [Google Scholar]
- [40].Bana L, Minniti S, Salvati E, Sesana S, Zambelli V, Cagnotto A, Orlando A, Cazzaniga E, Zwart R, Scheper W, Masserini M, Re F, Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect A?? aggregation features and cross the blood-brain-barrier: Implications for therapy of Alzheimer disease, Nanomedicine Nanotechnology, Biol. Med. 10 (2014) 1583–1590. doi: 10.1016/j.nano.2013.12.001. [DOI] [PubMed] [Google Scholar]
- [41].Balducci C, Mancini S, Minniti S, La Vitola P, Zotti M, Sancini G, Mauri M, Cagnotto A, Colombo L, Fiordaliso F, Grigoli E, Salmona M, Snellman A, Haaparanta-Solin M, Forloni G, Masserini M, Re F, Multifunctional Liposomes Reduce Brain -Amyloid Burden and Ameliorate Memory Impairment in Alzheimer’s Disease Mouse Models, J. Neurosci. 34 (2014) 14022–14031. doi: 10.1523/JNEUROSCI.0284-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li X, Wang L, Fan Y, Feng Q, Cui FZ, Biocompatibility and toxicity of nanoparticles and nanotubes, J. Nanomater. 2012. (2012). doi: 10.1155/2012/548389. [DOI] [Google Scholar]
- [43].Hung S-Y, Fu W-M, Drug candidates in clinical trials for Alzheimer’s disease, J. Biomed. Sci. 24 (2017)47. doi: 10.1186/sl2929-017-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Allen TM, Cullis PR, Liposomal drug delivery systems: From concept to clinical applications, Adv. Drug Deliv. Rev 65 (2013) 36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- [45].Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S, Advances and challenges of liposome assisted drug delivery, Front. Pharmacol. 6 (2015) 1–13. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bulbake U, Doppalapudi S, Kommineni N, Khan W, Liposomal formulations in clinical use: An updated review, Pharmaceutics. 9 (2017) 1–33. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Van Rooy I, Mastrobattista E, Storm G, Hennink WE, Schiffelers RM, Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain, J. Control. Release. 150 (2011) 30–36. doi: 10.1016/j.jconrel.2010.11.014. [DOI] [PubMed] [Google Scholar]
- [48].Salmaso S, Caliceti P, Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers, J. Drug Deliv. 2013 (2013) 1–19. doi: 10.1155/2013/374252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM, Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse., J. Pharmacol. Exp. Ther. 292 (2000) 1048–1052. [PubMed] [Google Scholar]
- [50].Stalmans S, Bracke N, Wynendaele E, Gevaert B, Peremans K, Burvenich C, Polis I, De Spiegeleer B, Cell-penetrating peptides selectively cross the blood-brain barrier in vivo, PLoS One. 10 (2015) 1–22. doi: 10.1371/joumal.pone.0139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ramsey JD, Flynn NH, Cell-penetrating peptides transport therapeutics into cells, Pharmacol. Ther. 154 (2015) 78–86. doi: 10.1016/j.pharmthera.2015.07.003. [DOI] [PubMed] [Google Scholar]
- [52].Kamei N, Morishita M, Eda Y, Ida N, Nishio R, Takayama K, Usefulness of cell- penetrating peptides to improve intestinal insulin absorption, J. Control. Release. 132 (2008) 21–25. doi: 10.1016/j.jconrel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- [53].Jain M, Chauhan SC, Singh AP, Venkatraman G, Colcher D, Batra SK, Penetratin improves tumor retention of single-chain antibodies: A novel step toward optimization of radioimmunotherapy of solid tumors, Cancer Res. 65 (2005) 7840–7846. doi: 10.1158/0008-5472.CAN-05-0662. [DOI] [PubMed] [Google Scholar]
- [54].Mao S, Sun W, Kissel T, Chitosan-based formulations for delivery of DNA and siRNA, Adv. DrugDeliv. Rev. 62 (2010) 12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [55].Mansouri S, Lavigne P, Corsi K, Benderdour M, Beaumont E, Fernandes JC, Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: Strategies to improve transfection efficacy, Eur. J. Pharm. Biopharm. (2004). doi: 10.1016/S0939-6411(03)00155-3. [DOI] [PubMed] [Google Scholar]
- [56].Lavertu M, Méthot S, Tran-Khanh N, Buschmann MD, High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation, Biomaterials. (2006). doi: 10.1016/j.biomaterials.2006.04.029. [DOI] [PubMed] [Google Scholar]
- [57].Lee S, Sato Y, Hyodo M, Harashima H, Topology of Surface Ligands on Liposomes : Characterization Based on the Terms, Incorporation Ratio, Surface Anchor Density, and Reaction Yield, 39 (2016) 1983–1994. doi: 10.1248/bpb.bl6-00462. [DOI] [PubMed] [Google Scholar]
- [58].Marqués-Gallego P, De Kroon AIPM, Ligation strategies for targeting liposomal nanocarriers, Biomed Res. Int. 2014. (2014). doi: 10.1155/2014/129458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nakamura K, Yamashita K, Itoh Y, Yoshino K, Nozawa S, Kasukawa H, Biochimica et Biophysica Acta Comparative studies of polyethylene glycol-modi fi ed liposomes prepared using different PEG-modi fi cation methods, BBA - Biomembr. 1818 (2012) 2801–2807. doi: 10.1016/j.bbamem.2012.06.019. [DOI] [PubMed] [Google Scholar]
- [60].Nag OK, Awasthi V, Surface engineering of liposomes for stealth behavior, Pharmaceutics. (2013). doi: 10.3390/pharmaceutics5040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wu X, Gehring W, Cellular uptake of the Antennapedia homeodomain polypeptide by macropinocytosis, Biochem. Biophys. Res. Commun. 443 (2014) 1136–1140. doi: 10.1016/j.bbrc.2013.12.062. [DOI] [PubMed] [Google Scholar]
- [62].Thevis M, Ogorzalek Loo RR, Loo JA, Mass spectrometric characterization of transferrins and their fragments derived by reduction of disulfide bonds, J. Am. Soc. Mass Spectrom. 14 (2003) 635–647. doi: 10.1016/S1044-0305(03)00199-5. [DOI] [PubMed] [Google Scholar]
- [63].Aparicio-Blanco J, Martin-Sabroso C, Torres-Suárez A-I, In vitro screening of nanomedicines through the blood brain barrier: A critical review., Biomaterials. 103 (2016) 229–55. doi: 10.1016/j.biomaterials.2016.06.051. [DOI] [PubMed] [Google Scholar]
- [64].Grabrucker AM, Ruozi B, Belletti D, Pederzoli F, Forni F, Vandelli MA, Tosi G, Grabrucker AM, Ruozi B, Belletti D, Pederzoli F, Forni F, Vandelli MA, Tosi G, Grabrucker AM, Ruozi B, Belletti D, Pederzoli F, Forni F, Vandelli MA, Tosi G, Nanoparticle transport across the blood brain barrier Nanoparticle transport across the blood brain barrier, 8370 (2016) 1–18. doi: 10.1080/21688370.2016.1153568. [DOI] [Google Scholar]
- [65].Qi P, Cao M, Song L, Chen C, Liu M, Li N, Wu D, Peng J, Hu G, Zhao J, The biological activity of cationic liposomes in drug delivery and toxicity test in animal models, Environ. Toxicol. Pharmacol. 47 (2016) 159–164. doi: 10.1016/j.etap.2016.09.015. [DOI] [PubMed] [Google Scholar]
- [66].Chou C-H, Sinden JD, Couraud P-O, Modo M, In Vitro Modeling of the Neurovascular Environment by Coculturing Adult Human Brain Endothelial Cells with Human Neural Stem Cells, PLoS One. 9 (2014) el06346. doi: 10.1371/journal.pone.0106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cecchelli R, Dehouck B, Descamps L, Fenart L, Buee-Scherrer V, Duhem C, Lundquist S, Rentfel M, Torpier G, Dehouck MP, In vitro model for evaluating drug transport across the blood-brain barrier, Adv. Drug Deliv. Rev 36 (1999) 165–178. doi: 10.1016/S0169-409X(98)00083-0. [DOI] [PubMed] [Google Scholar]
- [68].Burkhart A, Skjørringe T, Johnsen KB, Siupka P, Thomsen LB, Nielsen MS, Thomsen LL, Moos T, Expression of Iron-Related Proteins at the Neurovascular Unit Supports Reduction and Reoxidation of Iron for Transport Through the Blood- Brain Barrier, Mol. Neurobiol. 53 (2016) 7237–7253. doi: 10.1007/s12035-015-9582-7. [DOI] [PubMed] [Google Scholar]
- [69].Ponka P, Lok CN, The transferrin receptor: role in health and disease., Int. J. Biochem. Cell Biol 31 (1999) 1111–37. http://www.ncbi.nlm.nih.gov/pubmed/10582342 (accessed October 25, 2015). [DOI] [PubMed] [Google Scholar]
- [70].Vogel W, Bomford A, Young S, Williams R, Heterogeneous Distribution of Transferrin Receptors on Parenchymal and Nonparenchymal Liver Cells: Biochemical and Morphological Evidence, Blood. 69 (1987) 264–270. http://www.bloodjournal.Org/content/bloodjournal/69/l/264.full.pdf (accessed April 4, 2018). [PubMed] [Google Scholar]
- [71].Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T, Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors, J. Neurochem. 117 (2011) 333–345. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- [72].Qian ZM, To Y, Tang PL, Feng YM, Transferrin receptors on the plasma membrane of cultured rat astrocytes., Exp.Brain Res. 129 (1999) 473–476. [DOI] [PubMed] [Google Scholar]
- [73].Fotticchia I, Guarnieri D, Fotticchia T, Falanga AP, Vecchione R, Giancola C, Netti PA, Energetics of ligand-receptor binding affinity on endothelial cells: An in vitro model, Colloids Surfaces B Biointerfaces. 144 (2016) 250–256. doi: 10.1016/j.colsurfb.2016.04.018. [DOI] [PubMed] [Google Scholar]
- [74].Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ, Clearance systems in the brain implications for Alzheimer disease, Nat. Rev. Neurol. 11 (2015) 457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bakker ENTP, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AWJ, Weller RO, Carare RO, Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases, Cell. Mol. Neurobiol. 36(2016) 181–194. doi: 10.1007/s10571-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang W, Peng F, Zhou T, Huang Y, Zhang L, Ye P, Lu M, Yang G, Gai Y, Yang T, Ma X, Xiang G, Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes, Int. J. Nanomedicine. 10 (2015) 4825–4836. doi: 10.2147/UN.S79598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang T, Yin X, Lu Y, Shan W, Xiong S, Formulation, antileukemia mechanism, pharmacokinetics, and biodistribution of a novel liposomal emodin, Int. J. Nanomedicine. 7 (2012) 2325–2337. doi: 10.2147/UN.S31029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zheng C, Ma C, Bai E, Yang K, Xu R, Transferrin and cell-penetrating peptide dual-functioned liposome for targeted drug delivery to glioma, Int. J. Clin. Exp. Med. 8(2015)1658–1668. [PMC free article] [PubMed] [Google Scholar]
- [79].Girão Da Cruz MT, Simões S, Pedroso De Lima MC, Improving lipoplex-mediated gene transfer into C6 glioma cells and primary neurons, Exp. Neurol. 187 (2004) 65–75. doi: 10.1016/j.expneurol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- [80].Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME, Impact of tumor- specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging, Proc. Natl. Acad. Sci. 104 (2007) 15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lee SM, Kim J-S, Oh Y-K, Lee Y-B, Sah H, Biodistribution and Genotoxicity of Transferrin- Conjugated Liposomes/DNA Complexes in Mice, Macromol. Res. 13 (2005)218–222. [Google Scholar]
- [82].Suzuki R, Takizawa T, Kuwata Y, Mutoh M, Ishiguro N, Utoguchi N, Shinohara A, Eriguchi M, Yanagie H, Maruyama K, Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome, Int. J. Pharm. 346 (2008) 143–150. doi: 10.1016/j.ijpharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- [83].Gaillard PJ, Appeldoorn CCM, Dorland R, Van Kregten J, Manca F, Vugts DJ, Windhorst B, Van Dongen GAMS, De Vries HE, Maussang D, Van Tellingen O, Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3–101), PLoS One. 9 (2014). doi: 10.1371/journal.pone.0082331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Johnsen KB, Burkhart A, Melander F, Kempen PJ, Vejlebo JB, Siupka P, Nielsen MS, Andresen TL, Moos T, Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma, Sci. Rep. 7(2017) 1–13. doi: 10.1038/s41598-017-11220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Song Q, Huang M, Yao L, Wang X, Gu X, Chen J, Chen J, Huang J, Hu Q, Kang T, Rong Z, Qi H, Zheng G, Chen H, Gao X, Lipoprotein-based nanoparticles rescue the memory loss of mice with alzheimer’s disease by accelerating the clearance of amyloid-beta, ACS Nano. 8 (2014) 2345–2359. doi: 10.1021/nn4058215. [DOI] [PubMed] [Google Scholar]
- [86].Liu Y, Tseng Y, Huang L, Biodistribution Studies of Nanoparticles Using Fluorescence Imaging: A Qualitative or Quantitative Method?, Pharm. Res. 29 (2012) 3273–3277. doi: 10.1007/s11095-012-0818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Knudsen KB, Northeved H, Pramod Kumar EK, Permin A, Gjetting T, Andresen TL, Larsen S, Wegener KM, Lykkesfeldt J, Jantzen K, Loft S, Møller P, Roursgaard M, In vivo toxicity of cationic micelles and liposomes, Nanomedicine Nanotechnology, Biol. Med. 11 (2015)467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- [88].Gerets HHJ, Hanon E, Cornet M, Dhalluin S, Depelchin O, Canning M, Atienzar FA, Selection of cytotoxicity markers for the screening of new chemical entities in a pharmaceutical context: A preliminary study using a multiplexing approach, Toxicol. Vitr. 23 (2009) 319–332. doi: 10.1016/j.tiv.2008.11.012. [DOI] [PubMed] [Google Scholar]
- [89].Campion S, Aubrecht J, Boekelheide K, Brewster DW, Vaidya VS, Anderson L, Burt D, Dere E, Hwang K, Pacheco S, Saikumar J, Schomaker S, Sigman M, Goodsaid F, The current status of biomarkers for predicting toxicity., Expert Opin. Drug Metab. Toxicol. 9 (2013) 1391–408. doi: 10.1517/17425255.2013.827170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Siddiqi NJ, Abdelhalim MAK, El-Ansary AK, Alhomida AS, Ong WY, Identification of potential biomarkers of gold nanoparticle toxicity in rat brains, J. Neuroinflammation. 9 (2012) 656. doi: 10.1186/1742-2094-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kawata K, Liu CY, Merkel SF, Ramirez SH, Ryan T, Langford D, Angeles L, Neurosci Biobeha HHS Public Access Author manuscript Published in final edited form as: Blood biomarkers for brain injury: What are we measuring?, Neurosci. Biobehav. Rev 68 (2016) 460–473. doi: 10.1016/j.neubiorev.2016.05.009.Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Abu Lila AS, Kiwada H, Ishida T, The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage, J. Control. Release 172 (2013) 38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]


